Hydrogen Sensors Based on In2O3 Thin Films with Bimetallic Pt/Pd Catalysts on the Surface and Tin and Dysprosium Impurities in the Bulk
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
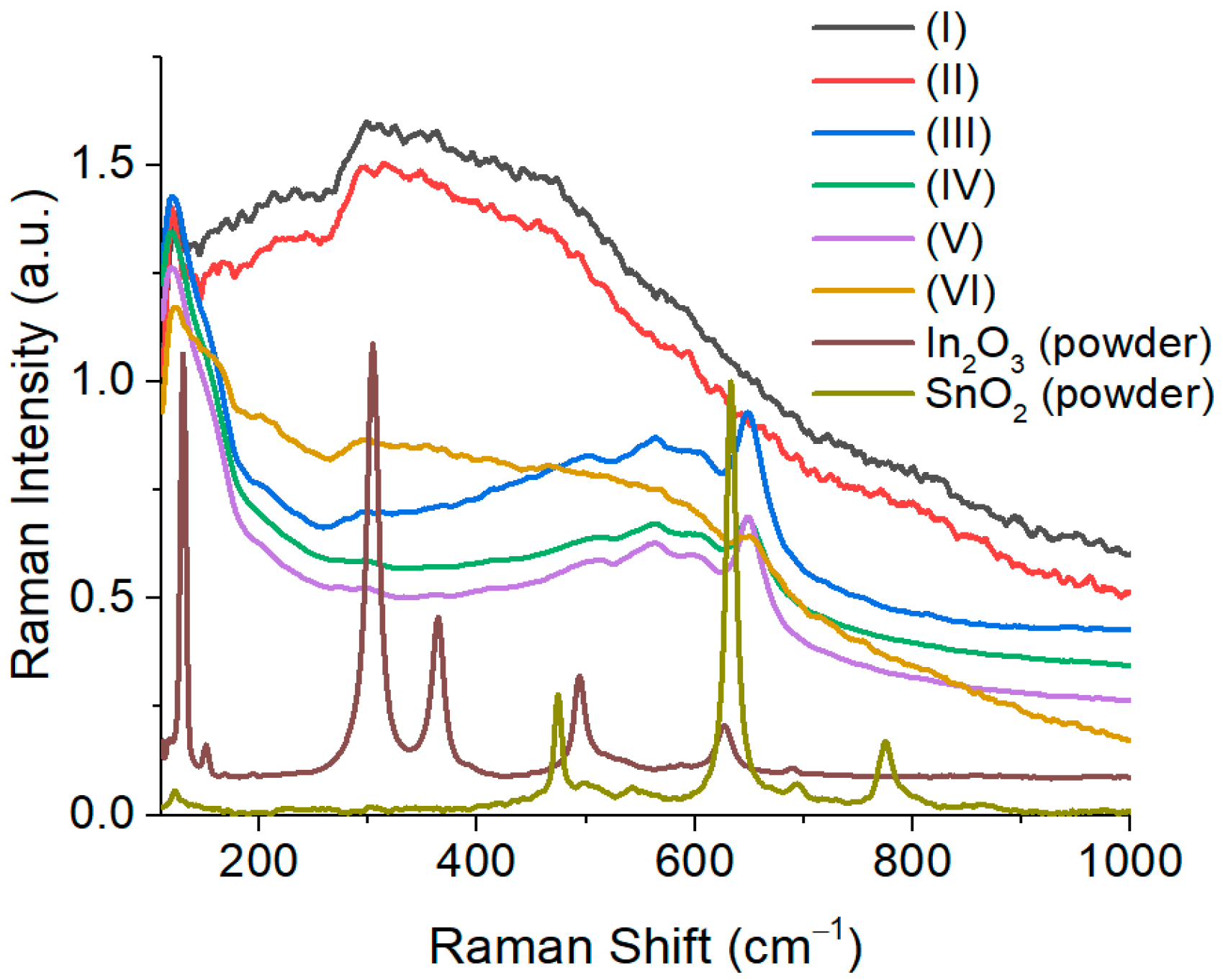
3.1. Raman Spectra of Pt/Pd/In2O3: Sn, Dy Films of Various Compositions
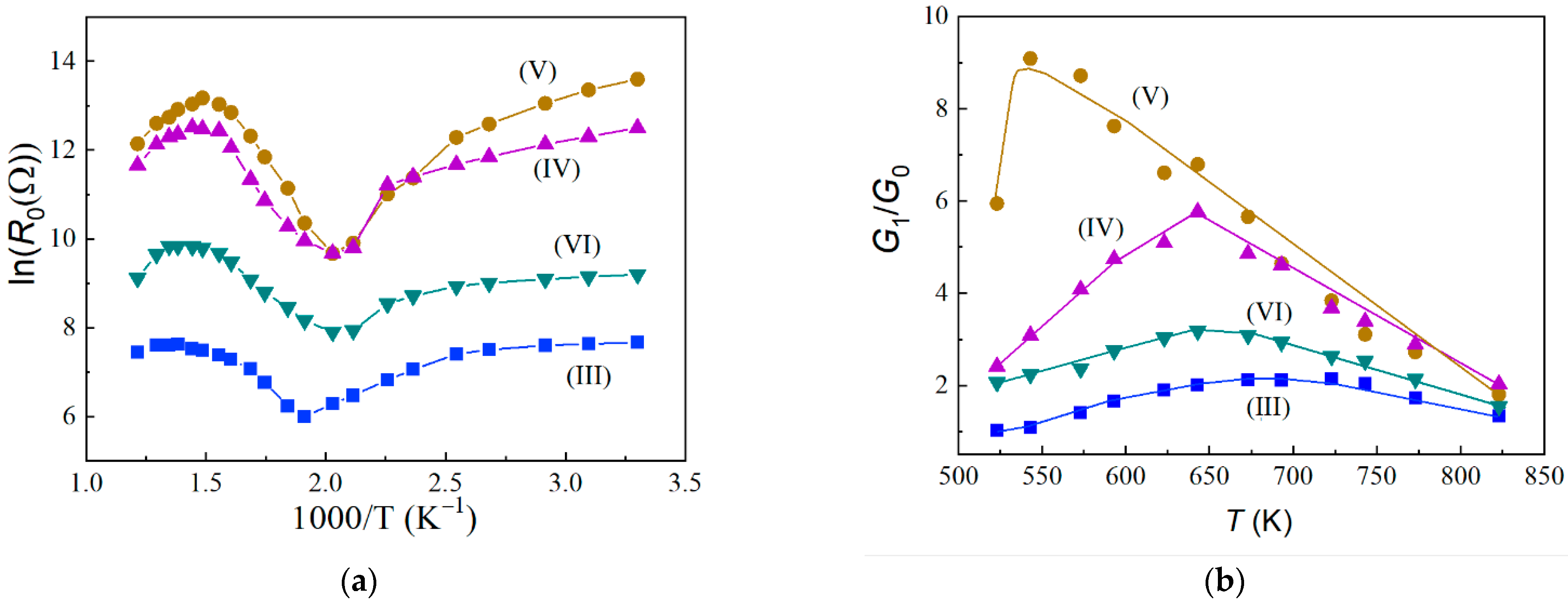
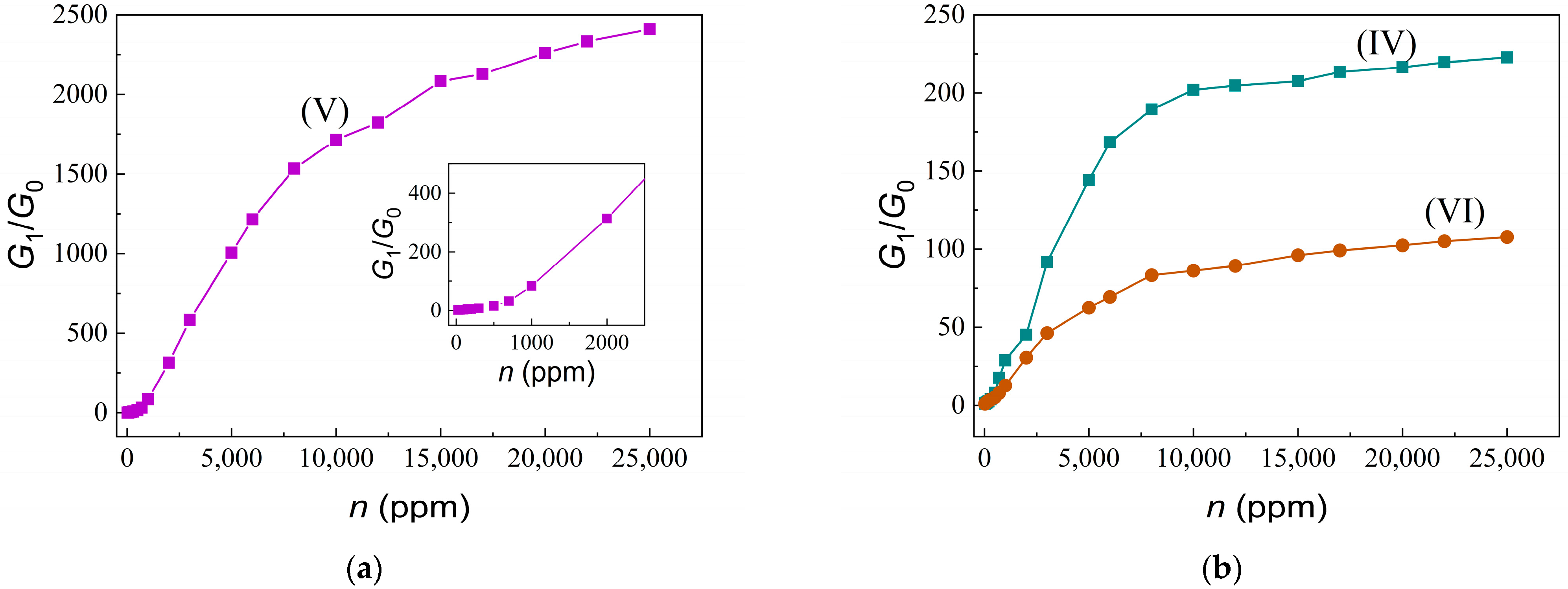
3.2. Electrical and Gas-Sensitive Properties of Sensors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sezemsky, P.; Burnat, D.; Kratochvil, J.; Wulff, H.; Kruth, A.; Lechowicz, K.; Janik, M.; Bogdanowicz, R.; Cada, M.; Hubicka, Z.; et al. Tailoring properties of indium tin oxide thin films for their work in both electrochemical and optical label-free sensing systems. Sens. Actuators B Chem. 2021, 343, 130173. [Google Scholar] [CrossRef]
- Frank, G.; Köstlin, H. Electrical properties and defect model of tin-doped indium oxide layer. Appl. Phys. A 1982, 27, 197–206. [Google Scholar] [CrossRef]
- Ray, S.; Banerjee, R.; Basu, N.; Batabyal, A.K.; Barua, A.K. Properties of tin doped indium oxide thin films prepared by magnetron sputtering. J. Appl. Phys. 1983, 54, 3497–3501. [Google Scholar] [CrossRef]
- Stadler, A. Transparent Conducting Oxides—An Up-To-Date Overview. Materials 2012, 5, 661–683. [Google Scholar] [CrossRef] [Green Version]
- Txintxurreta, J.; G-Berasategui, E.; Ortiz, R.; Hernández, O.; Mendizábal, L.; Barriga, J. Indium Tin Oxide Thin Film Deposi-tion by Magnetron Sputtering at Room Temperature for the Manufacturing of Efficient Transparent Heaters. Coatings 2021, 11, 92. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, W.; Shen, H.; Wang, J.; Han, J. New research progress of transparent conductive oxide films. Yadian Yu Shengguang Piezoelectrics Acoustooptics 2010, 32, 811–815. [Google Scholar]
- Hosono, H.; Ueda, K. Transparent Conductive Oxides. In Book Springer Handbook of Electronic and Photonic Materials, 2nd ed.; Kasap, S., Capper, P., Eds.; Springer Nature: Cham, Switzerland, 2017; pp. 1391–1404. [Google Scholar] [CrossRef]
- Mokrushin, A.S.; Fisenko, N.A.; Gorobtsov, P.Y.; Simonenko, T.L.; Glumov, O.V. Pen plotter printing of ITO thin film as a highly CO sensitive component of a resistive gas sensor. Talanta 2021, 221, 121455. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. The role of grain size on the thermal instability of nanostructured metal oxides used in gas sensor applications and approaches for grain-size stabilization. Prog. Cryst. Growth 2012, 58, 167–208. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, S.; Fan, S.; Hua, Z.; Wu, Y.; Tian, X.; Yuan, W. Selective detection of methane by Pd-In2O3 sensors with a catalyst filter film. Sens. Actuators B Chem. 2021, 328, 129030. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Boris, I.; Brinzari, V.; Han, S.H.; Cho, B.K.; Lychkovsky, Y.N. In2O3: Ga and In2O3: P-based one-electrode gas sensors: Comparative study. Ceram. Int. 2015, 41, 7478–7488. [Google Scholar] [CrossRef]
- Ullah, H.; Yamani, Z.H.; Qurashi, A.; Iqbal, J.; Safeen, K. Study of the optical and gas sensing properties of In2O3 nanoparticles synthesized by rapid sonochemical method. J. Mater. Sci. Mater. Electron. 2020, 31, 17474–17481. [Google Scholar] [CrossRef]
- Rahamim, G.; Mirilashvili, M.; Nanikashvili, P.; Greenberg, E.; Shpaisman, H.; Grinstein, D.; Welner, S.; Zitoun, D. Hydrogen sensors with high humidity tolerance based on indium-tin oxide colloids. Sens. Actuators B Chem. 2020, 310, 127845. [Google Scholar] [CrossRef]
- Anand, K.; Thangaraj, R.; Kohli, N.; Singh, R.C. Structural, Optical and Ethanol Gas Sensing Properties of In2O3 and Dy3+: In2O3 Nanoparticles. AIP Conf. Proc. 2014, 1591, 477–480. [Google Scholar] [CrossRef]
- Carreno, N.L.V.; Maciel, A.P.; Leite, E.R.; Lisboa-Filho, P.N.; Longo, E.; Valentino, A.; Probst, L.E.D.; Paiva-Santos, C.O.; Schreiner, W.H. The influence of cation segregation on the methanol decomposition on nanostructured SnO2. Sens. Actuators B Chem. 2002, 86, 185–192. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Metal oxide composites in conductometric gas sensors: Achievements and challenges. Sens. Actuators B Chem. 2017, 244, 182–210. [Google Scholar] [CrossRef]
- Leite, R.; Maciel, A.P.; Weber, I.T.; Lisboa-Filho, P.N.; Longo, E.; Paiva-Santos, C.O.; Andrade, A.V.C.; Pakoscimas, C.A.; Maniette, Y.; Schreiner, W.H. Development of metal oxide nanoparticles with high stability against particle growth using a metas solid solution. Adv. Mater. 2002, 14, 905–908. [Google Scholar] [CrossRef]
- Cheng, L.; Ma, S.Y.; Li, X.B.; Luo, J.; Li, W.Q.; Li, F.M.; Mao, Y.Z.; Wang, T.T.; Li, Y.F. Highly sensitive acetone sensors based on Y-doped SnO2 prismatic hollow nanofibers synthesized by electrospinning. Sens. Actuators B Chem. 2014, 200, 181–190. [Google Scholar] [CrossRef]
- Cheng, L.; Ma, S.Y.; Wang, T.T.; Luo, J.; Li, X.B.; Li, W.Q.; Mao, Y.Z.; Gz, D.J. Highly sensitive acetic acid gas sensor based on coral-like and Y-doped SnO2 nanoparticles prepared by electrospinning. Mater. Lett. 2014, 137, 265–268. [Google Scholar] [CrossRef]
- Kazuyoshi, I.; Koki, Y.; Masashi, K. Sputtering Target, Semiconductor Film and Semiconductor. Device. Patent US8333913B2, 18 December 2012. [Google Scholar]
- Maksimova, N.K.; Almaev, A.V.; Sevastyanov, E.Y.; Potekaev, A.I.; Chernikov, E.V.; Sergeychenko, N.V.; Korusenko, P.M.; Nesov, S.N. Effect of Additives Ag and Rare-Earth Elements Y and Sc on the Properties of Hydrogen Sensors Based on Thin SnO2 Films during Long-Term Testing. Coatings 2019, 9, 423. [Google Scholar] [CrossRef] [Green Version]
- Sevastyanov, E.Y.; Maksimova, N.K.; Novikov, V.A.; Rudov, F.V.; Sergeychenko, N.V.; Chernikov, E.V. Effect of Pt, Pd, Au additives on the surface and in the bulk of tin dioxide thin films on the electrical and gas-sensitive properties. Semiconductors 2012, 46, 801–809. [Google Scholar] [CrossRef]
- Maksimova, N.K.; Sevastyanov, E.Y.; Chernikov, E.V.; Korusenko, P.M.; Nesov, S.N.; Kim, S.V.; Biryukov, A.A.; Sergeychenko, N.V.; Davletkildeev, N.A.; Sokolov, D.V. Sensors based on tin dioxide thin films for the detection of pre-explosive hydrogen concentrations. Sens. Actuators B Chem. 2021, 341, 130020. [Google Scholar] [CrossRef]
- Maksimova, N.K.; Khludkova, L.S.; Biryukov, A.A.; Sevast’yanov, E.Y.; Chernikov, E.V.; Kushnarev, B.O. The Stability of Liquid Hydrocarbon Vapor Sensors Based on SnO2 Thin Films Modified with Various Catalysts. Tech. Phys. 2021, 66, 999–1008. [Google Scholar] [CrossRef]
- Gaman, V.I.; Sevast’yanov, E.Y.; Maksimova, N.K.; Almaev, A.V.; Sergeichenko, N.V. Characteristics of the semiconductor resistive hydrogen sensors in the thermo-cyclic operation mode. Russ. Phys. J. 2014, 56, 1427–1434. [Google Scholar] [CrossRef]
- Malinovskaya, T.; Ghyngazov, S.; Zhek, V. Thermal destruction of coprecipitated hydroxides of indium and dysprosium. J. Therm. Anal. Calorim. 2019, 138, 1871–1877. [Google Scholar] [CrossRef]
- Wang, C.Y.; Dai, Y.; Pezoldt, J.; Lu, B.; Kups, T.; Cimalla, V.; Ambacher, O. Phase stabilization and phonon properties of single crystalline rhombohedral indium oxide. Cryst. Growth Des. 2008, 8, 1257–1260. [Google Scholar] [CrossRef]
- Kranert, C.; Schmidt-Grund, R.; Grundmann, M. Raman active phonon modes of cubic In2O3. Phys. Status Solidi RRL 2014, 8, 554–559. [Google Scholar] [CrossRef]
- Gan, J.; Lu, X.; Wu, J.; Xie, S.; Zhai, T.; Yu, M.; Zhang, Z.; Mao, Y.; Wang, S.C.I.; Shen, Y.; et al. Oxygen vacancies promoting photochemical performance of In2O3 nanocubes. Sci. Rep. 2013, 3, 1021. [Google Scholar] [CrossRef] [Green Version]
- Berengue, O.M.; Rodrigues, A.D.; Dalmaschio, C.J.; Lanfredi, A.J.C.; Leite, E.R.; Chiquito, A.J. Structural characterization of indium oxide nanostructures: A Raman analysis. J. Phys. D 2010, 43, 045401. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Kim, B.; Gao, C.Y.; Choi, H.J.; Ko, J.H.; Seo, C.H.; Park, J. Raman spectroscopy study of solution processed In2O3 thin films: Effect of annealing temperature on the characteristics of In2O3 semiconductors and thin-film transistors. Mol. Cryst. Liq. Cryst. 2019, 679, 38–47. [Google Scholar] [CrossRef]
- Yadav, K.; Mehta, B.R.; Singh, J.P. Synthesis and Raman spectrum of crystalline indium oxide micro-rods with rectangular cross-section. Solid State Phys. AIP Conf. Proc. 2014, 1591, 409–410. [Google Scholar] [CrossRef]
- Rumyantseva, M.N.; Gaskov, A.M.; Rosman, N.; Pagnier, T.; Morante, J.R. Raman Surface Vibration Modes in Nanocrystalline SnO2: Correlation with Gas Sensor Performances Interfaces. Chem. Mater. 2005, 17, 893–901. [Google Scholar] [CrossRef]
- Luo, Y.R. Comprehensive Handbook of Chemical Bond Energies, 1st ed.; CRC Press: Boca Raton, FL, USA, 2007; Volume 1655. [Google Scholar] [CrossRef]
- FIGARO USA, Inc. Gas Sensors & Modules. Available online: https://www.figarosensor.com/product/sensor/ (accessed on 10 November 2022).
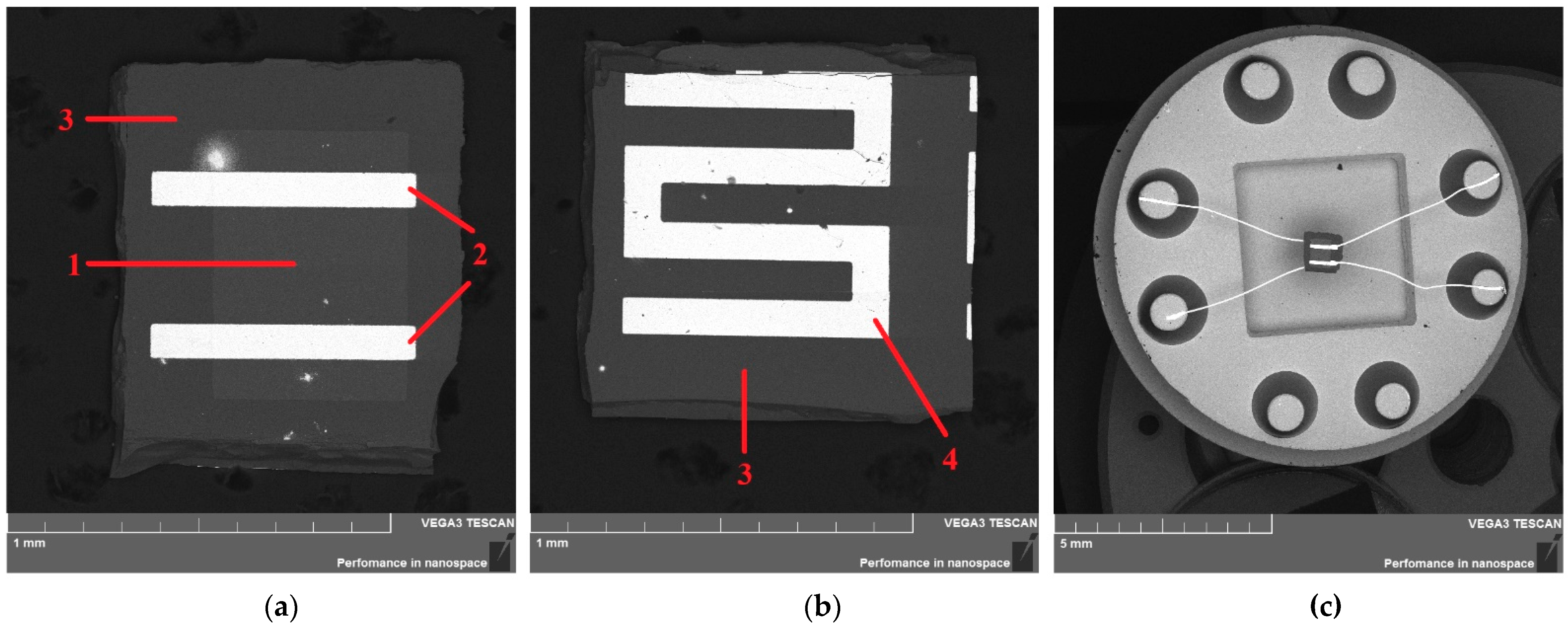

| Sample | R0 | ∆E1, eV | ∆E2, eV | Tmax, K | tr, s |
|---|---|---|---|---|---|
| (I) | 0.11 | 0.007–0.009 | 0.030–0.048 | 820 | 16 |
| (II) | 0.46 | 0.013–0.014 | 0.071–0.075 | 740 | 18 |
| (III) | 2.13 | 0.019–0.022 | 0.18–0.19 | 720 | 15 |
| (IV) | 694 | 0.09–0.18 | 0.38–0.46 | 640 | 24 |
| (V) | 803 | 0.044–0.15 | 0.58–0.61 | 540 | 30 |
| (VI) | 9.9 | 0.02–0.03 | 0.35–0.42 | 640 | 24 |
| Sample | eφS, eV | G1/G0 (1000 ppm) | G1/G0 (25,000 ppm) | G1/G0(T2) (1000 ppm) | G1/G0(T2) (25,000 ppm) |
|---|---|---|---|---|---|
| (I) 10 Sn | 0.04 | 1.7 | - | - | - |
| (II) 10 Sn | 0.04 | 3.0 | - | - | - |
| (III) 10 Sn + 4 Dy | 0.06 | 3.7 | - | - | - |
| (IV) 1 Sn + 4.95 Dy | 0.32 | 20.7 | 223 | 216 | 28,640 |
| (V) 0.5 Sn + 4.95 Dy | 0.37 | 31.2 | 2409 | 785 | 142,270 |
| (VI) 1 Sn + 2 Dy | 0.16 | 6.9 | 108 | 23 | 240 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maksimova, N.; Malinovskaya, T.; Zhek, V.; Sergeychenko, N.; Chernikov, E.; Lapin, I.; Svetlichnyi, V. Hydrogen Sensors Based on In2O3 Thin Films with Bimetallic Pt/Pd Catalysts on the Surface and Tin and Dysprosium Impurities in the Bulk. Chemosensors 2023, 11, 23. https://doi.org/10.3390/chemosensors11010023
Maksimova N, Malinovskaya T, Zhek V, Sergeychenko N, Chernikov E, Lapin I, Svetlichnyi V. Hydrogen Sensors Based on In2O3 Thin Films with Bimetallic Pt/Pd Catalysts on the Surface and Tin and Dysprosium Impurities in the Bulk. Chemosensors. 2023; 11(1):23. https://doi.org/10.3390/chemosensors11010023
Chicago/Turabian StyleMaksimova, Nadezhda, Tatyana Malinovskaya, Valentina Zhek, Nadezhda Sergeychenko, Evgenii Chernikov, Ivan Lapin, and Valery Svetlichnyi. 2023. "Hydrogen Sensors Based on In2O3 Thin Films with Bimetallic Pt/Pd Catalysts on the Surface and Tin and Dysprosium Impurities in the Bulk" Chemosensors 11, no. 1: 23. https://doi.org/10.3390/chemosensors11010023
APA StyleMaksimova, N., Malinovskaya, T., Zhek, V., Sergeychenko, N., Chernikov, E., Lapin, I., & Svetlichnyi, V. (2023). Hydrogen Sensors Based on In2O3 Thin Films with Bimetallic Pt/Pd Catalysts on the Surface and Tin and Dysprosium Impurities in the Bulk. Chemosensors, 11(1), 23. https://doi.org/10.3390/chemosensors11010023








