Exploring Deposition Techniques and Supramolecular Arrangement in Thin Films for Sensor Applications
Abstract
:1. Introduction
2. The Investigation of Molecular Organization
3. Supramolecular Thin Films Assembly Techniques
3.1. Langmuir-Blodgett and Langmuir-Schaefer Assembly
3.1.1. Graphene-Derivatives
3.1.2. One-Dimensional Materials (Nanowires and Carbon Nanotubes)
3.1.3. Nanoparticles
3.1.4. Other Materials
3.2. Layer-by-Layer Assembly
3.2.1. Graphene-Based Materials
3.2.2. Phthalocyanines (Pc)
3.2.3. Metal Nanoparticles
3.2.4. Biomolecules
3.3. Electrochemical Deposition
3.3.1. Polymers and Organic Films
3.3.2. Cilodextrins
3.3.3. Phthalocyanines (Pc)
3.3.4. Carbon Derivatives
3.3.5. Nanoparticles
3.4. Spray Pyrolysis
3.4.1. Effect of Deposition Parameters
- Solution flow rate: the flow rate of the spray solution affects the thickness and uniformity of the deposited film. Higher flow rates lead to thicker films resulting in less uniformity and more defects, on the other hand lower flows lead to thinner films and may result in no percolation. Therefore, it is important to optimize the flow rate to achieve the desired film thickness and uniformity. Yusuf et al. [319] investigated the influence of the solution flow rate on the structural, morphological, and electrical properties of molybdenum oxide films. The arriving spray on the silicon substrate was controlled by varying the solution flow rate (ranging from 3.0 to 1.5 mL/min) at the substrate temperature of 300 ± 5 °C. XRD analysis allowed verify the changes in the crystallite size as the flow rate decreased. A morphological study using FE-SEM and AFM demonstrated the reducing grain size with decreasing flow rate, which promotes the homogeneity and smoothness of the film. In a study conducted by Zargou et al. [320], thin films of ZnO were deposited on glass substrates using a spray solution volume of 60 mL at 300 °C and employing different spray rates of 5, 10, 15, and 20 mL/h. They observed that the solution flow rate had a significant impact on the physical and structural properties of the thin films. The ZnO thin film deposited at a solution flow rate of 15 mL/h exhibited remarkable characteristics displaying high transparency, with a transmission rate of 99.1%. Additionally, this film demonstrated the highest electrical conductivity in dark conditions. Moreover, it exhibited the lowest carrier concentration, activation energy, and resistivity among the tested deposition conditions. Overall, the findings emphasized the notable influence of the solution flow rate on the properties of the ZnO thin films, particularly highlighting the favourable attributes achieved at a flow rate of 15 mL/h.
- Solution composition: the composition of the spray solution, which includes the precursor materials and solvents [321], dramatically influences the chemical composition and stoichiometry of the resulting film. Modifying the concentration and ratio of precursors can alter the film’s elemental composition, doping levels, and crystalline structure [322].
- Pyrolysis temperature: the substrate temperature during the deposition in SP impacts the film’s crystallinity, grain size, and morphology [323]. Higher substrate temperatures promote crystallization and grain growth, producing films with improved crystallinity and larger grain sizes. Furthermore, uncontrolled high temperatures can lead to film degradation or damage to the substrate. Ebin et al. [324] reported the production of ZnO nanoparticles and porous particles using the ultrasonic SP method through zinc nitrate precursor at different temperatures under an air atmosphere. The ZnO particles were obtained in a hexagonal crystal structure, and the shape of the crystallites changed from spherical to hexagonal as the reaction temperature increased from 400 to 1000 °C. At the lowest reaction temperature, ZnO nanoparticles were obtained, while at higher temperatures, ZnO porous particles were formed through the aggregation of ZnO nanoparticles due to effective sintering. The results indicate that the reaction temperature can be readily adjusted to manipulate the size and morphology of ZnO nanostructures, highlighting the suitability of the SP method for large-scale production due to its controllability.Al Ghamdi et al. [325] investigated the influence of substrate temperature and solution concentration on the properties of copper oxide (CuO) thin films. They deposited a series of CuO thin films on glass substrates, varying the temperature between 400 and 650 °C and using two different concentrations of copper salt. The structure and morphology of the films were examined using XRD and SEM, while UV-visible spectroscopy was employed to analyse their optical properties. A significant influence of substrate temperature on the growth mechanisms and physical characteristics of the CuO films was verified: the films primarily exhibited a single phase of CuO with an observed reduction in crystallite size as the substrate temperature increased. Hameed et al. [326] fabricated nickel oxide (NiO) thin films on glass substrates using the SP technique at different temperatures (300, 400, and 500 °C). Analysis of the XRD data confirmed the presence of a cubic structure in the films, indicating their polycrystalline nature. Notably, it was observed that at a temperature of 500 °C, the lattice constant of the thin films closely approached the ideal lattice parameter of 4.176 Å for a cubic structure. Therefore, it can be concluded that the thin films exhibited a desirable lattice constant at 500 °C.
- Distance between the nozzle and substrate: the distance between the spray nozzle and the substrate affects the film’s morphology and surface roughness. Generally, its decrease results in a denser and smoother film due to the increased heat and mass transfer efficiency [327]. Omar [328] studied the influence of the distance between the nozzle and substrate on the morphological, structural, chemical composition, and optical properties of ZnO thin films deposited using the SP technique. The morphology of the thin film was examined using Scanning Tunnelling Microscopy (STM), and it was observed that reducing the nozzle-substrate distance led to an increased root mean square (rms) roughness. Specifically, the rms values were measured as 17.470 nm and 10.062 nm at 20 cm and 30 cm distances, respectively. The surface morphology strongly influenced the optical properties, including transmittance and the optical band gap, parameters of great importance to photovoltaic applications.
- Spray pressure: the spray pressure influences the droplet size, spray pattern, and film morphology. Higher pressure causes a lower solvent evaporation rate compared to lower pressure, and this allows the solute crystal to grow in a relatively longer time and thus larger nanoparticles are formed [327,329]. In addition, excessively high pressure can cause droplet fragmentation or spray pattern distortion.
- Deposition time: the deposition time must be controlled according to the desired consistency and film quality requirements in order to avoid defects or degradation. Hathot et al. [330] conducted a study to assess the influence of different deposition times (4, 8, and 12 min) on the crystallinity, absorption, and electrical characteristics of zinc sulfide thin films (ZSTFs). XRD analysis of the ZSTFs indicated the presence of both hexagonal and cubic phases. The average crystallite size of the thin films ranged from 27.81 to 31.54 nm, while the optical band gap energy varied between 2.7 and 3 eV. The thin films exhibited low transmittance in the visible and infrared regions, with the surface roughness playing a significant role in this characteristic. As the deposition time increased, the refractive indices of the films also increased while the extinction coefficients decreased. The thin films demonstrated excellent electrical and dielectric properties, making them suitable for various applications.
3.4.2. Sensing Applications
4. Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hulanicki, A.; Glab, S.; Ingman, F. Chemical Sensors Definitions and Classification. Pure Appl. Chem. 1991, 63, 1247–1250. [Google Scholar] [CrossRef]
- Paixao, T.R.L.C.; Reddy, S.M. Materials for Chemical Sensing; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 978-3-319-47833-3. [Google Scholar]
- Lehn, J.M. Supramolecular Chemistry. Science 1993, 260, 1762–1763. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Petty, M.C. Langmuir-Blodgett Films: An Introduction, 1st ed.; Cambridge University Press: Cambridge, UK, 1996; ISBN 9780521413961. [Google Scholar]
- Oliveira, O.N.; Caseli, L.; Ariga, K. The Past and the Future of Langmuir and Langmuir-Blodgett Films. Chem. Rev. 2022, 122, 6459–6513. [Google Scholar] [CrossRef] [PubMed]
- Rubira, R.J.G.; Aoki, P.H.B.; Constantino, C.J.L.; Alessio, P. Supramolecular Architectures of Iron Phthalocyanine Langmuir-Blodgett Films: The Role Played by the Solution Solvents. Appl. Surf. Sci. 2017, 416, 482–491. [Google Scholar] [CrossRef]
- Aroca, R.; Thedchanamoorthy, A. Vibrational Studies of Molecular Organization in Evaporated Phthalocyanine Thin Solid Films. Chem. Mater. 1995, 7, 69–74. [Google Scholar] [CrossRef]
- Alessio, P.; Apetrei, C.; Rubira, R.J.G.; Constantino, C.J.L.; Medina-Plaza, C.; De Saja, J.A.; Rodríguez-Méndez, M.L. Structural and Electrochemical Properties of Lutetium Bis-Octachloro-Phthalocyaninate Nanostructured Films. Application as Voltammetric Sensors. J. Nanosci. Nanotechnol. 2014, 14, 6754–6763. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.S.; Alessio, P.; Crespilho, F.N.; Constantino, C.J.L. Supramolecular Arrangement of Iron Phthalocyanine in Langmuir-Schaefer and Electrodeposited Thin Films. J. Nanosci. Nanotechnol. 2018, 18, 3206–3217. [Google Scholar] [CrossRef]
- Furini, L.N.; Martin, C.S.; Camacho, S.A.; Rubira, R.J.G.; Fernandes, J.D.; Silva, E.A.; Gomes, T.C.; Stunges, G.M.; Constantino, C.J.L.; Alessio, P. Electrochemical Properties of Nickel Phthalocyanine: The Effect of Thin Film Morphology Tuned by Deposition Techniques. Thin Solid Films 2020, 699, 137897. [Google Scholar] [CrossRef]
- Roy, D.; Das, N.M.; Shakti, N.; Gupta, P.S. Comparative Study of Optical, Structural and Electrical Properties of Zinc Phthalocyanine Langmuir-Blodgett Thin Film on Annealing. RSC Adv. 2014, 4, 42514–42522. [Google Scholar] [CrossRef]
- Greenler, R.G.; Slager, T.L. Method for Obtaining the Raman Spectrum of a Thin Film on a Metal Surface. Spectrochim. Acta Part A Mol. Spectrosc. 1973, 29, 193–201. [Google Scholar] [CrossRef]
- Greenler, R.G. Infrared Study of Adsorbed Molecules on Metal Surfaces by Reflection Techniques. J. Chem. Phys. 1966, 44, 310–315. [Google Scholar] [CrossRef]
- Hasegawa, T. Quantitative Infrared Spectroscopy for Understanding of a Condensed Matter; Springer: Tokyo, Japan, 2017; ISBN 9784431564935. [Google Scholar]
- Debe, M.K. Optical Probes of Organic Thin Films: Photons-in and Photons-Out. Prog. Surf. Sci. 1987, 24, 1–282. [Google Scholar] [CrossRef]
- Frątczak, E.Z.; Uznański, P.; Moneta, M.E. Characterization of Molecular Organization in Pentacene Thin Films on SiO2 Surface Using Infrared Spectroscopy, Spectroscopic Ellipsometry, and Atomic Force Microscopy. Chem. Phys. 2015, 456, 49–56. [Google Scholar] [CrossRef]
- Bieri, M.; Bürgi, T. Adsorption Kinetics, Orientation, and Self-Assembling of N-Acetyl-L-Cysteine on Gold: A Combined ATR-IR, PM-IRRAS, and QCM Study. J. Phys. Chem. B 2005, 109, 22476–22485. [Google Scholar] [CrossRef] [PubMed]
- Osawa, M.; Ataka, K.I.; Yoshii, K.; Yotsuyanagi, T. Surface-Enhanced Infrared ATR Spectroscopy for in Situ Studies of Electrode/Electrolyte Interfaces. J. Electron Spectros. Relat. Phenomena 1993, 64–65, 371–379. [Google Scholar] [CrossRef]
- Hutter, E.; Assiongbon, K.A.; Fendler, J.H.; Roy, D. Fourier Transform Infrared Spectroscopy Using Polarization Modulation and Polarization Selective Techniques for Internal and External Reflection Geometries: Investigation of Self-Assembled Octadecylmercaptan on a Thin Gold Film. J. Phys. Chem. B 2003, 107, 7812–7819. [Google Scholar] [CrossRef]
- Lang, P.; Hajlaoui, R.; Garnier, F.; Desbat, B.; Buffeteau, T.; Horowitz, G.; Yassar, A. IR Spectroscopy Evidence for a Substrate-Dependent Organization of Sexithiophene Thin Films Vacuum Evaporated onto SiH/Si and SiO2/Si. J. Phys. Chem. 1995, 99, 5492–5499. [Google Scholar] [CrossRef]
- Zanfolim, A.A.; Volpati, D.; Olivati, C.A.; Job, A.E.; Constantino, C.J.L. Structural and Electric-Optical Properties of Zinc Phthalocyanine Evaporated Thin Films: Temperature and Thickness Effects. J. Phys. Chem. C 2010, 114, 12290–12299. [Google Scholar] [CrossRef]
- Zawisza, I.; Wittstock, G.; Boukherroub, R.; Szunerits, S. Polarization Modulation Infrared Reflection Absorption Spectroscopy Investigations of Thin Silica Films Deposited on Gold. 2. Structural Analysis of a 1,2-Dimyristoyl-Sn-Glycero-3-Phosphocholine Bilayer. Langmuir 2008, 24, 3922–3929. [Google Scholar] [CrossRef]
- Umemura, J.; Kamata, T.; Kawai, T.; Takenaka, T. Quantitative Evaluation of Molecular Orientation in Thin Langmuir-Blodgett Films by FT-IR Transmission and Reflection-Absorption Spectroscopy. J. Phys. Chem. 1990, 94, 62–67. [Google Scholar] [CrossRef]
- Früh, A.; Rutkowski, S.; Akimchenko, I.O.; Tverdokhlebov, S.I.; Frueh, J. Orientation Analysis of Polymer Thin Films on Metal Surfaces via IR Absorbance of the Relative Transition Dipole Moments. Appl. Surf. Sci. 2022, 594, 153476. [Google Scholar] [CrossRef]
- Hasegawa, T.; Takeda, S.; Kawaguchi, A.; Umemura, J. Quantitative Analysis of Uniaxial Molecular Orientation in Langmuir-Blodgett Films by Infrared Reflection Spectroscopy. Langmuir 1995, 11, 1236–1243. [Google Scholar] [CrossRef]
- Shioya, N.; Fujiwara, R.; Tomita, K.; Shimoaka, T.; Hasegawa, T. Simultaneous Analysis of Molecular Orientation and Quantity Change of Constituents in a Thin Film Using PMAIRS. J. Phys. Chem. A 2020, 124, 2714–2720. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sharma, S.K.; Olaluwoye, O.S.; Alrashdi, S.A.; Hasegawa, T.; Leblanc, R.M. Conformation Change of α-Synuclein(61—95) at the Air-Water Interface and Quantitative Measurement of the Tilt Angle of the Axis of Its α-Helix by Multiple Angle Incidence Resolution Spectroscopy. Colloids Surf. B Biointerfaces 2019, 183, 110401. [Google Scholar] [CrossRef] [PubMed]
- Shioya, N.; Shimoaka, T.; Murdey, R.; Hasegawa, T. Accurate Molecular Orientation Analysis Using Infrared P-Polarized Multiple-Angle Incidence Resolution Spectrometry (PMAIRS) Considering the Refractive Index of the Thin Film Sample. Appl. Spectrosc. 2017, 71, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.A.; Caseli, L.; de Almeida Olivati, C. Organization of Polythiophenes at Ultrathin Films Mixed with Stearic Acid Investigated with Polarization-Modulation Infrared Reflection–Absorption Spectroscopy. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 628–633. [Google Scholar] [CrossRef]
- Brosseau, C.L.; Leitch, J.; Bin, X.; Chen, M.; Roscoe, S.G.; Lipkowski, J. Electrochemical and PM-IRRAS a Glycolipid-Containing Biomimetic Membrane Prepared Using Langmuir-Blodgett/Langmuir-Schaefer Deposition. Langmuir 2008, 24, 13058–13067. [Google Scholar] [CrossRef]
- Sang, L.; Mudalige, A.; Sigdel, A.K.; Giordano, A.J.; Marder, S.R.; Berry, J.J.; Pemberton, J.E. PM-IRRAS Determination of Molecular Orientation of Phosphonic Acid Self-Assembled Monolayers on Indium Zinc Oxide. Langmuir 2015, 31, 5603–5613. [Google Scholar] [CrossRef]
- Milosevic, M.; Berets, S.L.; Fadeev, A.Y. Single-Reflection Attenuated Total Reflection of Organic Monolayers on Silicon. Appl. Spectrosc. 2003, 57, 724–727. [Google Scholar] [CrossRef]
- Aroca, R. Surface-Enhanced Vibrational Spectroscopy; Wiley: Hoboken, NJ, USA, 2006; ISBN 9780471607311. [Google Scholar]
- Tanaka, M.; Young, R.J. Review Polarised Raman Spectroscopy for the Study of Molecular Orientation Distributions in Polymers. J. Mater. Sci. 2006, 41, 963–991. [Google Scholar] [CrossRef]
- Svenningsson, L.; Nordstierna, L. Polarized Raman Spectroscopy Strategy for Molecular Orientation of Polymeric Fibers with Raman Tensors Deviating from the Molecular Frame. ACS Appl. Polym. Mater. 2020, 2, 4809–4813. [Google Scholar] [CrossRef]
- Li, Z.; Young, R.J.; Wilson, N.R.; Kinloch, I.A.; Vallés, C.; Li, Z. Effect of the Orientation of Graphene-Based Nanoplatelets upon the Young’s Modulus of Nanocomposites. Compos. Sci. Technol. 2016, 123, 125–133. [Google Scholar] [CrossRef]
- Zhang, S.; Mao, N.; Zhang, N.; Wu, J.; Tong, L.; Zhang, J. Anomalous Polarized Raman Scattering and Large Circular Intensity Differential in Layered Triclinic ReS2. ACS Nano 2017, 11, 10366–10372. [Google Scholar] [CrossRef] [PubMed]
- Sereda, V.; Ralbovsky, N.M.; Vasudev, M.C.; Naik, R.R.; Igor, K.; States, U.; States, U.; Branch, M.M.; Directorate, M.; Air, W.; et al. Polarized Raman Spectroscopy for Determining the Orientation of Di-D-Phenylalanine Molecules in a Nanotube. J. Raman Spectrosc. 2016, 47, 1056–1062. [Google Scholar] [CrossRef]
- Liu, T.; Kumar, S. Quantitative Characterization of SWNT Orientation by Polarized Raman Spectroscopy. Chem. Phys. Lett. 2003, 378, 257–262. [Google Scholar] [CrossRef]
- Xu, B.; Mao, N.; Zhao, Y.; Tong, L.; Zhang, J. Polarized Raman Spectroscopy for Determining Crystallographic Orientation of Low-Dimensional Materials. J. Phys. Chem. Lett. 2021, 12, 7442–7452. [Google Scholar] [CrossRef]
- Li, Z.; Young, R.J.; Kinloch, I.A.; Wilson, N.R.; Marsden, A.J.; Raju, A.P.A. Quantitative Determination of the Spatial Orientation of Graphene by Polarized Raman Spectroscopy. Carbon N. Y. 2015, 88, 215–224. [Google Scholar] [CrossRef]
- Diego Fernandes, J.; Maximino, M.D.; Braunger, M.L.; Pereira, M.S.; De Almeida Olivati, C.; Constantino, C.J.L.; Alessio, P. Supramolecular Architecture and Electrical Conductivity in Organic Semiconducting Thin Films. Phys. Chem. Chem. Phys. 2020, 22, 13554–13562. [Google Scholar] [CrossRef]
- Garcia, M.P.B.; Martin, C.S.; Kavazoi, H.S.; Maximino, M.D.; Alessio, P. Perylene Nanostructured Films Optimization for Organic Electronics: Molecular, Structural, and Electrochemical Characterization. Thin Solid Films 2023, 777, 139895. [Google Scholar] [CrossRef]
- Hupfer, M.L.; Meyer, R.; Deckert-Gaudig, T.; Ghosh, S.; Skabeev, A.; Peneva, K.; Deckert, V.; Dietzek, B.; Presselt, M. Supramolecular Reorientation during Deposition Onto Metal Surfaces of Quasi-Two-Dimensional Langmuir Monolayers Composed of Bifunctional Amphiphilic, Twisted Perylenes. Langmuir 2021, 37, 11018–11026. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, B.; Wu, Y.; Huang, Y.; Chen, Y. The Production of Horizontally Aligned Single-Walled Carbon Nanotubes. Carbon N. Y. 2011, 49, 4098–4110. [Google Scholar] [CrossRef]
- Ackermann, T.; Neuhaus, R.; Roth, S. The Effect of Rod Orientation on Electrical Anisotropy in Silver Nanowire Networks for Ultra-Transparent Electrodes. Sci. Rep. 2016, 6, 34289. [Google Scholar] [CrossRef]
- Miao, J.; Lin, J.Y.S. Nanometer-Thick Films of Aligned ZnO Nanowires Sensitized with Au Nanoparticles for Few-Ppb-Level Acetylene Detection. ACS Appl. Nano Mater. 2020, 3, 9174–9184. [Google Scholar] [CrossRef]
- Bunaciu, A.A.; Udriştioiu, E.G.; Aboul-Enein, H.Y. X-Ray Diffraction: Instrumentation and Applications. Crit. Rev. Anal. Chem. 2015, 45, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Rivalta, A.; Albonetti, C.; Biancone, D.; Della Ciana, M.; d’Agostino, S.; Biniek, L.; Brinkmann, M.; Giunchi, A.; Salzillo, T.; Brillante, A.; et al. Growth, Morphology and Molecular Orientation of Controlled Indigo Thin Films on Silica Surfaces. Surf. Interfaces 2021, 24, 101058. [Google Scholar] [CrossRef]
- Dynarowicz-Łątka, P.; Dhanabalan, A.; Oliveira, O.N. Modern Physicochemical Research on Langmuir Monolayers. Adv. Colloid Interface Sci. 2001, 91, 221–293. [Google Scholar] [CrossRef]
- Riul, A.; Miyazaki, C.M.; Dantas, C.A.; Oliveira, O.N.; Lvova, L.; Kirsanov, D.; DiNatale, C.; Legin, A. The Use of Nanostructured Films in Sensing Applications. In Multisensor Systems for Chemical Analysis; Lvova, L., Kirsanov, D., Di Natale, C., Legin, A., Eds.; Jenny Stanford Publishing: Dubai, United Arab Emirates, 2014; pp. 320–349. ISBN 978-981-4411-15-8. [Google Scholar]
- Mendelsohn, R.; Mao, G.; Flach, C.R. Infrared Reflection-Absorption Spectroscopy: Principles and Applications to Lipid-Protein Interaction in Langmuir Films. Biochim. Biophys. Acta-Biomembr. 2010, 1798, 788–800. [Google Scholar] [CrossRef]
- Cohen Stuart, M.A.; Wegh, R.A.J.; Kroon, J.M.; Sudhölter, E.J.R. Design and Testing of a Low-Cost and Compact Brewster Angle Microscope. Langmuir 1996, 12, 2863–2865. [Google Scholar] [CrossRef]
- Ulman, A. An Introduction to Ultrathin Organic Films: From Langmuir–Blodgett to Self-Assembly; Academic Press: San Diego, CA, USA, 1991. [Google Scholar]
- Miyazaki, C.M.; De Barros, A.; Mascagni, D.B.T.; Graça, J.S.; Campos, P.P.; Ferreira, M. Self-Assembly Thin Films for Sensing; Springer: Cham, Switzerland, 2016; ISBN 9783319478357. [Google Scholar]
- Hussain, S.A.; Dey, B.; Bhattacharjee, D.; Mehta, N. Unique Supramolecular Assembly through Langmuir–Blodgett (LB) Technique. Heliyon 2018, 4, e01038. [Google Scholar] [CrossRef]
- Saha, S.; Dutta, B.; Ghosh, M.; Chowdhury, J. Adsorption of 4-Mercapto Pyridine with Gold Nanoparticles Embedded in the Langmuir-Blodgett Film Matrix of Stearic Acid: SERS, XPS Studies Aided by Born-Oppenheimer on the Fly Dynamics, Time-Resolved Wavelet Transform Theory, and DFT. ACS Omega 2022, 7, 27818–27830. [Google Scholar] [CrossRef] [PubMed]
- Swierczewski, M.; Bürgi, T. Langmuir and Langmuir-Blodgett Films of Gold and Silver Nanoparticles. Langmuir 2022, 39, 2135–2151. [Google Scholar] [CrossRef] [PubMed]
- Tahghighi, M.; Mannelli, I.; Janner, D.; Ignés-Mullol, J. Tailoring Plasmonic Response by Langmuir–Blodgett Gold Nanoparticle Templating for the Fabrication of SERS Substrates. Appl. Surf. Sci. 2018, 447, 416–422. [Google Scholar] [CrossRef]
- Tahghighi, M.; Janner, D.; Ignés-Mullol, J. Optimizing Gold Nanoparticle Size and Shape for the Fabrication of Sers Substrates by Means of the Langmuir–Blodgett Technique. Nanomaterials 2020, 10, 2264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, M.; Yang, Z.; Zhang, X. Highly Flexible and Stretchable Strain Sensors Based on Conductive Whisker Carbon Nanotube Films. Carbon N. Y. 2021, 176, 139–147. [Google Scholar] [CrossRef]
- Abdulla, S.; Pullithadathil, B. Unidirectional Langmuir-Blodgett-Mediated Alignment of Polyaniline-Functionalized Multiwalled Carbon Nanotubes for NH3gas Sensor Applications. Langmuir 2020, 36, 11618–11628. [Google Scholar] [CrossRef]
- Yin, Z.; Ding, A.; Zhang, H.; Zhang, W. The Relevant Approaches for Aligning Carbon Nanotubes. Micromachines 2022, 13, 1863. [Google Scholar] [CrossRef] [PubMed]
- Giancane, G.; Bettini, S.; Valli, L. State of Art in the Preparation, Characterisation and Applications of Langmuir-Blodgett Films of Carbon Nanotubes. Colloids Surfaces A Physicochem. Eng. Asp. 2010, 354, 81–90. [Google Scholar] [CrossRef]
- Solanki, S.; Soni, A.; Agrawal, V.V.; Pandey, M.K.; Sumana, G. Ultrasensitive Immunosensor Based on Langmuir-Blodgett Deposited Ordered Graphene Assemblies for Dengue Detection. Langmuir 2021, 37, 8705–8713. [Google Scholar] [CrossRef]
- Jaafar, M.M.; Ciniciato, G.P.M.K.; Ibrahim, S.A.; Phang, S.M.; Yunus, K.; Fisher, A.C.; Iwamoto, M.; Vengadesh, P. Preparation of a Three-Dimensional Reduced Graphene Oxide Film by Using the Langmuir-Blodgett Method. Langmuir 2015, 31, 10426–10434. [Google Scholar] [CrossRef]
- Kouloumpis, A.; Thomou, E.; Chalmpes, N.; Dimos, K.; Spyrou, K.; Bourlinos, A.B.; Koutselas, I.; Gournis, D.; Rudolf, P. Graphene/Carbon Dot Hybrid Thin Films Prepared by a Modified Langmuir-Schaefer Method. ACS Omega 2017, 2, 2090–2099. [Google Scholar] [CrossRef] [PubMed]
- Scholl, F.A.; Siqueira, J.R.; Caseli, L. Graphene Oxide Modulating the Bioelectronic Properties of Penicillinase Immobilized in Lipid Langmuir-Blodgett Films. Langmuir 2022, 38, 2372–2378. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Chowdhury, J. Sustained and Improved Enzymatic Activity of Trypsin Immobilized in the Langmuir Blodgett Film of DPPC: A Rapid Enzyme Sensor for the Detection of Azocasein. Mater. Chem. Phys. 2020, 243, 122647. [Google Scholar] [CrossRef]
- de Souza Furtado, F.A.; Caseli, L. Enzyme Activity Preservation for Galactose Oxidase Immobilized in Stearic Acid Langmuir-Blodgett Films. Thin Solid Films 2020, 709, 138253. [Google Scholar] [CrossRef]
- Sinha, R.; Das, S.K.; Ghosh, M.; Chowdhury, J. Self-Assembled Gold Nanoparticles on the Serpentine Networks of Calf Thymus-DNA Langmuir-Blodgett Films as Efficient SERS Sensing Platform: Fabrication and Its Application in Thiram Detection. Mater. Chem. Phys. 2023, 295, 127140. [Google Scholar] [CrossRef]
- Andrés, M.A.; Vijjapu, M.T.; Surya, S.G.; Shekhah, O.; Salama, K.N.; Serre, C.; Eddaoudi, M.; Roubeau, O.; Gascón, I. Methanol and Humidity Capacitive Sensors Based on Thin Films of MOF Nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 4155–4162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zheng, B.; Li, L.; Song, J.; Song, L.; Zhang, M. Fewer-Layer Conductive Metal-Organic Langmuir-Blodgett Films as Electrocatalysts Enable an Ultralow Detection Limit of H2O2. Appl. Surf. Sci. 2021, 539, 148255. [Google Scholar] [CrossRef]
- da Rocha Rodrigues, R.; da Silva, R.L.C.G.; Caseli, L.; Péres, L.O. Conjugated Polymers as Langmuir and Langmuir-Blodgett Films: Challenges and Applications in Nanostructured Devices. Adv. Colloid Interface Sci. 2020, 285, 102277. [Google Scholar] [CrossRef]
- Ly, T.N.; Park, S. Highly Sensitive Gas Sensor Using Hierarchically Self-Assembled Thin Films of Graphene Oxide and Gold Nanoparticles. J. Ind. Eng. Chem. 2018, 67, 417–428. [Google Scholar] [CrossRef]
- Ma, K.; Wang, R.; Rao, Y.; Zhao, W.; Liu, S.; Jiao, T. Langmuir-Blodgett Films of Two Chiral Perylene Bisimide-Based Molecules: Aggregation and Supramolecular Chirality. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 591, 124563. [Google Scholar] [CrossRef]
- Kouloumpis, A.; Vourdas, N.; Zygouri, P.; Chalmpes, N.; Potsi, G.; Kostas, V.; Spyrou, K.; Stathopoulos, V.N.; Gournis, D.; Rudolf, P. Controlled Deposition of Fullerene Derivatives within a Graphene Template by Means of a Modified Langmuir-Schaefer Method. J. Colloid Interface Sci. 2018, 524, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Chalmpes, N.; Patila, M.; Kouloumpis, A.; Alatzoglou, C.; Spyrou, K.; Subrati, M.; Polydera, A.C.; Bourlinos, A.B.; Stamatis, H.; Gournis, D. Graphene Oxide-Cytochrome c Multilayered Structures for Biocatalytic Applications: Decrypting the Role of Surfactant in Langmuir-Schaefer Layer Deposition. ACS Appl. Mater. Interfaces 2022, 14, 26204–26215. [Google Scholar] [CrossRef] [PubMed]
- Gür, B.; Ayhan, M.E.; Türkhan, A.; Gür, F.; Kaya, E.D. A Facile Immobilization of Polyphenol Oxidase Enzyme on Graphene Oxide and Reduced Graphene Oxide Thin Films: An Insight into in-Vitro Activity Measurements and Characterization. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 562, 179–185. [Google Scholar] [CrossRef]
- Cote, L.J.; Kim, F.; Huang, J. Langmuir-Blodgett Assembly of Graphite Oxide Single Layers. J. Am. Chem. Soc. 2009, 131, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, G.J.; Vecitis, C.D. Wrinkling and Periodic Folding of Graphene Oxide Monolayers by Langmuir-Blodgett Compression. Langmuir 2017, 33, 9880–9888. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Wang, S.; Feng, X.; Pauly, M.; Decher, G.; Long, Y. In-Plane Aligned Assemblies of 1D-Nanoobjects: Recent Approaches and Applications. Chem. Soc. Rev. 2020, 49, 509–553. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y.; Wang, Q.; Zou, L.; Ye, B. The Construction of Well-Aligned MWCNTs-PANI Langmuir-Blodgett Film Modified Glassy Carbon Electrode and Its Analytical Application. Sensors Actuators, B Chem. 2016, 228, 214–220. [Google Scholar] [CrossRef]
- Bonatout, N.; Muller, F.; Fontaine, P.; Gascon, I.; Konovalov, O.; Goldmann, M. How Exfoliated Graphene Oxide Nanosheets Organize at the Water Interface: Evidence for a Spontaneous Bilayer Self-Assembly. Nanoscale 2017, 9, 12543–12548. [Google Scholar] [CrossRef]
- Zheng, Q.; Ip, W.H.; Lin, X.; Yousefi, N.; Yeung, K.K.; Li, Z.; Kim, J.K. Consisting of Ultralarge Graphene Sheets Produced by Langmuir À Blodgett Assembly. ACS Nano 2011, 5, 6039–6051. [Google Scholar] [CrossRef]
- Mujica, M.L.; Zhang, Y.; Bédioui, F.; Gutiérrez, F.; Rivas, G. Label-Free Graphene Oxide–Based SPR Genosensor for the Quantification of MicroRNA21. Anal. Bioanal. Chem. 2020, 412, 3539–3546. [Google Scholar] [CrossRef]
- Liu, B.; Salgado, S.; Maheshwari, V.; Liu, J. DNA Adsorbed on Graphene and Graphene Oxide: Fundamental Interactions, Desorption and Applications. Curr. Opin. Colloid Interface Sci. 2016, 26, 41–49. [Google Scholar] [CrossRef]
- Gulotta, F.A.; Paz Zanini, V.I.; López de Mishima, B.A.; Martino, D.M.; Linarez Pérez, O.E.; Ferreyra, N.F. Electrostatically Mediated Layer-by-Layer Assembly of a Bioinspired Thymine Polycation and Gold Nanoparticles. J. Electroanal. Chem. 2021, 883, 114895. [Google Scholar] [CrossRef]
- Upan, J.; Youngvises, N.; Tuantranont, A.; Karuwan, C.; Banet, P.; Aubert, P.H.; Jakmunee, J. A Simple Label-Free Electrochemical Sensor for Sensitive Detection of Alpha-Fetoprotein Based on Specific Aptamer Immobilized Platinum Nanoparticles/Carboxylated-Graphene Oxide. Sci. Rep. 2021, 11, 13969. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, C.M.; Camilo, D.E.; Shimizu, F.M.; Ferreira, M. Improved Antibody Loading on Self-Assembled Graphene Oxide Films for Using in Surface Plasmon Resonance Immunosensors. Appl. Surf. Sci. 2019, 490, 502–509. [Google Scholar] [CrossRef]
- Miyazaki, C.M.; Mishra, R.; Kinahan, D.J.; Ferreira, M.; Ducrée, J. Polyethylene Imine/Graphene Oxide Layer-by-Layer Surface Functionalization for Significantly Improved Limit of Detection and Binding Kinetics of Immunoassays on Acrylate Surfaces. Colloids Surfaces B Biointerfaces 2017, 158, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Nangare, S.; Patil, P. Poly(Allylamine) Coated Layer-by-Layer Assembly Decorated 2D Carbon Backbone for Highly Sensitive and Selective Detection of Tau-441 Using Surface Plasmon Resonance Biosensor. Anal. Chim. Acta 2023, 1271, 341474. [Google Scholar] [CrossRef] [PubMed]
- Massey, M.K.; Pearson, C.; Zeze, D.A.; Mendis, B.G.; Petty, M.C. The Electrical and Optical Properties of Oriented Langmuir-Blodgett Films of Single-Walled Carbon Nanotubes. Carbon N. Y. 2011, 49, 2424–2430. [Google Scholar] [CrossRef]
- Baratto, C.; Golovanova, V.; Faglia, G.; Hakola, H.; Niemi, T.; Tkachenko, N.; Nazarchurk, B.; Golovanov, V. On the Alignment of ZnO Nanowires by Langmuir–Blodgett Technique for Sensing Application. Appl. Surf. Sci. 2020, 528, 146959. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Li, G.; Ye, B. A New Strategy for Enhancing Electrochemical Sensing from MWCNTs Modified Electrode with Langmuir-Blodgett Film and Used in Determination of Methylparaben. Sensors Actuators, B Chem. 2015, 211, 332–338. [Google Scholar] [CrossRef]
- He, Z.; Wang, J.L.; Chen, S.M.; Liu, J.W.; Yu, S.H. Self-Assembly of Nanowires: From Dynamic Monitoring to Precision Control. Acc. Chem. Res. 2022, 55, 1480–1491. [Google Scholar] [CrossRef]
- Bodik, M.; Maxian, O.; Hagara, J.; Nadazdy, P.; Jergel, M.; Majkova, E.; Siffalovic, P. Langmuir-Scheaffer Technique as a Method for Controlled Alignment of 1D Materials. Langmuir 2020, 36, 4540–4547. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Gupta, S.M.; Sharma, S.K. Carbon Nanotubes: Synthesis, Properties and Engineering Applications. Carbon Lett. 2019, 29, 419–447. [Google Scholar] [CrossRef]
- Rathinavel, S.; Priyadharshini, K.; Panda, D. A Review on Carbon Nanotube: An Overview of Synthesis, Properties, Functionalization, Characterization, and the Application. Mater. Sci. Eng. B 2021, 268, 115095. [Google Scholar] [CrossRef]
- Bian, R.; Meng, L.; Zhang, M.; Chen, L.; Liu, H. Aligning One-Dimensional Nanomaterials by Solution Processes. ACS Omega 2019, 4, 1816–1823. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, L.; Wang, X.; Shimoyama, I.; Sun, X.; Seo, W.K.; Dai, H. Langmuir-Blodgett Assembly of Densely Aligned Single-Walled Carbon Nanotubes from Bulk Materials. J. Am. Chem. Soc. 2007, 129, 4890–4891. [Google Scholar] [CrossRef] [PubMed]
- Tao, A.; Kim, F.; Hess, C.; Goldberger, J.; He, R.; Sun, Y.; Xia, Y.; Yang, P. Langmuir-Blodgett Silver Nanowire Monolayers for Molecular Sensing Using Surface-Enhanced Raman Spectroscopy. Nano Lett. 2003, 3, 1229–1233. [Google Scholar] [CrossRef]
- Sheng, S.Z.; Zheng, S.Q.; Feng, X.F.; Zhou, L.R.; Tao, Z.C.; Liu, J.W. Anisotropic Nanoparticle Arrays Guided by Ordered Nanowire Films Enhance Surface-Enhanced Raman Scattering. Adv. Opt. Mater. 2023, 11, 2201682. [Google Scholar] [CrossRef]
- Saha, S.; Ghosh, M.; Chowdhury, J. Infused Self-Assembly on Langmuir–Blodgett Film: Fabrication of Highly Efficient SERS Active Substrates with Controlled Plasmonic Aggregates. J. Raman Spectrosc. 2019, 50, 330–344. [Google Scholar] [CrossRef]
- Das, S.K.; Pal, K.; Bhattacharya, T.S.; Karmakar, P.; Chowdhury, J. Fabrication of SERS Active Langmuir–Blodgett Film Substrate for Screening Human Cancer Cell Lines: Experimental Observations Supported by Multivariate Data Analyses. Sens. Actuators B Chem. 2019, 299, 126962. [Google Scholar] [CrossRef]
- Tramonti, V.; Lofrumento, C.; Martina, M.R.; Lucchesi, G.; Caminati, G. Graphene Oxide/Silver Nanoparticles Platforms for the Detection and Discrimination of Native and Fibrillar Lysozyme: A Combined QCM and SERS Approach. Nanomaterials 2022, 12, 600. [Google Scholar] [CrossRef]
- Tim, B.; Błaszkiewicz, P.; Nowicka, A.B.; Kotkowiak, M. Optimizing SERS Performance through Aggregation of Gold Nanorods in Langmuir-Blodgett Films. Appl. Surf. Sci. 2022, 573, 151518. [Google Scholar] [CrossRef]
- Wu, Y.H.; Imae, T.; Ujihara, M. Surface Enhanced Plasmon Effects by Gold Nanospheres and Nanorods in Langmuir-Blodgett Films. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 532, 213–221. [Google Scholar] [CrossRef]
- Aleksandrovic, V.; Greshnykh, D.; Randjelovic, I.; Frömsdorf, A.; Kornowski, A.; Roth, S.V.; Klinke, C.; Weller, H. Preparation and Electrical Properties of Cobalt-Platinum Nanoparticle Monolayers Deposited by the Langmuir-Blodgett Technique. ACS Nano 2008, 2, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhao, Q.; Zhang, N.; Man, S.Q. Facile Fabrication of Large-Area and Uniform Silica Nanospheres Monolayer for Efficient Surface-Enhanced Raman Scattering. Appl. Surf. Sci. 2014, 308, 247–252. [Google Scholar] [CrossRef]
- Tang, J.; Zeng, C.; Wang, Y.; Lin, Z.; Man, S.Q. Langmuir–Blodgett Film of Esterifiable Silica Nanoparticles as Substrates for Surface-Enhanced Raman Scattering. Plasmonics 2015, 10, 563–568. [Google Scholar] [CrossRef]
- Pandey, C.M.; Pandey, M.K.; Sumana, G. Langmuir–Blodgett Based Ordered Deposition of Functionalized Iron Oxide Nanoparticles for Ultrasensitive Detection of Escherichia Coli O157: H7. Microchem. J. 2022, 181, 107708. [Google Scholar] [CrossRef]
- Saleh Khasraw, S.; Ghaderi, S.; Raza Saeed, S.; Hallaj, R.; Hassanzadeh, A. Observation of Nanodomains and Nanostripes in the Langmuir-Blodgett Monolayers of Fe3O4 Magnetic Nanoparticles. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2021, 273, 115402. [Google Scholar] [CrossRef]
- Ariga, K. Interfacial Nanoarchitectonics with Porphyrins and Related Molecules: Langmuir-Blodgett Method and Layer-by-Layer Assembly. J. Porphyr. Phthalocyanines 2023, 27, 924–945. [Google Scholar] [CrossRef]
- Maximino, M.D.; Martin, C.S.; Pereira, M.S.; Aléssio, P. Metallic Phthalocyanines: Impact of the Film Deposition Method on Its Supramolecular Arrangement and Sensor Performance. An. Acad. Bras. Cienc. 2019, 91, 1–14. [Google Scholar] [CrossRef]
- Bettini, S.; Pagano, R.; Borovkov, V.; Giancane, G.; Valli, L. The Role of the Central Metal Ion of Ethane-Bridged Bis-Porphyrins in Histidine Sensing. J. Colloid Interface Sci. 2019, 533, 762–770. [Google Scholar] [CrossRef]
- Bettini, S.; Grover, N.; Ottolini, M.; Mattern, C.; Valli, L.; Senge, M.O.; Giancane, G. Enantioselective Discrimination of Histidine by Means of an Achiral Cubane-Bridged Bis-Porphyrin. Langmuir 2021, 37, 13882–13889. [Google Scholar] [CrossRef] [PubMed]
- Ermakova, E.V.; Koroleva, E.O.; Shokurov, A.V.; Arslanov, V.V.; Bessmertnykh-Lemeune, A. Ultra-Thin Film Sensors Based on Porphyrin-5-Ylphosphonate Diesters for Selective and Sensitive Dual-Channel Optical Detection of Mercury(II) Ions. Dye. Pigment. 2021, 186, 108967. [Google Scholar] [CrossRef]
- Akyüz, D.; Koca, A. An Electrochemical Sensor for the Detection of Pesticides Based on the Hybrid of Manganese Phthalocyanine and Polyaniline. Sens. Actuators B Chem. 2019, 283, 848–856. [Google Scholar] [CrossRef]
- Miyazaki, C.M.C.M.; Shimizu, F.M.F.M.; Ferreira, M.; Mejía-Salazar, J.R.; Oliveira, O.N.; Ferreira, M. Surface Plasmon Resonance (SPR) for Sensors and Biosensors; William Andrew Publishing: Norwich, NY, USA, 2017; Volume 28, ISBN 9780323497794. [Google Scholar]
- de Lucena, N.C.; Miyazaki, C.M.; Shimizu, F.M.; Constantino, C.J.L.; Ferreira, M. Layer-by-Layer Composite Film of Nickel Phthalocyanine and Montmorillonite Clay for Synergistic Effect on Electrochemical Detection of Dopamine. Appl. Surf. Sci. 2018, 436, 957–966. [Google Scholar] [CrossRef]
- da Silva, V.N.C.; Farias, E.A.D.O.; Araújo, A.R.; Xavier Magalhães, F.E.; Neves Fernandes, J.R.; Teles Souza, J.M.; Eiras, C.; Alves da Silva, D.; Hugo do Vale Bastos, V.; Teixeira, S.S. Rapid and Selective Detection of Dopamine in Human Serum Using an Electrochemical Sensor Based on Zinc Oxide Nanoparticles, Nickel Phthalocyanines, and Carbon Nanotubes. Biosens. Bioelectron. 2022, 210, 114211. [Google Scholar] [CrossRef]
- Paula-Ayoub, F.; Caseli, L. Controlling the Molecular Architecture of Lactase Immobilized in Langmuir-Blodgett Films of Phospholipids to Modulate the Enzyme Activity. Colloids Surf. B Biointerfaces 2017, 150, 8–14. [Google Scholar] [CrossRef]
- Girard-Egrot, A.P.; Godoy, S.; Blum, L.J. Enzyme Association with Lipidic Langmuir-Blodgett Films: Interests and Applications in Nanobioscience. Adv. Colloid Interface Sci. 2005, 116, 205–225. [Google Scholar] [CrossRef] [PubMed]
- Araujo, F.T.; Peres, L.O.; Caseli, L. Conjugated Polymers Blended with Lipids and Galactosidase as Langmuir-Blodgett Films to Control the Biosensing Properties of Nanostructured Surfaces. Langmuir 2019, 35, 7294–7303. [Google Scholar] [CrossRef]
- Decher, G.; Hong, J.D.; Schmitt, J. Buildup of Ultrathin Multilayer Films by a Self-Assembly Process: III. Consecutively Alternating Adsorption of Anionic and Cationic Polyelectrolytes on Charged Surfaces. Thin Solid Films 1992, 210–211, 831–835. [Google Scholar] [CrossRef]
- Schönhoff, M. Layered Polyelectrolyte Complexes: Physics of Formation and Molecular Properties. J. Phys. Condens. Matter 2003, 15, R1781–R1808. [Google Scholar] [CrossRef]
- Behrens, S.H.; Grier, D.G. The Charge of Glass and Silica Surfaces. J. Chem. Phys. 2001, 115, 6716–6721. [Google Scholar] [CrossRef]
- Pujari, S.P.; Scheres, L.; Marcelis, A.T.M.; Zuilhof, H. Covalent Surface Modification of Oxide Surfaces. Angew. Chemie-Int. Ed. 2014, 53, 6322–6356. [Google Scholar] [CrossRef] [PubMed]
- Alessio, P.; Rodríguez-Méndez, M.L.; De Saja Saez, J.A.; Constantino, C.J.L. Iron Phthalocyanine in Non-Aqueous Medium Forming Layer-by-Layer Films: Growth Mechanism, Molecular Architecture and Applications. Phys. Chem. Chem. Phys. 2010, 12, 3972–3983. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.G.R.; Brazaca, L.C.; Rodríguez-Mendez, M.L.; de Saja, J.A.; Zucolotto, V. Immobilization of Lutetium Bisphthalocyanine in Nanostructured Biomimetic Sensors Using the LbL Technique for Phenol Detection. Biosens. Bioelectron. 2011, 26, 4715–4719. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Lu, S.; Jiang, S.P. Layer-by-Layer Self-Assembly in the Development of Electrochemical Energy Conversion and Storage Devices from Fuel Cells to Supercapacitors. Chem. Soc. Rev. 2012, 41, 7291–7321. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.; Ortega, F.; Rubio, R.G. Layer-by-Layer Materials for the Fabrication of Devices with Electrochemical Applications. Energies 2022, 15, 3399. [Google Scholar] [CrossRef]
- Oliveira, D.A.; Gasparotto, L.H.S.; Siqueira, J.R. Processing of Nanomaterials in Layer-by-Layer Films: Potential Applications in (Bio)Sensing and Energy Storage. An. Acad. Bras. Cienc. 2019, 91, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Iost, R.M.; Crespilho, F.N. Layer-by-Layer Self-Assembly and Electrochemistry: Applications in Biosensing and Bioelectronics. Biosens. Bioelectron. 2012, 31, 1–10. [Google Scholar] [CrossRef]
- Richardson, J.J.; Cui, J.; Björnmalm, M.; Braunger, J.A.; Ejima, H.; Caruso, F. Innovation in Layer-by-Layer Assembly. Chem. Rev. 2016, 116, 14828–14867. [Google Scholar] [CrossRef]
- Miyazaki, C.M.; Shimizu, F.M.; Ferreira, M. Layer-by-Layer Nanostructured Films for Electrochemical Sensors Fabrication. In Functionalized Nanomaterial-Based Electrochemical Sensors: Principles, Fabrication Methods, and Applications; Hussain, C.M., Manjunatha, J.G., Eds.; Woodhead Publishing: Sawston, UK, 2022; pp. 407–441. ISBN 9780128237885. [Google Scholar]
- Kurapati, R.; Groth, T.W.; Raichur, A.M. Recent Developments in Layer-by-Layer Technique for Drug Delivery Applications. ACS Appl. Bio Mater. 2019, 2, 5512–5527. [Google Scholar] [CrossRef]
- Guzmán, E.; Rubio, R.G.; Ortega, F. A Closer Physico-Chemical Look to the Layer-by-Layer Electrostatic Self-Assembly of Polyelectrolyte Multilayers. Adv. Colloid Interface Sci. 2020, 282, 102197. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.F.; Wong, W.T.; Rogach, A.L. Molecular Design of Layer-by-Layer Functionalized Liposomes for Oral Drug Delivery. ACS Appl. Mater. Interfaces 2020, 12, 43341–43351. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Hu, Y.; Li, C.; Qin, C.; Shi, M.; Ye, M. Layer-by-Layer Self-Assembly of Graphene Nanoplatelets. Langmuir 2009, 25, 6122–6128. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.A.; Molinnus, D.; Beging, S.; Siqueira, J.R.; Schöning, M.J. Biosensor Based on Self-Assembled Films of Graphene Oxide and Polyaniline Using a Field-Effect Device Platform. Phys. Status Solidi Appl. Mater. Sci. 2021, 218, 2000747. [Google Scholar] [CrossRef]
- Lipton, J.; Weng, G.M.; Rӧhr, J.A.; Wang, H.; Taylor, A.D. Layer-by-Layer Assembly of Two-Dimensional Materials: Meticulous Control on the Nanoscale. Matter 2020, 2, 1148–1165. [Google Scholar] [CrossRef]
- Rodrigues, G.H.S.; Miyazaki, C.M.; Rubira, R.J.G.; Constantino, C.J.L.; Ferreira, M. Layer-by-Layer Films of Graphene Nanoplatelets and Gold Nanoparticles for Methyl Parathion Sensing. ACS Appl. Nano Mater. 2019, 2, 1082–1091. [Google Scholar] [CrossRef]
- Mascagni, D.B.T.; Miyazaki, C.M.; da Cruz, N.C.; de Moraes, M.L.; Riul, A.; Ferreira, M. Layer-by-Layer Assembly of Functionalized Reduced Graphene Oxide for Direct Electrochemistry and Glucose Detection. Mater. Sci. Eng. C 2016, 68, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Hao, J.; Yang, S.; Sun, Y.; Wang, Y.; Zhang, W.; Mao, H.; Song, X.M. Layer-by-Layer Self-Assembly Film of PEI-Reduced Graphene Oxide Composites and Cholesterol Oxidase for Ultrasensitive Cholesterol Biosensing. Sens. Actuators B Chem. 2019, 298, 126856. [Google Scholar] [CrossRef]
- Oytun, F.; Basarir, F. Spin-Assisted Layer-by-Layer Assembled Oppositely Charged Reduced Graphene Oxide Films. Mater. Lett. 2019, 257, 126756. [Google Scholar] [CrossRef]
- Miyazaki, C.M.; Adriano, A.M.; Rubira, R.J.G.; Constantino, C.J.L.; Ferreira, M. Combining Electrochemically Reduced Graphene Oxide and Layer-by-Layer Films of Magnetite Nanoparticles for Carbofuran Detection. J. Environ. Chem. Eng. 2020, 8, 104294. [Google Scholar] [CrossRef]
- Oliveira, D.A.; Lutkenhaus, J.L.; Siqueira, J.R. Building up Nanostructured Layer-by-Layer Films Combining Reduced Graphene Oxide-Manganese Dioxide Nanocomposite in Supercapacitor Electrodes. Thin Solid Films 2021, 718, 138483. [Google Scholar] [CrossRef]
- Xu, X.; Huang, D.; Cao, K.; Wang, M.; Zakeeruddin, S.M.; Grätzel, M. Electrochemically Reduced Graphene Oxide Multilayer Films as Efficient Counter Electrode for Dye-Sensitized Solar Cells. Sci. Rep. 2013, 3, 1489. [Google Scholar] [CrossRef]
- Al-Hamry, A.; Lu, T.; Bai, J.; Adiraju, A.; Ega, T.K.; Paterno, L.G.; Pašti, I.A.; Kanoun, O. Versatile Sensing Capabilities of Layer-by-Layer Deposited Polyaniline-Reduced Graphene Oxide Composite-Based Sensors. Sens. Actuators B Chem. 2023, 390, 133988. [Google Scholar] [CrossRef]
- Al-Hamry, A.; Lu, T.; Bai, J.; Adiraju, A.; Ega, T.K.; Pašti, I.A.; Kanoun, O. Layer-by-Layer Deposited Multi-Modal PDAC/RGO Composite-Based Sensors. Foods 2023, 12, 268. [Google Scholar] [CrossRef] [PubMed]
- Kruk, T.; Socha, R.P.; Szyk-Warszyńska, L.; Warszyński, P. Flexible and Ultrathin Polyelectrolyte Conductive Coatings Formed with Reduced Graphene Oxide as a Base for Advanced New Materials. Appl. Surf. Sci. 2019, 484, 501–510. [Google Scholar] [CrossRef]
- Davia, F.G.; Johner, N.P.; Calvo, E.J.; Williams, F.J. Growth and Electrochemical Stability of a Layer-by-Layer Thin Film Containing Tetrasulfonated Fe Phthalocyanine. J. Electroanal. Chem. 2020, 877, 114485. [Google Scholar] [CrossRef]
- Ali, B.; McCormac, T.; Maccato, C.; Barreca, D.; Carraro, G. Multilayer Assemblies of a Cu-Phthalocyanine with Dawson Type Polyoxometalates (POMs) for the Electrocatalytic Reduction of Phosphate. J. Electroanal. Chem. 2020, 858, 113770. [Google Scholar] [CrossRef]
- Maciel, C.C.; de SM Freitas, A.; Medrades, J.P.; Ferreira, M. Simultaneous Determination of Catechol and Paraquat Using a Flexible Electrode of PBAT and Graphite Modified with Gold Nanoparticles and Copper Phthalocyanine (g-PBAT/AuNP/CuTsPc) LbL Film. J. Electrochem. Soc. 2022, 169, 027505. [Google Scholar] [CrossRef]
- de Lima, L.F.; Maciel, C.C.; Ferreira, A.L.; de Almeida, J.C.; Ferreira, M. Nickel (II) Phthalocyanine-Tetrasulfonic-Au Nanoparticles Nanocomposite Film for Tartrazine Electrochemical Sensing. Mater. Lett. 2020, 262, 127196. [Google Scholar] [CrossRef]
- de Lima, L.F.; Pereira, E.A.; Ferreira, M. Electrochemical Sensor for Propylparaben Using Hybrid Layer-by-Layer Films Composed of Gold Nanoparticles, Poly(Ethylene Imine) and Nickel(II) Phthalocyanine Tetrasulfonate. Sensors Actuators, B Chem. 2020, 310, 127893. [Google Scholar] [CrossRef]
- Ribeiro, C.D.L.; De Souza, J.R.; Pereira-Da-Silva, M.A.; Santos, V.O.; Paterno, L.G. Electrocatalytic Oxidation of Ethinyl Estradiol by an Iron Oxide Nanoparticle/Nickel Phthalocyanine Supramolecular Electrode. J. Phys. Chem. C 2020, 124, 19057–19069. [Google Scholar] [CrossRef]
- Furini, L.N.; Fernandes, J.D.; Vieira, D.H.; Morato, L.F.d.C.; Alves, N.; Constantino, C.J.L. Combining Impedance Spectroscopy and Information Visualization Methods to Optimize the Detection of Carbendazim Using Layer-by-Layer Films. Chemosensors 2023, 11, 213. [Google Scholar] [CrossRef]
- Belce, Y.; Cebeci, F. Investigation of PH and Concentration Influence on Layer-by-Layer Self-Assembly for Nickel(II)Phthalocyaninetetrasulfonic Acid Tetrasodium Salt Coatings. In Porphyrin Science By Women (In 3 Volumes); World Scientific Publishing Co.: Singapore, 2020; pp. 834–840. ISBN 9789811223556. [Google Scholar]
- de Oliveira, M.S.; Farias, E.A.d.O.; de Sousa, A.M.S.; Dionísio, N.A.; Teixeira, P.R.S.; Teixeira, A.S.d.N.M.; da Silva, D.A.; Eiras, C. Composite Films Based on Copper Nanoparticles and Nickel Phthalocyanine as Electrochemical Sensors for Serotonin Detection. Surf. Interfaces 2021, 25, 101245. [Google Scholar] [CrossRef]
- Coelho, M.K.L.; da Silva, D.N.; Pereira, A.C. Development of Electrochemical Sensor Based on Carbonaceal and Metal Phthalocyanines Materials for Determination of Ethinyl Estradiol. Chemosensors 2019, 7, 32. [Google Scholar] [CrossRef]
- Rangel, D.P.B.; Miyazaki, C.M.; Mascagni, D.B.T.; Ferreira, M. Layer-by-Layer Films of Gold Nanoparticles and Carbon Nanotubes for Improved Amperometric Detection of Cholesterol. J. Nanosci. Nanotechnol. 2019, 19, 5483–5488. [Google Scholar] [CrossRef]
- Basova, T.V.; Ray, A.K. Review—Hybrid Materials Based on Phthalocyanines and Metal Nanoparticles for Chemiresistive and Electrochemical Sensors: A Mini-Review. ECS J. Solid State Sci. Technol. 2020, 9, 061001. [Google Scholar] [CrossRef]
- Winiarski, J.P.; de Melo, D.J.; Santana, E.R.; Santos, C.S.; de Jesus, C.G.; Fujiwara, S.T.; Wohnrath, K.; Pessôa, C.A. Layer-By-Layer Films of Silsesquioxane and Nickel(II) Tetrasulphophthalocyanine as Glucose Oxidase Platform Immobilization: Amperometric Determination of Glucose in Kombucha Beverages. Chemosensors 2023, 11, 346. [Google Scholar] [CrossRef]
- Salvo-Comino, C.; González-Gil, A.; Rodriguez-Valentin, J.; Garcia-Hernandez, C.; Martin-Pedrosa, F.; Garcia-Cabezon, C.; Rodriguez-Mendez, M.L. Biosensors Platform Based on Chitosan/AuNPs/Phthalocyanine Composite Films for the Electrochemical Detection of Catechol. the Role of the Surface Structure. Sensors 2020, 20, 2152. [Google Scholar] [CrossRef]
- Fritea, L.; Banica, F.; Costea, T.O.; Moldovan, L.; Dobjanschi, L.; Muresan, M.; Cavalu, S. Metal Nanoparticles and Carbon-Based Nanomaterials for Improved Performances of Electrochemical (Bio)Sensors with Biomedical Applications. Materials 2021, 14, 6319. [Google Scholar] [CrossRef]
- Pang, X.; Li, F.; Huang, S.; Yang, Z.; Mo, Q.; Huang, L.; Xu, W.; Chen, L.; Li, X. Electrostatically Mediated Layer-by-Layer Assembly of Nitrogen-Doped Graphene/PDDA/Gold Nanoparticle Composites for Electrochemical Detection of Uric Acid. Anal. Bioanal. Chem. 2020, 412, 669–680. [Google Scholar] [CrossRef]
- de Almeida, J.C.; de Barros, A.; Odone Mazali, I.; Ferreira, M. Influence of Gold Nanostructures Incorporated into Sodium Montmorillonite Clay Based on LbL Films for Detection of Metal Traces Ions. Appl. Surf. Sci. 2020, 507, 144972. [Google Scholar] [CrossRef]
- Wu, H.; Chen, Z.; Guo, L.; Wang, Y.; Du, S.; Wu, Y.; Ren, Z. Direct Coupling of Phthalocyanine Cobalt(II) and Graphene via Self-Driven Layer-by-Layer Assembly for Efficient Electrochemical Detection of Catechol. J. Electrochem. Soc. 2020, 167, 027533. [Google Scholar] [CrossRef]
- Teixeira, P.R.; Machado, T.R.; Machado, F.; Sodré, F.F.; Silva, J.G.; Neto, B.A.D.; Paterno, L.G. Au Nanoparticle-Poly(Ionic Liquid) Nanocomposite Electrode for the Voltammetric Detection of Triclosan in Lake Water and Toothpaste Samples. Microchem. J. 2020, 152, 104421. [Google Scholar] [CrossRef]
- Teeparuksapun, K.; Hedström, M.; Mattiasson, B. A Sensitive Capacitive Biosensor for Protein a Detection Using Human Igg Immobilized on an Electrode Using Layer-by-Layer Applied Gold Nanoparticles. Sensors 2022, 22, 99. [Google Scholar] [CrossRef]
- Camilo, D.E.; Miyazaki, C.M.; Shimizu, F.M.; Ferreira, M. Improving Direct Immunoassay Response by Layer-by-Layer Films of Gold Nanoparticles–Antibody Conjugate towards Label-Free Detection. Mater. Sci. Eng. C 2019, 102, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Barsan, M.M.; Brett, C.M.A. Recent Advances in Layer-by-Layer Strategies for Biosensors Incorporating Metal Nanoparticles. TrAC-Trends Anal. Chem. 2016, 79, 286–296. [Google Scholar] [CrossRef]
- Medrades, J.d.P.; Maciel, C.C.; Moraes, A.d.S.; Leite, F.d.L.; Ferreira, M. Flavin Adenine Dinucleotide Functionalized Gold Nanoparticles for the Electrochemical Detection of Dopamine. Sens. Actuators Rep. 2022, 4, 100085. [Google Scholar] [CrossRef]
- Salvo-Comino, C.; Garcia-Hernandez, C.; Garcia-Cabezon, C.; Rodriguez-Mendez, M.L. Promoting Laccase Sensing Activity for Catechol Detection Using LBL Assemblies of Chitosan/Ionic Liquid/Phthalocyanine as Immobilization Surfaces. Bioelectrochemistry 2020, 132, 107407. [Google Scholar] [CrossRef]
- Martínez-Hernández, M.E.; Goicoechea, J.; Rivero, P.J.; Arregui, F.J. In-Situ Synthesis of Gold Nanoparticles in Layer-by-Layer Polymeric Coatings for the Fabrication of Optical Fiber Sensors. Polymers 2022, 14, 776. [Google Scholar] [CrossRef]
- Almeida, L.A.; Rodrigues, B.V.M.; Balogh, D.T.; Sanfelice, R.C.; Mercante, L.A.; Frade-Barros, A.F.; Pavinatto, A. Chitosan/Gold Nanoparticles Nanocomposite Film for Bisphenol A Electrochemical Sensing. Electrochem 2022, 3, 239–247. [Google Scholar] [CrossRef]
- Goicoechea, J.; Rivero, P.J.; Sada, S.; Arregui, F.J. Self-Referenced Optical Fiber Sensor for Hydrogen Peroxide Detection Based on LSPR of Metallic Nanoparticles in Layer-by-Layer Films. Sensors 2019, 19, 3872. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Lu, M.; Liang, Y.; Yu, L.; Peng, W. Polyelectrolyte-Enhanced Localized Surface Plasmon Resonance Optical Fiber Sensors: Properties Interrogation and Bioapplication. ACS Appl. Nano Mater. 2022, 5, 6171–6180. [Google Scholar] [CrossRef]
- Chia, K.K.; Cohen, R.E.; Rubner, M.F. Amine-Rich Polyelectrolyte Multilayer Nanoreactors for in Situ Gold Nanoparticle Synthesis. Chem. Mater. 2008, 20, 6756–6763. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, L.; Wang, D.; Liu, S.; Zhang, S. Preparation and Nanotribological Properties of Polyelectrolyte Multilayers with in Situ Au Nanoparticles. Prog. Org. Coatings 2015, 88, 164–171. [Google Scholar] [CrossRef]
- Guo, D.; Chen, X.; Cai, K.; Deng, P.; Zong, R. Analyzing the Molecular Orientation of Ultrathin Organic Films by Polarized Transmission and Grazing Incidence Reflection Absorption IR Spectroscopy. Mater. Focus 2013, 2, 231–238. [Google Scholar] [CrossRef]
- Rivero, P.J.; Goicoechea, J.; Arregui, F.J. Layer-by-Layer Nano-Assembly: A Powerful Tool for Optical Fiber Sensing Applications. Sensors 2019, 19, 683. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, Z.; Guo, J.; Wang, H.; Ma, Y.; Kong, X.; Fan, H.; Yu, Q. Layer-by-Layer Assembly of Polystyrene/Ag for a Highly Reproducible Sers Substrate and Its Use for the Detection of Food Contaminants. Polymers 2021, 13, 3270. [Google Scholar] [CrossRef] [PubMed]
- Kasztelan, M.; Słoniewska, A.; Gorzkowski, M.; Lewera, A.; Pałys, B.; Zoladek, S. Ammonia Modified Graphene Oxide–Gold Nanoparticles Composite as a Substrate for Surface Enhanced Raman Spectroscopy. Appl. Surf. Sci. 2021, 554, 149060. [Google Scholar] [CrossRef]
- Costa, Í.A.; Maciel, A.P.; Sales, M.J.A.; Moreira, S.G.C.; Paterno, L.G. The Role Played by Graphene Oxide in the Photodeposition and Surface-Enhanced Raman Scattering Activity of Plasmonic Ag Nanoparticle Substrates. Phys. Status Solidi Appl. Mater. Sci. 2020, 217, 1900965. [Google Scholar] [CrossRef]
- Karn-Orachai, K. Gap-Dependent Surface-Enhanced Raman Scattering (SERS) Enhancement Model of SERS Substrate-Probe Combination Using a Polyelectrolyte Nanodroplet as a Distance Controller. Langmuir 2021, 37, 10776–10785. [Google Scholar] [CrossRef]
- Mariani, S.; Paghi, A.; La Mattina, A.A.; Debrassi, A.; Dähne, L.; Barillaro, G. Decoration of Porous Silicon with Gold Nanoparticles via Layer-by-Layer Nanoassembly for Interferometric and Hybrid Photonic/Plasmonic (Bio)Sensing. ACS Appl. Mater. Interfaces 2019, 11, 43731–43740. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Yamauchi, Y.; Rydzek, G.; Ji, Q.; Yonamine, Y.; Kevin, C.W.; Hill, J.P. Layer-by-Layer Nanoarchitectonics: Invention, Innovation, and Evolution. Chem. Lett. 2014, 43, 36–68. [Google Scholar] [CrossRef]
- Guan, H.; Gong, D.; Song, Y.; Han, B.; Zhang, N. Biosensor Composed of Integrated Glucose Oxidase with Liposome Microreactors/Chitosan Nanocomposite for Amperometric Glucose Sensing. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 574, 260–267. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, G.C.; Wei, Y.; Feng, D. A Signal-on Photoelectrochemical Biosensor for Detecting Cancer Marker Type IV Collagenase by Coupling Enzyme Cleavage with Exciton Energy Transfer Biosensing. Anal. Methods 2019, 11, 5880–5885. [Google Scholar] [CrossRef]
- Si, Y.; Park, J.W.; Jung, S.; Hwang, G.S.; Park, Y.E.; Lee, J.E.; Lee, H.J. Voltammetric Layer-by-Layer Biosensor Featuring Purine Nucleoside Phosphorylase and Chitosan for Inosine in Human Serum Solutions. Sens. Actuators B Chem. 2019, 298, 126840. [Google Scholar] [CrossRef]
- Salem, S.R.; Sullivan, J.L.; Topham, P.D.; Tighe, B.J. Supramolecular Host–Guest Carrier Based on Maltose-Modified Hyperbranched Polymer and Polyelectrolyte Multilayers: Toward Stable and Reusable Glucose Biosensor. Polym. Bull. 2020, 77, 3143–3170. [Google Scholar] [CrossRef]
- Yokomichi, A.L.Y.; Rodrigues, V.d.C.; Moroz, A.; Bertanha, M.; Ribeiro, S.J.L.; Deffune, E.; Moraes, M.L. Detection of Factor VIII and D-Dimer Biomarkers for Venous Thromboembolism Diagnosis Using Electrochemistry Immunosensor. Talanta 2020, 219, 121241. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.C.; Soares, J.C.; Rodrigues, V.C.; Follmann, H.D.M.; Arantes, L.M.R.B.; Carvalho, A.C.; Melendez, M.E.; Fregnani, J.H.T.G.; Reis, R.M.; Carvalho, A.L.; et al. Microfluidic-Based Genosensor to Detect Human Papillomavirus (HPV16) for Head and Neck Cancer. ACS Appl. Mater. Interfaces 2018, 10, 36757–36763. [Google Scholar] [CrossRef]
- Wu, M.; Liu, S.; Qi, F.; Qiu, R.; Feng, J.; Ren, X.; Rong, S.; Ma, H.; Chang, D.; Pan, H. A Label-Free Electrochemical Immunosensor for CA125 Detection Based on CMK-3(Au/Fc@MgAl-LDH)n Multilayer Nanocomposites Modification. Talanta 2022, 241, 123254. [Google Scholar] [CrossRef]
- Ibáñez-Redín, G.; Materon, E.M.; Furuta, R.H.M.; Wilson, D.; do Nascimento, G.F.; Melendez, M.E.; Carvalho, A.L.; Reis, R.M.; Oliveira, O.N.; Gonçalves, D. Screen-Printed Electrodes Modified with Carbon Black and Polyelectrolyte Films for Determination of Cancer Marker Carbohydrate Antigen 19-9. Microchim. Acta 2020, 187, 417. [Google Scholar] [CrossRef]
- Soares, J.C.; Soares, A.C.; Popolin-Neto, M.; Paulovich, F.V.; Oliveira, O.N.; Mattoso, L.H.C. Detection of Staphylococcus Aureus in Milk Samples Using Impedance Spectroscopy and Data Processing with Information Visualization Techniques and Multidimensional Calibration Space. Sensors and Actuators Reports 2022, 4, 100083. [Google Scholar] [CrossRef]
- Bondancia, T.J.; Soares, A.C.; Popolin-Neto, M.; Gomes, N.O.; Raymundo-Pereira, P.A.; Barud, H.S.; Machado, S.A.S.; Ribeiro, S.J.L.; Melendez, M.E.; Carvalho, A.L.; et al. Low-Cost Bacterial Nanocellulose-Based Interdigitated Biosensor to Detect the P53 Cancer Biomarker. Biomater. Adv. 2022, 134, 112676. [Google Scholar] [CrossRef] [PubMed]
- Welden, M.; Poghossian, A.; Vahidpour, F.; Wendlandt, T.; Keusgen, M.; Wege, C.; Schöning, M.J. Capacitive Field-Effect Biosensor Modified with a Stacked Bilayer of Weak Polyelectrolyte and Plant Virus Particles as Enzyme Nanocarriers. Bioelectrochemistry 2023, 151, 108397. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an Environment Friendly Biomaterial—A Review on Recent Modifications and Applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.C.; Soares, J.C.; Shimizu, F.M.; Melendez, M.E.; Carvalho, A.L.; Oliveira, O.N. Controlled Film Architectures to Detect a Biomarker for Pancreatic Cancer Using Impedance Spectroscopy. ACS Appl. Mater. Interfaces 2015, 7, 25930–25937. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z.; Wang, Z.; Pan, H.; Lin, Y.; Chang, D. Sensitive Detection of Prostate-Specific Antigen Based on Dual Signal Amplification of Fc@MgAl-LDH and NH2-MIL-101(Fe). Biosens. Bioelectron. 2021, 190, 113437. [Google Scholar] [CrossRef] [PubMed]
- Mollarasouli, F.; Kurbanoglu, S.; Ozkan, S.A. The Role of Electrochemical Immunosensors in Clinical Analysis. Biosensors 2019, 9, 86. [Google Scholar] [CrossRef]
- Paunovic, M.; Schlesinger, M. Fundamentals of Electrochemical Deposition: Second Edition; Wiley: Hoboken, NJ, USA, 2005; ISBN 0471712213. [Google Scholar]
- Besra, L.; Liu, M. A Review on Fundamentals and Applications of Electrophoretic Deposition (EPD). Prog. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Cai, S.; Yang, H.; Chen, C.; Xu, J.; Zhao, P.; Liu, X.; Li, H.; Chen, L. Self-Assembled Deposition of Polyaniline/Cobalt Porphyrin Based on Flexible PET to Improve Sensing of Room-Temperature NH3 Sensor. J. Alloys Compd. 2023, 934, 167566. [Google Scholar] [CrossRef]
- Bia, F.; Gualandi, I.; Griebel, J.; Rasmussen, L.; Hallak, B.; Tonelli, D.; Kersting, B. Heterobimetallic Conducting Polymers Based on Salophen Complexes via Electrosynthesis. J. Mater. Chem. C 2023, 11, 2957–2969. [Google Scholar] [CrossRef]
- Kuzmin, S.M.; Chulovskaya, S.A.; Parfenyuk, V.I. Scan Rate Effect on Superoxide-Assisted Electrochemical Deposition of 2H-5,10,15,20-Tetrakis(3-Aminophenyl)Porphyrin Films. Electrochim. Acta 2022, 425, 140742. [Google Scholar] [CrossRef]
- Namsheer, K.; Rout, C.S. Conducting Polymers: A Comprehensive Review on Recent Advances in Synthesis, Properties and Applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar] [CrossRef]
- Raza, S.; Li, X.; Soyekwo, F.; Liao, D.; Xiang, Y.; Liu, C. A Comprehensive Overview of Common Conducting Polymer-Based Nanocomposites; Recent Advances in Design and Applications. Eur. Polym. J. 2021, 160, 110773. [Google Scholar] [CrossRef]
- Joice, E.K.; Rison, S.; Akshaya, K.B.; Varghese, A. Platinum Decorated Polythiophene Modified Stainless Steel for Electrocatalytic Oxidation of Benzyl Alcohol. J. Appl. Electrochem. 2019, 49, 937–947. [Google Scholar] [CrossRef]
- Ahumada, G.; Hamon, P.; Roisnel, T.; Dorcet, V.; Fuentealba, M.; Hernández, L.A.; Carrillo, D.; Hamon, J.-R.; Manzur, C. Spectroscopy, Molecular Structure, and Electropolymerization of Ni(II) and Cu(II) Complexes Containing a Thiophene-Appending Fluorinated Schiff Base Ligand. Dalt. Trans. 2023, 52, 4224–4236. [Google Scholar] [CrossRef] [PubMed]
- Oberhaus, F.V. Carbonyl Compounds and Inorganic Brønsted Acids as Catalysts for Electropolymerization of Conductive Polymers. Electrochim. Acta 2023, 449, 142237. [Google Scholar] [CrossRef]
- Fang, D.; Xu, T.; Fang, L.; Chen, H.; Huang, Y.; Zhang, H.; Miao, Z.; Mao, C.; Chi, B.; Xu, H. A Blood Compatible, High-Efficient Sensor for Detection of Cr(VI) in Whole Blood. Sens. Actuators B Chem. 2021, 329, 129219. [Google Scholar] [CrossRef]
- Keteklahijani, Y.Z.; Sharif, F.; Roberts, E.P.L.; Sundararaj, U. Enhanced Sensitivity of Dopamine Biosensors: An Electrochemical Approach Based on Nanocomposite Electrodes Comprising Polyaniline, Nitrogen-Doped Graphene, and DNA-Functionalized Carbon Nanotubes. J. Electrochem. Soc. 2019, 166, B1415–B1425. [Google Scholar] [CrossRef]
- Tavares, A.P.M.; Truta, L.A.A.N.A.; Moreira, F.T.C.; Carneiro, L.P.T.; Sales, M.G.F. Self-Powered and Self-Signalled Autonomous Electrochemical Biosensor Applied to Cancinoembryonic Antigen Determination. Biosens. Bioelectron. 2019, 140, 111320. [Google Scholar] [CrossRef]
- Wang, R.; Li, J.; Gao, L.; Yu, J. One-Step Electropolymerized Thieno[3,2-b]Thiophene-Based Bifunctional Electrode with Controlled Color Conversion for Electrochromic Energy Storage Application. Chem. Eng. J. 2022, 445, 136731. [Google Scholar] [CrossRef]
- Pineda, E.G.; Azpeitia, L.A.; Presa, M.J.R.; Bolzán, A.E.; Gervasi, C.A. Benchmarking Electrodes Modified with Bi-Doped Polypyrrole for Sensing Applications. Electrochim. Acta 2023, 444, 142011. [Google Scholar] [CrossRef]
- Tkach, V.V.; de Paiva Martins, J.I.F.; Ivanushko, Y.G.; Yagodynets, P.I. Dye Electropolymerization for Electrochemical Analysis. A Brief Review. Biointerface Res. Appl. Chem. 2022, 12, 4028–4047. [Google Scholar] [CrossRef]
- Kulikova, T.; Shiabiev, I.; Padnya, P.; Rogov, A.; Evtugyn, G.; Stoikov, I.; Porfireva, A. Impedimetric DNA Sensor Based on Electropolymerized N-Phenylaminophenothiazine and Thiacalix[4]Arene Tetraacids for Doxorubicin Determination. Biosensors 2023, 13, 513. [Google Scholar] [CrossRef] [PubMed]
- Dalkiran, B.; Brett, C.M.A. Polyphenazine and Polytriphenylmethane Redox Polymer/Nanomaterial–Based Electrochemical Sensors and Biosensors: A Review. Microchim. Acta 2021, 188, 178. [Google Scholar] [CrossRef] [PubMed]
- da Silva, W.; Queiroz, A.C.; Brett, C.M.A. Poly(Methylene Green)–Ethaline Deep Eutectic Solvent/Fe2O3 Nanoparticle Modified Electrode Electrochemical Sensor for the Antibiotic Dapsone. Sens. Actuators B Chem. 2020, 325, 128747. [Google Scholar] [CrossRef]
- Tsuruoka, N.; Soto, S.S.; Tahar, A.B.; Zebda, A.; Tsujimura, S. Mediated Electrochemical Oxidation of Glucose via Poly(Methylene Green) Grafted on the Carbon Surface Catalyzed by Flavin Adenine Dinucleotide-Dependent Glucose Dehydrogenase. Colloids Surf. B Biointerfaces 2020, 192, 111065. [Google Scholar] [CrossRef] [PubMed]
- Ghoorchian, A.; Madrakian, T.; Afkhami, A.; Bagheri, H. Spectroelectrochemical and Electrochromic Behavior of Poly(Methylene Blue) and Poly(Thionine)-Modified Multi-Walled Carbon Nanotubes. J. Solid State Electrochem. 2021, 25, 1217–1229. [Google Scholar] [CrossRef]
- Yin, C.; Zhuang, Q.; Xiao, Q.; Wang, Y.; Xie, J. Electropolymerization of Poly(Methylene Blue) on Flower-like Nickel-Based MOFs Used for Ratiometric Electrochemical Sensing of Total Polyphenolic Content in Chrysanthemum Tea. Anal. Methods 2021, 13, 1154–1163. [Google Scholar] [CrossRef]
- Mokhtari, Z.; Khajehsharifi, H.; Hashemnia, S.; Solati, Z.; Azimpanah, R.; Shahrokhian, S. Evaluation of Molecular Imprinted Polymerized Methylene Blue/Aptamer as a Novel Hybrid Receptor for Cardiac Troponin I (CTnI) Detection at Glassy Carbon Electrodes Modified with New Biosynthesized ZnONPs. Sens. Actuators B Chem. 2020, 320, 128316. [Google Scholar] [CrossRef]
- Liang, X.; Zhou, Y.; Brett, C.M.A. Electropolymerisation of Brilliant Cresyl Blue and Neutral Red on Carbon-Nanotube Modified Electrodes in Binary and Ternary Deep Eutectic Solvents. J. Electroanal. Chem. 2022, 919, 116557. [Google Scholar] [CrossRef]
- Liang, X.; Zhou, Y.; Almeida, J.M.S.; Brett, C.M.A. A Novel Electrochemical Acetaminophen Sensor Based on Multiwalled Carbon Nanotube and Poly(Neutral Red) Modified Electrodes with Electropolymerization in Ternary Deep Eutectic Solvents. J. Electroanal. Chem. 2023, 936, 117366. [Google Scholar] [CrossRef]
- Ağın, F.; Öztürk, G.; Kul, D. Voltammetric Analysis of Ephedrine in Pharmaceutical Dosage Forms and Urine Using Poly(Nile Blue A) Modified Glassy Carbon Electrode. Comb. Chem. High Throughput Screen. 2020, 24, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Olean-Oliveira, A.; Oliveira Brito, G.A.; Cardoso, C.X.; Teixeira, M.F.S. Role of Anion Size in the Electrochemical Performance of a Poly(Thionine) Redox Conductive Polymer Using Electrochemical Impedance Spectroscopy. Polymer 2022, 258, 125291. [Google Scholar] [CrossRef]
- Gribkova, O.L.; Kabanova, V.A.; Nekrasov, A.A. Electrodeposition of Thin Films of Polypyrrole-Polyelectrolyte Complexes and Their Ammonia-Sensing Properties. J. Solid State Electrochem. 2020, 24, 3091–3103. [Google Scholar] [CrossRef]
- Patois, T.; Sanchez, J.-B.; Berger, F.; Rauch, J.-Y.; Fievet, P.; Lakard, B. Ammonia Gas Sensors Based on Polypyrrole Films: Influence of Electrodeposition Parameters. Sens. Actuators B Chem. 2012, 171–172, 431–439. [Google Scholar] [CrossRef]
- Xie, X.; Tang, J.; Xing, Y.; Wang, Z.; Ding, T.; Zhang, J.; Cai, K. Intervention of Polydopamine Assembly and Adhesion on Nanoscale Interfaces: State-of-the-Art Designs and Biomedical Applications. Adv. Healthc. Mater. 2021, 10, 2002138. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, J.; Aguilar-Ferrer, D.; Coy, E. Polydopamine Films: Electrochemical Growth and Sensing Applications. Eur. Polym. J. 2022, 174, 111346. [Google Scholar] [CrossRef]
- Coy, E.; Iatsunskyi, I.; Colmenares, J.C.; Kim, Y.; Mrówczyński, R. Polydopamine Films with 2D-like Layered Structure and High Mechanical Resilience. ACS Appl. Mater. Interfaces 2021, 13, 23113–23120. [Google Scholar] [CrossRef]
- Szewczyk, J.; Pochylski, M.; Szutkowski, K.; Kempiński, M.; Mrówczyński, R.; Iatsunskyi, I.; Gapiński, J.; Coy, E. In-Situ Thickness Control of Centimetre-Scale 2D-Like Polydopamine Films with Large Scalability. Mater. Today Chem. 2022, 24, 100935. [Google Scholar] [CrossRef]
- Almeida, L.C.; Correia, R.D.; Squillaci, G.; Morana, A.; La Cara, F.; Correia, J.P.; Viana, A.S. Electrochemical Deposition of Bio-Inspired Laccase-Polydopamine Films for Phenolic Sensors. Electrochim. Acta 2019, 319, 462–471. [Google Scholar] [CrossRef]
- Healy, B.; Yu, T.; da Silva Alves, D.C.; Okeke, C.; Breslin, C.B. Cyclodextrins as Supramolecular Recognition Systems: Applications in the Fabrication of Electrochemical Sensors. Materials 2021, 14, 1668. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.C.; Oliveira, A.E.F.; Bettio, G.B. β-Cyclodextrin Electropolymerization: Mechanism, Electrochemical Behavior, and Optimization. Chem. Pap. 2019, 73, 1795–1804. [Google Scholar] [CrossRef]
- Chang, Z.; Zhu, B.; Liu, J.J.; Zhu, X.; Xu, M.; Travas-Sejdic, J. Electrochemical Aptasensor for 17β-Estradiol Using Disposable Laser Scribed Graphene Electrodes. Biosens. Bioelectron. 2021, 185, 113247. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.H.; Woi, P.M.; Alias, Y. The Selective Electrochemical Detection of Dopamine in the Presence of Ascorbic Acid and Uric Acid Using Electro-Polymerised-β-Cyclodextrin Incorporated f-MWCNTs/Polyaniline Modified Glassy Carbon Electrode. Microchem. J. 2019, 148, 322–330. [Google Scholar] [CrossRef]
- Harley, C.C.; Annibaldi, V.; Yu, T.; Breslin, C.B. The Selective Electrochemical Sensing of Dopamine at a Polypyrrole Film Doped with an Anionic Β−cyclodextrin. J. Electroanal. Chem. 2019, 855, 113614. [Google Scholar] [CrossRef]
- Annibaldi, V.; Breslin, C.B. Electrochemistry of Viologens at Polypyrrole Doped with Sulfonated β–Cyclodextrin. J. Electroanal. Chem. 2019, 832, 399–407. [Google Scholar] [CrossRef]
- Ghanbari, M.H.; Shahdost-fard, F.; Khoshroo, A.; Rahimi-Nasrabadi, M.; Ganjali, M.R.; Wysokowski, M.; Rębiś, T.; Żółtowska-Aksamitowska, S.; Jesionowski, T.; Rahimi, P.; et al. A Nanocomposite Consisting of Reduced Graphene Oxide and Electropolymerized β-Cyclodextrin for Voltammetric Sensing of Levofloxacin. Microchim. Acta 2019, 186, 438. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Bairagi, P.K.; Verma, N. Spermine Biomarker of Cancerous Cells Voltammetrically Detected on a Poly(β-Cyclodextrin)-Electropolymerized Carbon Film Dispersed with Cu-CNFs. Sens. Actuators B Chem. 2020, 313, 128055. [Google Scholar] [CrossRef]
- Wang, R.; Wang, S.; Qin, C.; Nie, Q.; Luo, Y.; Qin, Q.-P.; Wang, R.; Liu, B.; Luo, D. An Electrochemical Sensor Based on Electropolymerization of β-Cyclodextrin on Glassy Carbon Electrode for the Determination of Fenitrothion. Sensors 2022, 23, 435. [Google Scholar] [CrossRef]
- Ma, M.; Liang, J. Voltammetric Detection of 2-Aminoazotoluene Based on Electropolymerization of β-Cyclodextrin. Chem. Pap. 2023, 77, 2967–2976. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; Iglesias, B.A.; Martins, P.R.; Angnes, L. Recent Advances in Electroanalytical Drug Detection by Porphyrin/Phthalocyanine Macrocycles: Developments and Future Perspectives. Analyst 2021, 146, 365–381. [Google Scholar] [CrossRef] [PubMed]
- Gottfried, J.M. Surface Chemistry of Porphyrins and Phthalocyanines. Surf. Sci. Rep. 2015, 70, 259–379. [Google Scholar] [CrossRef]
- Rubio, R.; Suarez, M.B.; Pérez, M.E.; Heredia, D.A.; Morales, G.M.; Durantini, E.N.; Otero, L.; Gervaldo, M.; Durantini, J.E. Electropolymerized Porphyrin Films as Active Materials in Organic Supercapacitors. A Study of the Effect of Different Central Metals. Electrochim. Acta 2023, 458, 142552. [Google Scholar] [CrossRef]
- Napierała, S.; Kubicki, M.; Patroniak, V.; Wałęsa-Chorab, M. Electropolymerization of [2 × 2] Grid-Type Cobalt(II) Complex with Thiophene Substituted Dihydrazone Ligand. Electrochim. Acta 2021, 369, 137656. [Google Scholar] [CrossRef]
- Peng, R.; Offenhäusser, A.; Ermolenko, Y.; Mourzina, Y. Biomimetic Sensor Based on Mn(III) Meso-Tetra(N-Methyl-4-Pyridyl) Porphyrin for Non-Enzymatic Electrocatalytic Determination of Hydrogen Peroxide and as an Electrochemical Transducer in Oxidase Biosensor for Analysis of Biological Media. Sens. Actuators B Chem. 2020, 321, 128437. [Google Scholar] [CrossRef]
- Jiang, R.; Pang, Y.-H.; Yang, Q.-Y.; Wan, C.-Q.; Shen, X.-F. Copper Porphyrin Metal-Organic Framework Modified Carbon Paper for Electrochemical Sensing of Glyphosate. Sens. Actuators B Chem. 2022, 358, 131492. [Google Scholar] [CrossRef]
- Kuzmin, S.M.; Chulovskaya, S.A.; Parfenyuk, V.I. Effect of Substituent Structure on Formation and Properties of Poly-Hydroxyphenyl Porphyrin Films Obtained by Superoxide-Assisted Method. Electrochim. Acta 2020, 342, 136064. [Google Scholar] [CrossRef]
- Pavel, I.-A.; Lasserre, A.; Simon, L.; Rossignol, J.; Lakard, S.; Stuerga, D.; Lakard, B. Microwave Gas Sensors Based on Electrodeposited Polypyrrole–Nickel Phthalocyanine Hybrid Films. Sensors 2023, 23, 5550. [Google Scholar] [CrossRef]
- Martin, C.S.; Gouveia-Caridade, C.; Crespilho, F.N.; Constantino, C.J.L.; Brett, C.M.A. Iron Phthalocyanine Electrodeposited Films: Characterization and Influence on Dopamine Oxidation. J. Phys. Chem. C 2016, 120, 15698–15706. [Google Scholar] [CrossRef]
- Martin, C.S.; Alessio, P.; Crespilho, F.N.; Brett, C.M.A.; Constantino, C.J.L. Influence of the Supramolecular Arrangement of Iron Phthalocyanine Thin Films on Catecholamine Oxidation. J. Electroanal. Chem. 2019, 836, 7–15. [Google Scholar] [CrossRef]
- Luhana, C.; Mashazi, P. Simultaneous Detection of Dopamine and Paracetamol on Electroreduced Graphene Oxide–Cobalt Phthalocyanine Polymer Nanocomposite Electrode. Electrocatalysis 2023, 14, 406–417. [Google Scholar] [CrossRef]
- Adeniyi, O.; Nwahara, N.; Mwanza, D.; Nyokong, T.; Mashazi, P. High-Performance Non-Enzymatic Glucose Sensing on Nanocomposite Electrocatalysts of Nickel Phthalocyanine Nanorods and Nitrogen Doped-Reduced Graphene Oxide Nanosheets. Appl. Surf. Sci. 2023, 609, 155234. [Google Scholar] [CrossRef]
- Speranza, G. Carbon Nanomaterials: Synthesis, Functionalization and Sensing Applications. Nanomaterials 2021, 11, 967. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Nag, A.; Chandra Mukhopadhyay, S.; Xu, Y. Carbon Nanotubes and Its Gas-Sensing Applications: A Review. Sens. Actuators A Phys. 2019, 291, 107–143. [Google Scholar] [CrossRef]
- Norizan, M.N.; Moklis, M.H.; Ngah Demon, S.Z.; Halim, N.A.; Samsuri, A.; Mohamad, I.S.; Knight, V.F.; Abdullah, N. Carbon Nanotubes: Functionalisation and Their Application in Chemical Sensors. RSC Adv. 2020, 10, 43704–43732. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, T.; Gooding, J.J.; Liu, J. Review of Carbon and Graphene Quantum Dots for Sensing. ACS Sensors 2019, 4, 1732–1748. [Google Scholar] [CrossRef] [PubMed]
- Mushiana, T.; Mabuba, N.; Idris, A.O.; Peleyeju, G.M.; Orimolade, B.O.; Nkosi, D.; Ajayi, R.F.; Arotiba, O.A. An Aptasensor for Arsenic on a Carbon-gold Bi-Nanoparticle Platform. Sens. Bio-Sensing Res. 2019, 24, 100280. [Google Scholar] [CrossRef]
- Gallay, P.; Eguílaz, M.; Rivas, G. Designing Electrochemical Interfaces Based on Nanohybrids of Avidin Functionalized-Carbon Nanotubes and Ruthenium Nanoparticles as Peroxidase-like Nanozyme with Supramolecular Recognition Properties for Site-Specific Anchoring of Biotinylated Residues. Biosens. Bioelectron. 2020, 148, 111764. [Google Scholar] [CrossRef]
- Meskher, H.; Ragdi, T.; Thakur, A.K.; Ha, S.; Khelfaoui, I.; Sathyamurthy, R.; Sharshir, S.W.; Pandey, A.K.; Saidur, R.; Singh, P.; et al. A Review on CNTs-Based Electrochemical Sensors and Biosensors: Unique Properties and Potential Applications. Crit. Rev. Anal. Chem. 2023, 1–24. [Google Scholar] [CrossRef]
- Sen, S.; Sarkar, P. A Simple Electrochemical Approach to Fabricate Functionalized MWCNT-Nanogold Decorated PEDOT Nanohybrid for Simultaneous Quantification of Uric Acid, Xanthine and Hypoxanthine. Anal. Chim. Acta 2020, 1114, 15–28. [Google Scholar] [CrossRef]
- Taylor, I.M.; Patel, N.A.; Freedman, N.C.; Castagnola, E.; Cui, X.T. Direct in Vivo Electrochemical Detection of Resting Dopamine Using Poly(3,4-Ethylenedioxythiophene)/Carbon Nanotube Functionalized Microelectrodes. Anal. Chem. 2019, 91, 12917–12927. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Yang, T.; Wang, X.; He, Q.; He, P.; Jia, L.; Du, L.; Deng, H.; Zhang, H.; Jia, B.; et al. Spherical Phosphomolybdic Acid Immobilized on Graphene Oxide Nanosheets as an Efficient Electrochemical Sensor for Detection of Diphenylamine. Microchem. J. 2020, 158, 105158. [Google Scholar] [CrossRef]
- Zhao, P.; Huang, L.; Wang, H.; Wang, C.; Chen, J.; Yang, P.; Ni, M.; Chen, C.; Li, C.; Xie, Y.; et al. An Ultrasensitive High-Performance Baicalin Sensor Based on C3N4-SWCNTs/Reduced Graphene Oxide/Cyclodextrin Metal-Organic Framework Nanocomposite. Sens. Actuators B Chem. 2022, 350, 130853. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mohammadzadehsaliani, S.; Vafaei, S.; Ahmadi, L.; Iqbal, A.; Alreda, B.A.; Talib Al-Naqeeb, B.Z.; Kheradjoo, H. Synthesis and Electrochemical Study of Enzymatic Graphene Oxide-Based Nanocomposite as Stable Biosensor for Determination of Bevacizumab as a Medicine in Colorectal Cancer in Human Serum and Wastewater Fluids. Chemosphere 2023, 336, 139012. [Google Scholar] [CrossRef] [PubMed]
- Bolat, G.; Yaman, Y.T.; Abacı, S.; Seyyar, S. Poly-Arginine/Graphene Oxide Functionalized Disposable Sensor for Monitoring Fenitrothion Pesticide Residues in Water and Cucumber Samples. Mater. Today Chem. 2023, 30, 101517. [Google Scholar] [CrossRef]
- Kanagavalli, P.; Natchimuthu Karuppusamy, M.; Ganesan, V.S.; Saravanan, H.P.; Palanisamy, T.; Veerapandian, M. Electropolymerized Melamine on Electrochemically Reduced Graphene Oxide: Growth Mechanistics, Electrode Processing, and Amperometric Sensing of Acyclovir. Langmuir 2023, 39, 3512–3525. [Google Scholar] [CrossRef] [PubMed]
- Hssi, A.A.; Atourki, L.; Labchir, N.; Abouabassi, K.; Ouafi, M.; Mouhib, H.; Ihlal, A.; Elfanaoui, A.; Benmokhtar, S.; Bouabid, K. Structural and Optical Properties of Electrodeposited Cu2O Thin Films. Mater. Today Proc. 2020, 22, 89–92. [Google Scholar] [CrossRef]
- Ravichandiran, C.; Sakthivelu, A.; Davidprabu, R.; Deva Arun Kumar, K.; Valanarasu, S.; Kathalingam, A.; Ganesh, V.; Shkir, M.; AlFaify, S. The Effect of Rare Earth Nd3+doping on Physical Characteristics of Cu2O Thin Films Derived by Electrodeposition Technique. Thin Solid Films 2019, 683, 82–89. [Google Scholar] [CrossRef]
- Jose, S.; Das, S.; Vakamalla, T.R.; Sen, D. Electrochemical Glucose Sensing Using Molecularly Imprinted Polyaniline–Copper Oxide Coated Electrode. Surf. Eng. Appl. Electrochem. 2022, 58, 260–268. [Google Scholar] [CrossRef]
- Gowda, J.I.; Hanabaratti, R.M.; Hipparagi, S.S. Development of Manganese Oxide Nanoparticles Based Chemical Sensor for Sensitive Determination of an Antiviral Drug Valaciclovir. Results Chem. 2023, 5, 100801. [Google Scholar] [CrossRef]
- Amali, R.K.A.; Lim, H.N.; Ibrahim, I.; Zainal, Z.; Ahmad, S.A.A. A Copper-Based Metal–Organic Framework Decorated with Electrodeposited Fe2O3 Nanoparticles for Electrochemical Nitrite Sensing. Microchim. Acta 2022, 189, 356. [Google Scholar] [CrossRef]
- Dumore, N.S.; Mukhopadhyay, M. Electroanalytical Performance of Non-Enzymatical Electrochemical Sensor Based on PtNPs-SeNPs-SnO2NPs@BFTO Nanocomposites for the Detection of Hydrogen Peroxide. Electrocatalysis 2023, 14, 708–719. [Google Scholar] [CrossRef]
- Xiao, H.; Cao, L.; Qin, H.; Wei, S.; Gu, M.; Zhao, F.; Chen, Z. Non-Enzymatic Lactic Acid Sensor Based on AuPtNPs Functionalized MoS2 Nanosheet as Electrode Modified Materials. J. Electroanal. Chem. 2021, 903, 115806. [Google Scholar] [CrossRef]
- Hannula, P.-M.; Pletincx, S.; Janas, D.; Yliniemi, K.; Hubin, A.; Lundström, M. Controlling the Deposition of Silver and Bimetallic Silver/Copper Particles onto a Carbon Nanotube Film by Electrodeposition-Redox Replacement. Surf. Coatings Technol. 2019, 374, 305–316. [Google Scholar] [CrossRef]
- Tonelli, D.; Scavetta, E.; Gualandi, I. Electrochemical Deposition of Nanomaterials for Electrochemical Sensing. Sensors 2019, 19, 1186. [Google Scholar] [CrossRef]
- Wadie, M.; Abdel-Moety, E.M.; Rezk, M.R.; Mahmoud, A.M.; Marzouk, H.M. Electro-Polymerized Poly-Methyldopa as a Novel Synthetic Mussel-Inspired Molecularly Imprinted Polymeric Sensor for Darifenacin: Computational and Experimental Study. Appl. Mater. Today 2022, 29, 101595. [Google Scholar] [CrossRef]
- Atta, N.F.; Galal, A.; El-Said, D.M. Novel Design of a Layered Electrochemical Dopamine Sensor in Real Samples Based on Gold Nanoparticles/β-Cyclodextrin/Nafion-Modified Gold Electrode. ACS Omega 2019, 4, 17947–17955. [Google Scholar] [CrossRef]
- Gissawong, N.; Srijaranai, S.; Boonchiangma, S.; Uppachai, P.; Seehamart, K.; Jantrasee, S.; Moore, E.; Mukdasai, S. An Electrochemical Sensor for Voltammetric Detection of Ciprofloxacin Using a Glassy Carbon Electrode Modified with Activated Carbon, Gold Nanoparticles and Supramolecular Solvent. Microchim. Acta 2021, 188, 208. [Google Scholar] [CrossRef]
- Paramasivam, S.; Veerapandian, M.; Kumar, S.S. Rational Design of Effective Solid-State Electrochemiluminescence Platform of Gold@Polyluminol Nanocomposite as an Ultrasensitive Immuno-Probe for Selective Detection of Prostate Specific Antigen. Anal. Chim. Acta 2022, 1206, 339736. [Google Scholar] [CrossRef]
- Stoian, I.A.; Iacob, B.C.; Prates Ramalho, J.P.; Marian, I.O.; Chiș, V.; Bodoki, E.; Oprean, R. A Chiral Electrochemical System Based on L-Cysteine Modified Gold Nanoparticles for Propranolol Enantiodiscrimination: Electroanalysis and Computational Modelling. Electrochim. Acta 2019, 326, 134961. [Google Scholar] [CrossRef]
- Liu, C.; Wei, X.; Wang, X.; Shi, J.; Chen, Z.; Zhang, H.; Zhang, W.; Zou, X. Ratiometric Electrochemical Analysis on a Flexibly-Fabricated Vibratory Electrode Module for Reliable and Selective Determination of Imidacloprid. Sensors Actuators, B Chem. 2021, 329, 129228. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, J.; Di, J.; Tu, Y. Electrodeposition of Large Size Gold Nanoparticles on Indium Tin Oxide Glass and Application as Refractive Index Sensor. Electrochem. commun. 2009, 11, 1034–1037. [Google Scholar] [CrossRef]
- Ghanbari, M.H.; Mashhadizadeh, M.H.; Norouzi, Z.; Salehzadeh, H. Simultaneous Electrochemical Detection of Uric Acid and Xanthine Based on Electrodeposited B, N Co-Doped Reduced Graphene Oxide, Gold Nanoparticles and Electropolymerized Poly (L-Cysteine) Gradually Modified Electrode Platform. Microchem. J. 2022, 175, 107213. [Google Scholar] [CrossRef]
- Karthikeyan, M.; Dhinesh Kumar, M.; Kaniraja, G.; Ananthappan, P.; Sivasamy Vasantha, V.; Karunakaran, C. Gold Nanoparticles Enhanced Molecularly Imprinted Poly(3-Aminophenylboronic Acid) Sensor for Myo-Inositol Detection. Microchem. J. 2023, 189, 108536. [Google Scholar] [CrossRef]
- Ning, L.; Bai, Y.; Wang, Z.; Wen, W.; Wang, J. Label-Free Electrochemiluminescence Immunosensor Based on Conductive PANI to Synergistically Amplify Electrodeposited AuNPs Luminophore Signal for Ultrasensitive Detection of 3-Nitrotyrosine. Microchem. J. 2023, 190, 108619. [Google Scholar] [CrossRef]
- Atici, T.; Bilgi Kamaç, M.; Yilmaz, M.; Yilmaz Kabaca, A. Zinc Oxide Nanorod/Polymethylene Blue (Deep Eutectic Solvent)/Gold Nanoparticles Modified Electrode for Electrochemical Determination of Serotonin (5-HT). Electrochim. Acta 2023, 458, 142484. [Google Scholar] [CrossRef]
- Aydın, E.B.; Aydın, M.; Sezgintürk, M.K. A Label-Free Electrochemical Biosensor for Highly Sensitive Detection of GM2A Based on Gold Nanoparticles/Conducting Amino-Functionalized Thiophene Polymer Layer. Sens. Actuators B Chem. 2023, 392, 134025. [Google Scholar] [CrossRef]
- Zhe, T.; Sun, X.; Wang, Q.; Liu, Y.; Li, R.; Li, F.; Wang, L. A Screen Printed Carbon Electrode Modified with a Lamellar Nanocomposite Containing Dendritic Silver Nanostructures, Reduced Graphene Oxide, and β-Cyclodextrin for Voltammetric Sensing of Nitrite. Microchim. Acta 2019, 186, 319. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, G.; Chen, Y.; Li, X.; Wang, S.; Jiang, F.; Zhan, P.; Lu, C.; Cao, X.; Ye, Y.; et al. Electrochemical Detection of OmpA Gene of C. Sakazakii Based on Glucose-Oxidase-Mimicking Nanotags of Gold-Nanoparticles-Doped Copper Metal-Organic Frameworks. Sensors 2023, 23, 4396. [Google Scholar] [CrossRef]
- Rhys-Jones, T.N. Thermally Sprayed Coating Systems for Surface Protection and Clearance Control Applications in Aero Engines. Surf. Coatings Technol. 1990, 43–44, 402–415. [Google Scholar] [CrossRef]
- Jena, G.; Philip, J. A Review on Recent Advances in Graphene Oxide-Based Composite Coatings for Anticorrosion Applications. Prog. Org. Coatings 2022, 173, 107208. [Google Scholar] [CrossRef]
- Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. On Coating Techniques for Surface Protection: A Review. J. Manuf. Mater. Process. 2019, 3, 28. [Google Scholar] [CrossRef]
- Ma, S.; Sansoni, S.; Gatti, T.; Fino, P.; Liu, G.; Lamberti, F. Research Progress on Homogeneous Fabrication of Large-Area Perovskite Films by Spray Coating. Crystals 2023, 13, 216. [Google Scholar] [CrossRef]
- Workie, A.B.; Ningsih, H.S.; Shih, S.J. An Comprehensive Review on the Spray Pyrolysis Technique: Historical Context, Operational Factors, Classifications, and Product Applications. J. Anal. Appl. Pyrolysis 2023, 170, 105915. [Google Scholar] [CrossRef]
- Liao, T.Y.; Biesiekierski, A.; Berndt, C.C.; King, P.C.; Ivanova, E.P.; Thissen, H.; Kingshott, P. Multifunctional Cold Spray Coatings for Biological and Biomedical Applications: A Review. Prog. Surf. Sci. 2022, 97, 100654. [Google Scholar] [CrossRef]
- Sriram, S.R.; Parne, S.R.; Pothukanuri, N.; Edla, D.R. Prospects of Spray Pyrolysis Technique for Gas Sensor Applications—A Comprehensive Review. J. Anal. Appl. Pyrolysis 2022, 164, 105527. [Google Scholar] [CrossRef]
- Guild, C.; Biswas, S.; Meng, Y.; Jafari, T.; Gaffney, A.M.; Suib, S.L. Perspectives of Spray Pyrolysis for Facile Synthesis of Catalysts and Thin Films: An Introduction and Summary of Recent Directions. Catal. Today 2014, 238, 87–94. [Google Scholar] [CrossRef]
- Sawant, J.P.; Deokate, R.J.; Pathan, H.M.; Kale, R.B. Spray Pyrolytic Deposition of CuInS2 Thin Films: Properties and Applications. Eng. Sci. 2021, 13, 51–64. [Google Scholar] [CrossRef]
- Kate, R.S.; Pathan, H.M.; Kalubarme, R.; Kale, B.B.; Deokate, R.J. Spray Pyrolysis: Approaches for Nanostructured Metal Oxide Films in Energy Storage Application. J. Energy Storage 2022, 54, 105387. [Google Scholar] [CrossRef]
- Lampkin, C.M. Aerodynamics of Nozzles Used in Spray Pyrolysis. Prog. Cryst. Growth Charact. 1979, 1, 405–416. [Google Scholar] [CrossRef]
- Kastrinaki, G.; Lorentzou, S.; Karagiannakis, G.; Rattenbury, M.; Woodhead, J.; Konstandopoulos, A.G. Parametric Synthesis Study of Iron Based Nanoparticles via Aerosol Spray Pyrolysis Route. J. Aerosol Sci. 2018, 115, 96–107. [Google Scholar] [CrossRef]
- Abd-Alghafour, N.M.; Ahmed, N.M.; Hassan, Z. Fabrication and Characterization of V2O5 Nanorods Based Metal–Semiconductor–Metal Photodetector. Sens. Actuators A Phys. 2016, 250, 250–257. [Google Scholar] [CrossRef]
- Shahbazi, M.; Taherkhani, A. Study of Optical and Structural Properties of GO and MnO2-GO Hybrid Fabricated by Spray Pyrolysis Technique. Opt. Mater. 2022, 123, 111849. [Google Scholar] [CrossRef]
- Vijayalakshmi, K.; Renitta, A.; Monamary, A. Substantial Effect of Pd Incorporation on the Room Temperature Hydrogen Sensing Performance of ZnO/ITO Nanowires Prepared by Spray Pyrolysis Method. J. Mater. Sci. Mater. Electron. 2018, 29, 21023–21032. [Google Scholar] [CrossRef]
- Kundu, M.; Karunakaran, G.; Kolesnikov, E.; Sergeevna, V.E.; Kumari, S.; Gorshenkov, M.V.; Kuznetsov, D. Hollow NiCo2O4 Nano-Spheres Obtained by Ultrasonic Spray Pyrolysis Method with Superior Electrochemical Performance for Lithium-Ion Batteries and Supercapacitors. J. Ind. Eng. Chem. 2017, 59, 90–98. [Google Scholar] [CrossRef]
- Kaya, E.E.; Kaya, O.; Alkan, G.; Gürmen, S.; Stopic, S.; Friedrich, B. New Proposal for Size and Size-Distribution Evaluation of Nanoparticles Synthesized via Ultrasonic Spray Pyrolysis Using Search Algorithm Based on Image-Processing Technique. Materials 2020, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Abdulrahman, A.F.; Abd-Alghafour, N.M. Synthesis and Characterization of ZnO Nanoflowers by Using Simple Spray Pyrolysis Technique. Solid. State. Electron. 2022, 189, 108225. [Google Scholar] [CrossRef]
- Yusuf, B.; Hashim, M.R.; Pakhuruddin, M.Z.; Halim, M.M. Effect of Solution Flow Rate on the Physical Properties of Spray Pyrolyzed MoO3 Thin Films as Silicon-Based Heterojunction Device. Superlattices Microstruct. 2021, 164, 107111. [Google Scholar] [CrossRef]
- Zargou, S.; Chabane Sari, S.M.; Senoudi, A.R.; Aida, M.; Attaf, N.; Hakem, I.F. Effect of Solution Flow Rate on Growth and Characterization of Nanostructured ZnO Thin Films Deposited Using Spray Pyrolysis. J. Mater. Environ. Sci. 2016, 7, 3134–3147. [Google Scholar]
- Winkler, N.; Wibowo, R.A.; Kautek, W.; Dimopoulos, T. Influence of the Aqueous Solution Composition on the Morphology of Zn1−xMgxO Films Deposited by Spray Pyrolysis. J. Mater. Chem. C 2019, 7, 3889–3900. [Google Scholar] [CrossRef]
- Arca, E.; Fleischer, K.; Shvets, I.V. Influence of the Precursors and Chemical Composition of the Solution on the Properties of Zno Thin Films Grown by Spray Pyrolysis. J. Phys. Chem. C 2009, 113, 21074–21081. [Google Scholar] [CrossRef]
- Suresh, R.; Ponnuswamy, V.; Mariappan, R. Effect of Solvent and Substrate Temperature on Morphology of Cerium Oxide Thin Films by Simple Nebuliser Spray Pyrolysis Technique. Mater. Technol. 2015, 30, 12–22. [Google Scholar] [CrossRef]
- Ebin, B.; Arıg, E.; Özkal, B.; Gürmen, S. Production and Characterization of ZnO Nanoparticles and Porous Particles by Ultrasonic Spray Pyrolysis Using a Zinc Nitrate Precursor. Int. J. Miner. Metall. Mater. 2012, 19, 651–656. [Google Scholar] [CrossRef]
- Al Ghamdi, S.D.; Alzahrani, A.O.M.; Aida, M.S.; Abdel-wahab, M.S. Influence of Substrate Temperature and Solution Molarity on CuO Thin Films’ Properties Prepared by Spray Pyrolysis. J. Mater. Sci. Mater. Electron. 2022, 33, 14702–14710. [Google Scholar] [CrossRef]
- Hameed, S.A.; Kareem, M.M.; Khodair, Z.T.; Mohammed Saeed, I.M. The Influence of Deposition Temperatures on the Structural and Optical Properties for NiO Nanostructured Thin Films Prepared via Spray Pyrolysis Technique. Chem. Data Collect. 2021, 33, 100677. [Google Scholar] [CrossRef]
- Rahemi Ardekani, S.; Sabour Rouh Aghdam, A.; Nazari, M.; Bayat, A.; Yazdani, E.; Saievar-Iranizad, E. A Comprehensive Review on Ultrasonic Spray Pyrolysis Technique: Mechanism, Main Parameters and Applications in Condensed Matter. J. Anal. Appl. Pyrolysis 2019, 141, 104631. [Google Scholar] [CrossRef]
- Omar, H.; Omar, H. Substrate to Nozzle Distance Influence on the Properties of Zinc Oxide Films Formed with Chemical Spray Pyrolysis Technique. J. Eng. Technol. Appl. Sci. 2019, 4, 9–18. [Google Scholar] [CrossRef]
- Wang, W.N.; Itoh, Y.; Lenggoro, I.W.; Okuyama, K. Nickel and Nickel Oxide Nanoparticles Prepared from Nickel Nitrate Hexahydrate by a Low Pressure Spray Pyrolysis. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2004, 111, 69–76. [Google Scholar] [CrossRef]
- Hathot, S.F.; Abbas, S.I.; AlOgaili, H.A.T.; Salim, A.A. Influence of Deposition Time on Absorption and Electrical Characteristics of ZnS Thin Films. Optik (Stuttg). 2022, 260, 169056. [Google Scholar] [CrossRef]
- Saha, J.K.; Jang, J.; Billah, M.M.; Bukke, R.N.; Kim, Y.G.; Mude, N.N.; Siddik, A.B.; Islam, M.M.; Do, Y.; Choi, M. Highly Stable, Nanocrystalline, ZnO Thin-Film Transistor by Spray Pyrolysis Using High-K Dielectric. IEEE Trans. Electron Devices 2020, 67, 1021–1026. [Google Scholar] [CrossRef]
- Bae, J.; Ali, A.; Jang, J. Spray Pyrolyzed Amorphous InGaZnO for High Performance, Self-Aligned Coplanar Thin-Film Transistor Backplanes. Adv. Mater. Technol. 2023, 8, 2200726. [Google Scholar] [CrossRef]
- Ozório, M.S.; Vieira, D.H.; Nogueira, G.L.; Martin, C.S.; Alves, N.; Constantino, C.J.L. Effect of the Gate Electrodes/Water Interface on the Performance of ZnO-Based Water Gate Field-Effect Transistors. Mater. Sci. Semicond. Process. 2022, 151, 107045. [Google Scholar] [CrossRef]
- Morais, R.M.; Vieira, D.H.; Ozorio, M.D.S.; Pereira, L.; Martins, R.; Alves, N. UV-Assisted Annealing Effect on the Performance of an Electrolyte-Gated Transistor Based on Inkjet Printed ZnO Nanoparticles Blended with Zinc Nitrate. IEEE Trans. Electron Devices 2022, 69, 1538–1544. [Google Scholar] [CrossRef]
- Vieira, D.H.; Nogueira, G.L.; Ozório, M.S.; Fernandes, J.D.; Seidel, K.F.; Serbena, J.P.M.; Alves, N. A Performance Comparison between Honey and Water as Electrolytic Dielectrics for ZnO Liquid-Gated Transistors. Appl. Phys. A Mater. Sci. Process. 2023, 129, 272. [Google Scholar] [CrossRef]
- Ozório, M.S.; Nascimento, M.R.; Vieira, D.H.; Nogueira, G.L.; Martin, C.S.; Lima, S.A.M.; Alves, N. AZO Transparent Electrodes Grown in Situ during the Deposition of Zinc Acetate Dihydrate onto Aluminum Thin Film by Spray Pyrolysis. J. Mater. Sci. Mater. Electron. 2019, 30, 13454–13461. [Google Scholar] [CrossRef]
- Bertoldo, L.H.T.; Nogueira, G.L.; Vieira, D.H.; Klem, M.S.; Ozório, M.S.; Alves, N. Analytical Study of a Solution-Processed Diode Based on ZnO Nanoparticles Using Multi-Walled Carbon Nanotubes as Schottky Contact. J. Mater. Sci. Mater. Electron. 2022, 33, 14508–14518. [Google Scholar] [CrossRef]
- Vijayan, K.; Vijayachamundeeswari, S.P.; Sivaperuman, K.; Ahsan, N.; Logu, T.; Okada, Y. A Review on Advancements, Challenges, and Prospective of Copper and Non-Copper Based Thin-Film Solar Cells Using Facile Spray Pyrolysis Technique. Sol. Energy 2022, 234, 81–102. [Google Scholar] [CrossRef]
- Shahiduzzaman, M.; Hossain, M.I.; Visal, S.; Kaneko, T.; Qarony, W.; Umezu, S.; Tomita, K.; Iwamori, S.; Knipp, D.; Tsang, Y.H.; et al. Spray Pyrolyzed TiO2 Embedded Multi-Layer Front Contact Design for High-Efficiency Perovskite Solar Cells. Nano-Micro Lett. 2021, 13, 36. [Google Scholar] [CrossRef]
- Lv, L.; Wang, Y.; Cheng, P.; Zhang, B.; Dang, F.; Xu, L. Ultrasonic Spray Pyrolysis Synthesis of Three-Dimensional ZnFe2O4-Based Macroporous Spheres for Excellent Sensitive Acetone Gas Sensor. Sens. Actuators B Chem. 2019, 297, 126755. [Google Scholar] [CrossRef]
- Filipovic, L.; Selberherr, S. Spray Pyrolysis Deposition for Gas Sensor Integration in the Backend of Standard CMOS Processes. In Proceedings of the 2014 IEEE 12th International Conference on Solid-State and Integrated Circuit Technology, ICSICT 2014, Guilin, China, 28–31 October 2014; pp. 1–4. [Google Scholar]
- Tombak, A.; Ocak, Y.S.; Bayansal, F. Cu/SnO2 Gas Sensor Fabricated by Ultrasonic Spray Pyrolysis for Effective Detection of Carbon Monoxide. Appl. Surf. Sci. 2019, 493, 1075–1082. [Google Scholar] [CrossRef]
- Sankar ganesh, R.; Navaneethan, M.; Mani, G.K.; Ponnusamy, S.; Tsuchiya, K.; Muthamizhchelvan, C.; Kawasaki, S.; Hayakawa, Y. Influence of Al Doping on the Structural, Morphological, Optical, and Gas Sensing Properties of ZnO Nanorods. J. Alloys Compd. 2017, 698, 555–564. [Google Scholar] [CrossRef]
- Kundu, S.; Majumder, R.; Ghosh, R.; Pal Chowdhury, M. Superior Positive Relative Humidity Sensing Properties of Porous Nanostructured Al:ZnO Thin Films Deposited by Jet-Atomizer Spray Pyrolysis Technique. J. Mater. Sci. Mater. Electron. 2019, 30, 4618–4625. [Google Scholar] [CrossRef]
- Xiao, B.; Jiao, A.; Zhang, Y.; Yang, L.; Zhao, X.; Wu, C.; Guo, D.; Zhan, R.; Lin, H. Mixed Potential Type Ammonia Sensor Using Fe-Substituted LaCoO3 Sensing Electrode Prepared by Flame Spray Pyrolysis. Sens. Actuators B Chem. 2021, 346, 130470. [Google Scholar] [CrossRef]
- Jang, H.D.; Kim, S.K.; Chang, H.; Jo, E.H.; Roh, K.M.; Choi, J.H.; Choi, J.W. Synthesis of 3D Silver-Graphene-Titanium Dioxide Composite via Aerosol Spray Pyrolysis for Sensitive Glucose Biosensor. Aerosol Sci. Technol. 2015, 49, 538–546. [Google Scholar] [CrossRef]
- Martínez-Saucedo, G.; Ugalde-Reygadas, M.; Peña, J.J.A.; Lastra-Medina, G.; Márquez-Marín, J.; Torres-Delgado, G.; Castanedo-Pérez, R.; Chávez-Urbiola, I.R. Non-Enzymatic Glucose Sensor Using Nanostructured Copper Oxide Thin Films Deposited by Spray Pyrolysis. Surf. Interfaces 2023, 37, 102702. [Google Scholar] [CrossRef]
- Dong, F.; Zhang, L.; Li, R.; Qu, Z.; Zou, X.; Jia, S. Electrochemical Non-Enzymatic Biosensor for Tyramine Detection in Food Based on Silver-Substituted ZnO Nano-Flower Modified Glassy Carbon Electrode. Int. J. Electrochem. Sci. 2021, 16, 210234. [Google Scholar] [CrossRef]
- Kumar, A.; Park, G.D.; Patel, S.K.S.; Kondaveeti, S.; Otari, S.; Anwar, M.Z.; Kalia, V.C.; Singh, Y.; Kim, S.C.; Cho, B.K.; et al. SiO2 Microparticles with Carbon Nanotube-Derived Mesopores as an Efficient Support for Enzyme Immobilization. Chem. Eng. J. 2019, 359, 1252–1264. [Google Scholar] [CrossRef]
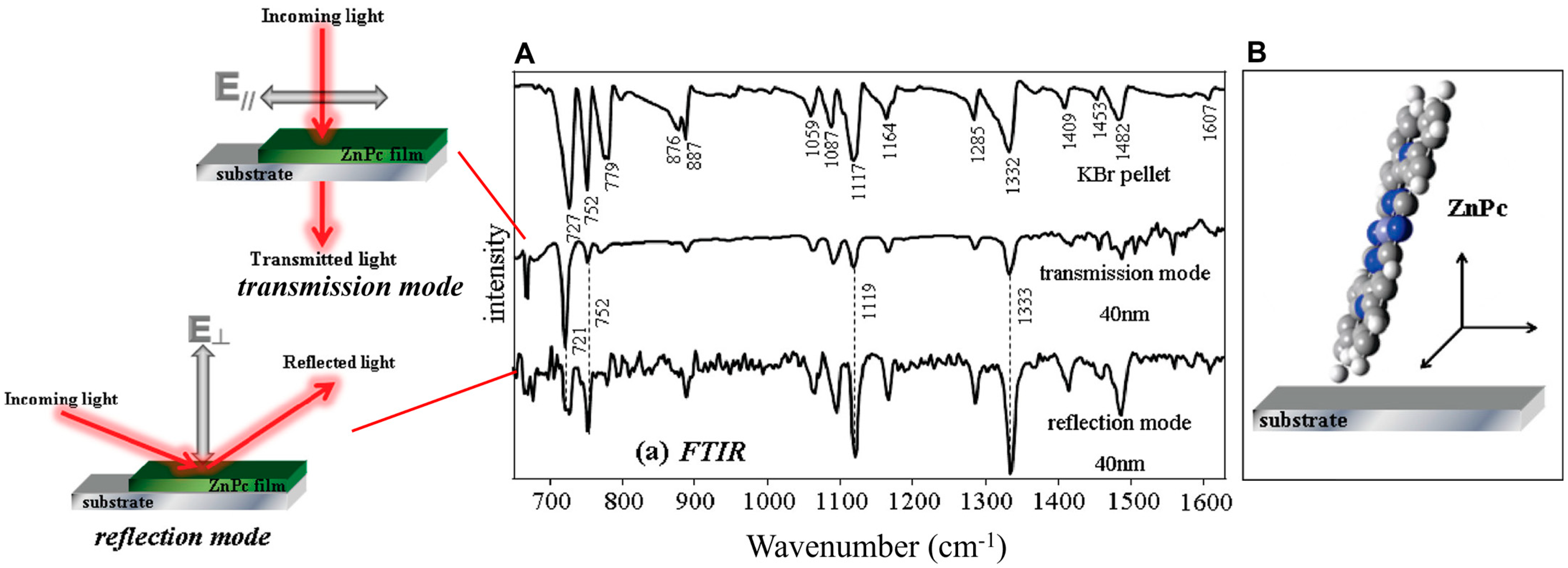
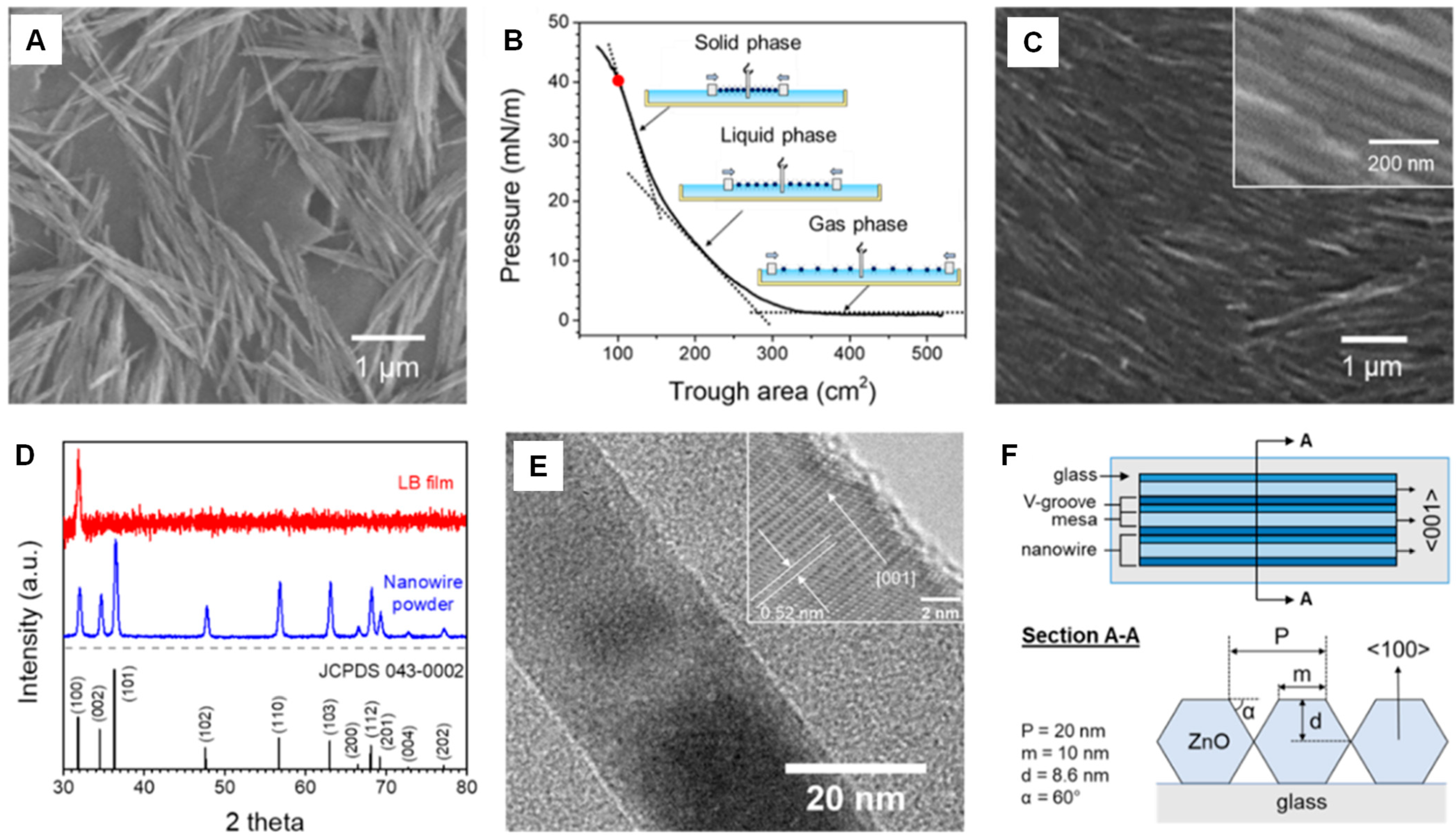

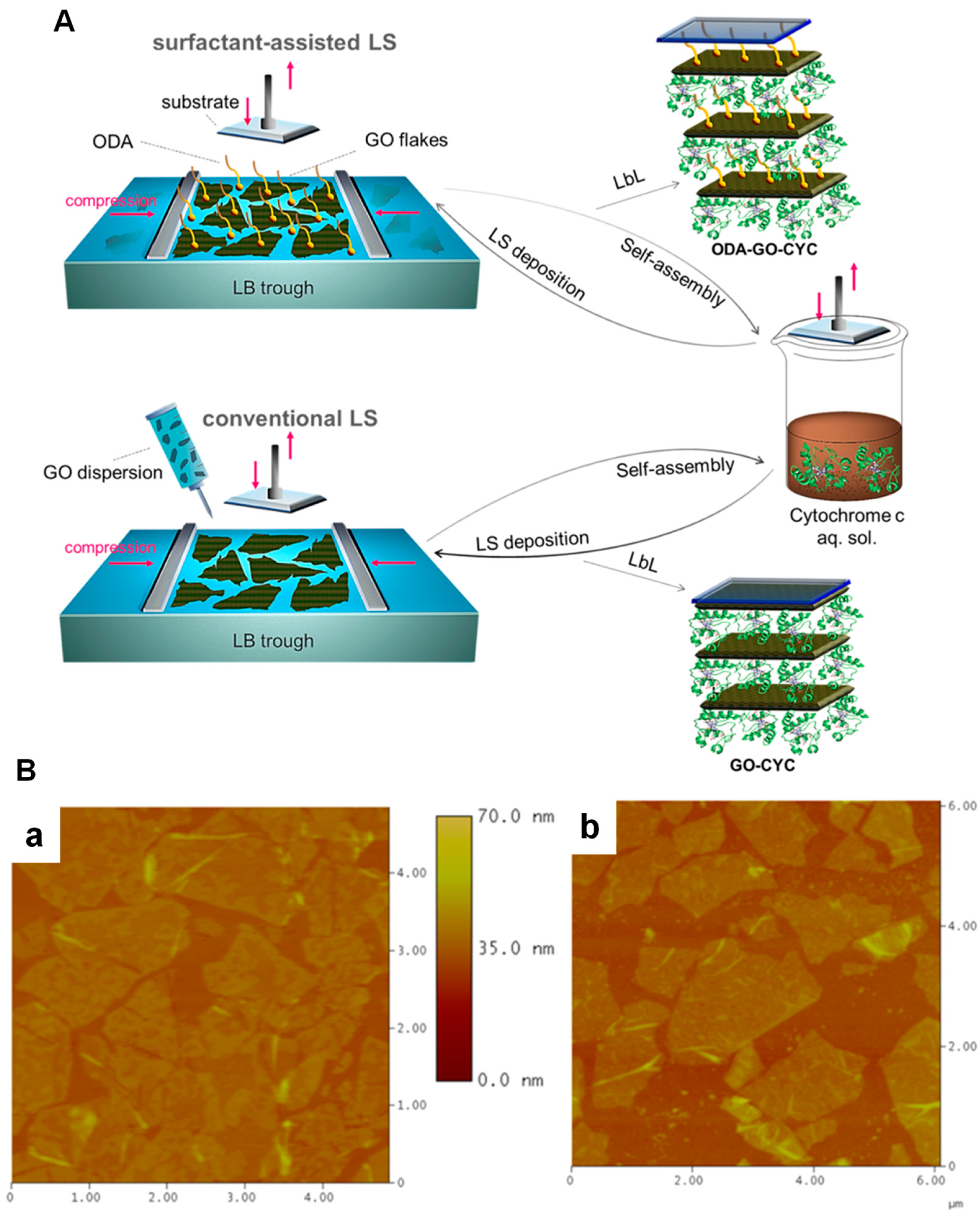
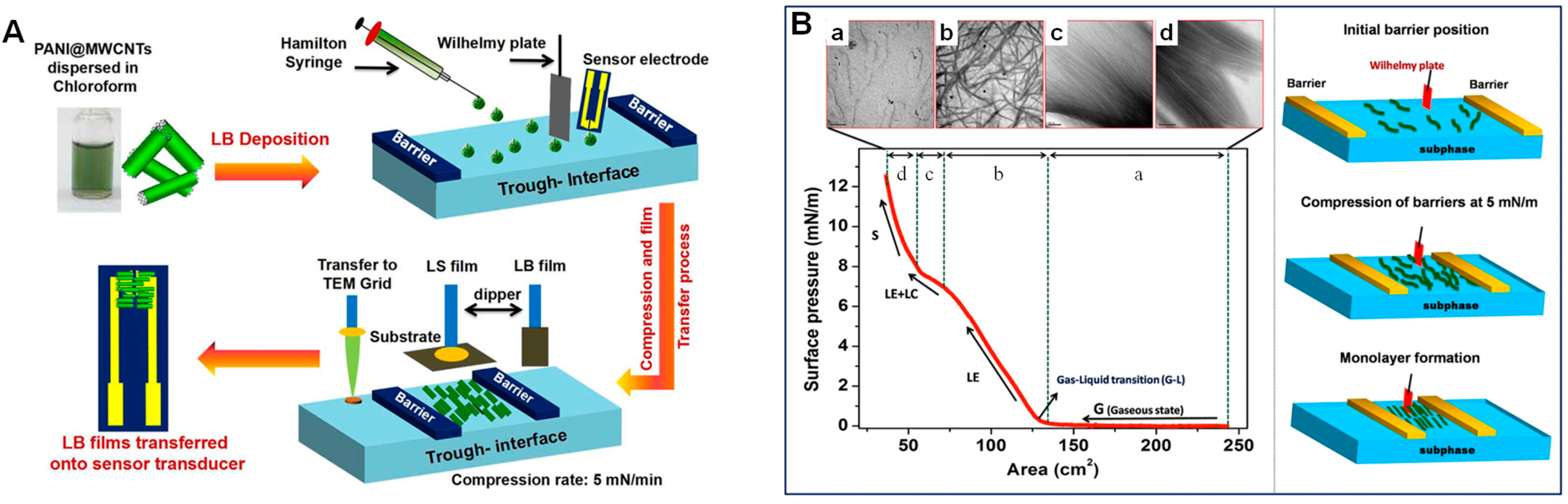
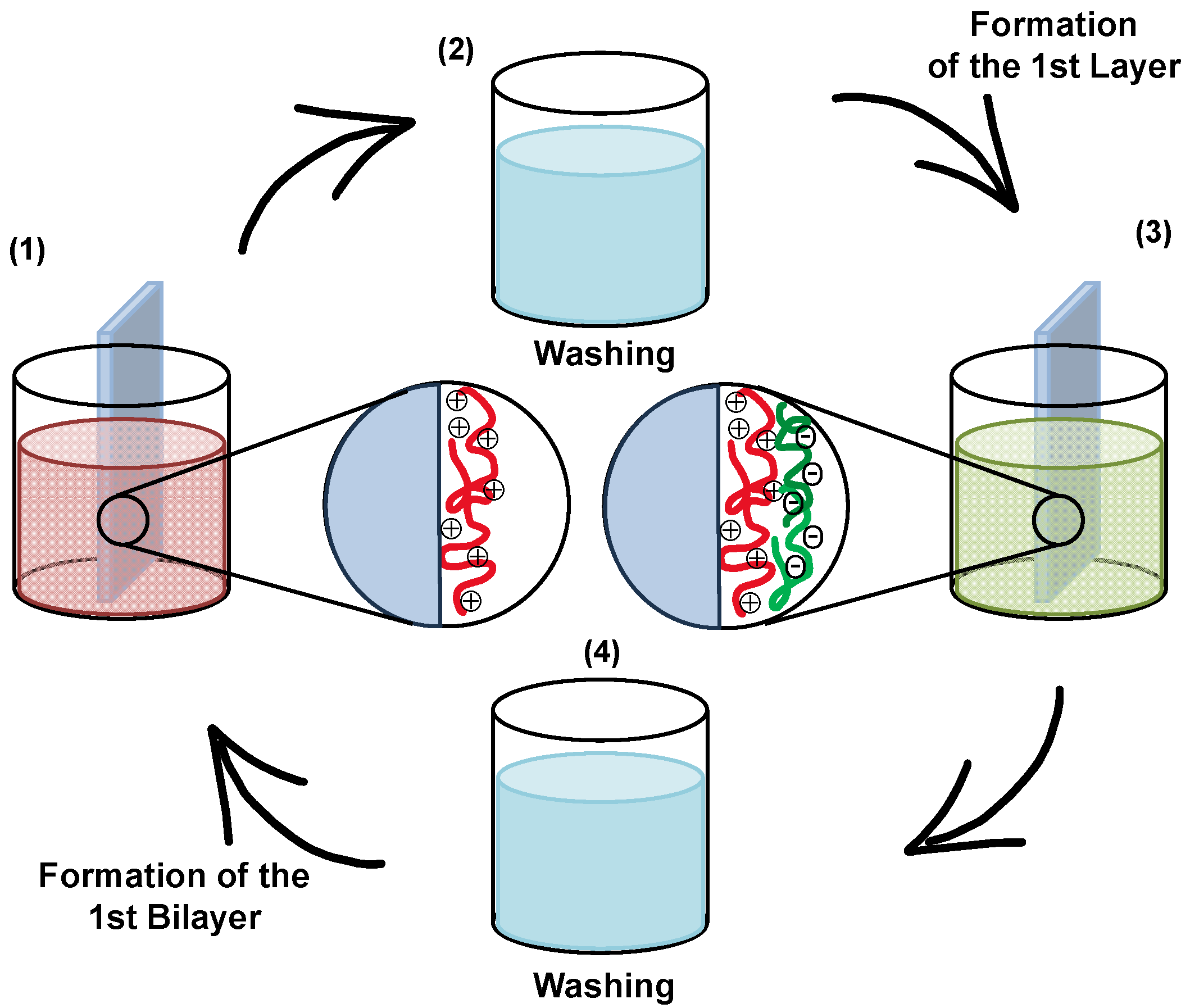
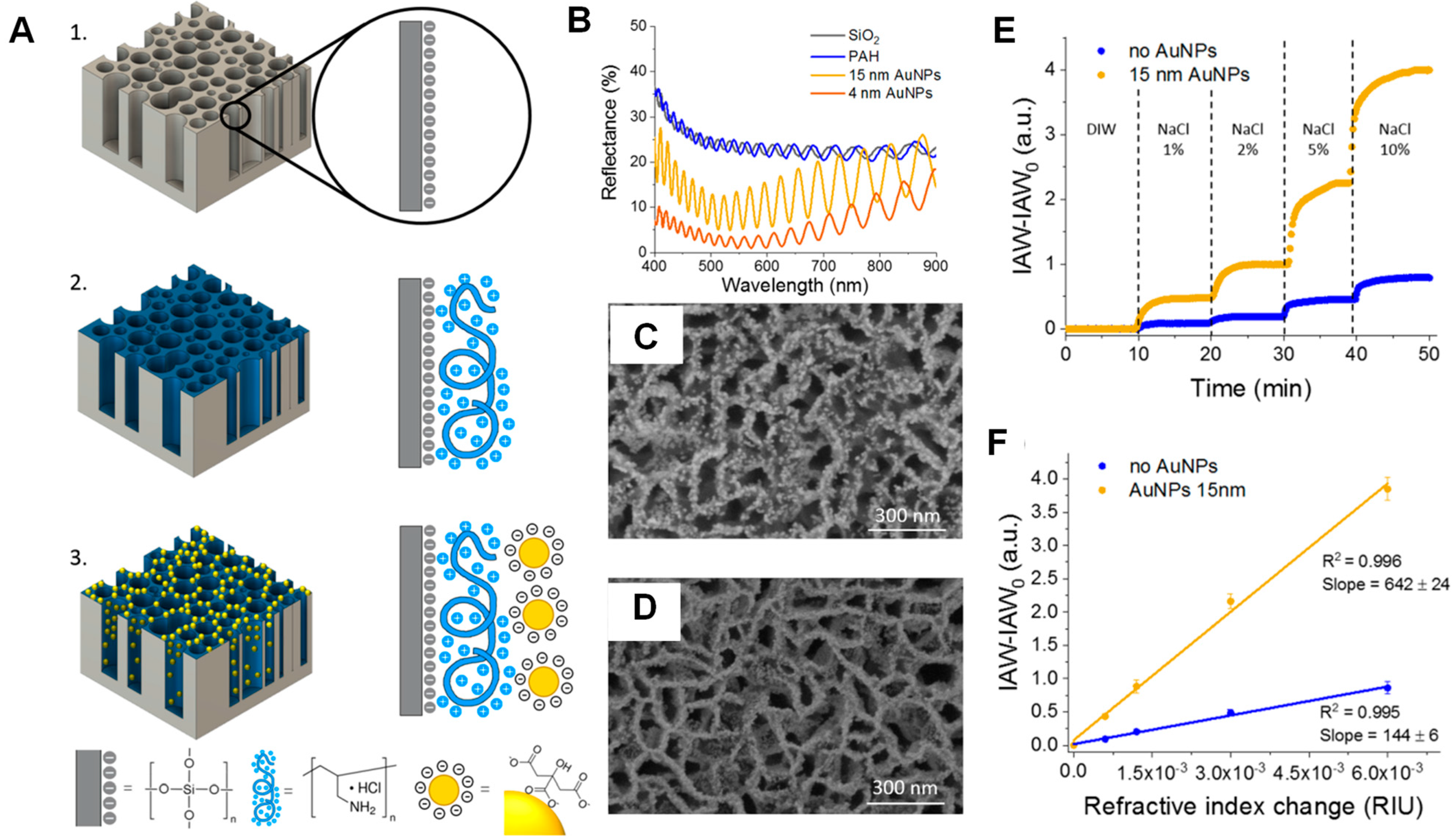
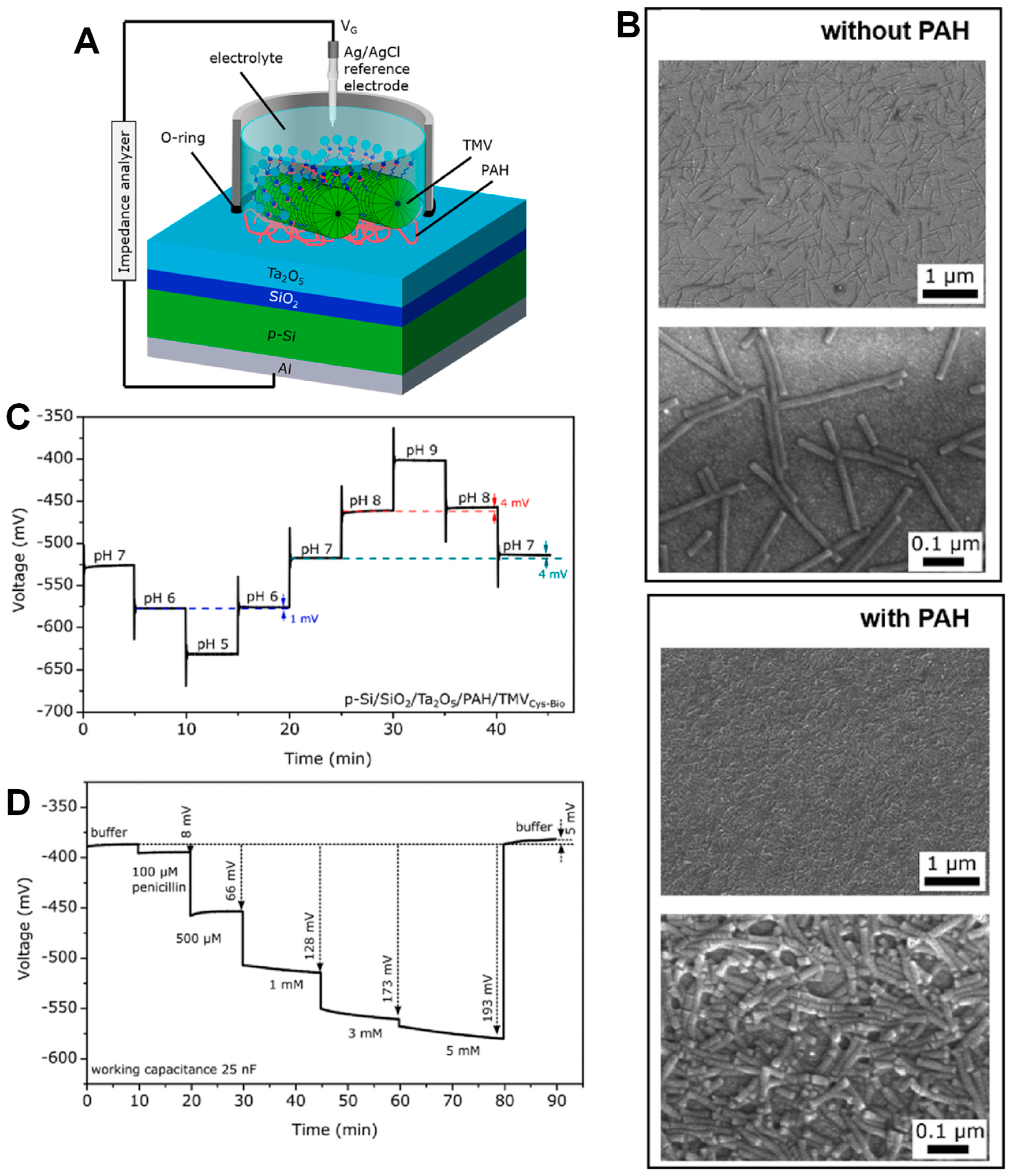
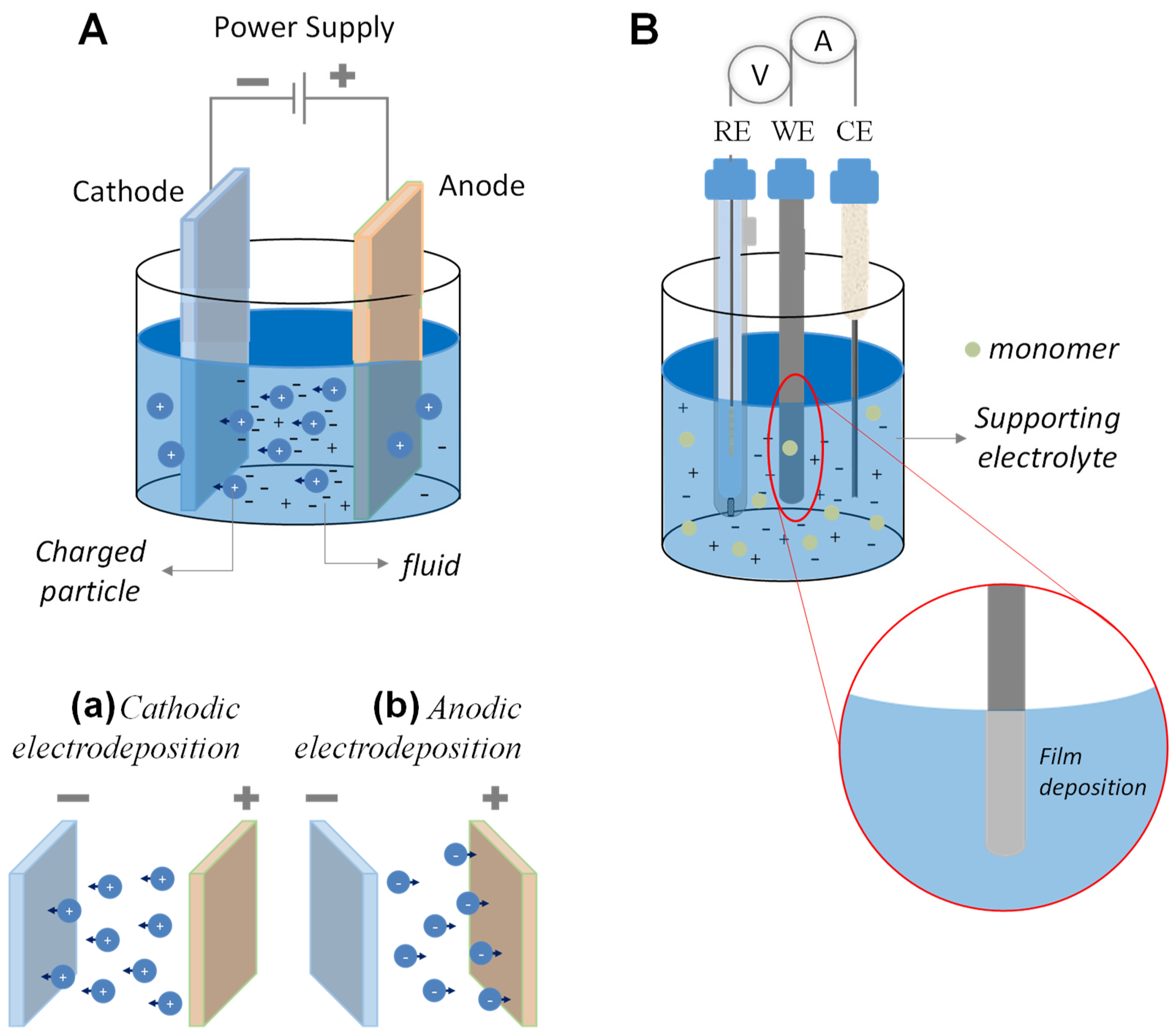
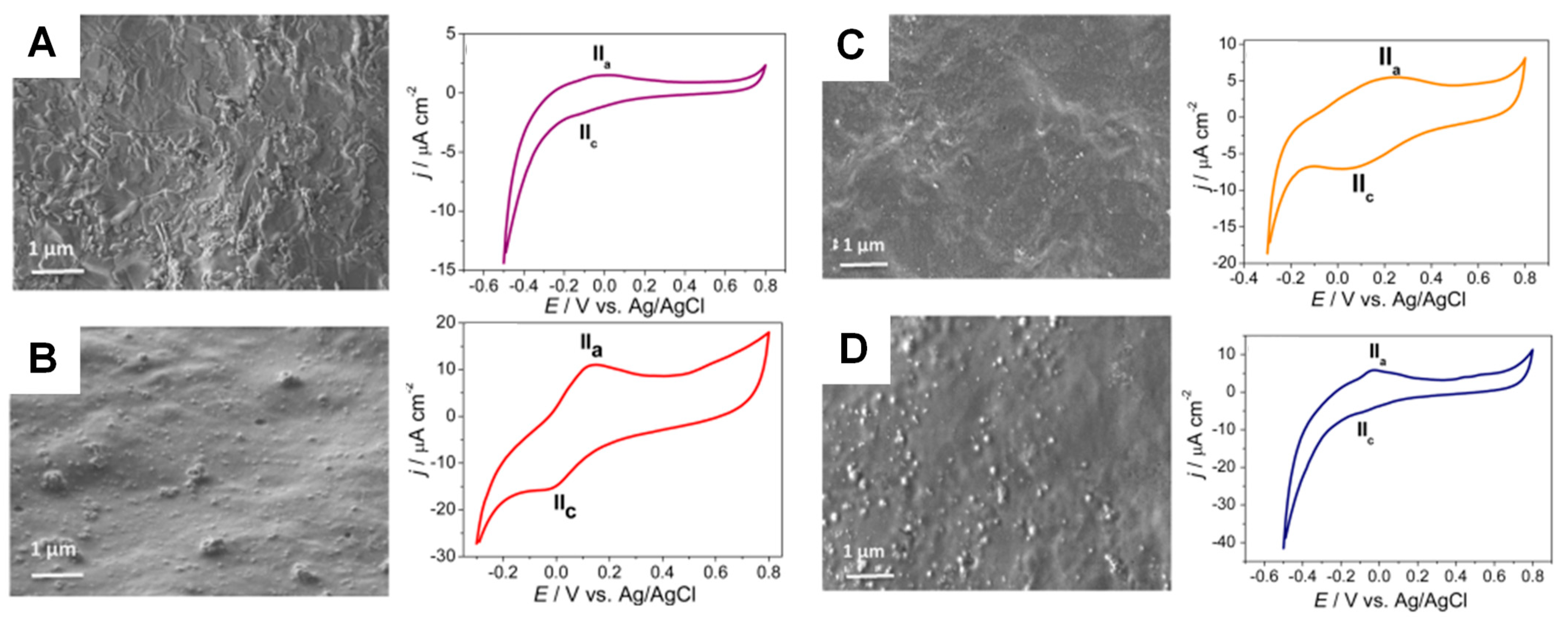
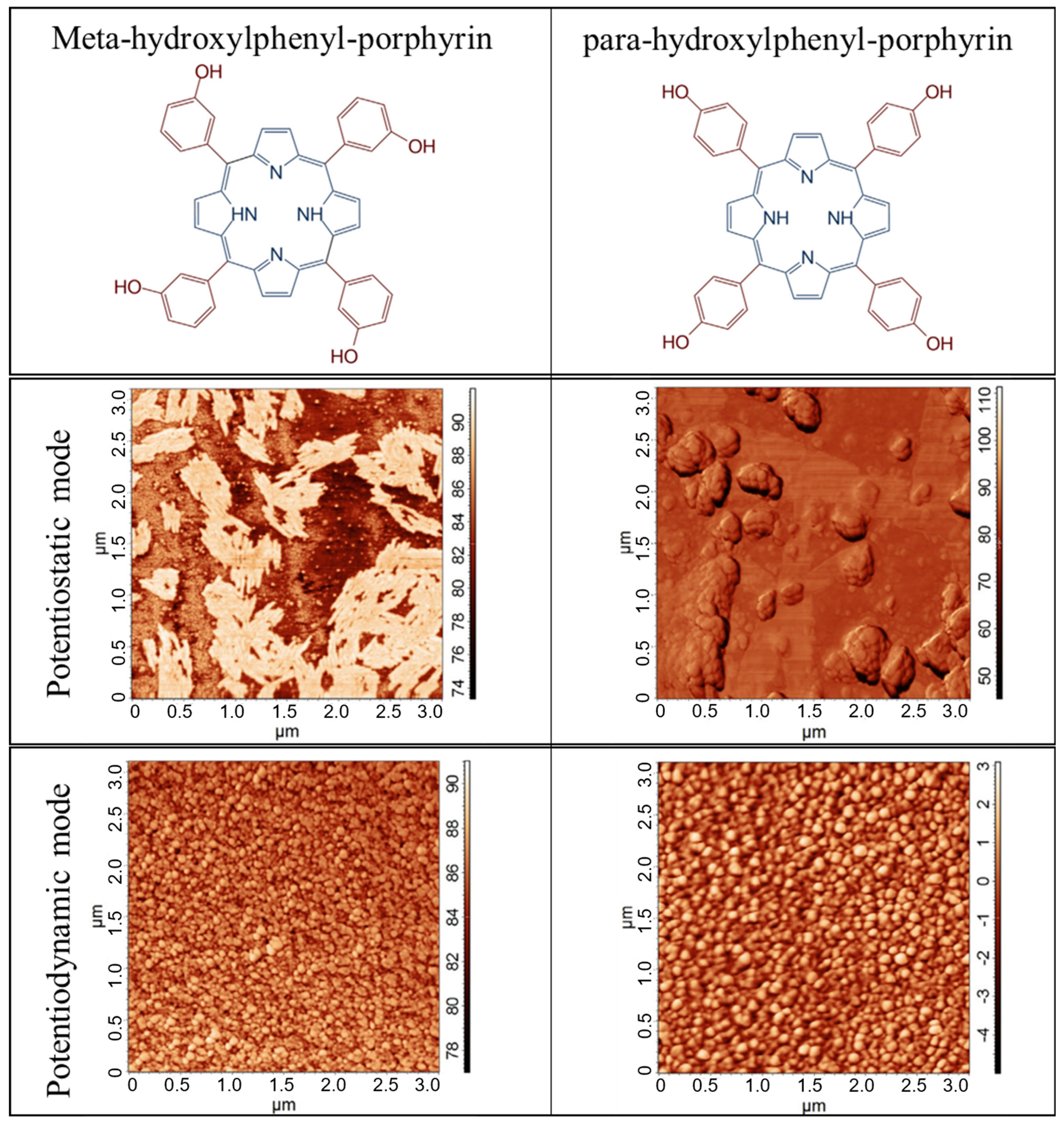


| Substrate | Solution | ED Conditions | Size/Shape | Application/LOD | Ref. |
|---|---|---|---|---|---|
| Carbon paper | HAuCl4⋅3H2O (0.1%) solution | −0.2 V for 120 s | 400 nm/spherical | Voltammetric sensor for glyphosate/0.03 μmol/L | [257] |
| MoS2/SPE | 1:1 (v:v) of HAuCl4 and H2PtCl6 in 0.2 mol/L Na2SO4 | −0.5 V for 100 s a | 110−130 nm/spherical | Voltametrica sensor for latic acid/0.33 μmol/L | [284] |
| AC/GCE | 5.0 mmol/L HAuCl4⋅3H2O in 0.05 mol/L H2SO4 | −1.0 V for 30 s | 125 nm/non-uniform | Voltammetric sensor for ciprofloxacin/0.20 nmol/L | [289] |
| FPC | 0.5 mmol/L HAuCl4⋅3H2O in in 0.5 mol/L H2SO4 | −0.9 to 200 s | typical mellow and full shape | Voltammetric sensor for imidacloprid/61 nmol/L | [292] |
| N-rGO/GCE | 0.1 mmol/L HAuCl4 in 0.1 mol/L PBS (pH 6.0) | −0.2 to −1.3 V, 20 cycles, 50 mV/s | spherical | Simultaneous voltammetric detection of uric acid/0.9 nmol/L and xanthine/90 pmol/L | [294] |
| PMD/PGEs | 0.5 mmol/L HAuCl4⋅3H2O in 0.1 mol/L KCl | −1.0 to 0.2 V, 10 cycles, 100 mV/s | 50 nm/spherical | Voltammetric sensor for darifenacin/0.94 μmol/L | [287] |
| NF/CD | 6.0 mmol/L HAuCl4 in 0.1 mol/L KNO3 | −0.8 to 0.4 V, 20 cycles, 50 mV/s | clusters | Voltammetric sensor for dopamine/0.6 nmol/L | [288] |
| GCE | 0.25 mmol/L HAuCl4+5 mmol/L luminol in 0.5 mol/L H2SO4 | 0 to 1.0 V, 20 cycles, 100 mV/s b | 5 nm/spherical | Immuno sensor for prostate antigen/0.45 fg/mL | [290] |
| CNPs/GCE | 5.0 mmol/L HAuCl4 | −0.3 to 1.2 V, 10 cycles, 50 V/s | spherical | Voltammetric sensor for arsenic/0.092 ppb | [268] |
| SPCE * | - | 0.9 to 0 V, 5 cycles, 100 mV/s | spherical | Voltammetric sensor for myo-inositol/1 nmol/L | [295] |
| PANI/GCE | HAuCl4 (1%) | −0.2 V for 30 s | clusters | electrochemiluminescence immunosensor for 3-nitrotyrosine/2.57 pmol/L | [296] |
| SPCE/ZnONR/ PMBDES | 4.0 mmol/L HAuCl4 in 0.05 mol/L PBS (pH 7.0) | −1.3 to 0.2 V, 10 cycles, 50 mV/s | spherical | Voltammetric sensor for serotonin (5-HT)/1.91 nmol/L | [297] |
| ITO | 0.05 mol/L HAuCl4 | −0.2 to 1.3 V, 10 cycles, 50 mV/s | 5.8 nm/spherical | Electrochemical biosensor for GM2A/5.8 fg/mL | [298] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyazaki, C.M.; Martin, C.S.; Ozório, M.S.; Kavazoi, H.S.; Constantino, C.J.L.; Aléssio, P. Exploring Deposition Techniques and Supramolecular Arrangement in Thin Films for Sensor Applications. Chemosensors 2023, 11, 524. https://doi.org/10.3390/chemosensors11100524
Miyazaki CM, Martin CS, Ozório MS, Kavazoi HS, Constantino CJL, Aléssio P. Exploring Deposition Techniques and Supramolecular Arrangement in Thin Films for Sensor Applications. Chemosensors. 2023; 11(10):524. https://doi.org/10.3390/chemosensors11100524
Chicago/Turabian StyleMiyazaki, Celina M., Cibely S. Martin, Maíza S. Ozório, Henry S. Kavazoi, Carlos J. L. Constantino, and Priscila Aléssio. 2023. "Exploring Deposition Techniques and Supramolecular Arrangement in Thin Films for Sensor Applications" Chemosensors 11, no. 10: 524. https://doi.org/10.3390/chemosensors11100524
APA StyleMiyazaki, C. M., Martin, C. S., Ozório, M. S., Kavazoi, H. S., Constantino, C. J. L., & Aléssio, P. (2023). Exploring Deposition Techniques and Supramolecular Arrangement in Thin Films for Sensor Applications. Chemosensors, 11(10), 524. https://doi.org/10.3390/chemosensors11100524








