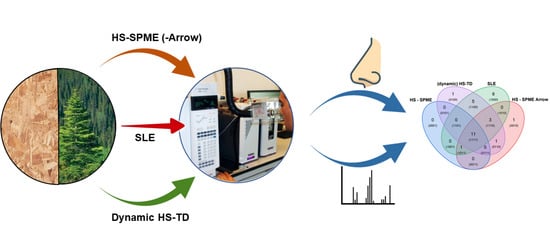
Method Comparison for the Identification and Characterization of Odorants from Scots Pine (Pinus sylvestris L.) and Oriented Strand Boards (OSB) Made Thereof by GC-MS and GC-FID/O Using Different Headspace Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. GC and Thermal Desorption Methods
2.3. Data Analysis
2.4. Extraction Methods
2.4.1. Solid Liquid Extraction (SLE)
2.4.2. Dynamic Headspace Extraction Thermal Desorption ((Dynamic)-HS-TD)
2.4.3. Headspace Solid Phase Microextraction (HS–SPME)
2.4.4. Headspace Solid Phase Microextraction Arrow (HS–SPME ARROW)
3. Results
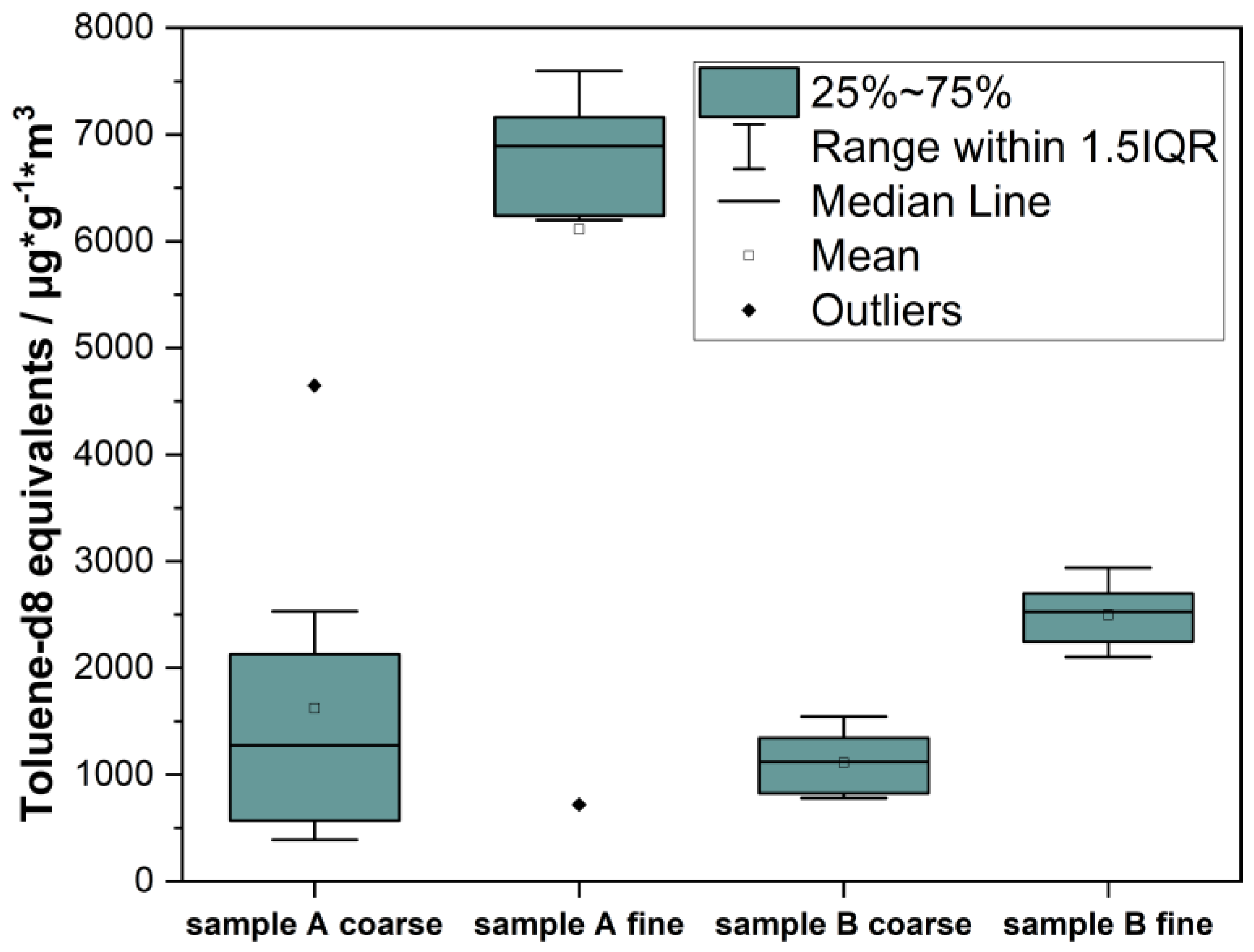
3.1. Method Optimization of Sample Homogeneity
3.2. Optimization of Extraction Times for Conventional SPME
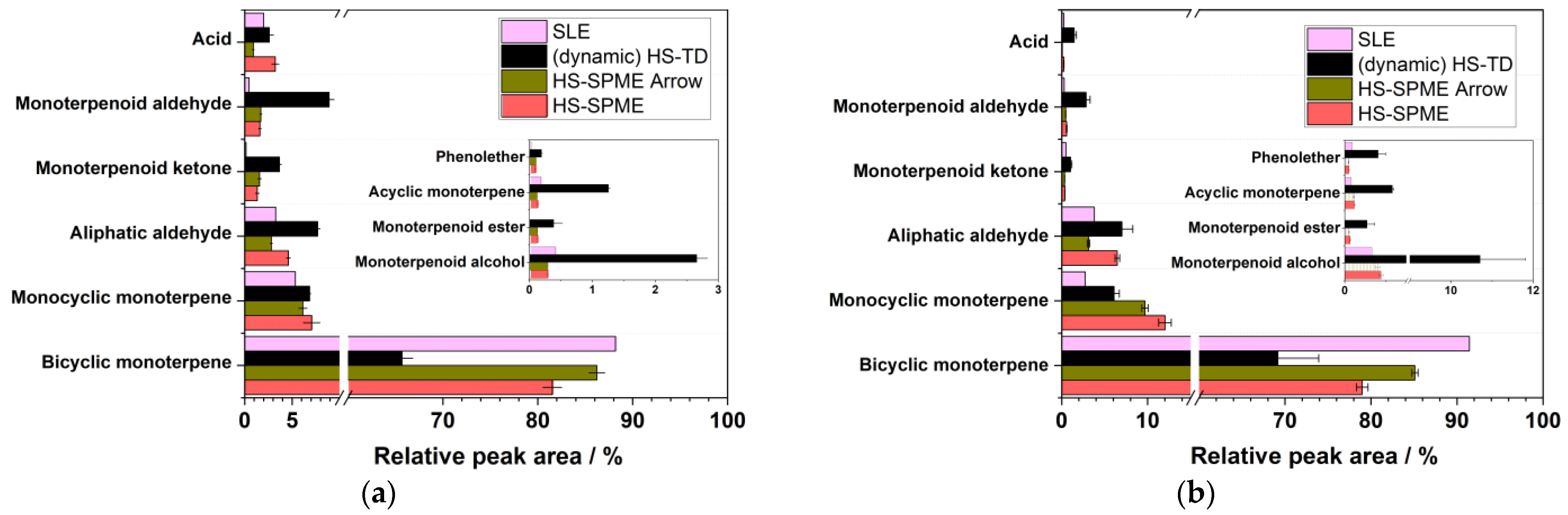
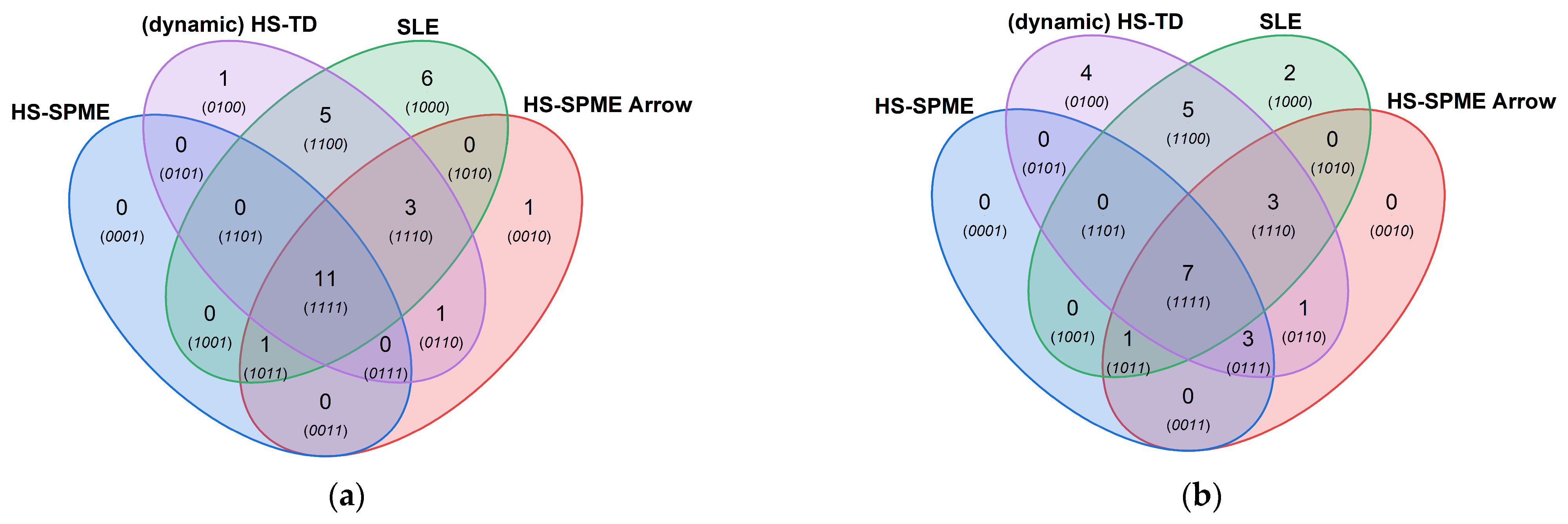
3.3. Comparison of Extraction Methods
3.4. Olfactometric Detection of Odor Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Wu, J.; Lam, F.; Zhang, C.; Kang, J.; Xu, H. Effect of the Degree of Wood Use on the Visual Psychological Response of Wooden Indoor Spaces. Wood Sci. Technol. 2021, 55, 1485–1508. [Google Scholar] [CrossRef]
- Watchman, M.; Potvin, A.; Demers, C.M.H. A Post-Occupancy Evaluation of the Influence of Wood on Environmental Comfort. Bioresources 2017, 12, 8704–8724. [Google Scholar] [CrossRef]
- Rowell, R.M.; Pettersen, R.; Tshabalala, M.A. Cell Wall Chemistry. In Handbook of Wood Chemistry and Wood Composites, 2nd ed.; CRC-Press: Boca Raton, FL, USA, 2016; Volume 110, pp. 33–71. [Google Scholar]
- Leon, M.; Johnson, B.A. Olfactory Coding in the Mammalian Olfactory Bulb. Brain Res. Rev. 2003, 42, 23–32. [Google Scholar] [CrossRef]
- Zhuang, B.; Cloutier, A.; Koubaa, A. Physical and Mechanical Properties of Oriented Strand Board Made from Eastern Canadian Softwood Species. Forests 2022, 13, 523. [Google Scholar] [CrossRef]
- Jandl, R. Climate-Induced Challenges of Norway Spruce in Northern Austria. Trees For. People 2020, 1, 100008. [Google Scholar] [CrossRef]
- Kovačević, M.; Rieder-Gradinger, C.; Teischinger, A.; Srebotnik, E. Volatile Organic Compounds Emitted from Scots Pine and Norway Spruce Wood. Eur. J. Wood Wood Prod. 2022, 81, 699–712. [Google Scholar] [CrossRef]
- Delahunty, C.M.; Eyres, G.; Dufour, J.P. Gas Chromatography-Olfactometry. J. Sep. Sci. 2006, 29, 2107–2125. [Google Scholar] [CrossRef]
- Culleré, L.; Fernández de Simón, B.; Cadahía, E.; Ferreira, V.; Hernández-Orte, P.; Cacho, J. Characterization by Gas Chromatography-Olfactometry of the Most Odor-Active Compounds in Extracts Prepared from Acacia, Chestnut, Cherry, Ash and Oak Woods. LWT-Food Sci. Technol. 2013, 53, 240–248. [Google Scholar] [CrossRef]
- Faix, O.; Meier, D.; Fortmann, I. Thermal Degradation Products of Wood. Holz Roh Werkst. 1990, 48, 281–285. [Google Scholar] [CrossRef]
- Ishino, K.; Wakita, C.; Shibata, T.; Toyokuni, S.; Machida, S.; Matsuda, S.; Matsuda, T.; Uchida, K. Lipid Peroxidation Generates Body Odor Component Trans-2-Nonenal Covalently Bound to Protein In Vivo. J. Biol. Chem. 2010, 285, 15302–15313. [Google Scholar] [CrossRef]
- Song, S.; Zheng, F.; Tian, X.; Feng, T.; Yao, L.; Sun, M.; Shi, L. Evolution Analysis of Free Fatty Acids and Aroma-Active Compounds during Tallow Oxidation. Molecules 2022, 27, 352. [Google Scholar] [CrossRef]
- Granström, K.M. Wood Processing as a Source of Terpene Emissions Compared to Natural Sources. WIT Trans. Ecol. Environ. 2007, 101, 263–272. [Google Scholar]
- Breitmaier, E. Terpene: Aroma, Düfte Pharmaka, Pheromone, 2nd ed.; Wiley-VCH: Hoboken, NJ, USA, 2005; pp. 3–9. [Google Scholar]
- Rosenkranz, M.; Chen, Y.; Zhu, P.; Vlot, A.C. Volatile Terpenes—Mediators of Plant-to-Plant Communication. Plant J. 2021, 108, 617–631. [Google Scholar] [CrossRef]
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and Terpenoids as Main Bioactive Compounds of Essential Oils, Their Roles in Human Health and Potential Application as Natural Food Preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Schreiner, L.; Bauer, P.; Buettner, A. Resolving the Smell of Wood—Identification of Odour-Active Compounds in Scots Pine (Pinus sylvestris L.). Sci. Rep. 2018, 8, 8294. [Google Scholar] [CrossRef]
- Schreiner, L.; Loos, H.M.; Buettner, A. Identification of Odorants in Wood of Calocedrus decurrens (Torr.) Florin by Aroma Extract Dilution Analysis and Two-Dimensional Gas Chromatography–Mass Spectrometry/Olfactometry. Anal. Bioanal. Chem. 2017, 409, 3719–3729. [Google Scholar] [CrossRef]
- Belinato, J.R.; Dias, F.F.G.; Caliman, J.D.; Augusto, F.; Hantao, L.W. Opportunities for Green Microextractions in Comprehensive Two-Dimensional Gas Chromatography/Mass Spectrometry-Based Metabolomics—A Review. Anal. Chim. Acta 2018, 1040, 1–18. [Google Scholar] [CrossRef]
- Mayol, A.R.; Acree, T.E. Advances in Gas Chromatography-Olfactometry. In Gas Chromatography-Olfactometry: The State of the Art, 1st ed.; Leland, J.V., Schieberle, P., Buettner, A., Acree, T.E., Eds.; American Chemical Society: Washington, DC, USA, 2001; Volume 782, pp. 1–10. [Google Scholar]
- Sell, C.S. Chemistry and the Sense of Smell, 1st ed.; Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 32–51. [Google Scholar]
- Nicolotti, L.; Cordero, C.; Bicchi, C.; Rubiolo, P.; Sgorbini, B.; Liberto, E. Volatile Profiling of High Quality Hazelnuts (Corylus avellana L.): Chemical Indices of Roasting. Food Chem. 2013, 138, 1723–1733. [Google Scholar] [CrossRef]
- Rega, B.; Fournier, N.; Guichard, E. Solid Phase Microextraction (SPME) of Orange Juice Flavor: Odor Representativeness by Direct Gas Chromatography Olfactometry (D-GC-O). J. Agric. Food Chem. 2003, 51, 7092–7099. [Google Scholar] [CrossRef]
- Ferracane, A.; Manousi, N.; Tranchida, P.Q.; Zachariadis, G.A.; Mondello, L.; Rosenberg, E. Exploring the Volatile Profile of Whiskey Samples Using Solid-Phase Microextraction Arrow and Comprehensive Two-Dimensional Gas Chromatography-Mass Spectrometry. J. Chromatogr. A 2022, 1676, 463241. [Google Scholar] [CrossRef]
- Engel, W.; Schieberle, P.; Bahr, W.; Bahr, G.; Engel, W.; Bahr, W.; Schieberle, P. Solvent assisted flavour evaporation—A new and versatile technique for the careful and direct isolation of aroma compounds from complex food matrices. Eur. Res. Technol. 1999, 209, 237–241. [Google Scholar] [CrossRef]
- Sharma, L.K.; Agarwal, D.; Rathore, S.S.; Malhotra, S.K.; Saxena, S.N. Effect of Cryogenic Grinding on Volatile and Fatty Oil Constituents of Cumin (Cuminum cyminum L.) Genotypes. J. Food Sci. Technol. 2016, 53, 2827–2834. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheng, Z.; Mannion, D.T.; O’sullivan, M.G.; Miao, S.; Kerry, J.P.; Kilcawley, K.N. Comparison of Automated Extraction Techniques for Volatile Analysis of Whole Milk Powder. Foods 2021, 10, 2061. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.C.; Owen, C.M.; Patterson, J. Solid Phase Microextraction (SPME) Combined with Gas-Chromatography and Olfactometry-Mass Spectrometry for Characterization of Cheese Aroma Compounds. LWT-Food Sci. Technol. 2004, 37, 139–154. [Google Scholar] [CrossRef]
- SPME Fiber Assembly Divinylbenzene/Carboxen/Polydimethylsiloxane (DVB/CAR/PDMS). Available online: https://www.sigmaaldrich.com/AT/en/product/supelco/57348u (accessed on 30 August 2023).
- SPME Fibers and Arrows. Available online: https://at.vwr.com/store/product/22785727/spme-fibers-and-arrows (accessed on 30 August 2023).
- Alfeeli, B.; Jain, V.; Johnson, R.K.; Beyer, F.L.; Heflin, J.R.; Agah, M. Characterization of Poly(2,6-Diphenyl-p-Phenylene Oxide) Films as Adsorbent for Microfabricated Preconcentrators. Microchem. J. 2011, 98, 240–245. [Google Scholar] [CrossRef]
- Elmore, J.S.; Erbahadir, M.A.; Mottram, D.S. Comparison of Dynamic Headspace Concentration on Tenax with Solid Phase Microextraction for the Analysis of Aroma Volatiles. J. Agric. Food Chem. 1997, 45, 2638–2641. [Google Scholar] [CrossRef]
- Schreiner, L.; Ortner, E.; Buettner, A. Nosy Confirmation: Reconstitution of the Characteristic Odor of Softwood via Quantitative Analysis and Human Sensory Evaluation. Anal. Bioanal. Chem. 2020, 412, 1137–1149. [Google Scholar] [CrossRef]
- Allenspach, M.; Valder, C.; Flamm, D.; Grisoni, F.; Steuer, C. Verification of Chromatographic Profile of Primary Essential Oil of Pinus sylvestris L. Combined with Chemometric Analysis. Molecules 2020, 25, 2973. [Google Scholar] [CrossRef]
- Note 100: Volatile and Semi-Volatile Profile Comparison of Whole Versus Cracked Versus Dry Homogenized Barley Grains by Direct Thermal Extraction. Available online: https://www.sisweb.com/referenc/applnote/app-100.htm (accessed on 27 August 2023).
- Ankney, E.; Swor, K.; Satyal, P.; Setzer, W.N. Essential Oil Compositions of Pinus Species (P. contorta Subsp. contorta, P. ponderosa Var. ponderosa, and P. flexilis); Enantiomeric Distribution of Terpenoids in Pinus Species. Molecules 2022, 27, 5658. [Google Scholar] [CrossRef]
- Szmigielski, R.; Cieslak, M.; Rudziński, K.J.; Maciejewska, B. Identification of Volatiles from Pinus Silvestris Attractive for Monochamus Galloprovincialis Using a SPME-GC/MS Platform. Environ. Sci. Pollut. Res. 2012, 19, 2860–2869. [Google Scholar] [CrossRef][Green Version]
- Komenda, M.; Kobel, K.; Koppmann, R.; Wildt, J. Comparability of Biogenic VOC Emission Rate Measurements under Laboratory and Ambient Conditions at the Example of Monoterpene Emissions from Scots pine (Pinus sylvestris). J. Atmos. Chem. 2003, 45, 1–23. [Google Scholar] [CrossRef]
- Alapieti, T.; Castagnoli, E.; Salo, L.; Mikkola, R.; Pasanen, P.; Salonen, H. The Effects of Paints and Moisture Content on the Indoor Air Emissions from Pinewood (Pinus sylvestris) Boards. Indoor Air 2021, 31, 1563–1576. [Google Scholar] [CrossRef] [PubMed]
- Dragomanova, S.; Tancheva, L.; Georgieva, M. A review: Biological activity of myrtenal and some myrtenal containing plant essential. Scr. Sci. Pharm. 2018, 5, 22–33. [Google Scholar] [CrossRef]
- Noma, Y.; Asakawa, Y. 3.19—Biotransformation of Monoterpenoids. In Comprehensive Natural Products II, 1st ed.; Liu, H.W., Mander, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 3, pp. 669–801. [Google Scholar]
- Niu, Y.; Ma, Y.; Xiao, Z.; Zhu, J.; Xiong, W.; Chen, F. Characterization of the Key Aroma Compounds of Three Kinds of Chinese Representative Black Tea and Elucidation of the Perceptual Interactions of Methyl Salicylate and Floral Odorants. Molecules 2022, 27, 1631. [Google Scholar] [CrossRef] [PubMed]
- Kesen, S.; Kelebek, H.; Sen, K.; Ulas, M.; Selli, S. GC-MS-Olfactometric Characterization of the Key Aroma Compounds in Turkish Olive Oils by Application of the Aroma Extract Dilution Analysis. Food Res. Int. 2013, 54, 1987–1994. [Google Scholar] [CrossRef]
- Sharp, M.-E.E. A Comprehensive Screen for Volatile Organic Compounds in Biological Fluids. J. Anal. Toxicol. 2001, 25, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Welke, J.E.; Manfroi, V.; Zanus, M.; Lazarotto, M.; Alcaraz Zini, C. Characterization of the Volatile Profile of Brazilian Merlot Wines through Comprehensive Two Dimensional Gas Chromatography Time-of-Flight Mass Spectrometric Detection. J. Chromatogr. A 2012, 1226, 124–139. [Google Scholar] [CrossRef]
- Giardina, M.; Mccurry, J.D. Comparison of Temperature Programmable Split/Splitless and Cool On-Column Inlets for the Determination of Glycerol and Glycerides in Biodiesel by Gas Chromatography with Flame Ionization Detection. J. Chromatogr. Sci. 2016, 55, 683–688. [Google Scholar] [CrossRef][Green Version]
- Tsuzuki, S. Higher Straight-Chain Aliphatic Aldehydes: Importance as Odor-Active Volatiles in Human Foods and Issues for Future Research. J. Agric. Food Chem. 2019, 67, 4720–4725. [Google Scholar] [CrossRef]
- Sheridan, B.A.; Curran, T.P.; Dodd, V.A. Biofiltration of N-Butyric Acid for the Control of Odour. Bioresour. Technol. 2003, 89, 199–205. [Google Scholar] [CrossRef]
- Reyes, J.; Toledo, M.; Michán, C.; Siles, J.A.; Alhama, J.; Martín, M.A. Biofiltration of Butyric Acid: Monitoring Odor Abatement and Microbial Communities. Environ. Res. 2020, 190, 110057. [Google Scholar] [CrossRef] [PubMed]
- Toshio, M.; Mlchele Gallagher; George, P.; Wise, P.M. Odor Detection of Mixtures of Homologous Carboxylic Acids and Coffee Aroma Compounds by Humans. J. Agric. Food Chem. 2009, 57, 9895–9901. [Google Scholar]
- Wang, G.; Jing, S.; Song, X.; Zhu, L.; Zheng, F.; Sun, B. Reconstitution of the Flavor Signature of Laobaigan-Type Baijiu Based on the Natural Concentrations of Its Odor-Active Compounds and Nonvolatile Organic Acids. J. Agric. Food Chem. 2022, 70, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Clausen, P.A.; Knudsen, H.N.; Larsen, K.; Kofoed-Sørensen, V.; Wolkoff, P.; Wilkins, C.K. Use of Thermal Desorption Gas Chromatography–Olfactometry/Mass Spectrometry for the Comparison of Identified and Unidentified Odor Active Compounds Emitted from Building Products Containing Linseed Oil. J. Chromatogr. A 2008, 1210, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Cai, Y.; Sun-Waterhouse, D.; Cui, C.; Su, G.; Lin, L.; Zhao, M. Approaches of Aroma Extraction Dilution Analysis (AEDA) for Headspace Solid Phase Microextraction and Gas Chromatography-Olfactometry (HS-SPME-GC-O): Altering Sample Amount, Diluting the Sample or Adjusting Split Ratio? Food Chem. 2015, 187, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Grosch, W. Evaluation of the Key Odorants of Foods by Dilution Experiments, Aroma Models and Omission. Chem. Senses 2001, 26, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Stöppelmann, F.; Zhu, L.; Liang, J.; Rigling, M.; Wang, X.; Jin, Q.; Zhang, Y. A Comparative Study on Flavor Trapping Techniques from the Viewpoint of Odorants of Hot-Pressed Rapeseed Oil. Food Chem. 2023, 426, 136617. [Google Scholar] [CrossRef]



| Method | Abbreviation |
|---|---|
| Solid-liquid extraction | SLE |
| Dynamic headspace extraction thermal desorption | (dynamic) HS-TD |
| Headspace solid-phase microextraction | HS–SPME |
| Headspace solid-phase microextraction Arrow | HS–SPME ARROW |
| Sample | Mean | Standard Deviation TVOC | RSD/% |
|---|---|---|---|
| A coarse | 1.622 | 1.425 | 88 |
| A fine | 6.883 | 0.505 | 7 |
| B coarse | 1.112 | 0.2871 | 26 |
| B fine | 2.497 | 0.2877 | 12 |
| Technique | Phase Chemistry | Phase Volume/µL |
|---|---|---|
| HS–SPME | DVB/CAR/PDMS | 2.2 |
| HS-SPME Arrow | DVB/CAR/PDMS | 7.4 |
| Compound | LRI | Chemical Group | HS–SPME | HS–SPME ARROW | (Dynamic) HS-TD | SLE | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (%) | STD (%) | RSD (%) | Mean (%) | STD (%) | RSD (%) | Mean (%) | STD (%) | RSD (%) | (%) | |||
| Acetic acid | 655 | 6 | 2.05 | 0.24 | 11.50 | 0.24 | 0.07 | 27.73 | 1.16 | 0.15 | 13.29 | 0.00 |
| Pentanal | 704 | 3 | 0.26 | 0.01 | 3.30 | 0.06 | 0.00 | 6.82 | 0.26 | 0.01 | 3.93 | 0.05 |
| Propanoic acid | 731 | 6 | 0.63 | 0.07 | 11.17 | 0.19 | 0.02 | 10.50 | 0.87 | 0.07 | 8.25 | 1.08 |
| Butanoic acid | 804 | 6 | 0.45 | 0.06 | 12.85 | 0.34 | 0.03 | 8.15 | 0.36 | 0.11 | 32.25 | 0.74 |
| Hexanal | 813 | 3 | 3.55 | 0.14 | 3.98 | 1.79 | 0.11 | 6.19 | 3.45 | 0.13 | 3.73 | 0.75 |
| Pentanoic acid | 900 | 6 | 0.10 | 0.01 | 14.17 | 0.14 | 0.01 | 8.95 | 0.21 | 0.13 | 59.36 | 0.18 |
| Heptanal | 907 | 3 | 0.14 | 0.01 | 8.04 | 0.13 | 0.01 | 4.12 | 0.28 | 0.01 | 4.79 | 0.02 |
| α-Pinene | 942 | 1 | 40.15 | 1.40 | 3.48 | 46.20 | 0.89 | 1.93 | 38.28 | 1.10 | 2.88 | 48.61 |
| β-Pinene | 984 | 1 | 1.49 | 0.01 | 0.47 | 1.79 | 0.07 | 4.07 | 0.99 | 0.02 | 2.20 | 2.07 |
| β-Myrcene | 993 | 9 | 0.13 | 0.02 | 15.87 | 0.12 | 0.01 | 5.07 | 1.25 | 0.03 | 2.07 | 0.17 |
| Octanal | 1011 | 3 | 0.23 | 0.02 | 7.97 | 0.27 | 0.02 | 6.44 | 0.72 | 0.03 | 3.77 | 0.03 |
| 3-Carene | 1020 | 1 | 39.88 | 0.84 | 2.12 | 38.23 | 0.15 | 0.40 | 26.43 | 0.25 | 0.96 | 37.50 |
| α-Terpinene | 1023 | 2 | 0.12 | 0.01 | 12.39 | 0.09 | 0.00 | 5.55 | 0.06 | 0.00 | 6.25 | 0.25 |
| p-Cymene | 1030 | 2 | 3.86 | 0.48 | 12.52 | 3.13 | 0.30 | 9.69 | 2.20 | 0.15 | 6.77 | 2.91 |
| D-Limonene | 1035 | 2 | 1.88 | 0.25 | 13.39 | 1.70 | 0.10 | 5.73 | 0.90 | 0.02 | 2.51 | 1.72 |
| γ-Terpinene | 1064 | 2 | 0.14 | 0.02 | 11.04 | 0.15 | 0.01 | 7.48 | 0.14 | 0.00 | 2.60 | 0.02 |
| 2-Octenal | 1065 | 3 | 0.02 | 0.00 | 10.35 | 0.03 | 0.00 | 12.66 | 0.05 | 0.01 | 13.65 | 2.01 |
| α-Terpinolene | 1094 | 2 | 0.68 | 0.09 | 13.70 | 0.87 | 0.08 | 9.08 | 0.54 | 0.01 | 2.65 | 0.13 |
| p-Cymenene | 1095 | 2 | 0.37 | 0.02 | 4.80 | 0.19 | 0.02 | 11.30 | 3.01 | 0.07 | 2.24 | 0.32 |
| L-Fenchone | 1096 | 4 | 0.31 | 0.06 | 20.39 | 0.34 | 0.05 | 14.42 | 0.20 | 0.01 | 2.78 | 0.04 |
| Nonanal | 1109 | 3 | 0.32 | 0.02 | 7.02 | 0.38 | 0.02 | 5.91 | 2.63 | 0.13 | 4.91 | 0.13 |
| α-Campholenal | 1138 | 5 | 0.79 | 0.07 | 8.36 | 0.79 | 0.04 | 4.79 | 4.63 | 0.31 | 6.66 | 0.23 |
| Camphor | 1161 | 4 | 0.45 | 0.06 | 13.58 | 0.58 | 0.06 | 10.61 | 2.19 | 0.13 | 6.01 | 0.00 |
| 2-Nonenal | 1173 | 3 | 0.09 | 0.01 | 14.84 | 0.11 | 0.01 | 13.04 | 0.04 | 0.00 | 10.77 | 0.22 |
| Pinocarvone | 1178 | 4 | 0.57 | 0.07 | 12.29 | 0.65 | 0.07 | 10.76 | 1.28 | 0.07 | 5.69 | 0.10 |
| endo-Borneol | 1181 | 7 | 0.14 | 0.01 | 7.71 | 0.14 | 0.00 | 1.90 | 1.21 | 0.12 | 9.88 | 0.18 |
| α-Terpineol | 1198 | 7 | 0.15 | 0.02 | 12.85 | 0.15 | 0.01 | 4.94 | 1.56 | 0.06 | 4.11 | 0.23 |
| Myrtenal | 1208 | 5 | 0.83 | 0.07 | 8.19 | 0.92 | 0.06 | 6.28 | 4.26 | 0.25 | 5.78 | 0.23 |
| 2-Methoxy-p-cymene | 1244 | 10 | 0.08 | 0.01 | 13.05 | 0.09 | 0.01 | 13.89 | 0.09 | 0.01 | 10.91 | 0.00 |
| 2-Decenal | 1276 | 3 | - | - | - | 0.02 | 0.00 | 21.82 | 0.27 | 0.01 | 3.64 | 0.04 |
| Bornylacetate | 1296 | 8 | 0.12 | 0.01 | 9.89 | 0.13 | 0.01 | 9.81 | 0.38 | 0.14 | 36.67 | 0.00 |
| (E,E)-2,4-Decadienal | 1301 | 3 | - | - | - | - | - | - | - | - | - | 0.02 |
| Methyleugenol | 1364 | 10 | 0.01 | 0.00 | 25.15 | 0.02 | 0.00 | 7.06 | 0.10 | - | 3.15 | 0.02 |
| Compound | LRI | Chemical Group | HS–SPME | HS–SPME ARROW | (Dynamic) HS-TD | SLE | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (%) | STD (%) | RSD (%) | Mean (%) | STD (%) | RSD (%) | Mean (%) | STD (%) | RSD (%) | (%) | |||
| Acetic acid | 655 | 6 | 0.17 | 0.01 | 8.15 | - | - | - | 0.61 | 0.02 | 3.98 | - |
| Pentanal | 704 | 3 | 0.40 | 0.02 | 5.24 | 0.09 | 0.01 | 5.61 | 0.24 | 0.05 | 21.31 | 0.12 |
| Propanoic acid | 731 | 6 | - | - | - | - | - | - | 0.32 | 0.14 | 43.62 | - |
| Butanoic acid | 804 | 6 | - | - | - | - | - | - | 0.24 | 0.14 | 60.29 | - |
| Hexanal | 813 | 3 | 5.18 | 0.23 | 4.39 | 2.22 | 0.10 | 4.46 | 3.10 | 0.41 | 13.36 | 1.35 |
| Pentanoic acid | 900 | 6 | 0.09 | 0.01 | 13.53 | 0.07 | 0.01 | 8.90 | 0.27 | 0.04 | 15.27 | 0.24 |
| Heptanal | 907 | 3 | 0.16 | 0.00 | 2.30 | 0.11 | 0.01 | 4.70 | 0.22 | 0.03 | 14.20 | 0.06 |
| α-Pinene | 942 | 1 | 35.00 | 1.74 | 4.96 | 42.80 | 0.89 | 2.07 | 41.21 | 4.53 | 10.99 | 56.92 |
| β-Pinene | 984 | 1 | 1.15 | 0.06 | 5.08 | 1.59 | 0.04 | 2.70 | 0.91 | 0.06 | 6.27 | 1.60 |
| β-Myrcene | 993 | 9 | 0.23 | 0.01 | 6.42 | 0.22 | 0.01 | 4.21 | 1.16 | 0.02 | 1.74 | 0.16 |
| Octanal | 1011 | 3 | 0.22 | 0.01 | 3.40 | 0.20 | 0.01 | 4.16 | 0.59 | 0.13 | 22.03 | 0.09 |
| 3-Carene | 1020 | 1 | 42.83 | 1.18 | 2.75 | 40.71 | 0.60 | 1.47 | 27.04 | 0.58 | 2.15 | 32.93 |
| α-Terpinene | 1023 | 2 | 0.45 | 0.04 | 8.66 | 0.38 | 0.02 | 5.06 | 0.13 | 0.02 | 12.19 | 0.19 |
| p-Cymene | 1030 | 2 | 4.62 | 0.27 | 5.93 | 3.13 | 0.15 | 4.64 | 1.40 | 0.23 | 16.58 | 1.30 |
| D-Limonene | 1035 | 2 | 2.16 | 0.12 | 5.44 | 1.87 | 0.09 | 4.74 | 0.83 | 0.07 | 8.06 | 0.90 |
| γ-Terpinene | 1064 | 2 | 0.86 | 0.07 | 7.77 | 0.83 | 0.04 | 4.43 | 0.34 | 0.04 | 11.15 | 0.11 |
| 2-Octenal | 1065 | 3 | 0.06 | 0.00 | 7.54 | 0.07 | 0.00 | 5.96 | 0.10 | 0.02 | 15.96 | 1.76 |
| α-Terpinolene | 1094 | 2 | 3.20 | 0.23 | 7.09 | 3.09 | 0.12 | 3.86 | 1.25 | 0.12 | 9.41 | 0.19 |
| p-Cymenene | 1095 | 2 | 0.71 | 0.03 | 4.92 | 0.39 | 0.01 | 3.57 | 2.13 | 0.17 | 8.02 | 0.03 |
| L-Fenchone | 1096 | 4 | 0.10 | 0.01 | 8.68 | 0.08 | 0.00 | 3.86 | 0.09 | 0.03 | 30.34 | 0.24 |
| Nonanal | 1109 | 3 | 0.36 | 0.02 | 5.14 | 0.33 | 0.02 | 5.42 | 2.15 | 0.53 | 24.82 | 0.22 |
| α-Campholenal | 1138 | 5 | 0.38 | 0.03 | 7.19 | 0.29 | 0.02 | 5.56 | 1.54 | 0.31 | 20.17 | 0.09 |
| Camphor | 1161 | 4 | 0.19 | 0.01 | 4.55 | 0.19 | 0.01 | 4.48 | 0.72 | 0.08 | 11.22 | 0.02 |
| 2-Nonenal | 1173 | 3 | 0.06 | 0.01 | 10.02 | 0.06 | 0.00 | 5.03 | 0.05 | 0.01 | 19.69 | 0.04 |
| Pinocarvone | 1178 | 4 | 0.08 | 0.00 | 5.02 | 0.06 | 0.00 | 4.15 | 0.23 | 0.04 | 19.54 | 0.23 |
| endo-Borneol | 1181 | 7 | 0.38 | 0.02 | 6.43 | 0.32 | 0.02 | 5.83 | 3.61 | 0.95 | 26.25 | 0.53 |
| α-Terpineol | 1198 | 7 | 0.50 | 0.04 | 7.18 | 0.48 | 0.03 | 6.68 | 6.30 | 1.29 | 20.40 | 0.13 |
| Myrtenal | 1208 | 5 | 0.19 | 0.01 | 3.78 | 0.17 | 0.01 | 5.96 | 1.30 | 0.14 | 10.89 | 0.21 |
| 2-Methoxy-p-cymene | 1244 | 10 | 0.07 | 0.00 | 6.60 | 0.06 | 0.00 | 5.16 | 0.21 | 0.05 | 21.59 | 0.08 |
| 2-Decenal | 1276 | 3 | 0.02 | 0.00 | 7.24 | 0.04 | 0.00 | 9.56 | 0.47 | 0.08 | 17.69 | 0.06 |
| Bornylacetate | 1296 | 8 | 0.13 | 0.01 | 5.04 | 0.10 | 0.00 | 4.56 | 0.54 | 0.18 | 33.12 | 0.00 |
| (E,E)-2,4-Decadienal | 1301 | 3 | - | - | - | - | - | - | 0.09 | 0.01 | 15.85 | 0.10 |
| Methyleugenol | 1364 | 10 | 0.03 | 0.00 | 11.55 | 0.04 | 0.00 | 6.40 | 0.59 | 0.15 | 25.18 | 0.10 |
| Pine Strands | OSB | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compound | Odor Characteristics | LRI | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 |
| Acetic acid | acidic, fresh | 655 | x | |||||||
| Pentanal | fresh, pungent | 704 | x | x | ||||||
| Propanoic acid | acidic, putrid | 731 | x | x | ||||||
| Hexanal | green, grassy | 804 | x | x | x | x | x | x | x | x |
| Butanoic acid | acidic, putrid | 813 | x | x | x | |||||
| Pentanoic acid | acidic, cheese-like | 900 | x | |||||||
| Heptanal | green, oily, grassy | 907 | x | x | ||||||
| α-Pinene | fresh, resinous | 942 | x | x | x | x | x | x | x | x |
| β-Pinene | fresh, resinous | 984 | x | x | x | x | x | x | x | |
| β-Myrcene | sweet, mushroom-like | 993 | x | x | x | x | x | x | x | x |
| Octanal | fresh, fatty | 1011 | x | |||||||
| 3-Carene | terpenoid, solvent-like | 1020 | x | x | x | x | x | x | x | x |
| α-Terpinene | woody, pine, sweet | 1023 | x | x | x | |||||
| p-Cymene | spicy, pungent, solvent-like | 1030 | x | x | x | x | x | x | x | x |
| D-Limonene | citrus, fresh | 1035 | x | x | x | |||||
| γ-Terpinene | sweet, green | 1064 | x | x | x | x | x | x | ||
| 2-Octenal | fatty, oily | 1065 | x | x | x | x | ||||
| 1-Octanol | flowery, citrus | 1079 | x | |||||||
| α-Terpinolene | lemon, floral | 1094 | x | x | x | x | x | x | ||
| p-Cymenene | phenolic, coffee | 1095 | x | x | x | x | x | |||
| L-Fenchone | herbal, woody | 1096 | x | x | x | |||||
| Nonanal | cucumber, sweet | 1109 | x | x | x | x | x | x | x | x |
| α-Campholenal | green, spicy, leafy | 1138 | x | x | x | x | ||||
| Camphor | medicinal, camphorous | 1161 | x | x | x | x | x | x | x | |
| 2-Nonenal | fatty, cucumber | 1173 | x | x | ||||||
| Pinocarvone | sweet, herbal | 1178 | x | x | x | x | ||||
| endo-Borneol | camphorous, spicy | 1181 | x | x | x | x | ||||
| α-Terpineol | resinous, flowery, citrus | 1198 | x | x | ||||||
| Myrtenal | sweet, cool, spicy | 1208 | x | x | x | x | x | x | x | |
| 2-Methoxy-p-cymene | smokey, phenolic | 1244 | x | x | x | x | x | x | ||
| 2-Decenal | fatty, fruity | 1276 | x | x | x | x | x | |||
| Bornylacetate | spicy, metholic | 1296 | x | |||||||
| (E,E)-2,4-Decadienal | fatty, oily | 1301 | x | |||||||
| Methyleugenol | clove, spicy | 1364 | x | x | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schierer, V.; Rieder-Gradinger, C.; Rosenberg, E. Method Comparison for the Identification and Characterization of Odorants from Scots Pine (Pinus sylvestris L.) and Oriented Strand Boards (OSB) Made Thereof by GC-MS and GC-FID/O Using Different Headspace Techniques. Chemosensors 2023, 11, 543. https://doi.org/10.3390/chemosensors11100543
Schierer V, Rieder-Gradinger C, Rosenberg E. Method Comparison for the Identification and Characterization of Odorants from Scots Pine (Pinus sylvestris L.) and Oriented Strand Boards (OSB) Made Thereof by GC-MS and GC-FID/O Using Different Headspace Techniques. Chemosensors. 2023; 11(10):543. https://doi.org/10.3390/chemosensors11100543
Chicago/Turabian StyleSchierer, Valentin, Cornelia Rieder-Gradinger, and Erwin Rosenberg. 2023. "Method Comparison for the Identification and Characterization of Odorants from Scots Pine (Pinus sylvestris L.) and Oriented Strand Boards (OSB) Made Thereof by GC-MS and GC-FID/O Using Different Headspace Techniques" Chemosensors 11, no. 10: 543. https://doi.org/10.3390/chemosensors11100543
APA StyleSchierer, V., Rieder-Gradinger, C., & Rosenberg, E. (2023). Method Comparison for the Identification and Characterization of Odorants from Scots Pine (Pinus sylvestris L.) and Oriented Strand Boards (OSB) Made Thereof by GC-MS and GC-FID/O Using Different Headspace Techniques. Chemosensors, 11(10), 543. https://doi.org/10.3390/chemosensors11100543