Scanning Electron Microscopy and Raman Spectroscopy Characterization of Structural Changes Induced by Thermal Treatment in Innovative Bio-Based Polyamide Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
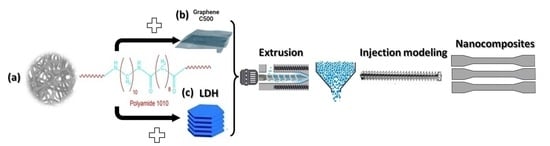
2.2. Preparation of Nanocomposites and Samples
2.3. Thermal Treatment
2.4. Scanning Electron Microscopy
2.5. Raman Spectroscopy
3. Results and Discussion
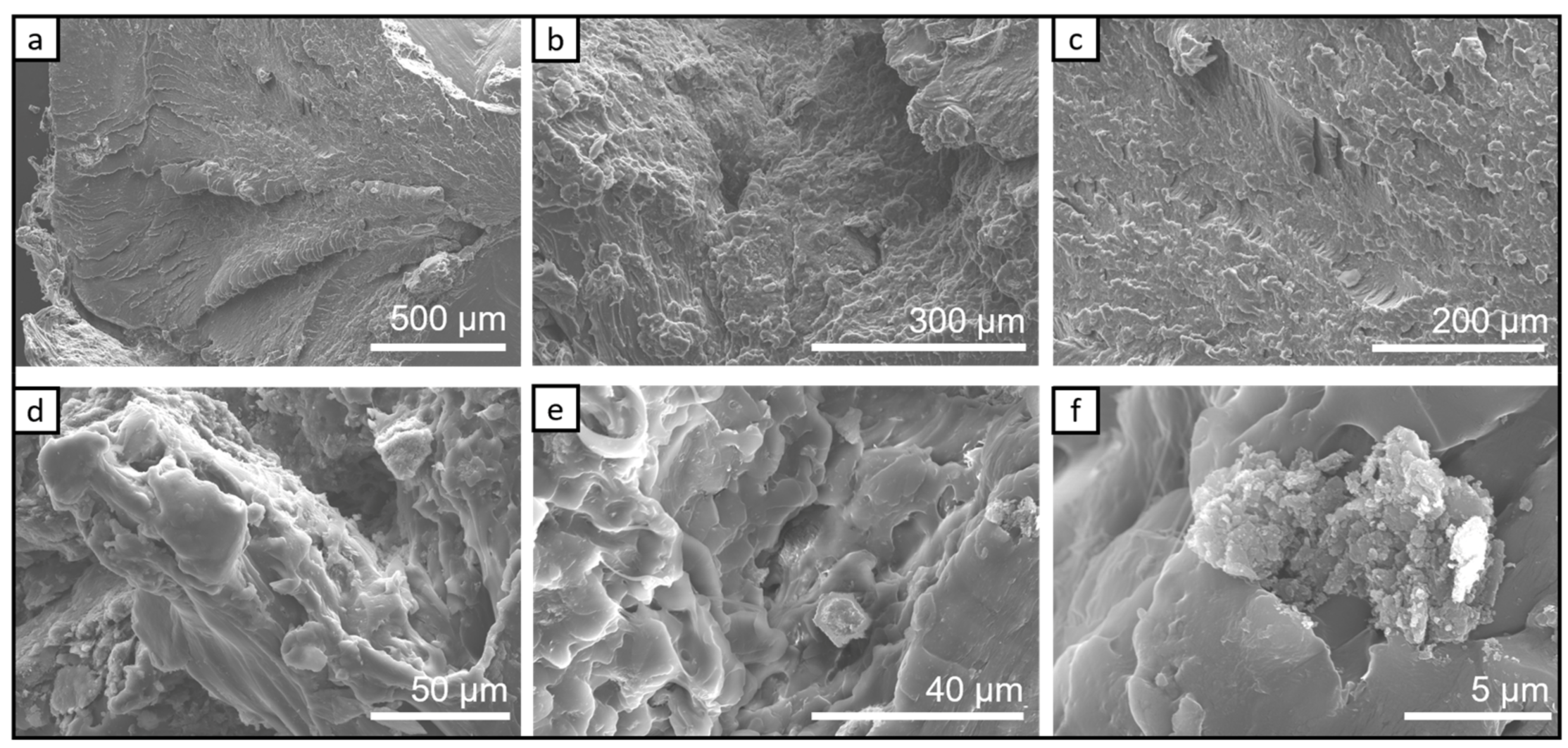
3.1. SEM Characterization
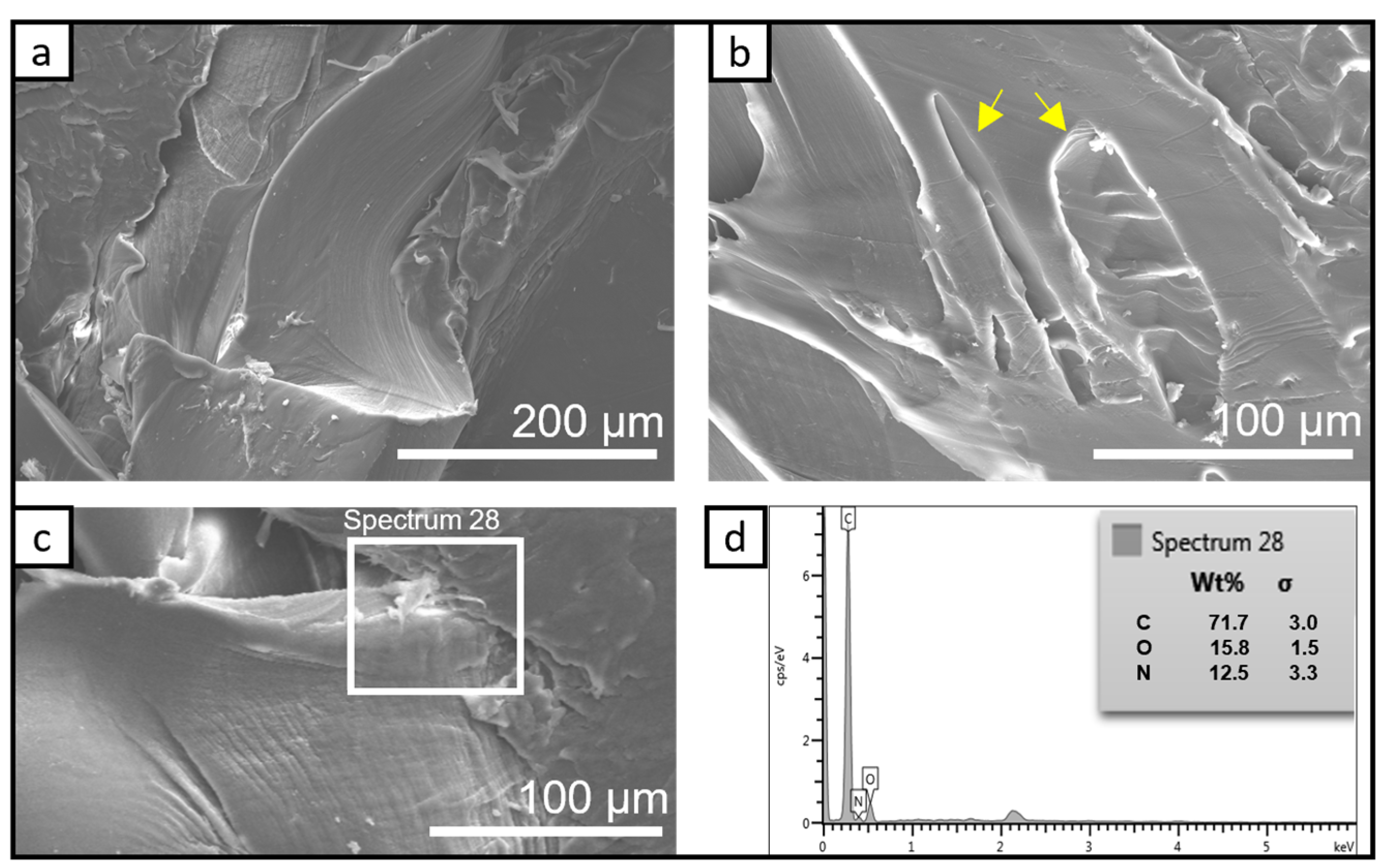
3.1.1. SEM Investigation of the Bio-PA1010 and Nanocomposites
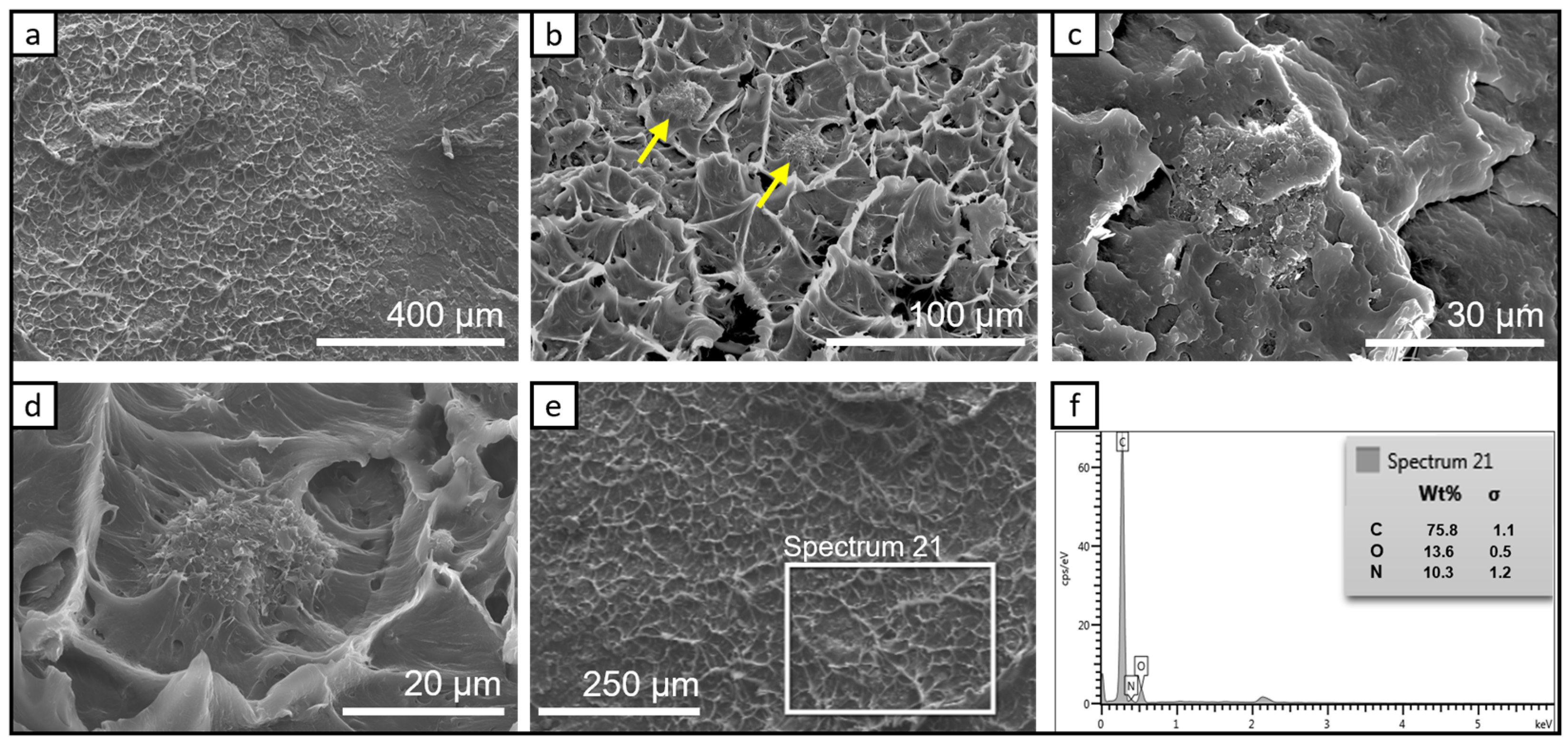
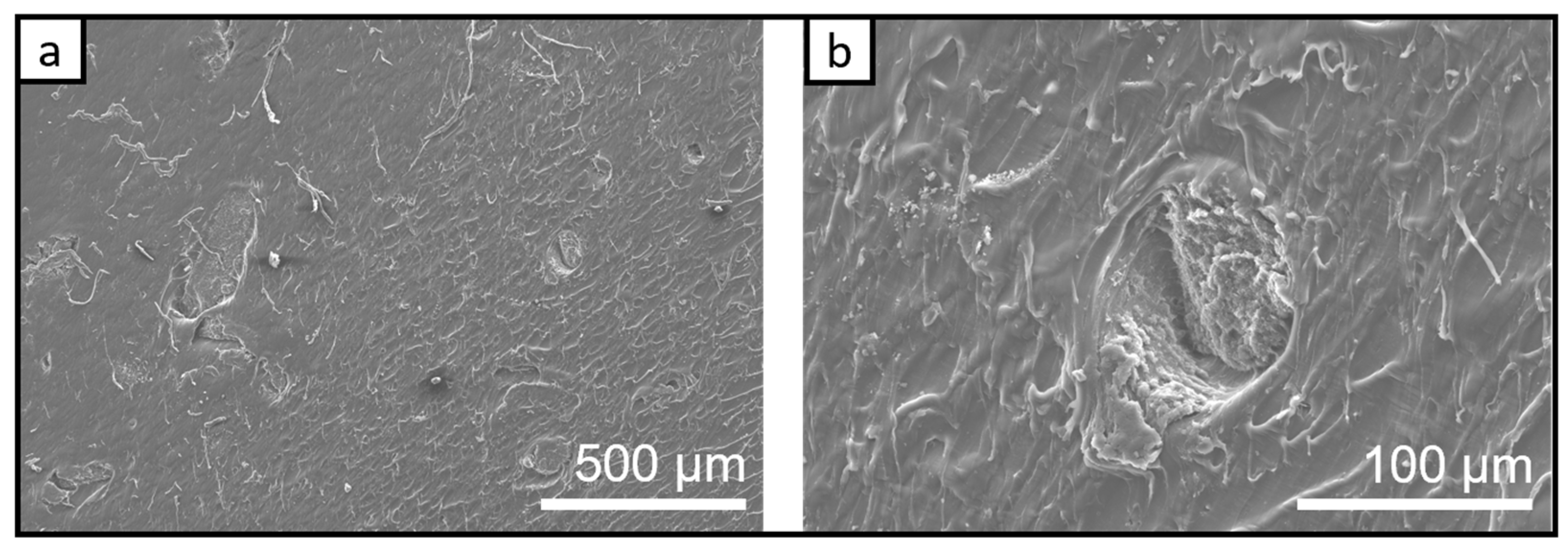
3.1.2. SEM Investigation of the Bio-PA1010 Nanocomposites Filled with Graphene Nanoplatelets C500-4% and LDH-5% after Thermal Treatment at 80 °C
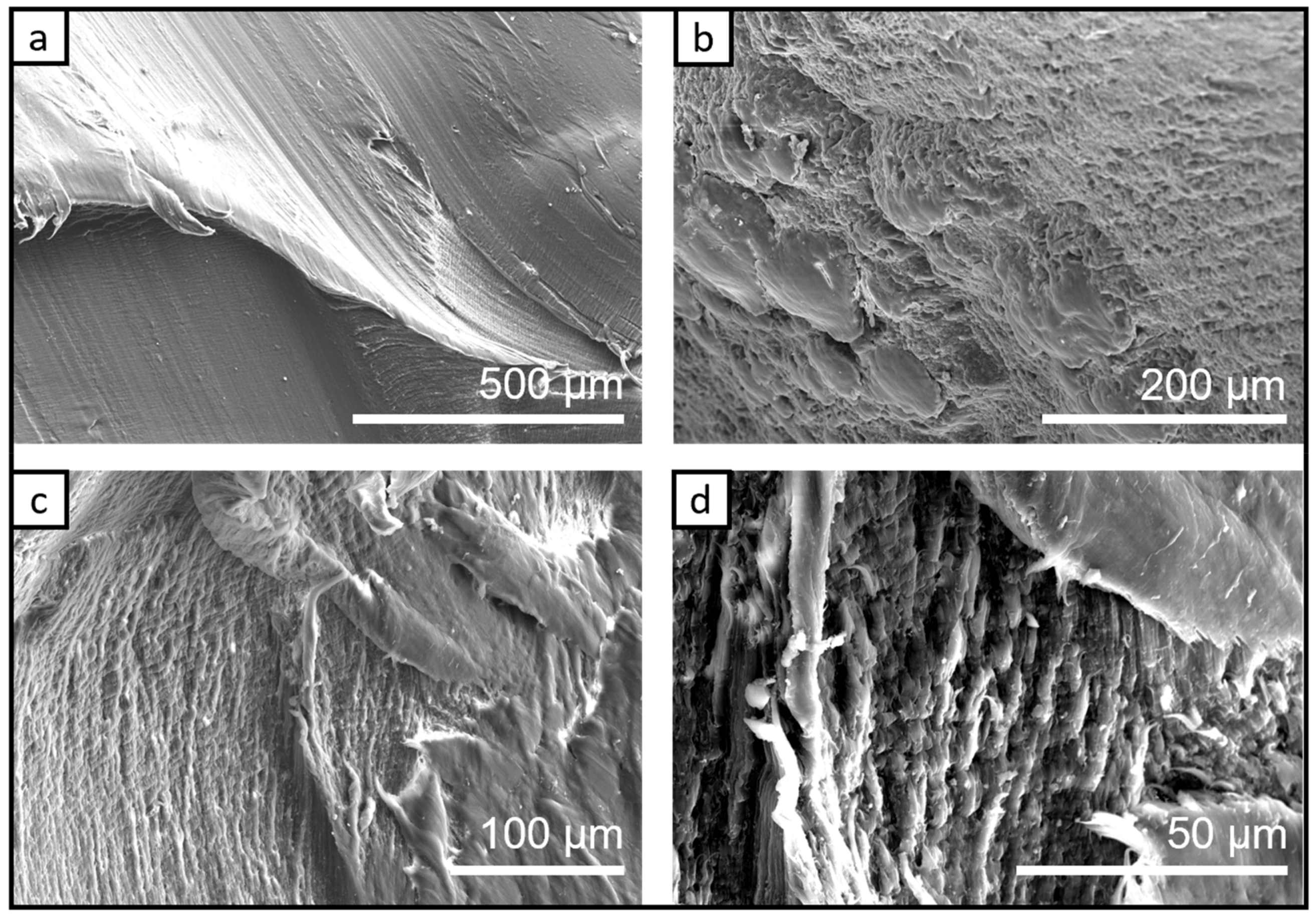
3.1.3. The SEM Analysis/Investigation of the Bio-PA1010 Nanocomposites filled with Graphene Nanoplatelets C500-4% and LDH-5% Thermal Treated at 145 °C
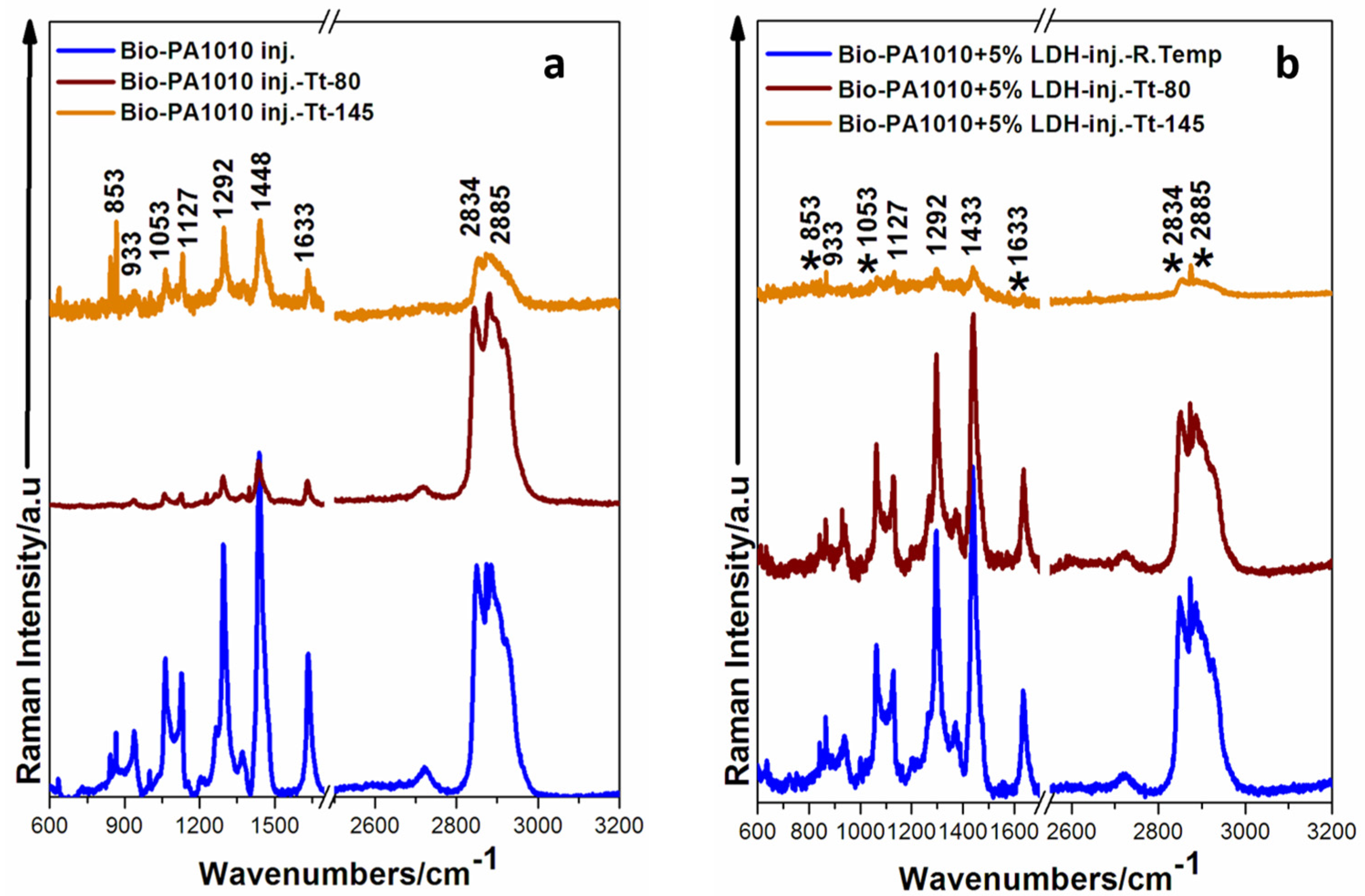
3.2. Raman Analysis
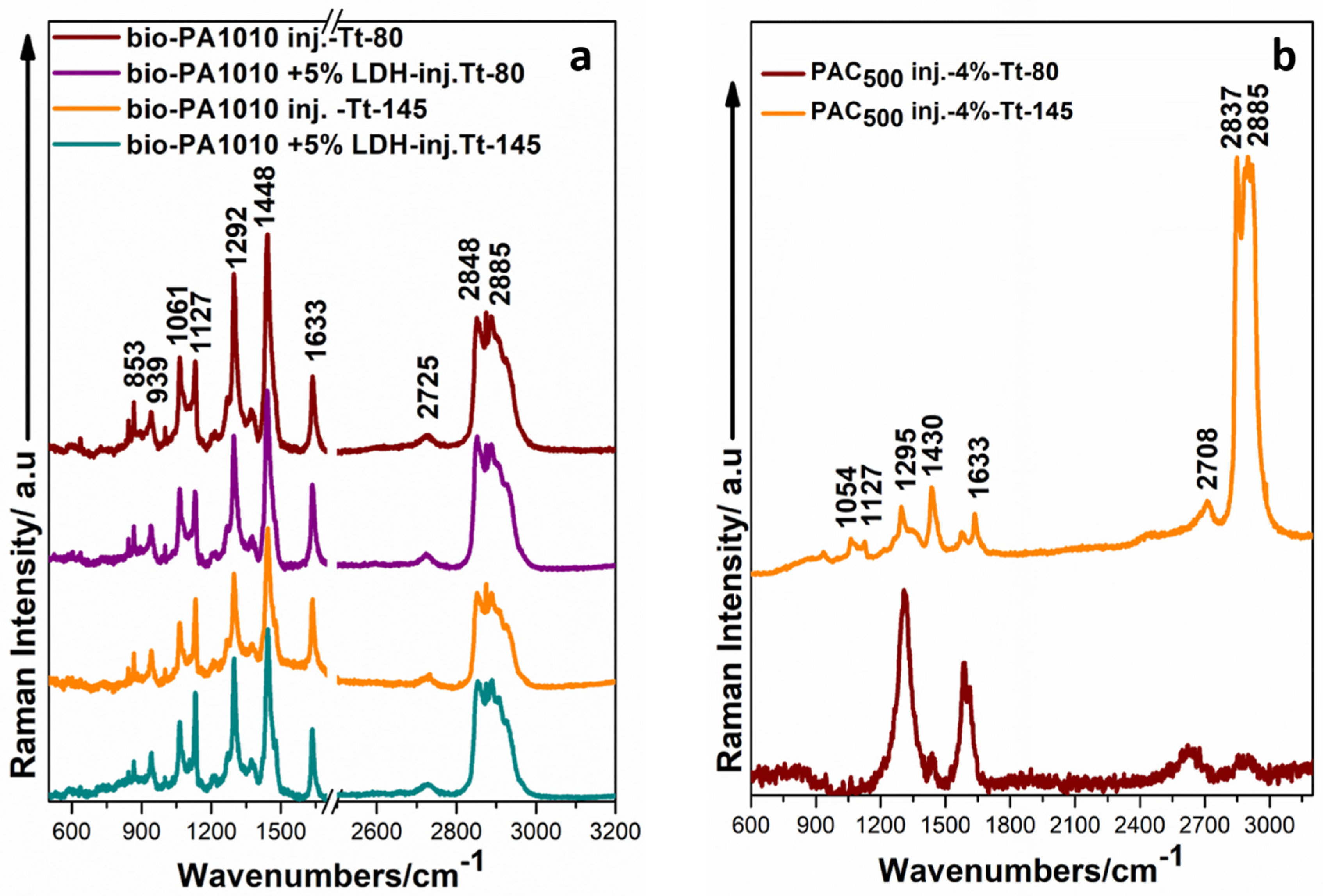
3.2.1. Raman Spectra of Bio-PA1010 Sample and Bio-PA1010 Filled with LDH before and after the Thermal Treatment
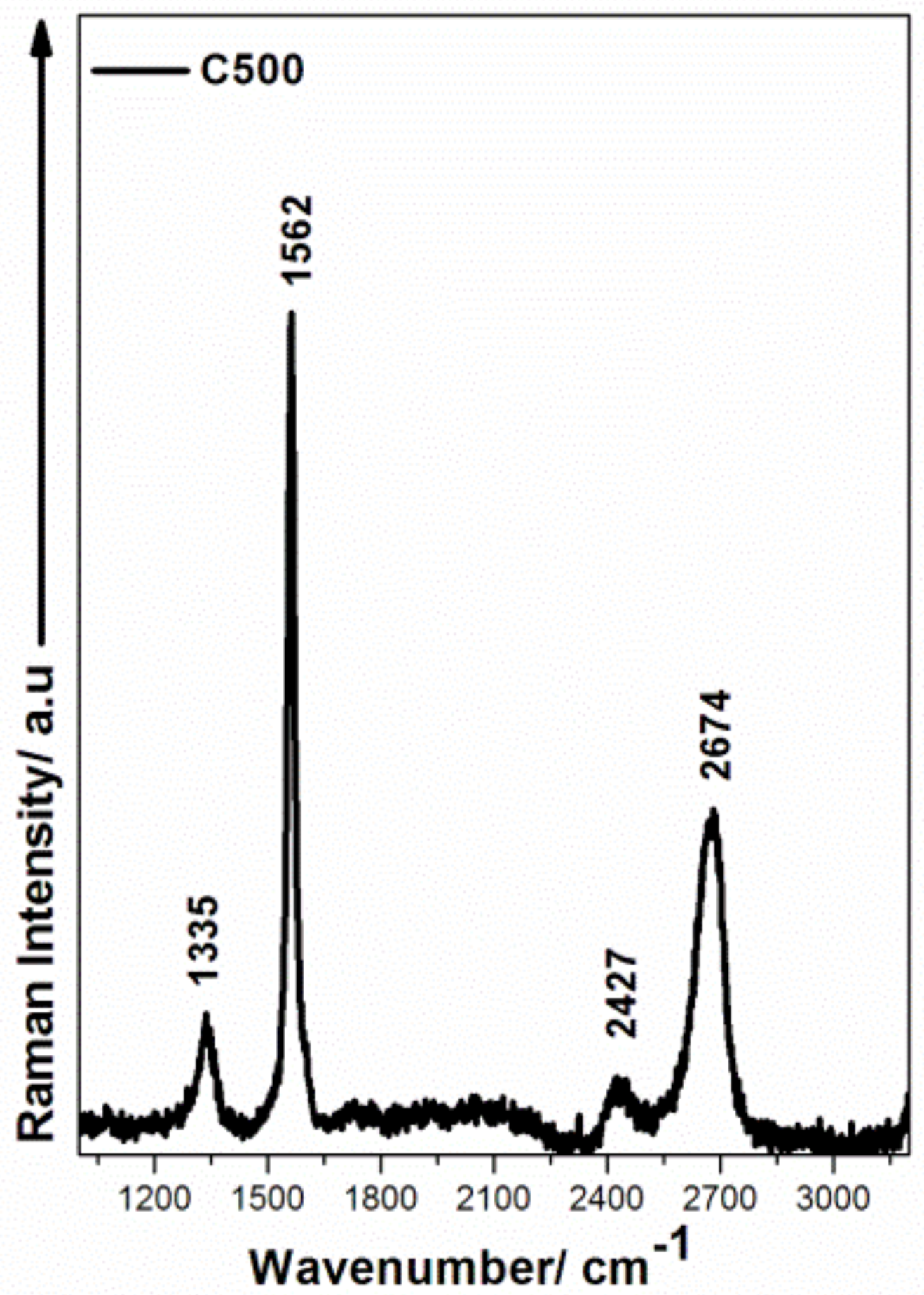
3.2.2. Raman Analysis of Bio-PA1010 Filled with C500 Graphene before and after the Thermal Treatment
3.2.3. Raman Spectra of Bio-PA1010 Polyamide Filled with LDH and C500-4% Measured 1 Month after the Thermal Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jia, Y.; He, H.; Peng, X.; Meng, S.; Chen, J.; Geng, Y. Preparation of a new filament based on polyamide-6 for three-dimensional printing. Polym. Eng. Sci. 2017, 57, 1322–1328. [Google Scholar] [CrossRef]
- Marchildon, K. Polyamides—Still strong after seventy years. Macromol. React. Eng. 2011, 5, 22–54. [Google Scholar] [CrossRef]
- Russo, S.; Casazza, E. 4.14—Ring-opening polymerization of cyclic amides (lactams). In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 331–396. [Google Scholar]
- Schmitz, K.; Schepers, U. Polyamides as artificial transcription factors: Novel tools for molecular medicine? Angew. Chem. Int. Ed. 2004, 43, 2472–2475. [Google Scholar] [CrossRef] [PubMed]
- Rulkens, R.; Koning, C. 5.18—Chemistry and technology of polyamides. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 431–467. [Google Scholar]
- Nguyen, X.H.; Honda, T.; Wang, Y.; Yamamoto, R. Eco-Materials; University of Tokyo: Tokyo, Japan, 2010. [Google Scholar]
- Winnacker, M.; Rieger, B. Biobased polyamides: Recent advances in basic and applied research. Macromol. Rapid Commun. 2016, 37, 1391–1413. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [Green Version]
- Taylor, K. EU Set to Adopt Mandatory Recycled Content Targets in New Packaging Law. Available online: https://www.euractiv.com/section/energy-environment/news/eu-set-to-adopt-mandatory-recycled-content-targets-in-new-packaging-law/ (accessed on 15 November 2022).
- Rydz, J.; Sikorska, W.; Kyulavska, M.; Christova, D. Polyester-based (bio)degradable polymers as environmentally friendly materials for sustainable development. Int. J. Mol. Sci. 2015, 16, 564–596. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Zhou, P.; Yan, D. Preparation and properties of nylon-1010/montmorillonite nanocomposites by melt intercalation. J. Appl. Polym. Sci. 2004, 91, 1834–1841. [Google Scholar] [CrossRef]
- Zeng, H.; Gao, C.; Wang, Y.; Watts, P.C.; Kong, H.; Cui, X.; Yan, D. In situ polymerization approach to multiwalled carbon nanotubes-reinforced nylon 1010 composites: Mechanical properties and crystallization behavior. Polymer 2006, 47, 113–122. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb carbon: A review of graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Loh, K.P.; Bao, Q.; Ang, P.K.; Yang, J. The chemistry of graphene. J. Mater. Chem. 2010, 20, 2277–2289. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.-B.; Yan, Q.; Zheng, W.-G.; He, Z.; Yu, Z.-Z. Tough graphene−polymer microcellular foams for electromagnetic interference shielding. ACS Appl. Mater. Interfaces 2011, 3, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Barjasteh, E.; Sutanto, C.; Nepal, D. Conductive polyamide–graphene composite fabric via interface engineering. Langmuir 2019, 35, 2261–2269. [Google Scholar] [CrossRef] [PubMed]
- Kausar, A. Trends in graphene reinforced polyamide nanocomposite for functional application: A review. Polym. Plast. Technol. Mater. 2019, 58, 917–933. [Google Scholar] [CrossRef]
- Francisco, D.L.; Paiva, L.B.; Aldeia, W. Advances in polyamide nanocomposites: A review. Polym. Compos. 2019, 40, 851–870. [Google Scholar] [CrossRef]
- Wang, R.; Wu, L.; Zhuo, D.; Zhang, J.; Zheng, Y. Fabrication of polyamide 6 nanocomposite with improved thermal conductivity and mechanical properties via incorporation of low graphene content. Ind. Eng. Chem. Res. 2018, 57, 10967–10976. [Google Scholar] [CrossRef]
- Sisti, L.; Totaro, G.; Vannini, M.; Giorgini, L.; Ligi, S.; Celli, A. Bio-based PA11/graphene nanocomposites prepared by in situ polymerization. J. Nanosci. Nanotechnol. 2018, 18, 1169–1175. [Google Scholar] [CrossRef]
- Zheng, D.; Tang, G.; Zhang, H.-B.; Yu, Z.-Z.; Yavari, F.; Koratkar, N.; Lim, S.-H.; Lee, M.-W. In situ thermal reduction of graphene oxide for high electrical conductivity and low percolation threshold in polyamide 6 nanocomposites. Compos. Sci. Technol. 2012, 72, 284–289. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Q.; O’Hare, D.; Sun, L. Preparation of two dimensional layered double hydroxide nanosheets and their applications. Chem. Soc. Rev. 2017, 46, 5950–5974. [Google Scholar] [CrossRef]
- Basu, D.; Das, A.; Heinrich, G. Layered double hydroxide (LDH)-based rubber nanocomposites. In Encyclopedia of Polymeric Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–7. [Google Scholar]
- Sinha Ray, S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar] [CrossRef]
- Paul, D.R.; Robeson, L.M. Polymer nanotechnology: Nanocomposites. Polymer 2008, 49, 3187–3204. [Google Scholar] [CrossRef] [Green Version]
- Mayoral, B.; Harkin-Jones, E.; Khanam, P.N.; AlMaadeed, M.A.; Ouederni, M.; Hamilton, A.R.; Sun, D. Melt processing and characterisation of polyamide 6/graphene nanoplatelet composites. RSC Adv. 2015, 5, 52395–52409. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dresselhaus, M.S.; Jorio, A.; Hofmann, M.; Dresselhaus, G.; Saito, R. Perspectives on carbon nanotubes and graphene Raman spectroscopy. Nano Lett. 2010, 10, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Bokobza, L. Spectroscopic techniques for the characterization of polymer nanocomposites: A review. Polymers 2018, 10, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bokobza, L.; Couzi, M.; Bruneel, J.-L. Raman spectroscopy of polymer–carbon nanomaterial composites. Rubber Chem. Technol. 2017, 90, 37–59. [Google Scholar] [CrossRef]
- Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Graphene-based polymer nanocomposites. Polymer 2011, 52, 5–25. [Google Scholar] [CrossRef] [Green Version]
- Ye, K.; Li, K.; Lu, Y.; Guo, Z.; Ni, N.; Liu, H.; Huang, Y.; Ji, H.; Wang, P. An overview of advanced methods for the characterization of oxygen vacancies in materials. TrAC Trends Anal. Chem. 2019, 116, 102–108. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, D.; Zhang, J.; Balogun, M.T.; Wang, P.; Tong, Y.; Huang, Y. Charge relays via dual carbon-actions on nanostructured BiVO4 for high performance photoelectrochemical water splitting. Adv. Funct. Mater. 2022, 32, 2112738. [Google Scholar] [CrossRef]
- Stamate, A.-E.; Zăvoianu, R.; Pavel, O.D.; Birjega, R.; Matei, A.; Dumitru, M.; Brezeștean, I.; Osiac, M.; Marcu, I.-C. The influence of the preparation method on the physico-chemical properties and catalytic activities of Ce-modified LDH structures used as catalysts in condensation reactions. Molecules 2021, 26, 6191. [Google Scholar] [CrossRef] [PubMed]
- Stamate, A.-E.; Pavel, O.D.; Zăvoianu, R.; Brezeştean, I.; Ciorȋță, A.; Bȋrjega, R.; Neubauer, K.; Koeckritz, A.; Marcu, I.-C. Ce-containing MgAl-layered double hydroxide-graphene oxide hybrid materials as multifunctional catalysts for organic transformations. Materials 2021, 14, 7457. [Google Scholar] [CrossRef]
- Levinta, N.; Corobea, M.C.; Vuluga, Z.; Nicolae, C.-A.; Gabor, A.R.; Raditoiu, V.; Osiac, M.; Teodorescu, G.-M.; Teodorescu, M. Bio-based polyamide 1010 with a halogen-free flame retardant based on melamine–gallic acid complex. Polymers 2020, 12, 1482. [Google Scholar] [CrossRef] [PubMed]
- Cho, L.-L. Identification of textile fiber by Raman microspectroscopy. Forensic Sci. J. 2007, 6, 55–62. [Google Scholar]
- Birjega, R.; Matei, A.; Mitu, B.; Ionita, M.; Filipescu, M.; Stokker-Cheregi, F.; Luculescu, C.; Dinescu, M.; Zăvoianu, R.; Pavel, O.; et al. Layered double hydroxides/polymer thin films grown by matrix assisted pulsed laser evaporation. Thin Solid Film. 2013, 543, 63–68. [Google Scholar] [CrossRef]
- Birjega, R.; Matei, A.; Marascu, V.; Vlad, A.; Ionita, M.; Dinescu, M.; Zăvoianu, R.; Corobea, M. Stearic acid/layered double hydroxides composite thin films deposited by combined laser techniques. Molecules 2020, 25, 4097. [Google Scholar] [CrossRef]
- Kim, H.S.; Bae, H.S.; Yu, J.; Kim, S.Y. Thermal conductivity of polymer composites with the geometrical characteristics of graphene nanoplatelets. Sci. Rep. 2016, 6, 26825. [Google Scholar] [CrossRef] [Green Version]
- Schuepfer, D.B.; Badaczewski, F.; Guerra-Castro, J.M.; Hofmann, D.M.; Heiliger, C.; Smarsly, B.; Klar, P.J. Assessing the structural properties of graphitic and non-graphitic carbons by Raman spectroscopy. Carbon 2020, 161, 359–372. [Google Scholar] [CrossRef]
- Hendra, P.; Maddams, W.; Royaud, I.; Willis, H.; Zichy, V. The application of Fourier transform Raman spectroscopy to the identification and characterization of polyamides—I. Single number nylons. Spectrochim. Acta Part A 1990, 46, 747–756. [Google Scholar] [CrossRef]



| BIO-PA1010 (cm−1) | Nanocomposite (cm−1) | Intensity | Vibration Modes | Spectral Assignment [39,42,44] |
|---|---|---|---|---|
| 650 | Amido IV C=O mode | |||
| 853 | - | Very Strong | Finger print | C-C stretching |
| 931 | - | Strong | Finger print | C-C stretching |
| 1057 | - | Medium | C-O stretching | |
| 1127 | - | Very Strong | CH3 rocking | |
| 1292 | - | Strong | Amide II | C-H+C-O-C |
| - | 1335 | Very Strong | D band | Double resonance effects in sp2 carbon attributable of the presence of graphene |
| 1448 | 1444 | Strong | CH2 bending | |
| - | 1570 | Medium | G- band | Raman active mode of graphite |
| 1632 | - | Weak | Amide I | C=O mixed with NH deformation |
| - | 2704 | Strong | Overtones C-H | |
| 2724 | - | Medium | Overtones C-H | |
| 2850 | 2847 | Strong | G′ band | Overtones of G band C-H |
| 2941 | 2941 | Medium | G′ band | Overtones of G band |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brezeștean, I.A.; Marconi, D.; Colniță, A.; Ciorîță, A.; Tripon, S.C.; Vuluga, Z.; Corobea, M.C.; Dina, N.E.; Turcu, I. Scanning Electron Microscopy and Raman Spectroscopy Characterization of Structural Changes Induced by Thermal Treatment in Innovative Bio-Based Polyamide Nanocomposites. Chemosensors 2023, 11, 28. https://doi.org/10.3390/chemosensors11010028
Brezeștean IA, Marconi D, Colniță A, Ciorîță A, Tripon SC, Vuluga Z, Corobea MC, Dina NE, Turcu I. Scanning Electron Microscopy and Raman Spectroscopy Characterization of Structural Changes Induced by Thermal Treatment in Innovative Bio-Based Polyamide Nanocomposites. Chemosensors. 2023; 11(1):28. https://doi.org/10.3390/chemosensors11010028
Chicago/Turabian StyleBrezeștean, Ioana Andreea, Daniel Marconi, Alia Colniță, Alexandra Ciorîță, Septimiu Cassian Tripon, Zina Vuluga, Mihai Cosmin Corobea, Nicoleta Elena Dina, and Ioan Turcu. 2023. "Scanning Electron Microscopy and Raman Spectroscopy Characterization of Structural Changes Induced by Thermal Treatment in Innovative Bio-Based Polyamide Nanocomposites" Chemosensors 11, no. 1: 28. https://doi.org/10.3390/chemosensors11010028