External-Cavity Quantum Cascade Laser-Based Gas Sensor for Sulfur Hexafluoride Detection
Abstract
:1. Introduction
2. Theory of Laser Absorption Spectroscopy and Quartz Tuning Fork Detector
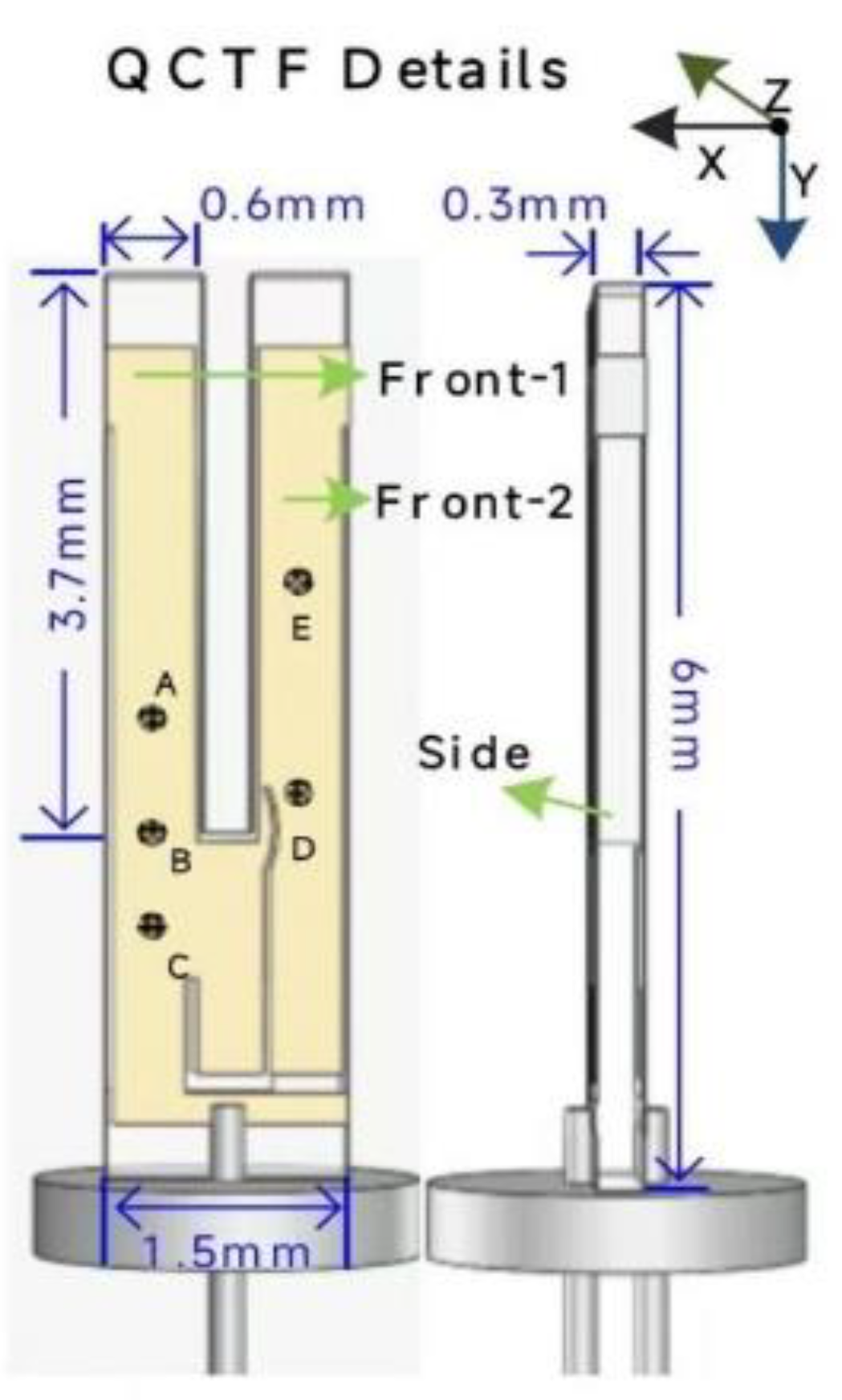
3. Experimental Details
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, N.; Xu, L.; Zhou, S.; Zhang, L.; Li, J. Soil respiration analysis using a mid-infrared quantum cascade laser and calibration-free WMS-based dual-gas sensor. Analyst 2021, 146, 3841–3851. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Parchatka, U.; Fischer, H. Development of field-deployable real time QCL spectrometer for simultaneous detection of ambient N2O and CO. Sens. Actuators B 2013, 182, 659–667. [Google Scholar] [CrossRef]
- Li, J.; Chen, W.; Fischer, H. Quantum cascade laser spectrometry techniques: A new trend in atmospheric chemistry. Appl. Spectrosc. Rev. 2013, 48, 523–559. [Google Scholar] [CrossRef]
- KöSter, J.R.; Well, R.; Tuzson, B.; Bol, R.; Dittert, K.; Giesemann, A.; Emmenegger, L.; Manninen, A.; Cárdenas, L.; Mohn, J. Novel laser spectroscopic technique for continuous analysis of N2O isotopomers-application and intercomparison with isotope ratio mass spectrometry. Rapid Commun. Mass Spectrom. 2013, 27, 216–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Liu, N.; Ding, J.; Zhou, S.; He, T.; Zhang, L. Piezoelectric effect-based detector for spectroscopic application. Opt. Lasers Eng. 2019, 115, 141–148. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology (NIST) Database. Available online: https://www.nist.gov (accessed on 21 July 2009).
- Li, J.; Yu, B.; Zhao, W.; Chen, W. A review of signal enhancement and noise reduction techniques for Tunable Diode Laser Absorption Spectroscopy. Appl. Spectrosc. Rev. 2014, 49, 666–691. [Google Scholar] [CrossRef]
- Sun, J.; Deng, H.; Liu, N.; Wang, H.; Yu, B.; Li, J. Mid-infrared gas absorption sensor based on a broadband external cavity quantum cascade laser. Rev. Sci. Instrum. 2016, 87, 123101. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhou, S.; Zhang, L.; Yu, B.; Fischer, H.; Ren, W.; Li, J. Standoff detection of VOCs using external cavity quantum cascade laser spectroscopy. Laser Phys. Lett. 2018, 15, 085701. [Google Scholar] [CrossRef]
- Cheng, G.; Cao, Y.; Liu, K.; Cao, Y.; Tian, X.; Chen, J.; Yang, G.; Gao, X. Modal Simulation Calculation and Research of Tuning Fork Based on QEPAS System. Spectrosc. Spectr. Anal. 2019, 39, 31–38. [Google Scholar]
- Chapados, C.; Birnbaum, G. Infrared absorption of SF6 from 32 to 3000 cm−1 in the gaseous and liquid states. J. Mol. Spectrosc. 1988, 132, 323–335. [Google Scholar] [CrossRef]
- Werle, P.W.; Mazzinghi, P.; D’Amato, F.; De Rosa, M.; Maurer, K.; Slemr, F. Signal processing and calibration procedures for in situ diode-laser absorption spectroscopy. Spectrochim. Acta Part A 2004, 60, 1685–1705. [Google Scholar] [CrossRef] [PubMed]
- Werle, P.; Mucke, R.; Slemr, F. The limits of signal averaging in atmospheric trace-gas monitoring by tunable diode-laser absorption spectroscopy (TDLAS). Appl. Phys. B 1993, 57, 131–139. [Google Scholar] [CrossRef]
- Wan, F.; Zhou, F.; Hu, J.; Wang, P.; Wang, J.; Chen, W.; Zhu, C.; Liu, Y. Highly Sensitive and Precise Analysis of SF6 Decomposition Component CO by Multi-comb Optical-feedback Cavity Enhanced Absorption Spectroscopy with a 2.3 μm Diode Laser. Sci. Rep. 2019, 9, 9690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waßmuth, B.; Fuchs, G.; Zimmermann, H.; Giesen, T. Concept and application of a linearized ring multipass optics configuration. Appl. Opt. 2021, 60, 10273–10281. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.; Sthel, M.; Lima, G.; Silva, M.; Schramm, D.; Miklós, A.; Vargas, H. A Sulfur Hexafluoride Sensor Using Quantum Cascade and CO2 Laser-Based Photoacoustic Spectroscopy. Sensors 2010, 10, 9359–9368. [Google Scholar] [CrossRef] [PubMed]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, X.; Zhang, Y.; Zeng, J.; Zhang, M.; Li, J. External-Cavity Quantum Cascade Laser-Based Gas Sensor for Sulfur Hexafluoride Detection. Chemosensors 2023, 11, 30. https://doi.org/10.3390/chemosensors11010030
Pan X, Zhang Y, Zeng J, Zhang M, Li J. External-Cavity Quantum Cascade Laser-Based Gas Sensor for Sulfur Hexafluoride Detection. Chemosensors. 2023; 11(1):30. https://doi.org/10.3390/chemosensors11010030
Chicago/Turabian StylePan, Xingyu, Yifan Zhang, Jiayu Zeng, Minghui Zhang, and Jingsong Li. 2023. "External-Cavity Quantum Cascade Laser-Based Gas Sensor for Sulfur Hexafluoride Detection" Chemosensors 11, no. 1: 30. https://doi.org/10.3390/chemosensors11010030




