Highly Sensitive SF6 Decomposition Byproducts Sensing Platform Based on CuO/ZnO Heterojunction Nanofibers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
2.2. Materials Characterization
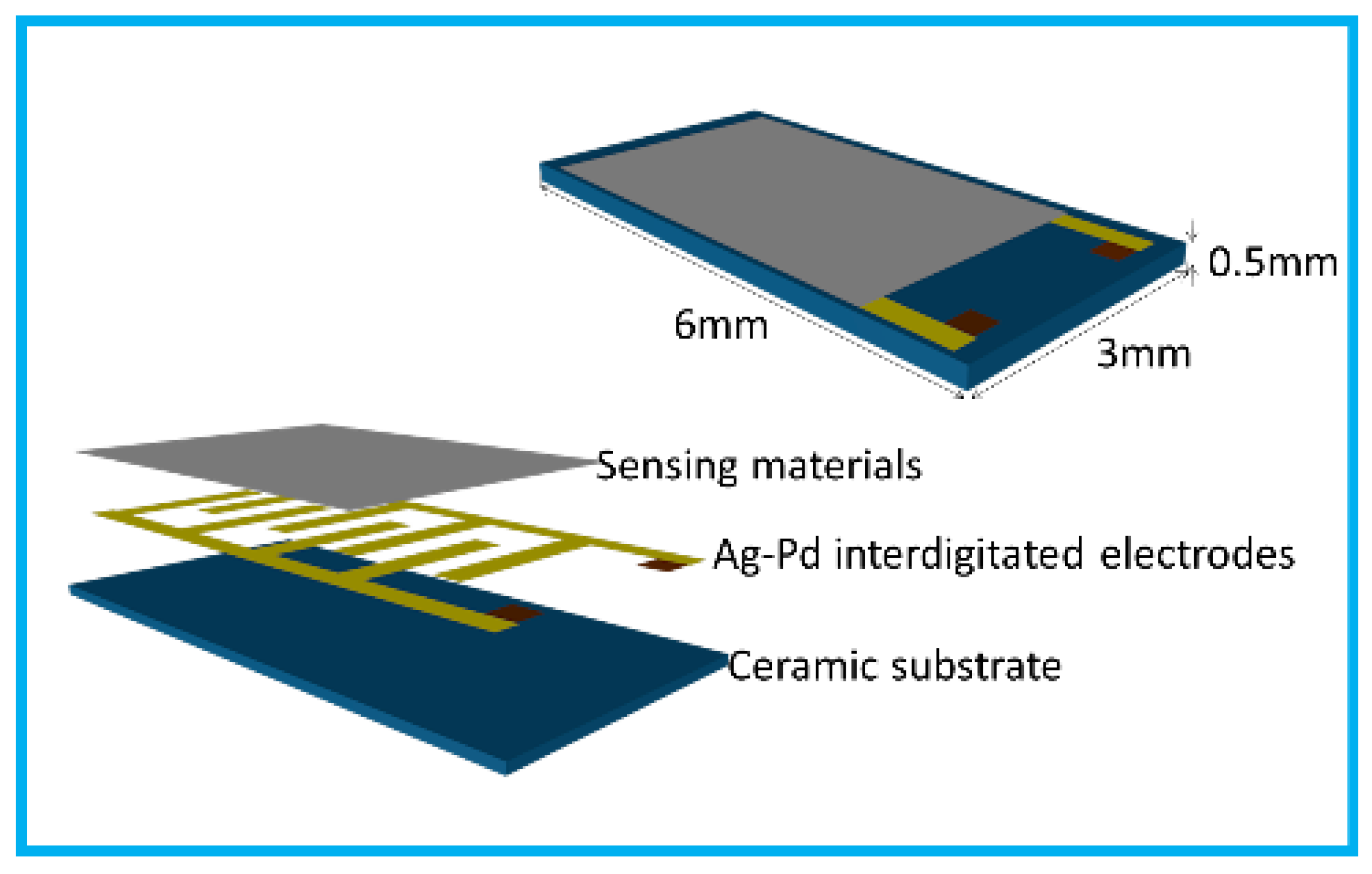
2.3. Fabrication and Measurement of Gas Sensors
3. Results and Discussion
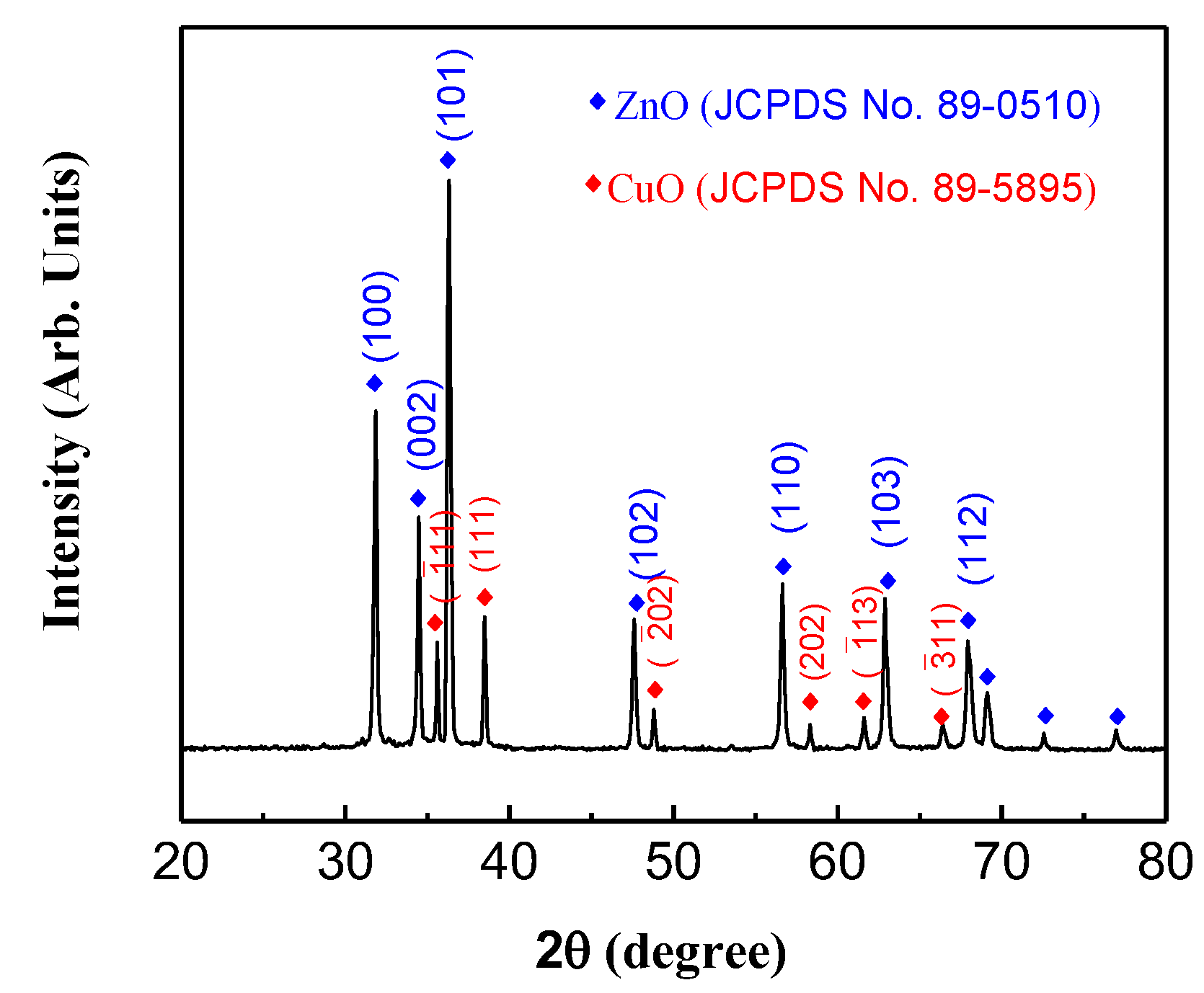
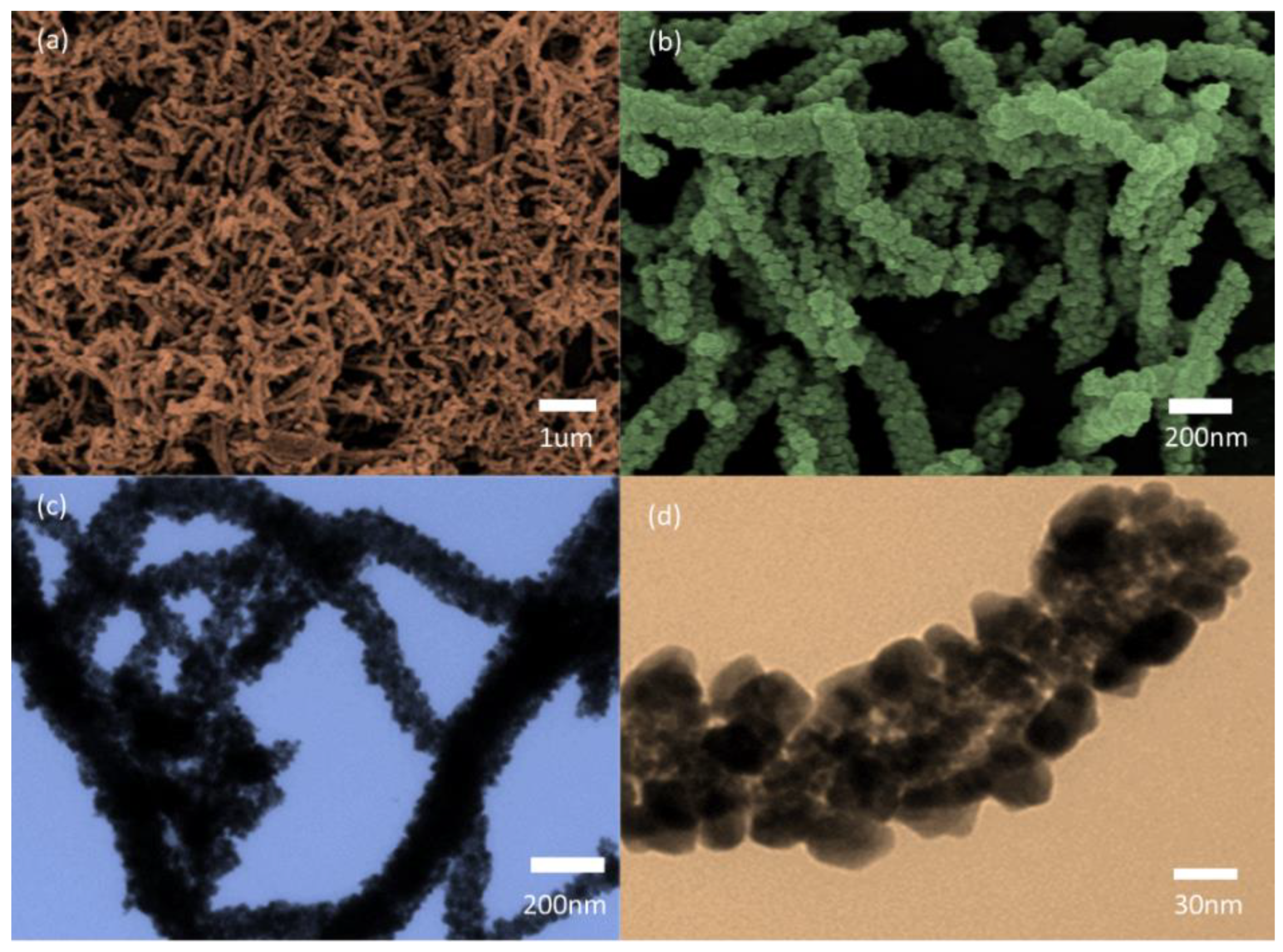
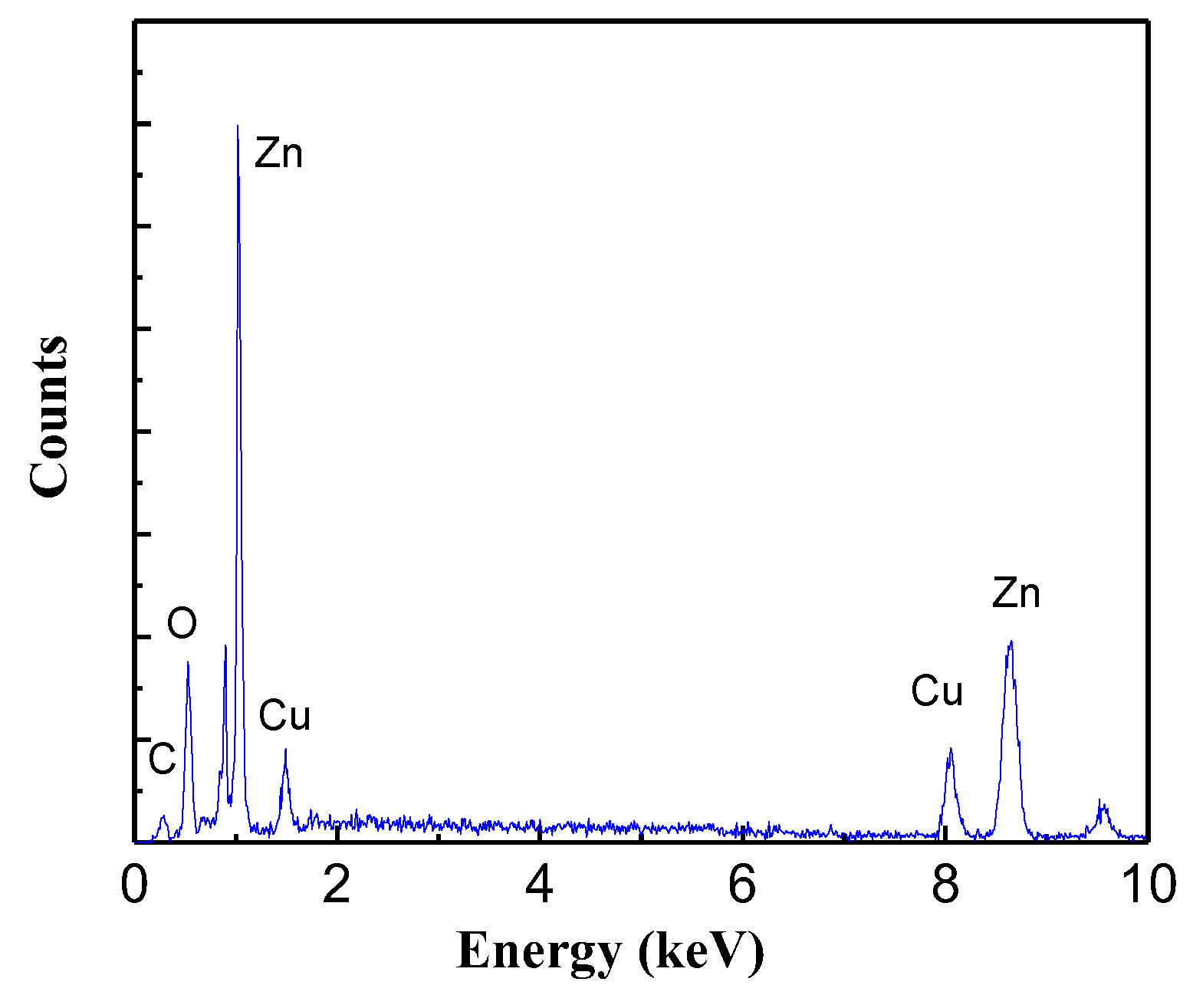
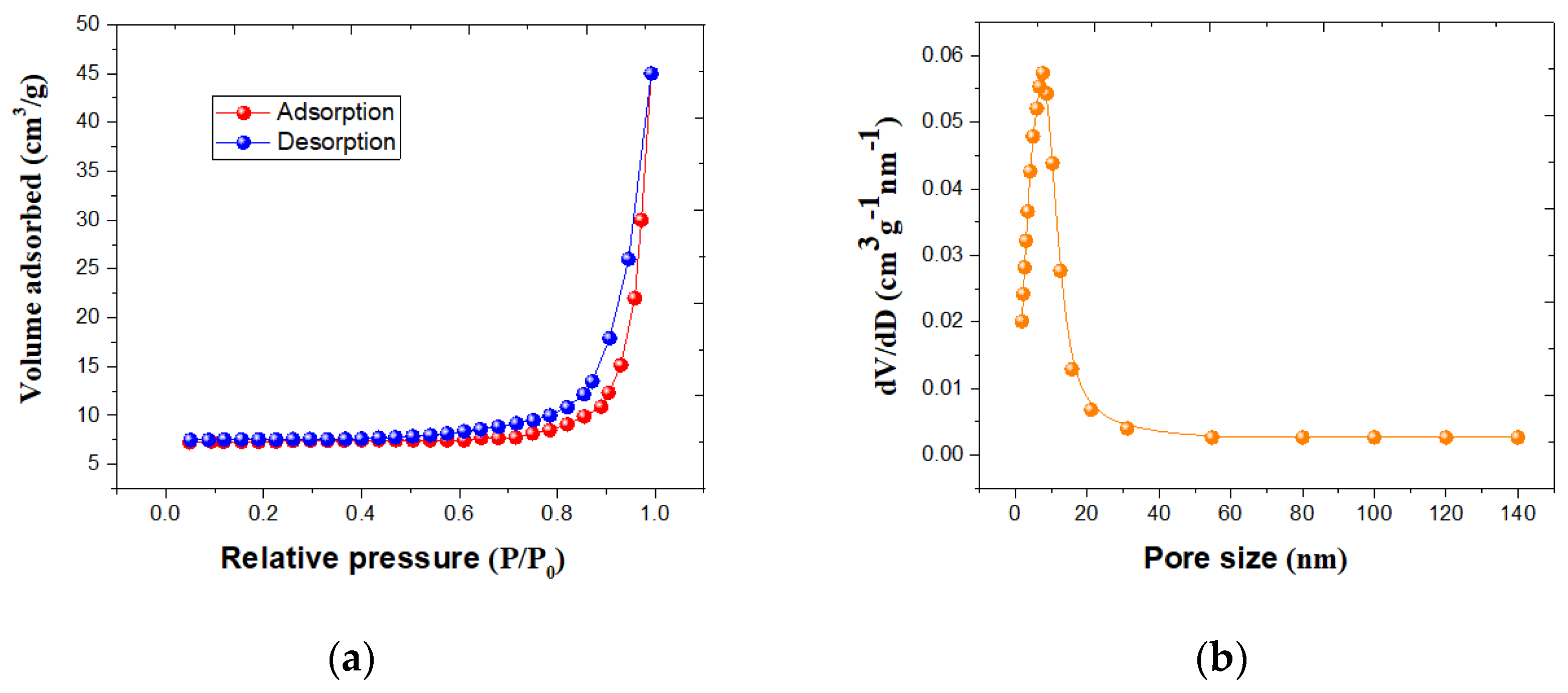
3.1. Nanomaterials Characterizations
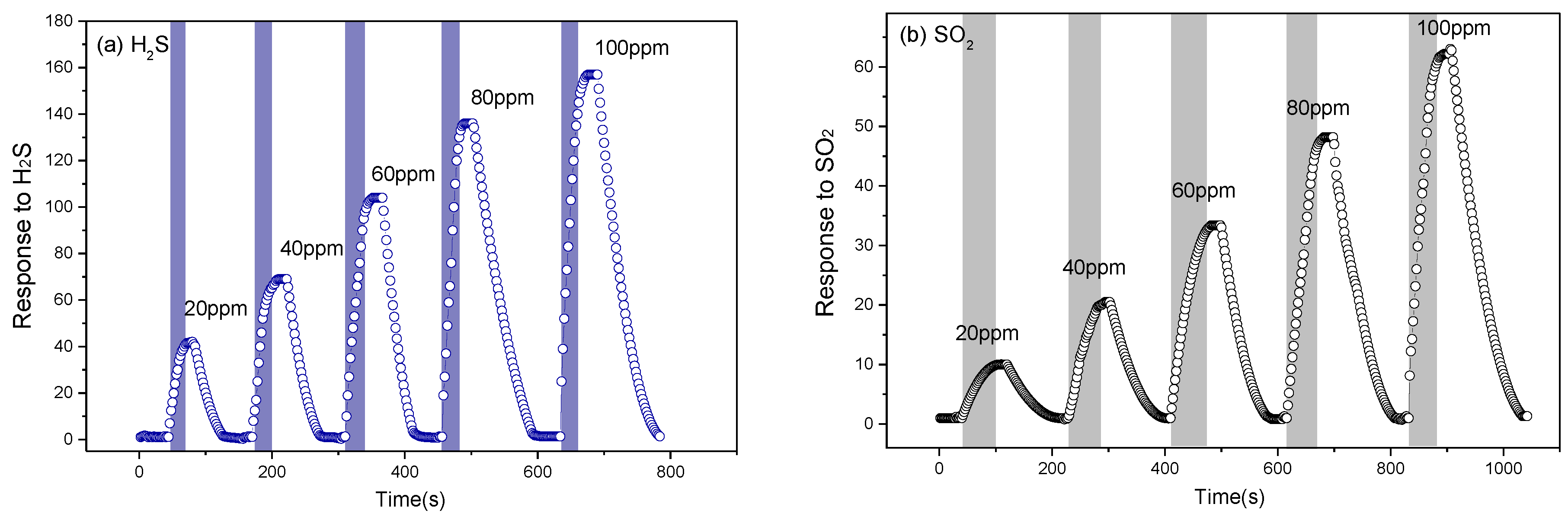
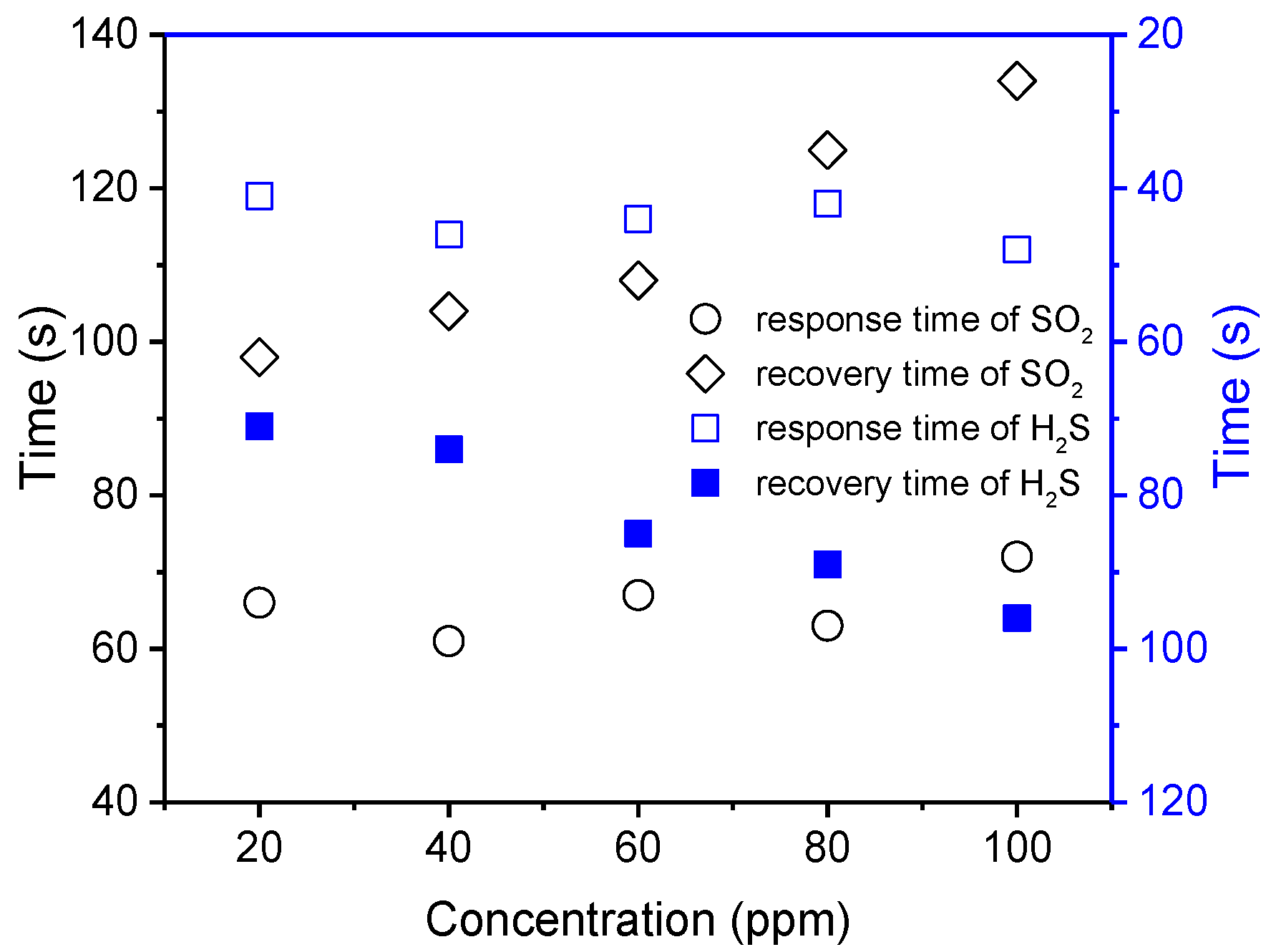
3.2. Gas Sensing Studies
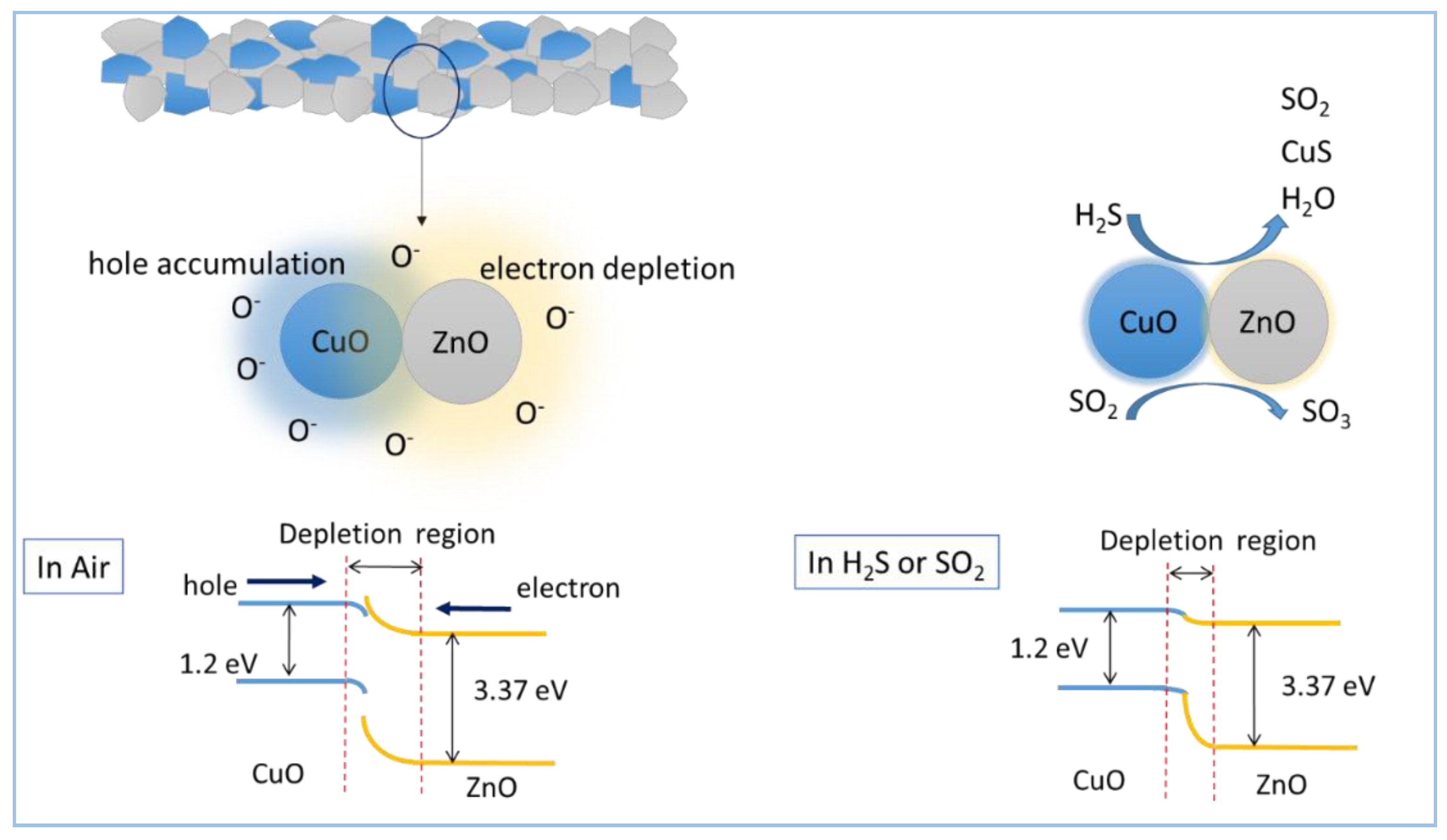
3.3. Gas Sensing Mechanism
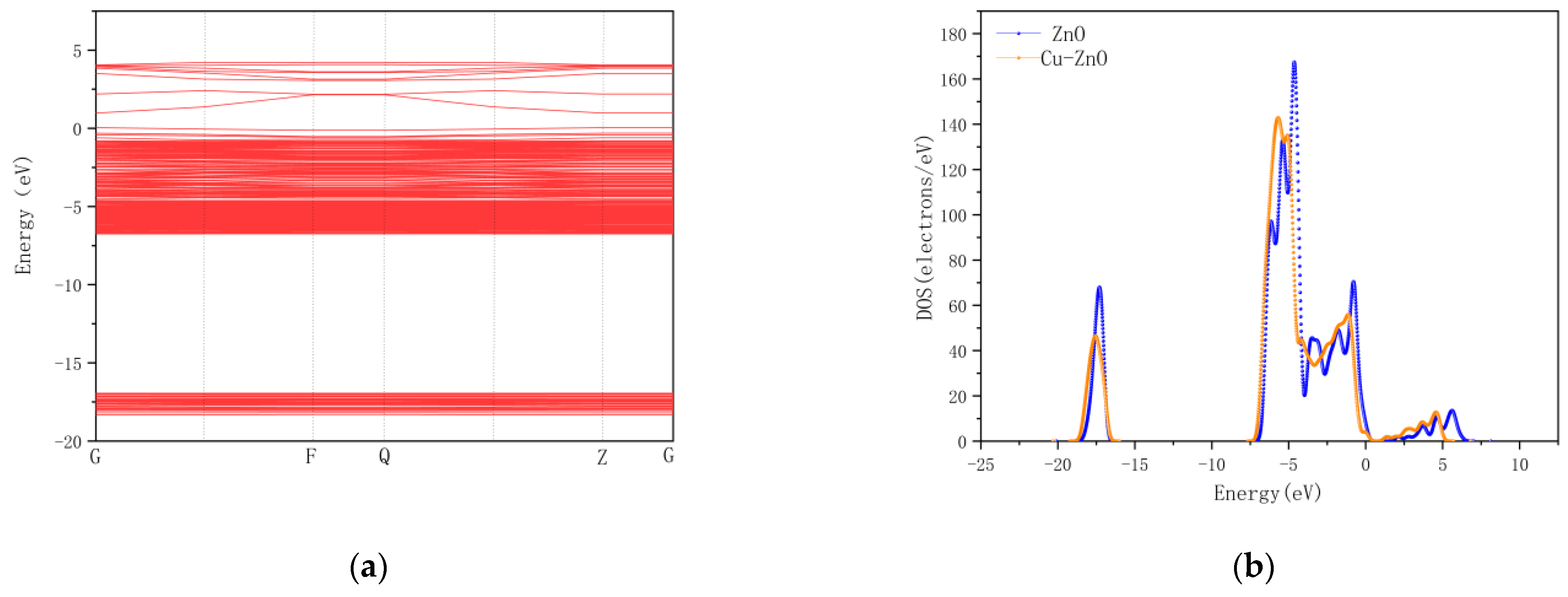
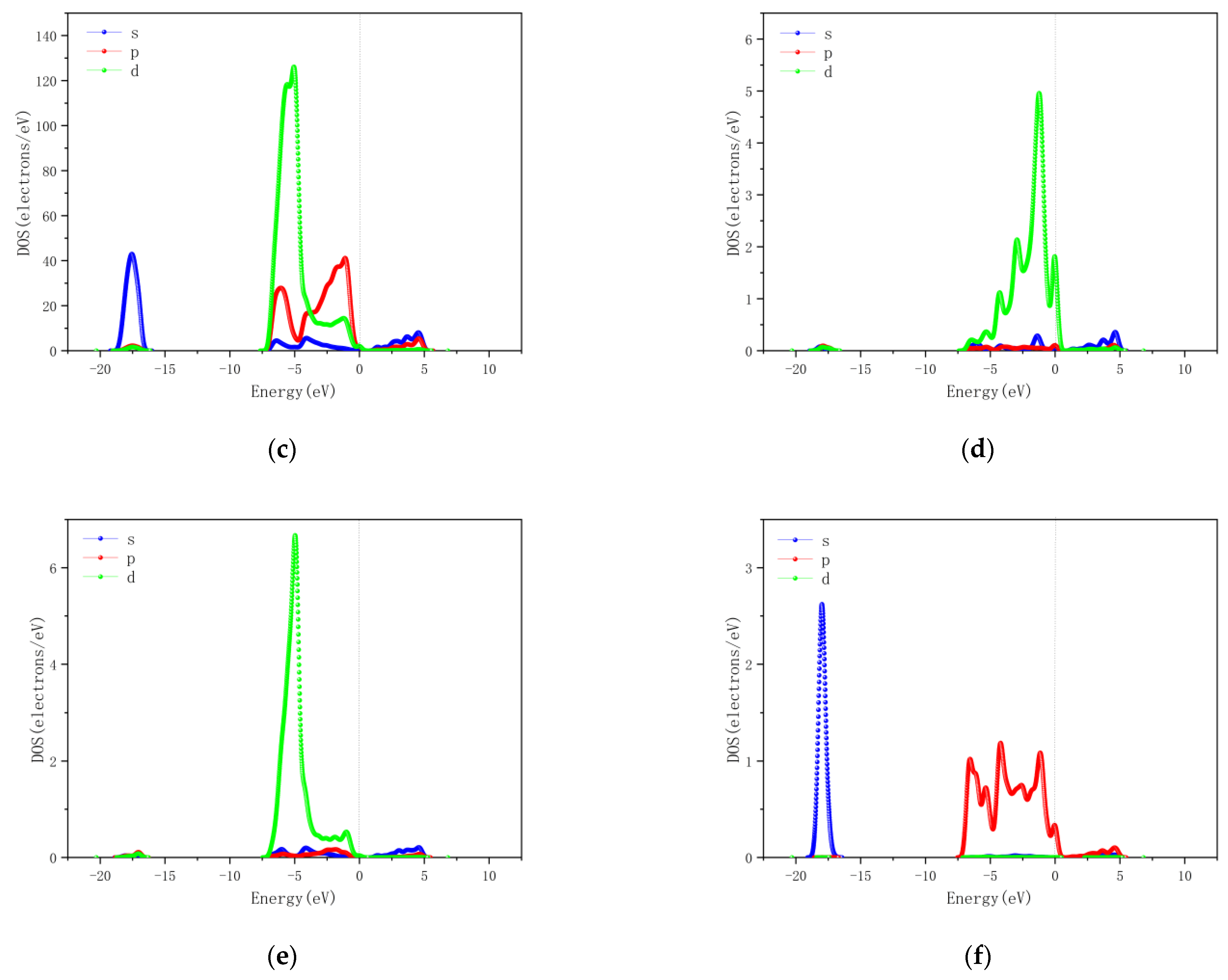
4. First-Principles Based Simulation Analysis
4.1. Computational Details
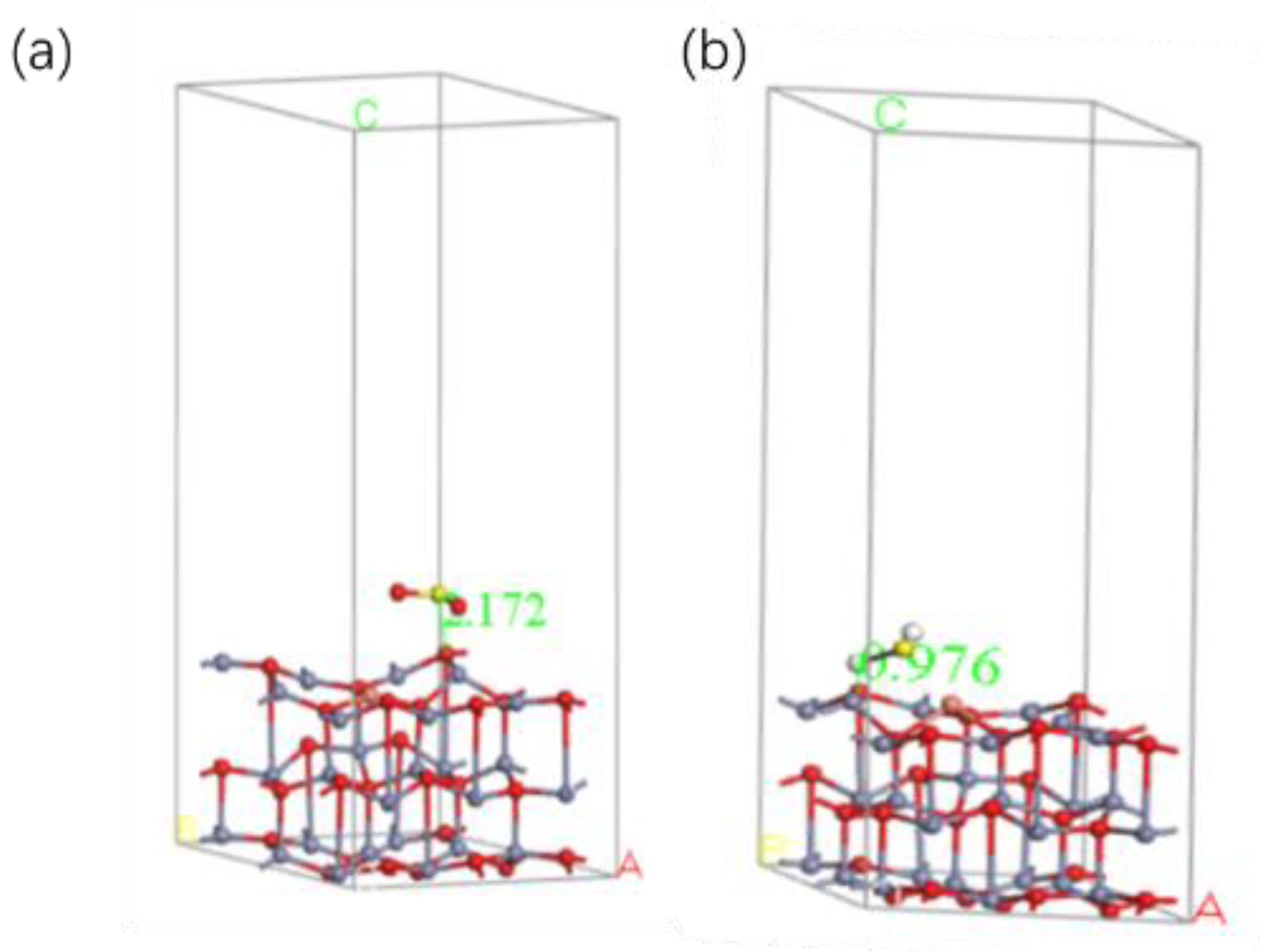
4.2. Cu/ZnO-Based SO2, H2S Gas Sensing Mechanism Based on the First Principles
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, J.; Zhou, Q.; Jia, L.; Cui, X.; Zeng, W. The gas-sensing mechanism of Pt3 cluster doped SnS2 monolayer for SF6 decomposition: A DFT study. Appl. Surf. Sci. 2022, 597, 153693. [Google Scholar] [CrossRef]
- Li, Z.; Jia, L.; Chen, J.; Cui, X.; Zeng, W.; Zhou, Q. Ag-modified hexagonal GaN monolayer as an innovative gas detector toward SF6 decomposed species: Insights from the first-principles computations. Appl. Surf. Sci. 2022, 589, 153000. [Google Scholar] [CrossRef]
- Gao, X.; Zhou, Q.; Wang, J.; Xu, L.; Zeng, W. DFT study on the selective adsorption properties of modified graphene for SF6 decompositions. IEEE Sens. J. 2021, 21, 3193–3200. [Google Scholar] [CrossRef]
- Chu, J.; Wang, Q.; Liu, Y.; Pan, J.; Yuan, H.; Yang, A.; Wang, X.; Rong, M. Fault Diagnosis of SF6-insulated Equipment by Micro Gas Sensor Array. IEEE Trans. Power Deliv. 2022, 1–9. [Google Scholar] [CrossRef]
- Chu, J.; Li, W.; Yang, X.; Wu, Y.; Wang, D.; Yang, A.; Yuan, H.; Wang, X.; Li, Y.; Rong, M. Identification of gas mixtures via sensor array combining with neural networks. Sens. Actuators B Chem. 2021, 329, 129090. [Google Scholar] [CrossRef]
- Luo, J.; Fang, Y.H.; Su, Z.X.; Li, D.C.; Zhao, Y.D.; Wang, A.J.; Wu, J.; Cui, F.X.; Li, Y.Y. The research of temperature properties of photoacoustic spectroscopy detection for SF6 decomposition products in gas insulated switchgear. Anal. Methods 2015, 7, 3806–3813. [Google Scholar] [CrossRef]
- Jong, E.C.; Macek, P.V.; Perera, I.E.; Luxbacher, K.D.; McNair, H.M. An Ultra-Trace Analysis Technique for SF6 Using Gas Chromatography with Negative Ion Chemical Ionization Mass Spectrometry. J. Chromatogr. Sci. 2015, 53, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Zhang, C.; Ren, M.; Albarracín, R.; Ye, R. Electrochemical and Infrared Absorption Spectroscopy Detection of SF6 Decomposition Products. Sensors 2017, 17, 2627. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, Q.; Zeng, W. Theoretical study on adsorption of SF6 decomposition gas in GIS gas cell based on intrinsic and Ni-doped MoTe2 monolayer. Appl. Surf. Sci. 2022, 591, 153167. [Google Scholar] [CrossRef]
- Li, B.; Zhou, Q.; Peng, R.; Liao, Y.; Zeng, W. Adsorption of SF6 decomposition gases (H2S, SO2, SOF2 and SO2F2) on Sc-doped MoS2 surface: A DFT study. Appl. Surf. Sci. 2021, 549, 149271. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, X.; Gui, Y. Theoretical and experimental study on competitive adsorption of SF6 decomposed components on Au-modified anatase (101) surface. Appl. Surf. Sci. 2016, 387, 437–445. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Jia, Y.; Xiao, P.; Tang, J. TiO2 Nanotube Array Sensor for Detecting the SF6 Decomposition Product SO2. Sensors 2012, 12, 3302–3313. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, L.; Wang, B.; Sun, P.; Wang, Q.; Gao, Y.; Liang, X.; Zhang, T.; Lu, G. Acetone gas sensor based on NiO/ZnO hollow spheres: Fast response and recovery, and low (ppb) detection limit. J. Colloid Interface Sci. 2017, 495, 207–215. [Google Scholar] [CrossRef]
- Hou, L.; Zhang, C.; Li, L.; Du, C.; Li, X.; Kang, X.-F.; Chen, W. CO gas sensors based on p-type CuO nanotubes and CuO nanocubes: Morphology and surface structure effects on the sensing performance. Talanta 2018, 188, 41–49. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, W.; Xu, L.; Kumar, R.; Gui, Y.; Zhao, Z.; Tang, C.; Zhu, S. Highly sensitive carbon monoxide (CO) gas sensors based on Ni and Zn doped SnO2 nanomaterials. Ceram. Int. 2018, 44, 4392–4399. [Google Scholar] [CrossRef]
- Bai, S.; Fu, H.; Zhao, Y.; Tian, K.; Luo, R.; Li, D.; Chen, A. On the construction of hollow nanofibers of ZnO-SnO2 heterojunctions to enhance the NO2 sensing properties. Sens. Actuators B Chem. 2018, 266, 692–702. [Google Scholar] [CrossRef]
- Luo, L.; Xu, W.; Xia, Z.; Fei, Y.; Zhu, J.; Chen, C.; Lu, Y.; Wei, Q.; Qiao, H.; Zhang, X. Electrospun ZnO–SnO2 composite nanofibers with enhanced electrochemical performance as lithium-ion anodes. Ceram. Int. 2016, 42, 10826–10832. [Google Scholar] [CrossRef]
- Zhu, L.; Li, Y.; Zeng, W. Enhanced ethanol sensing and mechanism of Cr-doped ZnO nanorods: Experimental and computational study. Ceram. Int. 2017, 43, 14873–14879. [Google Scholar] [CrossRef]
- Kong, M.; Liu, Q.C.; Zhu, B.H.; Yang, J.; Li, L.; Zhou, Q.; Ren, S. Synergy of KCl and Hg-el on selective catalytic reduction of NO with NH3 over V2O5-WO3/TiO2 catalysts. Chem. Eng. J. 2015, 264, 815–823. [Google Scholar] [CrossRef]
- Long, H.; Zeng, W.; Zhang, H. Synthesis of WO3 and its gas sensing: A review. J. Mater. Sci. Mater. Electron. 2015, 26, 4698–4707. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, Z.; Chen, C.; Wen, T.; Zhao, X.; Xie, L. Highly sensitive H2S gas sensors based on Pd-doped CuO nanoflowers with low operating temperature. Sens. Actuators B Chem. 2017, 253, 809–817. [Google Scholar] [CrossRef]
- Choi, S.-W.; Park, J.Y.; Kim, S.S. Growth behavior and sensing properties of nanograins in CuO nanofibers. Chem. Eng. J. 2011, 172, 550–556. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Q.; Wei, Z.; Xu, L.; Zeng, W. Experimental and theoretical studies of Zn-doped MoO3 hierarchical microflower with excellent sensing performances to carbon monoxide. Ceram. Int. 2020, 46, 29222–29232. [Google Scholar] [CrossRef]
- Gao, X.; Li, Y.; Zeng, W.; Zhang, C.; Wei, Y. Hydrothermal synthesis of agglomerating TiO2 nanoflowers and its gas sensing. J. Mater. Sci. Mater. Electron. 2017, 28, 18781–18786. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, T.; Zhou, Y.; Meng, C.; Zhu, W.; Liu, L. TiO2-Based Nanoheterostructures for Promoting Gas Sensitivity Performance: Designs, Developments, and Prospects. Sensors 2017, 17, 1971. [Google Scholar] [CrossRef]
- Zhou, Q.; Xu, L.; Umar, A.; Chen, W.; Kumar, R. Pt nanoparticles decorated SnO2 nanoneedles for efficient CO gas sensing applications. Sens. Actuators B Chem. 2018, 256, 656–664. [Google Scholar] [CrossRef]
- Zhou, Q.; Zeng, W.; Chen, W.G.; Xu, L.N.; Kumar, R.; Umar, A. High sensitive and low-concentration sulfur dioxide (SO2) gas sensor application of heterostructure NiO-ZnO nanodisks. Sens. Actuators B Chem. 2019, 298, 126870. [Google Scholar] [CrossRef]
- Yan, S.; Ma, S.; Li, W.; Xu, X.; Cheng, L.; Song, H.; Liang, X. Synthesis of SnO2–ZnO heterostructured nanofibers for enhanced ethanol gas-sensing performance. Sens. Actuators B Chem. 2015, 221, 88–95. [Google Scholar] [CrossRef]
- Zhang, B.; Fu, W.; Li, H.; Fu, X.; Wang, Y.; Bala, H.; Wang, X.; Sun, G.; Cao, J.; Zhang, Z. Synthesis and enhanced gas sensing properties of flower-like ZnO nanorods decorated with discrete CuO nanoparticles. Mater. Lett. 2016, 176, 13–16. [Google Scholar] [CrossRef]
- Qu, Z.; Fu, Y.; Yu, B.; Deng, P.; Xing, L.; Xue, X. High and fast H2S response of NiO/ZnO nanowire nanogenerator as a self-powered gas sensor. Sens. Actuators B Chem. 2016, 222, 78–86. [Google Scholar] [CrossRef]
- Bonyani, M.; Zebarjad, S.M.; Janghorban, K.; Kim, J.-Y.; Kim, H.W.; Kim, S.S. Au-Decorated Polyaniline-ZnO Electrospun Composite Nanofiber Gas Sensors with Enhanced Response to NO2 Gas. Chemosensors 2022, 10, 388. [Google Scholar] [CrossRef]
- Huang, B.; Zeng, W.; Li, Y. Synthesis of ZIF-8 Coating on ZnO Nanorods for Enhanced Gas-Sensing Performance. Chemosensors 2022, 10, 297. [Google Scholar] [CrossRef]
- Katoch, A.; Choi, S.-W.; Kim, J.-H.; Lee, J.H.; Lee, J.-S.; Kim, S.S. Importance of the nanograin size on the H2S-sensing properties of ZnO–CuO composite nanofibers. Sens. Actuators B Chem. 2015, 214, 111–116. [Google Scholar] [CrossRef]
- Qin, W.; Xu, L.; Song, J.; Xing, R.; Song, H. Highly enhanced gas sensing properties of porous SnO2–CeO2 composite nanofibers prepared by electrospinning. Sens. Actuators B Chem. 2013, 185, 231–237. [Google Scholar] [CrossRef]
- GB/T 8905-2012; The Guide for Management and Measuring SF6 Gas in Electrical Equipment. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China; Standardization Administration of the People’s Republic of China: Beijing, China, 2012.
- Zhou, X.; Lin, X.; Yang, S.; Zhu, S.; Chen, X.; Dong, B.; Bai, X.; Wen, X.; Geyu, L.; Song, H. Highly dispersed Metal–Organic-Framework-Derived Pt nanoparticles on three-dimensional macroporous ZnO for trace-level H2S sensing. Sens. Actuators B Chem. 2020, 309, 127802. [Google Scholar] [CrossRef]
- Rumyantseva, M.N.; Vladimirova, S.A.; Platonov, V.B.; Chizhov, A.S.; Batuk, M.; Hadermann, J.; Khmelevsky, N.O.; Gaskov, A.M. Sub-ppm H2S sensing by tubular ZnO-Co3O4 nanofibers. Sens. Actuators B Chem. 2020, 307, 127624. [Google Scholar] [CrossRef]
- Nadargi, D.Y.; Tamboli, M.S.; Patil, S.S.; Dateer, R.B.; Mulla, I.S.; Choi, H.; Suryavanshi, S.S. Microwave-Epoxide-Assisted Hydrothermal Synthesis of the CuO/ZnO Heterojunction: A Highly Versatile Route to Develop H2S Gas Sensors. ACS Omega 2020, 5, 8587–8595. [Google Scholar] [CrossRef]
- Sun, M.; Yu, H.; Dong, X.; Xia, L.; Yang, Y. Sedum lineare flower-like ordered mesoporous In2O3/ZnO gas sensing materials with high sensitive response to H2S at room temperature prepared by self-assembled of 2D nanosheets. J. Alloys Compd. 2020, 844, 156170. [Google Scholar] [CrossRef]
- Behera, B.; Chandra, S. An innovative gas sensor incorporating ZnO-CuO nanoflakes in planar MEMS technology. Sens. Actuators B Chem. 2016, 229, 414–424. [Google Scholar] [CrossRef]
- Lupan, O.; Ursaki, V.; Chai, G.; Chow, L.; Emelchenko, G.; Tiginyanu, I.; Gruzintsev, A.; Redkin, A. Selective hydrogen gas nanosensor using individual ZnO nanowire with fast response at room temperature. Sens. Actuators B Chem. 2010, 144, 56–66. [Google Scholar] [CrossRef]
- Zhang, D.Z.; Jiang, C.X.; Li, P.; Sun, Y.E. Layer-by-layer self-assembly of Co3O4 nanorod-decorated MoS2 nanosheet based nanocomposite toward high performance ammonia detection. ACS Appl. Mater. Interfaces 2017, 9, 6462–6471. [Google Scholar] [CrossRef]
- Liu, H.C.; Zhou, Q.; Zhang, Q.Y.; Hong, C.X.; Xu, L.N.; Jin, L.F.; Chen, W.G. Synthesis, characterization and enhanced sensing properties of a NiO/ZnO p-n junctions sensor for the SF6 decomposition byproducts SO2, SO2F2, and SOF2. Sensors 2017, 17, 913. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Qin, L.; Zhao, P.; Zhang, Y.; Liu, D.; Liu, F.; Kang, B.; Wang, Y.; Song, H.; Zhang, T.; et al. Preparation and gas-sensing performances of ZnO-CuO rough nanotubular arrays for low-working temperature H2S detection. Sens. Actuators B Chem. 2018, 254, 834–841. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.; Kheel, H.; Hyun, S.K.; Jin, C.; Lee, C. Enhanced H2S gas sensing performance of networked CuO-ZnO composite nanoparticle sensor. Mater. Res. Bull. 2016, 82, 130–135. [Google Scholar] [CrossRef]
- Vuong, N.M.; Chinh, N.D.; Huy, B.T.; Lee, Y.I. CuO-Decorated ZnO Hierarchical nanostructures as efficient and established sensing materials for H2S gas sensors. Sci. Rep. 2016, 6, 26736. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, Q.; Mi, H.; Wang, J.; Zeng, W. Gas-sensing mechanism of Cr doped SnP3 monolayer to SF6 partial discharge decomposition components. Appl. Surf. Sci. 2021, 546, 149084. [Google Scholar] [CrossRef]
- Li, Z.; Liao, Y.; Liu, Y.; Zeng, W.; Zhou, Q. Room temperature detection of nitrogen dioxide gas sensor based on Pt-modified MoSe2 nanoflowers: Experimental and theoretical analysis. Appl. Surf. Sci. 2023, 610, 155527. [Google Scholar] [CrossRef]





| Element | Norm. C [wt%] | Atom. C [at.%] |
|---|---|---|
| C | 3.36 | 12.48 |
| O | 13.31 | 37.15 |
| Zn | 65.39 | 44.65 |
| Cu | 17.94 | 5.72 |
| Material | Response | T (°C) | Concentration | Ref. |
|---|---|---|---|---|
| Pt/ZnO | 51.2 | 320 | 20 ppm | [36] |
| Co3O4/ZnO CuO/ZnO | 1.8 | 270 | 0.2 ppm | [37] |
| In2O3/ZnO | 1.9 | 250 | 25 ppm | [38] |
| Ni/ZnO | 10.2 | 25 | 100 ppm | [39] |
| CuO/ZnO nanofibers | 11.35 | 200 | 5 ppm | this work |
| Gas | Adsorption Distance Å | Adsorption Energy (eV) | Charge Transfer (e) |
|---|---|---|---|
| SO2 | 2.172 | −1.2217 | −0.248 |
| H2S | 0.976 | −2.2152 | 0.322 |
| Adsorption Systems | HOMO (ha) | LUMO (ha) | Energy Gap (eV) |
|---|---|---|---|
| Cu/ZnO | −0.160075 | −0.115594 | 1.2104 |
| Cu/ZnO/SO2 | −0.165323 | −0.121176 | 1.2013 |
| Cu/ZnO/H2S | −0.153723 | −0.11019 | 1.1846 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, X.; Lu, Z.; Wang, Z.; Zeng, W.; Zhou, Q. Highly Sensitive SF6 Decomposition Byproducts Sensing Platform Based on CuO/ZnO Heterojunction Nanofibers. Chemosensors 2023, 11, 58. https://doi.org/10.3390/chemosensors11010058
Cui X, Lu Z, Wang Z, Zeng W, Zhou Q. Highly Sensitive SF6 Decomposition Byproducts Sensing Platform Based on CuO/ZnO Heterojunction Nanofibers. Chemosensors. 2023; 11(1):58. https://doi.org/10.3390/chemosensors11010058
Chicago/Turabian StyleCui, Xiaosen, Zhaorui Lu, Zhongchang Wang, Wen Zeng, and Qu Zhou. 2023. "Highly Sensitive SF6 Decomposition Byproducts Sensing Platform Based on CuO/ZnO Heterojunction Nanofibers" Chemosensors 11, no. 1: 58. https://doi.org/10.3390/chemosensors11010058
APA StyleCui, X., Lu, Z., Wang, Z., Zeng, W., & Zhou, Q. (2023). Highly Sensitive SF6 Decomposition Byproducts Sensing Platform Based on CuO/ZnO Heterojunction Nanofibers. Chemosensors, 11(1), 58. https://doi.org/10.3390/chemosensors11010058









