Preparation and Application of a Fast, Naked-Eye, Highly Selective, and Highly Sensitive Fluorescent Probe of Schiff Base for Detection of Cu2+
Abstract
:1. Introduction
2. Experiments
2.1. Reagents and Chemicals
2.2. Synthesis of MPMC
2.2.1. Synthesis of Edaravone
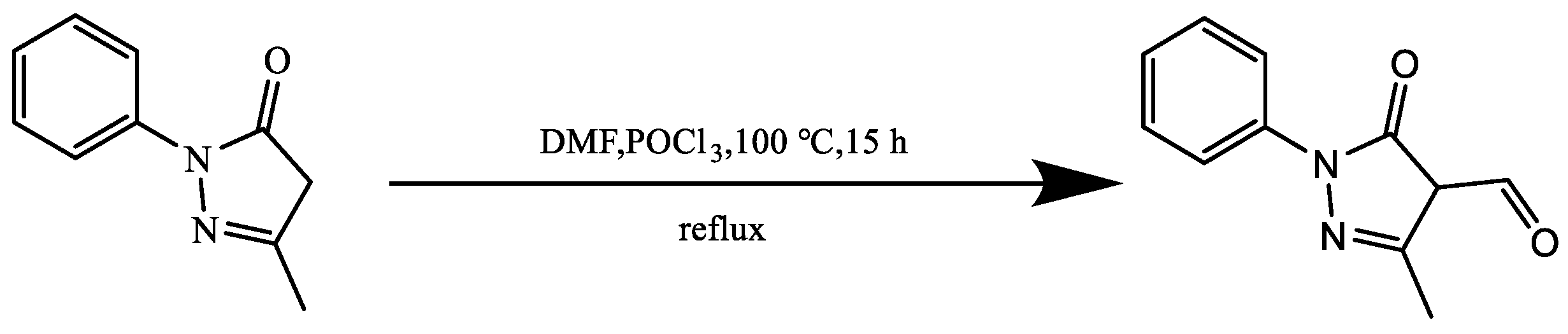
2.2.2. Synthesis of Edaravone Aldehyde Derivative
2.2.3. Synthesis of Ethyl Coumarin-3-Carboxylate
2.2.4. Synthesis of Coumarin-3-Carbohydrazide
2.2.5. Synthesis of MPMC Probe
2.3. General Procedure for the Spectrum Measurement
3. Results and Discussion
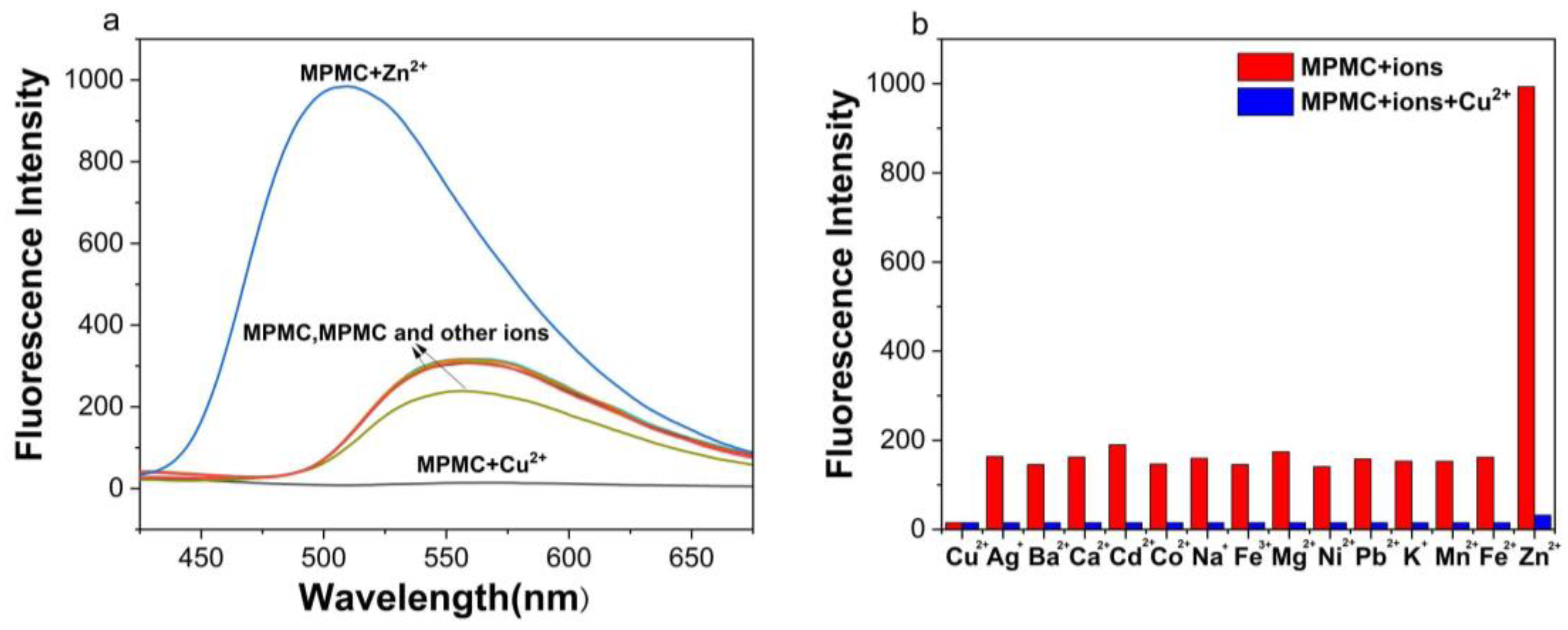
3.1. Fluorescence Spectra of the MPMC Probe for Selectivity and Anti-Interference Detection
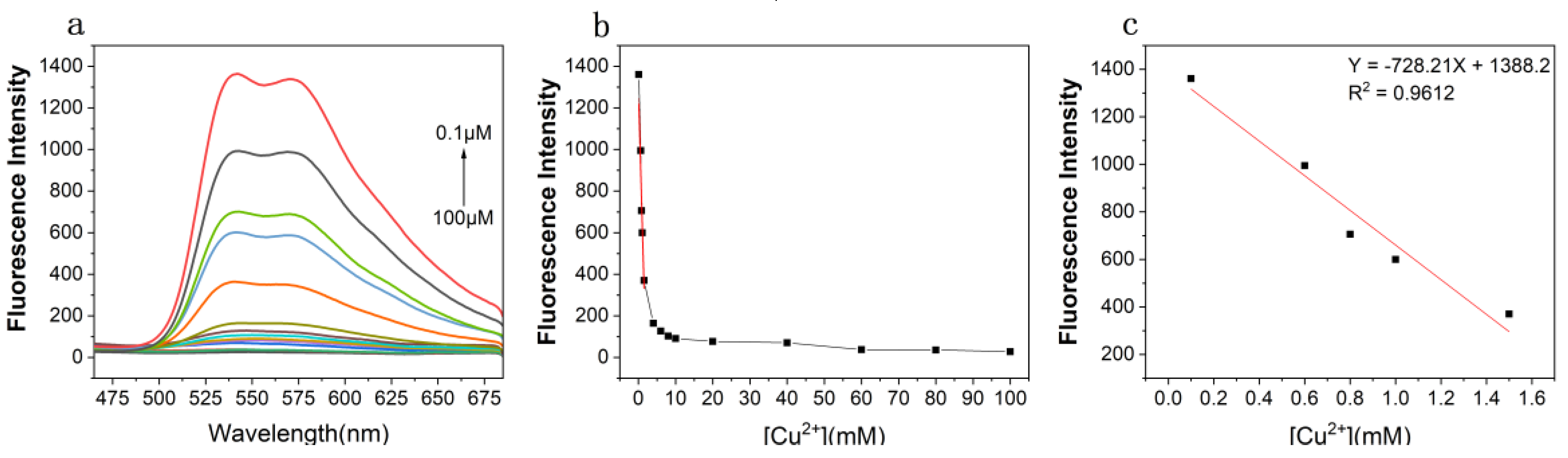
3.2. Titration Experiment of the MPMC Probe to Cu2+
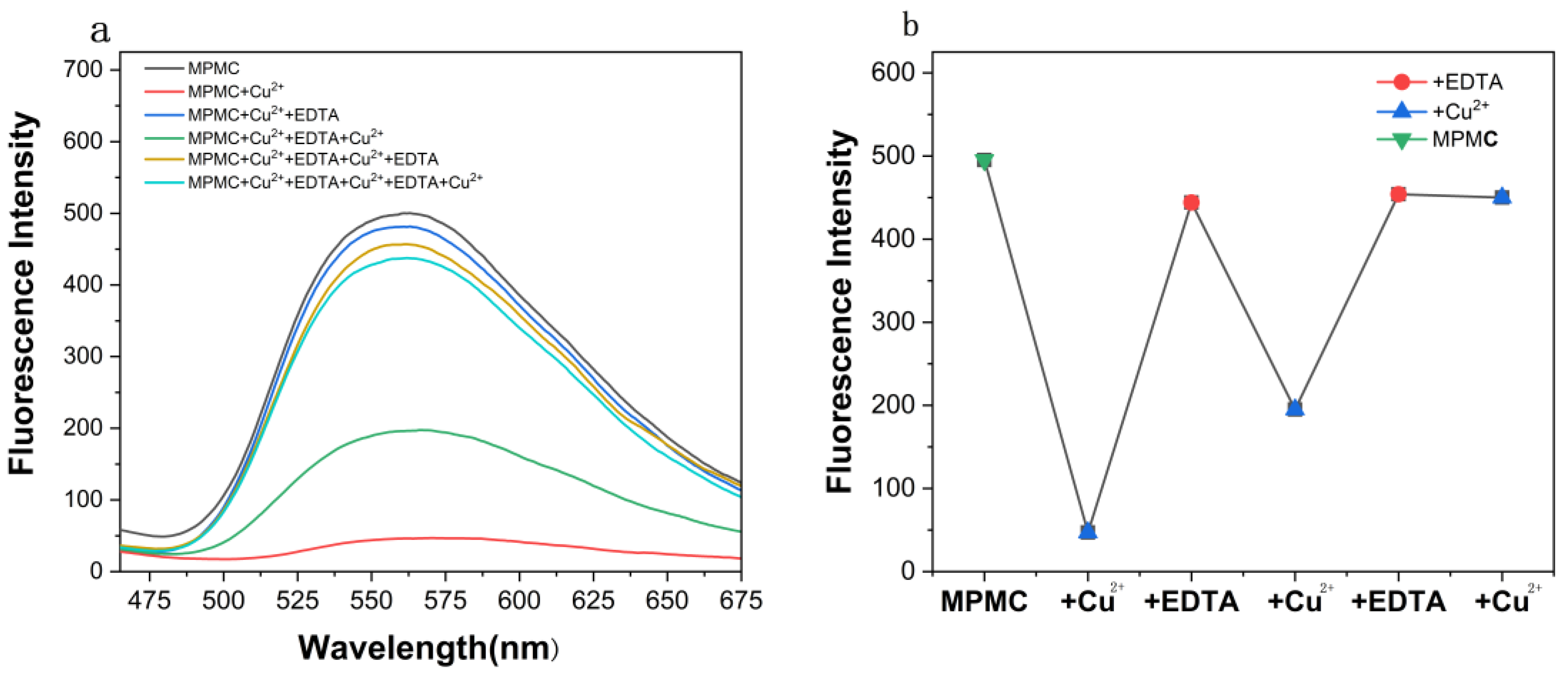
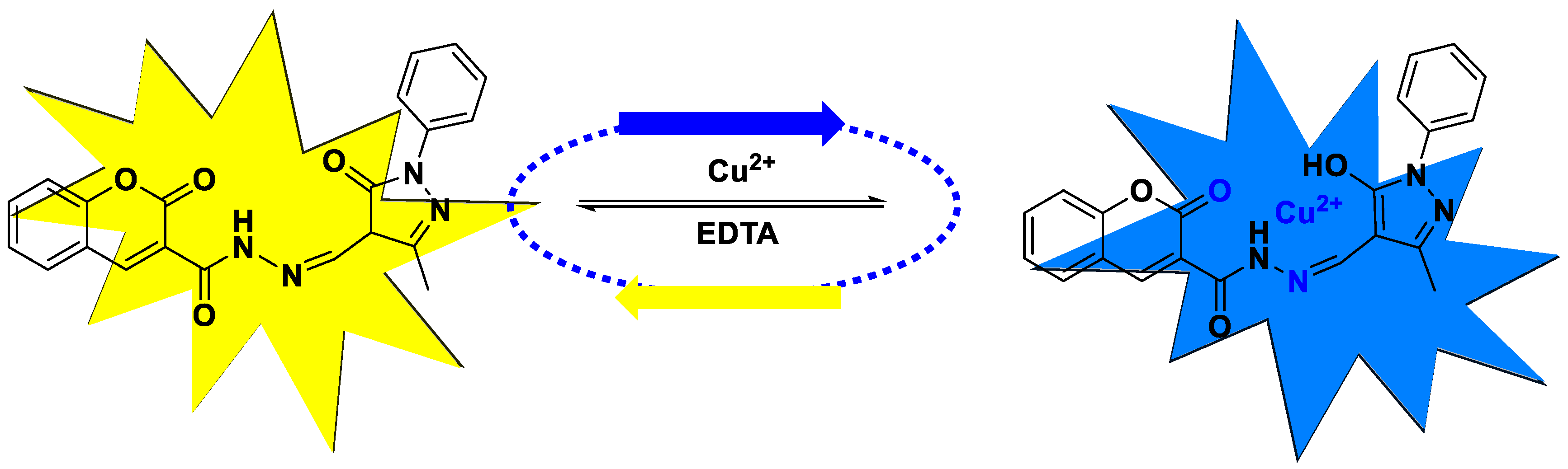
3.3. Study of EDTA Effect of MPMC Probe on Cu2+
3.4. Study of pH Effect of MPMC Probe to Cu2+
3.5. Study of the Response Time of MPMC Probe to Cu2+
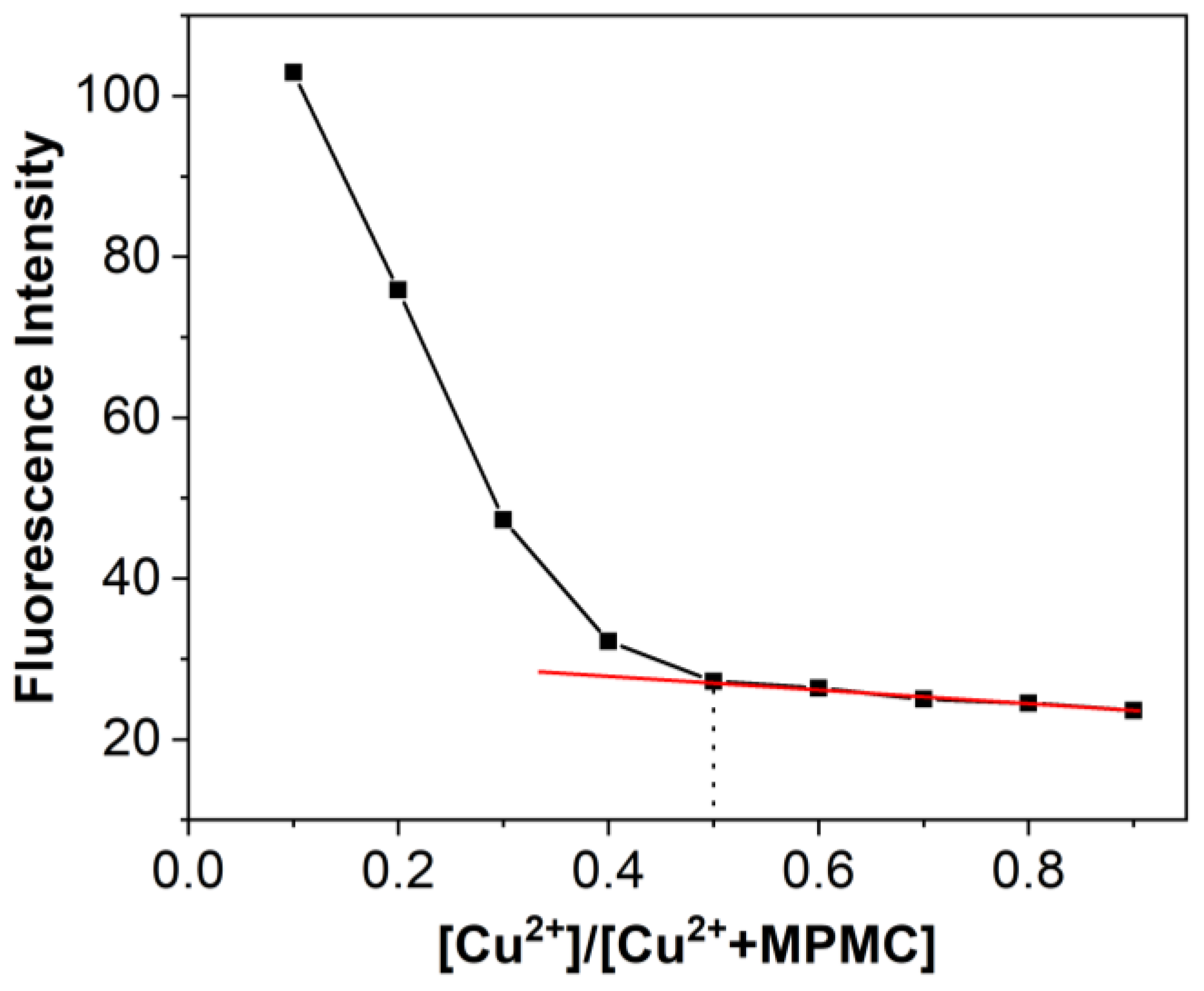
3.6. Contact Mode Detection between MPMC Probe and Cu2+
3.7. The Test Paper for Cu2+ Ions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peers, G.; Price, N.M. Copper-containing plastocyanin used for electron transport by an oceanic diatom. Nature 2006, 441, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Uauy, R.; Olivares, M.; Gonzalez, M. Essentiality of copper in humans. Am. J. Clin. Nutr. 1998, 67, 952S–959S. [Google Scholar] [CrossRef] [PubMed]
- Linder, M.C.; Hazegh-Azam, M. Copper biochemistry and molecular biology. Am. J. Clin. Nutr. 1996, 63, 797S–811S. [Google Scholar] [CrossRef] [PubMed]
- Gaggelli, E.; Kozlowski, H.; Valensin, D.; Valensin, G. Copper homeostasis and neurodegenerative disorders (Alzheimer’s, prion, and Parkinson’s diseases and amyotrophic lateral sclerosis). Chem. Rev. 2006, 106, 1995–2044. [Google Scholar] [CrossRef] [PubMed]
- Strausak, D.; Mercer, J.F.B.; Dieter, H.H.; Stremmel, W.; Multhaup, G. Copper in disorders with neurological symptoms: Alzheimer’s, Menkes, and Wilson diseases. Brain Res. Bull. 2001, 55, 175–185. [Google Scholar] [CrossRef]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef]
- Sun, J.Z.; Xu, X.; Yu, G.H.; Li, W.N.; Shi, J.S. Coumarin-based tripodal chemosensor for selective detection of Cu(II) ion and resultant complex as anion probe through a Cu(II) displacement approach. Tetrahedron 2018, 74, 987–991. [Google Scholar] [CrossRef]
- Meng, X.J.; Cao, D.L.; Hu, Z.Y.; Han, X.H.; Li, Z.C.; Liang, D.; Ma, W.B. A coumarin based highly selective fluorescent chemosensor for sequential recognition of Cu2+ and PPi. Tetrahedron Lett. 2018, 59, 4299–4304. [Google Scholar] [CrossRef]
- Amjadi, M.; Abolghasemi-Fakhri, Z. Gold nanostar-enhanced chemiluminescence probe for highly sensitive detection of Cu(II) ions. Sens. Actuators B Chem. 2018, 257, 629–634. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Yu, C.; Yin, T.X.; Ou, S.S.; Sun, X.Y.; Wen, X.R.; Zhang, L.; Tang, D.Q.; Yin, X.X. Functionalized poly (ionic liquid) as the support to construct a ratiometric electrochemical biosensor for the selective determination of copper ions in AD rats. Biosens. Bioelectron. 2017, 87, 278–284. [Google Scholar] [CrossRef]
- Al-Nidawi, M.; Alshana, U. Reversed-phase switchable-hydrophilicity solvent liquid-liquid microextraction of copper prior to its determination by smartphone digital image colorimetry. J. Food Compos. Anal. 2021, 104, 104140. [Google Scholar] [CrossRef]
- Gamela, R.R.; Costa, V.C.; Pereira, E.R. Multivariate Optimization of Ultrasound-Assisted Extraction Procedure for the Determination of Ca, Fe, K, Mg, Mn, P, and Zn in Pepper Samples by ICP OES. Food Anal. Method. 2020, 13, 69–77. [Google Scholar] [CrossRef]
- Bonin, M.; Lariviere, D.; Povinec, P.P. Detection of radium at the attogram per gram level in copper by inductively coupled plasma mass spectrometry after cation-exchange chromatography. Anal. Methods 2020, 12, 2272–2278. [Google Scholar] [CrossRef]
- Zhou, Z.L.; Tang, H.H.; Chen, S.Y.; Huang, Y.H.; Zhu, X.H.; Li, H.T.; Zhang, Y.Y.; Yao, S.Z. A turn-on red-emitting fluorescent probe for determination of copper(II) ions in food samples and living zebrafish. Food Chem. 2021, 343, 128513. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Cai, Y.H.; He, Y.H.; Lin, Q.L.; Ren, J.L.; Cao, D.S.; Zhang, L. A label-free fluorescent peptide probe for sensitive and selective determination of copper and sulfide ions in aqueous systems. RSC Adv. 2021, 11, 7426–7435. [Google Scholar] [CrossRef]
- Zeng, X.D.; Gao, S.; Jiang, C.; Duan, Q.X.; Ma, M.S.; Liu, Z.G.; Chen, J. Rhodol-derived turn-on fluorescent probe for copper ions with high selectivity and sensitivity. Luminescence 2021, 36, 1761–1766. [Google Scholar] [CrossRef]
- Liu, J.Q.; Liu, Z.C.; Wang, W.K.; Tian, Y. Real-time Tracking and Sensing of Cu+ and Cu2+ with a Single SERS Probe in the Live Brain: Toward Understanding Why Copper Ions Were Increased upon Ischemia. Angew. Chem. Int. Ed. 2021, 60, 21351–21359. [Google Scholar] [CrossRef]
- Sun, X.Y.; Liu, T.; Sun, J.; Wang, X.J. Synthesis and application of coumarin fluorescence probes. RSC Adv. 2020, 10, 10826–10847. [Google Scholar] [CrossRef]
- Wang, K.N.; Sun, P.Z.; Chao, X.J.; Cao, D.X.; Mao, Z.W.; Liu, Z.Q. A coumarin Schiff’s base two-photon fluorescent probe for hypochlorite in living cells and zebrafish. RSC Adv. 2018, 8, 6904–6909. [Google Scholar] [CrossRef]
- He, G.J.; Hua, X.B.; Yang, N.; Li, L.L.; Xu, J.H.; Yang, L.L.; Wang, Q.Z.; Ji, L.G. Synthesis and application of a “turn on” fluorescent probe for glutathione based on a copper complex of coumarin hydrazide Schiff base derivative. Bioorg. Chem. 2019, 91, 103176. [Google Scholar] [CrossRef]
- Yan, L.Q.; Hu, C.J.; Li, J.P. A fluorescence turn-on probe for rapid monitoring of hypochlorite based on coumarin Schiff base. Anal. Bioanal. Chem. 2018, 410, 7457–7464. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, X.H.; Liang, L.X.; Gao, L.Y.; Ren, X.M.; Wu, Y.G.; Zhao, H.C. A coumarin-containing Schiff base fluorescent probe with AIE effect for the copper(ii) ion. RSC Adv. 2020, 10, 6109–6113. [Google Scholar] [CrossRef]
- Mani, K.S.; Rajamanikandan, R.; Ravikumar, G.; Pandiyan, B.V.; Kolandaivel, P.; Ilanchelian, M.; Rajendran, S.P. Highly Sensitive Coumarin-Pyrazolone Probe for the Detection of Cr3+ and the Application in Living Cells. ACS Omega 2018, 3, 17212–17219. [Google Scholar] [CrossRef]
- Babur, B.; Seferoglu, N.; Seferoglu, Z. A coumarin-pyrazolone based fluorescent probe for selective colorimetric and fluorimetric fluoride detection: Synthesis, spectroscopic properties and DFT calculations. J. Mol. Struct. 2018, 1161, 218–225. [Google Scholar] [CrossRef]
- Babur, B.; Seferoglu, N.; Ocal, M.; Sonugur, G.; Akbulut, H.; Seferoglu, Z. A novel fluorescence turn-on coumarin-pyrazolone based monomethine probe for biothiol detection. Tetrahedron 2016, 72, 4498–4502. [Google Scholar] [CrossRef]
- Honglei, L.; Pengyu, C.; Juan, L.; Sai, C.; Meng, W. Coumarin Pyrazolone Fluorescent Probe as Well as Preparation Method and Application Thereof. CN202210882392.3[P]. 11 November 2022. Available online: https://d.wanfangdata.com.cn/patent/ChJQYXRlbnROZXdTMjAyMzA5MDESEENOMjAyMjEwODgyMzkyLjMaCGxzemJpOXd5 (accessed on 1 November 2023).
- Vijay, K.; Nandi, C.; Samant, S.D. Synthesis of a dihydroquinoline based merocyanine as a ‘naked eye’ and ‘fluorogenic’ sensor for hydrazine hydrate in aqueous medium and hydrazine gas. RSC Adv. 2014, 4, 30712–30717. [Google Scholar] [CrossRef]
- Surati, K.R.; Thaker, B.T. Synthesis, spectroscopic and thermal investigation of Schiff-base complexes of Cu(II) derived from heterocyclic beta-diketone with various primary amines. J. Coord. Chem. 2006, 59, 1191–1202. [Google Scholar] [CrossRef]
- Naik, M.D.; Bodke, Y.D.; Kumar, V.M.; Revanasiddappa, B.C. An efficient one-pot synthesis of coumarin-amino acid derivatives as potential anti-inflammatory and antioxidant agents. Synth. Commun. 2020, 50, 1210–1216. [Google Scholar] [CrossRef]
- Taha, M.; Shah, S.A.A.; Afifi, M.; Imran, S.; Sultan, S.; Rahim, F.; Khan, K.M. Synthesis, alpha-glucosidase inhibition and molecular docking study of coumarin based derivatives. Bioorg. Chem. 2018, 77, 586–592. [Google Scholar] [CrossRef]
- Xia, X.C.; Fu, Y.; Tang, H.; Li, Y.; Wang, F.Y.; Ren, J. A self-assembled micellar nanoprobe for specific recognition of hydrazine in vitro and in vivo. Sens. Actuators B Chem. 2018, 272, 479–484. [Google Scholar] [CrossRef]
- Yin, J.; Wang, Z.; Zhao, F.; Yang, H.; Li, M.; Yang, Y. A novel dual functional pyrene-based turn-on fluorescent probe for hypochlorite and copper (II) ion detection and bioimaging applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 239, 118470. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Ai, Y.; Zhang, Y.L.; Ren, Y.Y.; Wang, J.L.; Yao, F.Y.; Li, W.Y.; Zhou, Y.; Sun, Y.N.; Liu, J.L.; et al. A new probe with high selectivity and sensitivity for detecting copper ions in traditional Chinese medicine and water sample. Inorg. Chem. Commun. 2021, 128, 108563. [Google Scholar] [CrossRef]
- Wang, M.M.; Wang, C.; Wang, M.; Sun, T.M.; Huang, Y.; Tang, Y.F.; Ju, J.F.; Shen, L.J.; Hu, Y.Y.; Zhu, J.L. A Dual-Functional “On-Off-On” Relay Fluorescent Probe for the Highly Sensitive Detection of Copper(II) and Phosphate Ions. ChemistrySelect 2020, 5, 1331–1334. [Google Scholar] [CrossRef]
- Anand, T.; Sivaraman, G.; Chellappa, D. Quinazoline copper(II) ensemble as turn-on fluorescence sensor for cysteine and chemodosimeter for NO. J. Photochem. Photobiol. A 2014, 281, 47–52. [Google Scholar] [CrossRef]
- Arslan, F.N.; Geyik, G.A.; Koran, K.; Ozen, F.; Aydin, D.; Elmas, S.N.K.; Gorgulu, A.O.; Yilmaz, I. Fluorescence “Turn On-Off” Sensing of Copper (II) Ions Utilizing Coumarin-Based Chemosensor: Experimental Study, Theoretical Calculation, Mineral and Drinking Water Analysis. J. Fluoresc. 2020, 30, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Choi, D.; Kim, C. A Benzothiazole-Based Fluorescence Turn-on Sensor for Copper(II). J. Fluoresc. 2021, 31, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Silpcharu, K.; Soonthonhut, S.; Sukwattanasinitt, M.; Rashatasakhon, P. Fluorescent Sensor for Copper(II) and Cyanide Ions via the Complexation-Decomplexation Mechanism with Di(bissulfonamido)spirobifluorene. ACS Omega 2021, 6, 16696–16703. [Google Scholar] [CrossRef]
- Tamil Selvan, G.; Varadaraju, C.; Tamil Selvan, R.; Enoch, I.; Mosae Selvakumar, P. On/Off Fluorescent Chemosensor for Selective Detection of Divalent Iron and Copper Ions: Molecular Logic Operation and Protein Binding. ACS Omega 2018, 3, 7985–7992. [Google Scholar] [CrossRef]
- Rupa, S.A.; Patwary, M.A.M.; Ghann, W.E.; Abdullahi, A.; Uddin, A.K.M.R.; Mahmud, M.M.; Haque, M.A.; Uddin, J.; Kazi, M. Synthesis of a novel hydrazone-based compound applied as a fluorescence turn-on chemosensor for iron(iii) and a colorimetric sensor for copper(ii) with antimicrobial, DFT and molecular docking studies. RSC Adv. 2023, 13, 23819–23828. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Teng, Q.; Yang, Y.S.; Guo, H.C.; Xue, J.J. A novel coumarin-based pyrazoline fluorescent probe for detection of Fe3+ and its application in cells. Inorg. Chim. Acta 2021, 525, 120469. [Google Scholar] [CrossRef]
- Zong, L.Y.; Wang, C.; Song, Y.C.; Hu, J.; Li, Q.Q.; Li, Z. A fluorescent and colorimetric probe based on naphthalene diimide and its high sensitivity towards copper ions when used as test strips. RSC Adv. 2019, 9, 12675–12680. [Google Scholar] [CrossRef] [PubMed]

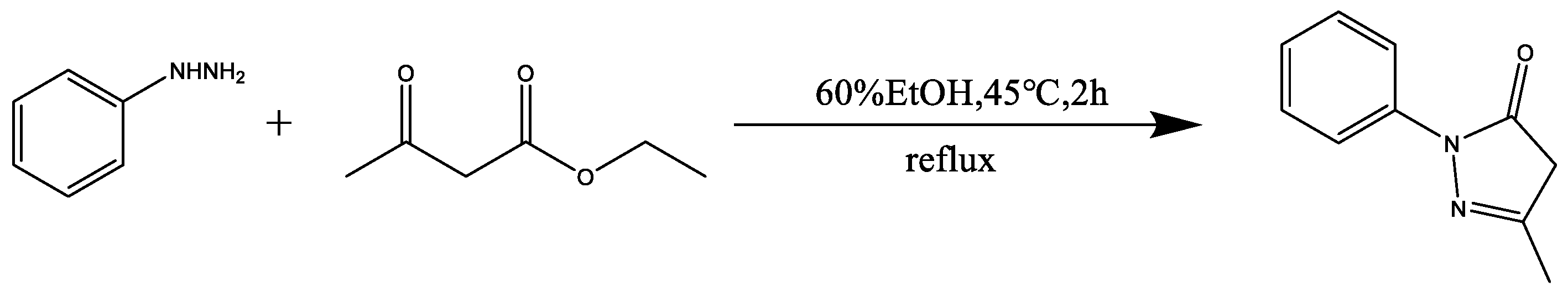
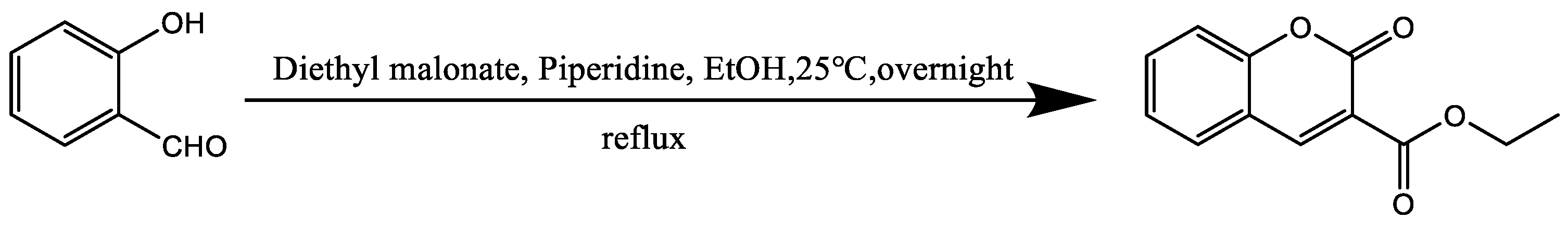





| Compound | Molecular Formula | Solvent | Repeatability | Detection Limit | Reference |
|---|---|---|---|---|---|
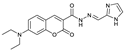 | C18H19N5O3 | CH3CN:HEPES (3:2, v/v) | No | 0.12 μM | [20] |
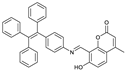 | C37H27NO3 | THF/H2O | No | 0.36 μM | [22] |
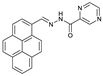 | C22H14N4O | EtOH-HEPES | No | 157 nM | [32] |
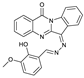 | C23H16N4O3 | Tris-HCl buffer solution | Yes | 84 nM | [33] |
 | C23H16N2O5 | EtOH-HEPES (80:20, v/v) | No | 6.14 × 10−7 M | [34] |
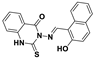 | C19H13N3O2S | DMSO:H2O (1:9, v/v) | No | 20.0 nM | [35] |
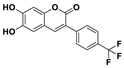 | C16H9F3O4 | CH3CN/HEPES (95/5, v/v) | No | 24.5 nM | [36] |
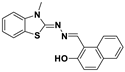 | C19H15N3OS | DMSO | No | 3.3 μM | [37] |
 | C45H36N4O8S4 | 50% CH3CN and 20 mM HEPES | No | 390 nM | [38] |
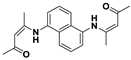 | C20H22N2O2 | Water/DMF (9.9/0.1) | No | - | [39] |
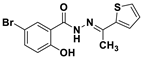 | C13H11BrN2O2S | DMSO | No | 2.35 μM | [40] |
 | C21H16N4O4 | EtOH/H2O (1:1, v/v) | Yes | 10.23 nM | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Cheng, P.-Y.; Chen, S.; Wang, M.; Wei, K.; Li, Y.; Cao, Y.-Y.; Wang, X.; Li, H.-L. Preparation and Application of a Fast, Naked-Eye, Highly Selective, and Highly Sensitive Fluorescent Probe of Schiff Base for Detection of Cu2+. Chemosensors 2023, 11, 556. https://doi.org/10.3390/chemosensors11110556
Liu J, Cheng P-Y, Chen S, Wang M, Wei K, Li Y, Cao Y-Y, Wang X, Li H-L. Preparation and Application of a Fast, Naked-Eye, Highly Selective, and Highly Sensitive Fluorescent Probe of Schiff Base for Detection of Cu2+. Chemosensors. 2023; 11(11):556. https://doi.org/10.3390/chemosensors11110556
Chicago/Turabian StyleLiu, Juan, Peng-Yu Cheng, Sai Chen, Meng Wang, Kai Wei, Yuan Li, Yao-Yao Cao, Xing Wang, and Hong-Lei Li. 2023. "Preparation and Application of a Fast, Naked-Eye, Highly Selective, and Highly Sensitive Fluorescent Probe of Schiff Base for Detection of Cu2+" Chemosensors 11, no. 11: 556. https://doi.org/10.3390/chemosensors11110556





