Solid-Phase Microextraction (SPME) and Gas Chromatographic/Mass Spectrometry in Chronic Obstructive Pulmonary Disease (COPD): A Chemometric Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Exhaled Air Collection
2.3. Solid-Phase Microextraction (SPME)
2.4. Gas Chromatography-Mass Spectrometry (GC/MS) Analysis
2.5. GC-MS Data Preprocessing
2.6. Statistical Analysis
3. Results
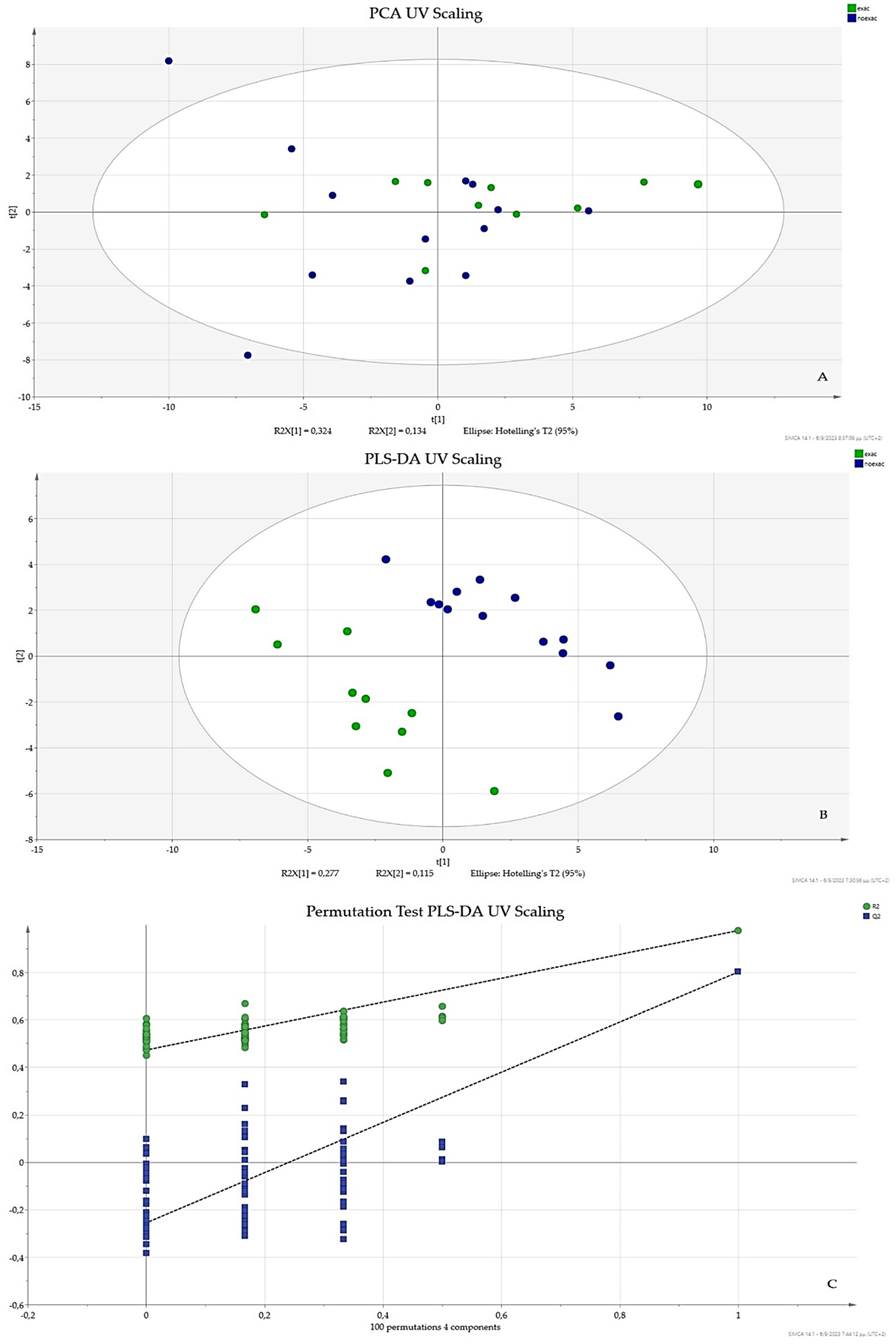
3.1. UV Scaling
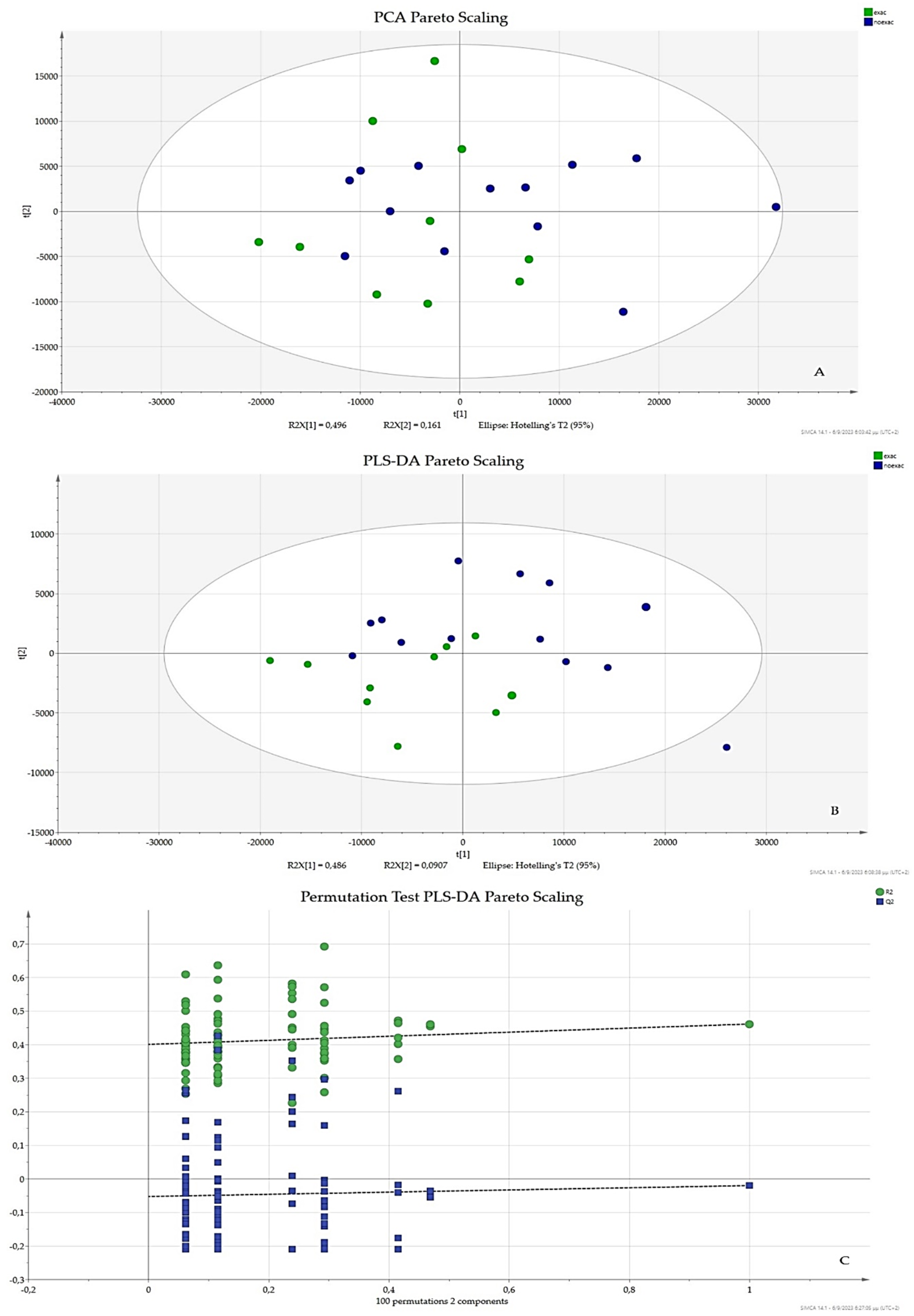
3.2. Pareto Scaling
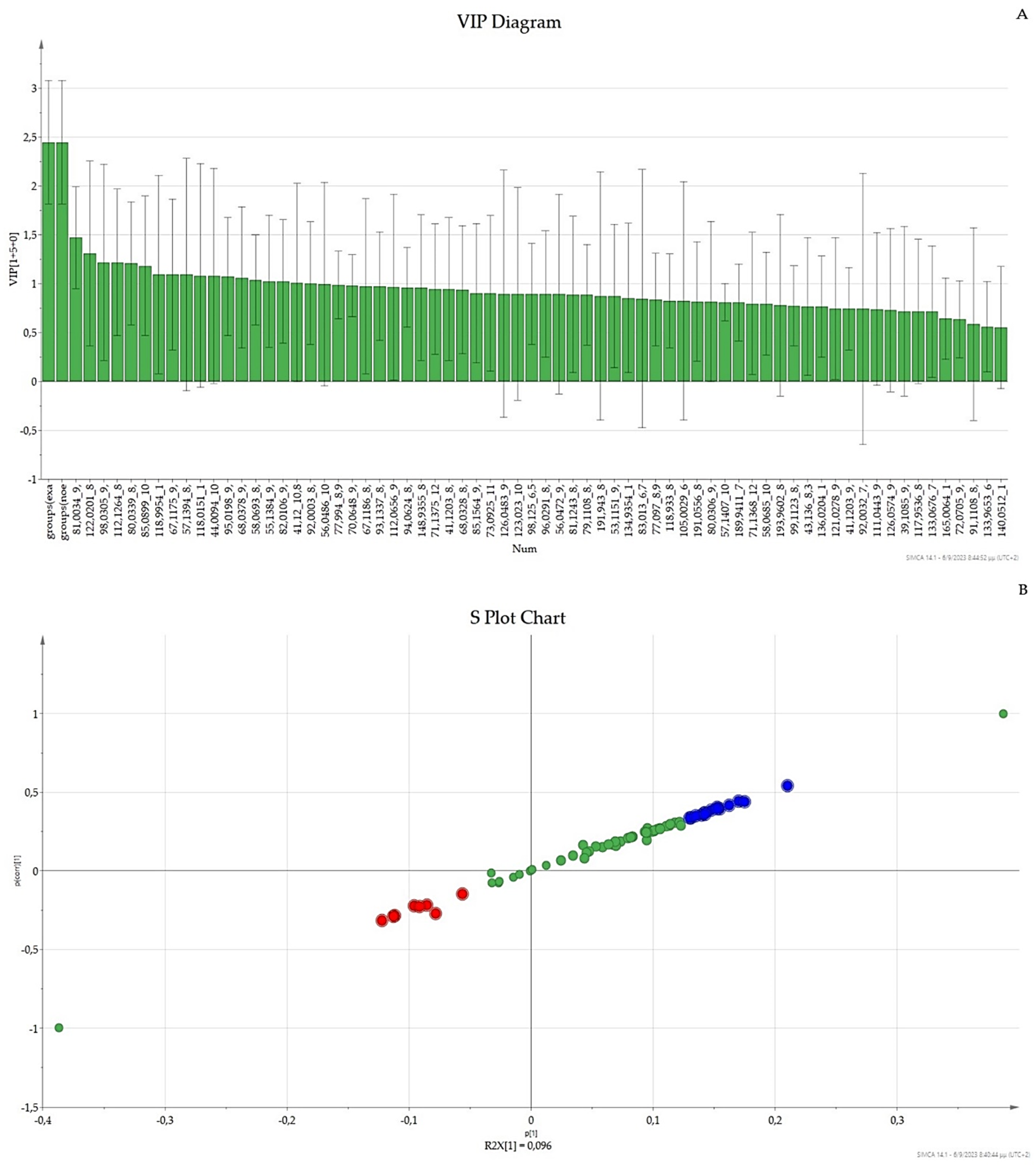
3.3. OPLS-DA UV Scaling, VIP, and S-Plot
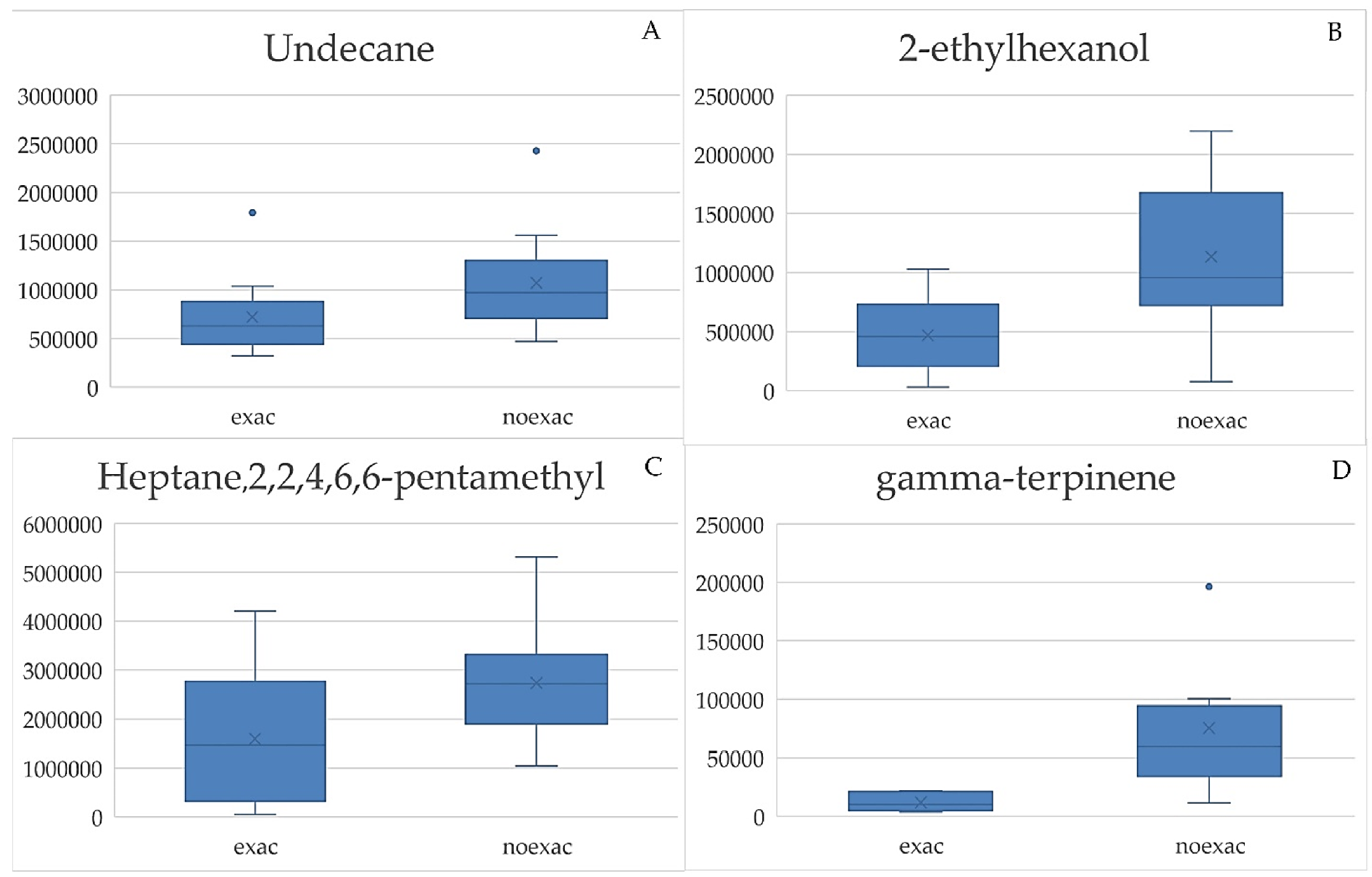
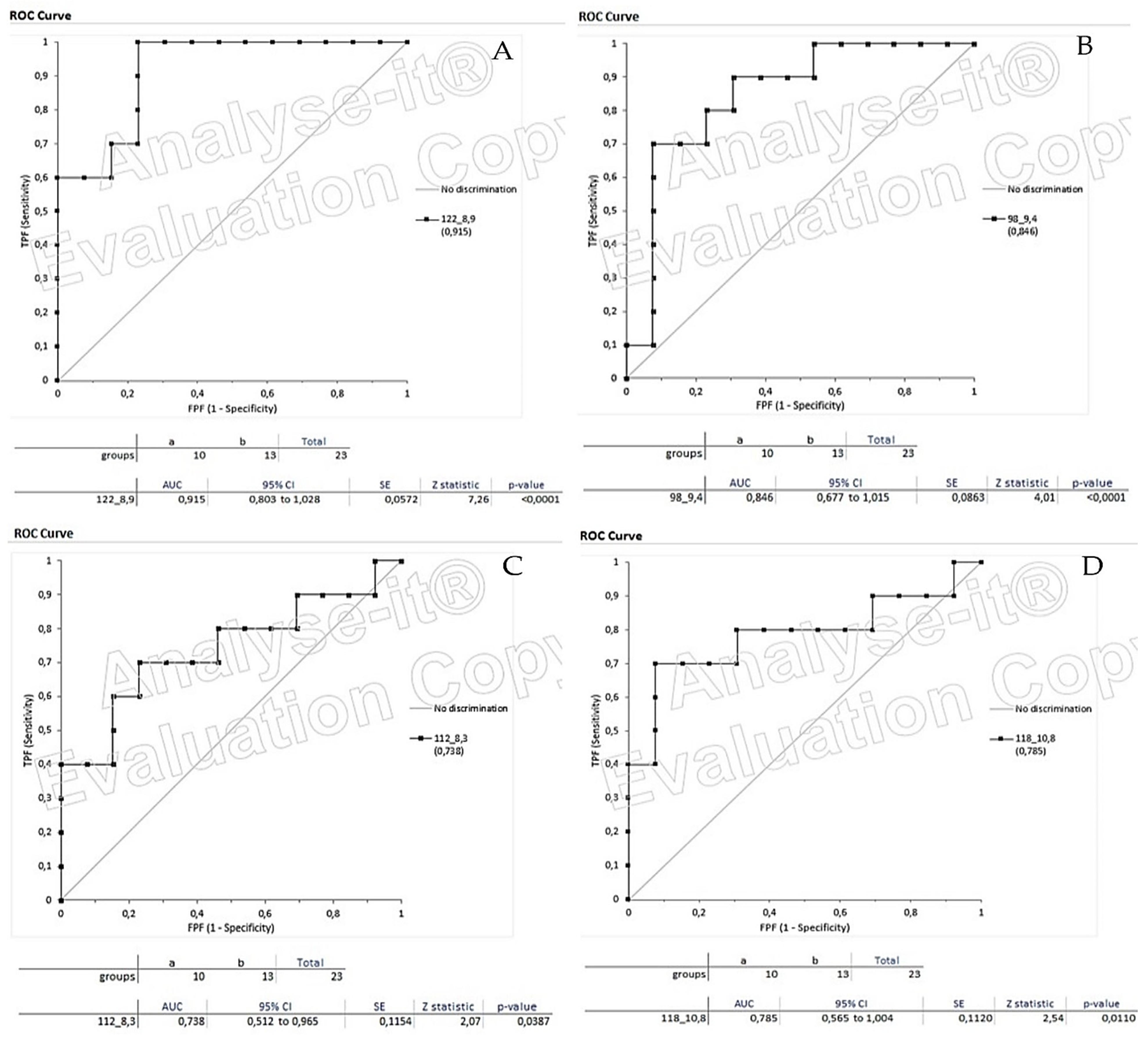
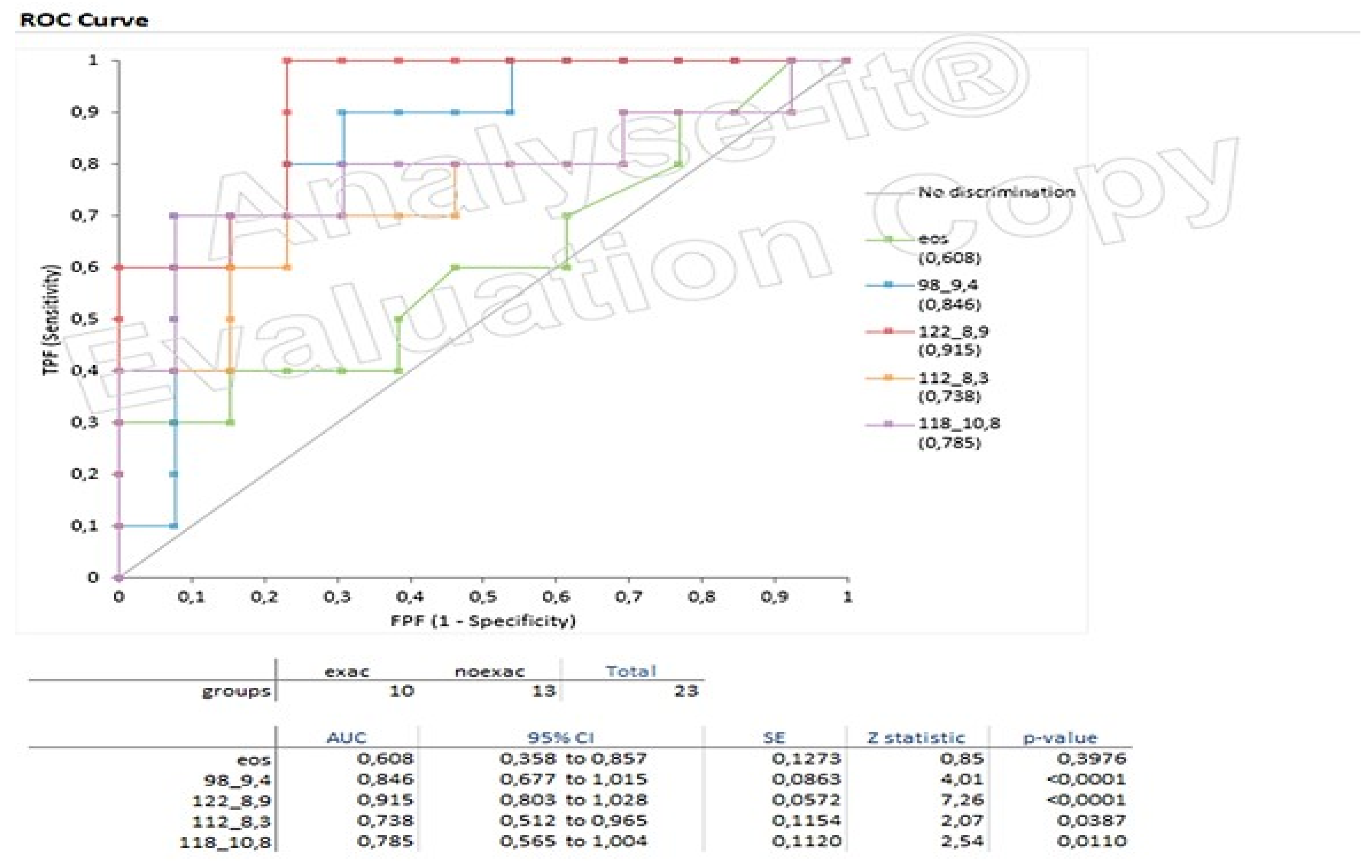
3.4. Box Plots and ROC Curves
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adeloye, D.; Song, P.; Zhu, Y.; Campbell, H.; Sheikh, A.; Rudan, I.; NIHR RESPIRE Global Respiratory Health Unit. Global, Regional, and National Prevalence of, and Risk Factors for, Chronic Obstructive Pulmonary Disease (COPD) in 2019: A Systematic Review and Modelling Analysis. Lancet Respir. Med. 2022, 10, 447–458. [Google Scholar] [CrossRef]
- Vos, T.; Barber, R.; Bell, B.; Bertozzi-Villa, A.; Biryukov, S.; Bolliger, I.; Charlson, F. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 301 Acute and Chronic Diseases and Injuries in 188 Countries, 1990–2013: A Systematic Analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 743–800. [Google Scholar] [CrossRef]
- Kim, V.; Aaron, S.D. What Is a COPD Exacerbation? Current Definitions, Pitfalls, Challenges and Opportunities for Improvement. Eur. Respir. J. 2018, 52, 1801261. [Google Scholar] [CrossRef] [PubMed]
- Stockley, R.A.; Halpin, D.M.G.; Celli, B.R.; Singh, D. Chronic Obstructive Pulmonary Disease Biomarkers and Their Interpretation. Am. J. Respir. Crit. Care Med. 2019, 199, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Signe, V.K.; Nielsen, S.F.; Lange, P.; Vestbo, J.; Nordestgaard, B.G. Blood Eosinophils and Exacerbations in Chronic Obstructive Pulmonary Disease. The Copenhagen General Population Study. Am. J. Respir. Crit. Care Med. 2016, 193, 965–974. [Google Scholar] [CrossRef]
- Yun, J.H.; Lamb, A.; Chase, R.; Singh, D.; Parker, M.; Saferali, A.; Vestbo, J. Blood Eosinophil Count Thresholds and Exacerbations in Patients with Chronic Obstructive Pulmonary Disease. J. Allergy Clin. Immunol. 2018, 141, 2037–2047. [Google Scholar] [CrossRef] [PubMed]
- Higham, A.; Beech, A.; Wolosianka, S.; Jackson, N.; Long, G.; Kolsum, U.; Southworth, T. Type 2 Inflammation in Eosinophilic Chronic Obstructive Pulmonary Disease. Allergy 2021, 76, 1861–1864. [Google Scholar] [CrossRef] [PubMed]
- Agusti, A.; Bel, E.; Thomas, M.; Vogelmeier, C.; Brusselle, G.; Holgate, S.; Humbert, M. Treatable Traits: Toward Precision Medicine of Chronic Airway Diseases. Eur. Respir. J. 2016, 47, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Benet, J.G.; Seo, M.; Khine, M.; Padró, J.G.; Martnez, A.P.; Kurdahi, F. Breast Cancer Detection by Analyzing the Volatile Organic Compound (VOC) Signature in Human Urine. Sci. Rep. 2022, 12, 14873. [Google Scholar] [CrossRef] [PubMed]
- Schleich, F.N.; Zanella, D.; Stefanuto, P.R.; Bessonov, K.; Smolinska, A.; Dallinga, J.W.; Henket, M. Exhaled Volatile Organic Compounds Are Able to Discriminate between Neutrophilic and Eosinophilic Asthma. Am. J. Respir. Crit. Care Med. 2019, 200, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Nakhleh, M.K.; Haick, H.; Humbert, M.; Cohen-Kaminsky, S. Volatolomics of Breath as an Emerging Frontier in Pulmonary Arterial Hypertension. Eur. Respir. J. 2017, 49, 1601897. [Google Scholar] [CrossRef] [PubMed]
- Plantier, L.; Smolinska, A.; Fijten, R.; Flamant, M.; Dallinga, J.; Mercadier, J.J.; Pachen, D.; d’Ortho, M.P.; van Schooten, F.J.; Crestani, B.; et al. The Use of Exhaled Air Analysis in Discriminating Interstitial Lung Diseases: A Pilot Study. Respir. Res. 2022, 23, 12. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sun, B.; Guo, L.; Wang, X.; Ke, C.; Liu, S.; Zhao, W.; Luo, S.; Guo, Z.; Zhang, Y.; et al. Volatile organic metabolites identify patients with breast cancer, cyclomastopathy, and mammary gland fibroma. Sci. Rep. 2014, 4, 5383. [Google Scholar] [CrossRef] [PubMed]
- Westerhuis, J.A.; Hoefsloot, H.C.J.; Smit, S.; Vis, D.J.; Smilde, A.K.; Velzen, E.J.J.; Duijnhoven, J.P.M.; Dorsten, F.A. Assessment of PLSDA cross validation. Metabolomics 2008, 4, 81–89. [Google Scholar] [CrossRef]
- Blasco, H.; Błaszczyński, J.; Billaut, J.C.; Nadal-Desbarats, L.; Pradat, P.F.; Devos, D.; Moreau, C.; Andres, C.R.; Emond, P.; Corcia, P.; et al. Comparative analysis of targeted metabolomics: Dominance-based rough set approach versus orthogonal partial least square-discriminant analysis. J. Biomed. Inform. 2015, 53, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Farrés, M.; Platikanov, S.; Tsakovski, S.; Tauler, R. Comparison of the variable importance in projection (VIP) and of the selectivity ratio (SR) methods for variable selection and interpretation. J. Chemom. 2015, 29, 528–531. [Google Scholar] [CrossRef]
- Ratiu, I.A.; Ligor, T.; Bocos-Bintintan, V.; Mayhew, C.A.; Buszewski, B. Volatile organic compounds in exhaled breath as fingerprints of lung cancer, asthma, and COPD. J. Clin. Med. 2021, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Mule, N.M.; Patil, D.D.; Kaur, M. A comprehensive survey on investigation techniques of exhaled breath (EB) for diagnosis of diseases in human body. Inform. Med. Unlocked 2021, 26, 100715. [Google Scholar] [CrossRef]

| Patients Code | Age | Number of Exacerbations | EOS Count per μL |
|---|---|---|---|
| exac1 | 66 | 3 | 450 |
| noexac1 | 66 | 0 | 380 |
| noexac2 | 60 | 0 | 30 |
| noexac3 | 53 | 0 | 160 |
| noexac4 | 77 | 0 | 170 |
| exac2 | 79 | 2 | 180 |
| noexac5 | 62 | 0 | 150 |
| noexac6 | 77 | 0 | 310 |
| noexac7 | 60 | 0 | 210 |
| noexac8 | 78 | 0 | 420 |
| exac3 | 77 | 3 | 190 |
| exac4 | 70 | 2 | 360 |
| exac5 | 70 | 4 | 500 |
| noexac9 | 69 | 0 | 70 |
| exac6 | 58 | 2 | 160 |
| noexac10 | 71 | 0 | 170 |
| exac7 | 73 | 3 | 120 |
| exac8 | 72 | 1 | 150 |
| exac9 | 61 | 1 | 310 |
| noexac11 | 69 | 0 | 330 |
| noexac12 | 59 | 0 | 180 |
| noexac13 | 73 | 0 | 150 |
| noexac14 | 68 | 0 | 60 |
| noexac15 | 70 | 0 | 80 |
| exac10 | 70 | 4 | 480 |
| MZ_RT | VIP Values | MZ_RT | VIP Values |
|---|---|---|---|
| 81_9.4 | 1.64496 | 58_8.3 | 1.12807 |
| 122_8.9 | 1.40979 | 41_10.8 | 1.11455 |
| 98_9.4 | 1.35878 | 55_9.4 | 1.09555 |
| 80_8.9 | 1.34396 | 112_10 | 1.09417 |
| 112_8,3 | 1.33589 | 82_9.4 | 1.09209 |
| 85_10 | 1.29344 | 92_8.9 | 1.06967 |
| 55_6.5 | 1.27024 | 67_8.3 | 1.06094 |
| 119_10 | 1.24997 | 77_8.9 | 1.05121 |
| 57_8.3 | 1.24264 | 93_8.9 | 1.05057 |
| 67_9.4 | 1.23331 | 56_10.8 | 1.04641 |
| 118_10.8 | 1.21833 | 94_8.9 | 1.04191 |
| 44_10.8 | 1.20416 | 70_9.4 | 1.0409 |
| 95_9.4 | 1.18957 | 71_12 | 1.03677 |
| 68_9.4 | 1.17337 | 123_10.8 | 1.01216 |
| MZ_RT | MZ_RT |
|---|---|
| 98_9.4 | 67_9.4 |
| 80_8.9 | 112_8.3 |
| 55_6.5 | 44_10.8 |
| 122_8.9 | 95_9.4 |
| 68_9.4 | 118_10.8 |
| 85_10 | 119_10 |
| 81_9.4 |
| RT (min) | Characteristics (M/Z) |
|---|---|
| 6.5 | 55 |
| 8.3 | 57, 58, 67, 112 |
| 8.9 | 77, 80, 92, 93, 94, 122 |
| 9.4 | 55, 67, 68, 70, 81, 82, 95, 98 |
| 10 | 85, 112, 119 |
| 10.8 | 41, 44, 56, 118, 123 |
| 12 | 71 |
| RT (min) | M/Z | p-Value | Volatiles |
|---|---|---|---|
| 8.3 | 57, 58, 67, 112 | 0.0387 | Heptane,2,2,4,6,6-pentamethyl |
| 8.9 | 77, 80, 92, 93, 94, 122 | 0.0001 | gamma-terpinene(1-methyl-4-propan-2-ylcyclohexa-1,4-diene) |
| 9.4 | 55, 67, 68, 70, 81, 82, 95, 98 | 0.0001 | 2-ethylhexanol |
| 10.8 | 41, 44, 56, 118, 123 | 0.0110 | Undecane |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lypirou, L.; Chronis, C.; Exarchos, K.; Kostikas, K.; Sakkas, V. Solid-Phase Microextraction (SPME) and Gas Chromatographic/Mass Spectrometry in Chronic Obstructive Pulmonary Disease (COPD): A Chemometric Approach. Chemosensors 2023, 11, 542. https://doi.org/10.3390/chemosensors11100542
Lypirou L, Chronis C, Exarchos K, Kostikas K, Sakkas V. Solid-Phase Microextraction (SPME) and Gas Chromatographic/Mass Spectrometry in Chronic Obstructive Pulmonary Disease (COPD): A Chemometric Approach. Chemosensors. 2023; 11(10):542. https://doi.org/10.3390/chemosensors11100542
Chicago/Turabian StyleLypirou, Loukia, Christos Chronis, Konstantinos Exarchos, Konstantinos Kostikas, and Vasilios Sakkas. 2023. "Solid-Phase Microextraction (SPME) and Gas Chromatographic/Mass Spectrometry in Chronic Obstructive Pulmonary Disease (COPD): A Chemometric Approach" Chemosensors 11, no. 10: 542. https://doi.org/10.3390/chemosensors11100542
APA StyleLypirou, L., Chronis, C., Exarchos, K., Kostikas, K., & Sakkas, V. (2023). Solid-Phase Microextraction (SPME) and Gas Chromatographic/Mass Spectrometry in Chronic Obstructive Pulmonary Disease (COPD): A Chemometric Approach. Chemosensors, 11(10), 542. https://doi.org/10.3390/chemosensors11100542








