Pyrene-Based Fluorescent Probe for “Off-on-Off” Sequential Detection of Cu2+ and CN− with HeLa Cells Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Preparation of Metal Ion and Anion Solutions for UV-vis and Fluorescence Studies
2.3. Quantum Yield (QY) Calculation
2.4. NMR Titrations
2.5. Synthesis of Probe L
2.6. Cytotoxicity Assay
2.7. Living Cell Culture and Confocal Laser Scanning Microscopy Imaging of Probe L
2.8. Theoretical Calculations [31,32,33]
3. Results and Discussion
3.1. Synthesis
3.2. Effect of Solvents and Aggregation-Induced Emissions (AIEs) from Schiff Bases
3.3. Selectivity Analysis of Probe L by Metal Ions
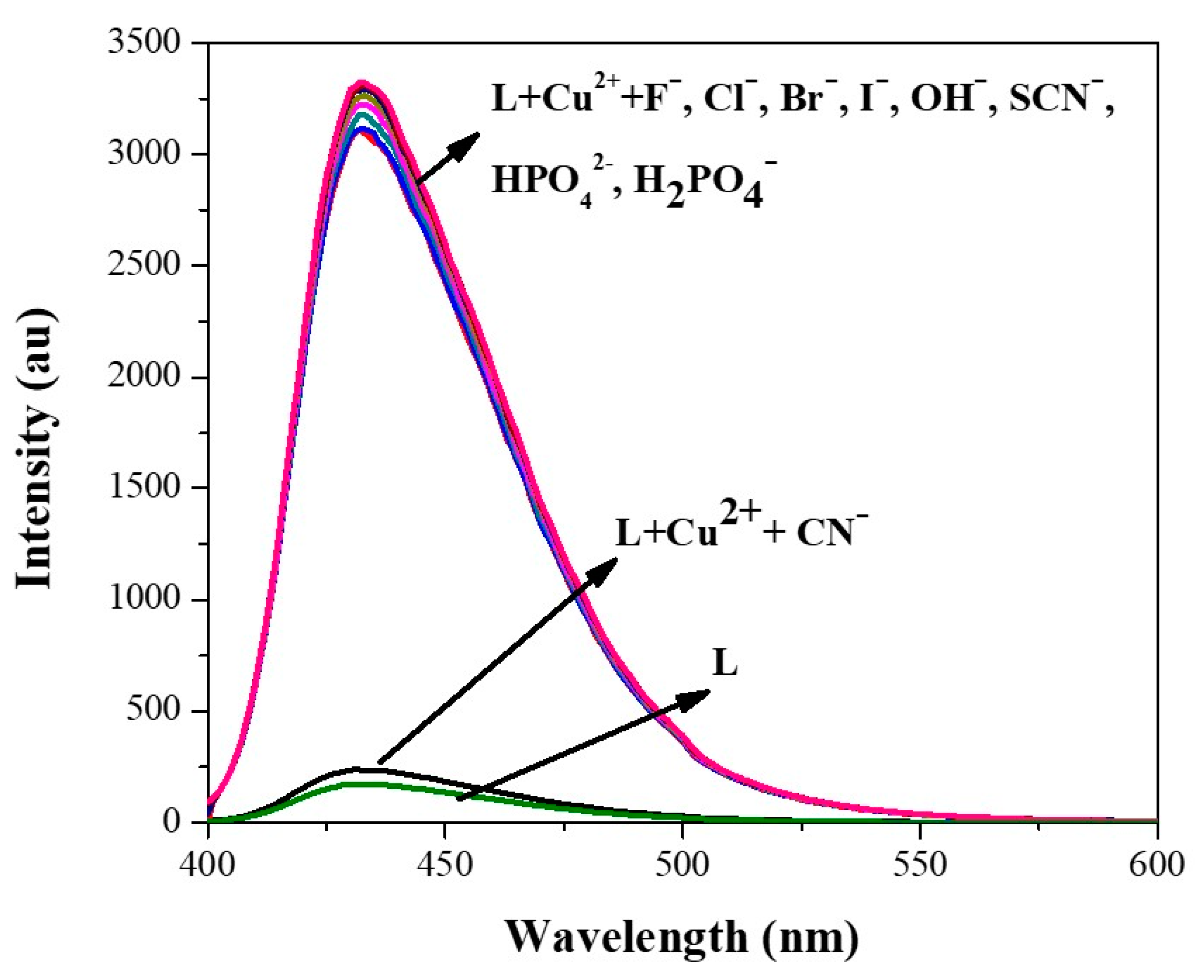
3.4. Changes in Excimer Emission of L–Cu2+ with Anions
3.5. 1H NMR, Mass and DFT Investigations on Complex Formation
3.6. pH Effect
3.7. Probing the Interaction on TLC Plate and Polymer Membrane
3.7.1. Emission Color Modulation on a TLC Plate
3.7.2. Emission Color Modulation on a Polymer Membrane
3.8. Bio Image of Living Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, C.; Chen, G.; Zhao, Y.; Liu, P.; Duan, X.; Gu, L.; Fu, Y.; Yuan, G.; Zheng, N. Interfacing with silica boosts the catalysis of copper. Nat. Commun. 2018, 9, 3367. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Q.; Feng, W.; Li, F. Luminescent Chemodosimeters for Bioimaging. Chem. Rev. 2013, 113, 192–270. [Google Scholar] [CrossRef]
- Li, X.; Pan, L.; Yang, F.; Yang, L. Blue Carbon Dot-Based Portable Smartphone Platform for Visualization of Copper (II). ACS Appl. Nano Mater. 2022, 5, 9252–9259. [Google Scholar] [CrossRef]
- Kizilgun, M.; Takci, S.; Erkekoglu, P.; Asci, A.; Balci, A.; Yigit, S.; Gumusel, B.K. Copper, zinc and iron levels in premature infants following red blood cell transfusion. J. Trace Elem. Med. Biol. 2016, 38, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Sparling, T.M.; Deeney, M.; Cheng, B.; Han, X.; Lier, C.; Lin, Z.; Offner, C.; Santoso, M.V.; Pfeiffer, E.; Emerson, J.A.; et al. Systematic evidence and gap map of research linking food security and nutrition to mental health. Nat. Commun. 2022, 13, 4608. [Google Scholar] [CrossRef]
- Viles, J.H. Metal ions and amyloid fiber formation in neurodegenerative diseases. Copper, zinc and iron in Alzheimer’s, Parkinson’s and prion diseases. Coord. Chem. Rev. 2012, 256, 2271–2284. [Google Scholar] [CrossRef]
- Cacciatore, I.; Turkez, H.; Rienzo, A.D.; Ciulla, M.; Mardinoglu, A.; Stefano, A.D. Boron-based hybrids as novel scaffolds for the development of drugs with neuroprotective properties. RSC Med. Chem. 2021, 12, 1944–1949. [Google Scholar] [CrossRef]
- Scheiber, I.F.; Mercer, J.F.B.; Dringen, R. Metabolism and functions of copper in brain. Prog. Neurobiol. 2014, 116, 33–57. [Google Scholar]
- Ahuja, A.; Dev, K.; Tanwar, R.S.; Selwal, K.K.; Tyagi, P.K. Copper mediated neurological disorder: Visions into amyotrophic lateral sclerosis, Alzheimer and Menkes disease. J. Trace Elem. Med. Biol. 2015, 29, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Haddad, M.R.; Macri, C.J.; Holmes, C.S.; Goldstein, D.S.; Jacobson, B.E.; Centeno, J.A.; Popek, E.J.; Gahl, W.A.; Kaler, S.G. In utero copper treatment for Menkes disease associated with a severe ATP7A mutation. Mol. Genet. Metab. 2012, 107, 222–228. [Google Scholar] [CrossRef]
- Ugarte, M.; Osborne, N.N.; Brown, L.A.; Bishop, P.N. Iron, zinc, and copper in retinal physiology and disease. Surv. Ophthalmol. 2013, 58, 585–609. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Bok, K.H.; Kim, J.A.; Kim, S.Y.; Kim, C. Simultaneous detection of Cu2+ and Cr3+ by a simple Schiff-base colorimetric chemosensor bearing NBD (7-nitrobenzo-2-oxa-1,3-diazolyl) and julolidine moieties. Tetrahedron 2016, 72, 5563–5570. [Google Scholar] [CrossRef]
- Wang, P.; Xue, S.; Zhou, D.; Guo, Z.; Wang, Q.; Guo, B.; Yang, X.; Wu, J. Peptide-based colorimetric and fluorescent dual-functional probe for sequential detection of copper(Ⅱ) and cyanide ions and its application in real water samples, test strips and living cells. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 276, 121222. [Google Scholar] [CrossRef] [PubMed]
- Mayurachayakul, P.; Chantarasriwong, O.; Yotapan, N.; Kamkaew, A.; Mingvanish, W.; Srisuwannaket, C.; Sukwattanasinitt, M.; Niamnont, N. Novel selective “on–off” fluorescence sensor based on julolidine hydrazone-Al3+ complex for Cu2+ ion: DFT study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 279, 21382. [Google Scholar]
- Nantapon, T.; Naweephattana, P.; Surawatanawong, P.; Saetear, P.; Chantarojsiri, T.; Ruangsupapichat, N. Amino-coumarin-based colorimetric and fluorescent chemosensors capable of discriminating Co2+, Ni2+, and Cu2+ ions in solution and potential utilization as a paper-based device. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 282, 121662. [Google Scholar] [CrossRef]
- Yang, W.; Chen, X.; Su, H.; Fang, W.; Zhang, Y. The fluorescence regulation mechanism of the paramagnetic metal in a biological HNO sensor. Chem. Commun. 2015, 51, 9616. [Google Scholar] [CrossRef]
- Su, H.; Chen, X.; Fang, W. ON–OFF Mechanism of a Fluorescent Sensor for the Detection of Zn(II), Cd(II), and Cu(II)Transition Metal Ions. Anal. Chem. 2014, 86, 891–899. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, X.; Kim, H.N.; Yoon, J. Sensors for the optical detection of cyanide ion. Chem. Soc. Rev. 2010, 39, 127–137. [Google Scholar] [CrossRef]
- Chen, Z.Z.; Li, R.Y.; Zhang, W.Z.; Zhang, Y.; Dong, W.K. A new salamo-based colorimetric and fluorescent turn-on sensor with aggregation-induced emission for the rapid and highly sensitive detection of cyanide in real samples. New J. Chem. 2020, 44, 21038–21048. [Google Scholar] [CrossRef]
- Wu, L.; Liu, Y.; Wu, X.; Li, Y.; Du, J.; Qi, S.; Yang, Q.; Xu, H.; Li, Y. A novel Near-Infrared fluorescent probe for Zn2+ and CN– double detection based on dicyanoisfluorone derivatives with highly sensitive and selective, and its application in Bioimaging. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 267, 120621. [Google Scholar] [CrossRef]
- Behymer, M.M.; Mo, H.; Fujii, N.; Suresh, V.; Chan, A.; Lee, J.; Nath, A.K.; Saha, K.; Mahon, S.B.; Brenner, M.; et al. Identification of Platinum (II) Sulfide Complexes Suitable as Intramuscular Cyanide Countermeasures. Chem. Res. Toxicol. 2022, 35, 1983–1996. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.; Chen, M.; Guo, W. A novel fluorescent and chromogenic probe for cyanide detection in water based on the nucleophilic addition of cyanide to imine group. Talanta 2009, 80, 996–1000. [Google Scholar] [CrossRef]
- Song, Y.F.; Wu, W.N.; Zhao, X.L.; Wang, Y.; Fan, Y.C.; Dong, X.Y.; Xu, Z.H. A simple colorimetric and fluorometric probe for rapid detection of CN– with large emission shift. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 280, 121540. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, H.; Guan, R.; Cao, D.; Wu, Q.; Yu, X. Synthesis and cyanide anion recognition of a new displacement fluorescence chemosensor based on two-branched aurone. Fibers Polym. 2016, 17, 181–185. [Google Scholar] [CrossRef]
- Robbins, T.F.; Qian, H.; Su, X.; Hughes, R.P.; Aprahamian, I. Cyanide Detection Using a Triazolopyridinium Salt. Org. Lett. 2013, 15, 2386–2389. [Google Scholar] [CrossRef] [PubMed]
- Shellaiah, M.; Chen, Y.T.; Thirumalaivasan, N.; Aazaad, B.; Awasthi, K.; Sun, K.W.; Wu, S.P.; Lin, M.C.; Ohta, N. Pyrene-Based AIEE Active Nanoprobe for Zn2+ and Tyrosine Detection Demonstrated by DFT, Bioimaging, and Organic Thin-Film Transistor. ACS Appl. Mater. Interfaces 2021, 13, 28610–28626. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, P.; Wu, S.P. A turn-on fluorescent pyrene-based chemosensor for Cu(ii) with live cell application. Rsc Adv. 2015, 5, 42591–42596. [Google Scholar]
- Balamurugan, G.; Venkatesan, P.; Wu, S.P.; Velmathi, S. Novel ratiometric turn-on fluorescent probe for selective sensing of cyanide ions, effect of substitution and bio-imaging studies. Rsc Adv. 2016, 6, 24229–24235. [Google Scholar] [CrossRef]
- Kowser, Z.; Jin, C.C.; Jiang, X.; Rahman, S.; Georghiou, P.E.; Ni, X.L.; Zeng, X.; Redshaw, C.; Yamato, T. Fluorescent turn-on sensors based on pyrene-containing Schiff base derivatives for Cu2+ recognition: Spectroscopic and DFT computational studies. Tetrahedron 2016, 72, 4575–4581. [Google Scholar] [CrossRef]
- Wei, L.F.; Chen, C.Y.; Lai, C.K.; Thirumalaivasan, N.; Wu, S.P. A nano-molar fluorescent turn-on probe for copper (II) detection in living cells. Methods 2019, 168, 18–23. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Exchange-Energy Approximation with Correct Asymptotic Behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Shellaiah, M.; Thirumalaivasan, N.; Aazaad, B.; Awasthi, K.; Sun, K.W.; Wu, S.P.; Lin, M.C.; Ohta, N. An AIEE Active Anthracene-Based Nanoprobe for Zn2+ and Tyrosine Detection Validated by Bioimaging Studies. Chemosensors 2022, 10, 381. [Google Scholar] [CrossRef]
- Mahata, S.; Dey, S.; Mandal, B.B.; Manivannan, V. 3-(2-Hydroxyphenyl)imidazo[5, 1-a] isoquinoline as Cu(II) sensor, its Cu(II) complex for selective detection of CN− ion and biological compatibility. J. Photochem. Photobiol. A 2022, 427, 113795. [Google Scholar] [CrossRef]
- Long, C.; Hu, J.H.; Fu, Q.Q.; Ni, P.W. A new colorimetric and fluorescent probe based on Rhodamine B hydrazone derivatives for cyanide and Cu2+ in aqueous media and its application in real life. Spectrochim. Acta A 2019, 219, 297–306. [Google Scholar] [CrossRef]
- Zulfajri, M.; Dhandabani, G.K.; Chen, H.F.; Wang, J.J.; Huang, G.G. A Simple Visible Recognition Method for Copper Ions Using Dibenzo[b,j][1,10]Phenanthroline Scaffold as a Colorimetric Sensor. Chemosensors 2021, 9, 7. [Google Scholar] [CrossRef]
- Saikia, G.; Dwivedi, A.K.; Iyer, P.K. Development of solution, film and membrane based fluorescent sensor for the detection of fluoride anions from water. Anal. Methods 2012, 4, 3180. [Google Scholar] [CrossRef]
- Silpcharu, K.; Soonthonhut, S.; Sukwattanasinitt, M.; Rashatasakhon, P. Fluorescent Sensor for Copper (II) and Cyanide Ions via the Complexation−Decomplexation Mechanism with Di(bissulfonamido)spirobifluorene. ACS Omega 2021, 6, 16696–16703. [Google Scholar] [CrossRef]
- Mohammadi, A.; Ghasemi, Z. A simple pyrimidine based colorimetric and fluorescent chemosensor for sequential detection of copper (II) and cyanide ions and its application in real samples. Spectrochim. Acta A Mol. 2020, 228, 117730. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, J.; Shen, J.; Zhang, C.; Wu, Z.; Zhou, H. A colorimetric quinoline-based chemosensor for sequential detection of copper ion and cyanide anions. Tetrahedron 2017, 73, 5715–5719. [Google Scholar] [CrossRef]
- Mohammadi, A.; Kianfar, M. A simple colorimetric chemosensor with highly performance for detection of cyanide and copper ions and its practical application in real samples. J. Photochem. Photobiol. A Chem. 2018, 367, 22–31. [Google Scholar] [CrossRef]
- You, G.R.; Park, G.J.; Lee, J.J.; Kim, C. A colorimetric sensor for the sequential detection of Cu2+ and CN− in fully aqueous media: Practical performance of Cu2+. Dalton Trans. 2015, 44, 9120–9129. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Ghorai, A.; Patra, G.K. colorimetric and fluorescent ‘on–off-on’ sensor for sequential detection of copper (II) and cyanide ions in aqueous solution. Sens. Actuators B Chem. 2018, 255, 701–711. [Google Scholar] [CrossRef]
- Jo, T.G.; Na, Y.J.; Lee, J.J.; Lee, M.M.; Lee, S.Y.; Kim, C. A multifunctional colorimetric chemosensor for cyanide and copper (II) ions. Sens. Actuators B Chem. 2015, 211, 498–506. [Google Scholar] [CrossRef]
- Kang, J.H.; Lee, S.Y.; Ahn, H.M.; Kim, C. Sequential detection of copper (II) and cyanide by a simple colorimetric chemosensor. Inorg. Chem. Commun. 2016, 74, 62–65. [Google Scholar] [CrossRef]
- Kaushik, R.; Ghosh, A.; Singh, A.; Gupta, P.; Mittal, A.; Jose, D.A. Selective detection of cyanide in water and biological samples by an off-the-shelf compound. ACS Sens. 2016, 10, 1265–1271. [Google Scholar] [CrossRef]
- Park, G.J.; Hwang, I.H.; Song, E.J.; Kim, H.; Kim, C. A colorimetric and fluorescent sensor for sequential detection of copper ion and cyanide. Tetrahedron 2014, 70, 2822–2828. [Google Scholar] [CrossRef]
- Ho, F.C.; Huang, Y.J.; Weng, C.C.; Wu, C.H.; Li, Y.K.; Wu, J.I.; Lin, H.C. Efficient FRET Approaches toward Copper (II) and Cyanide Detections via Host–Guest Interactions of Photo-Switchable [2] Pseudo-Rotaxane Polymers Containing Naphthalimide and Merocyanine Moieties. ACS Appl. Mater. Interfaces 2020, 47, 53257–53273. [Google Scholar] [CrossRef]












| Derivative Probes | Method of Detection | Analytes | LOD | Applications | Ref. |
|---|---|---|---|---|---|
| Spirobifluorene | Fluorescent | Cu2+ and CN− | 98.2 nM and 390 nM | NA | [38] |
| Pyrimidine-based | Colorimetric | Cu2+ and CN− | 2.40 × 10−7 M and 0.320 µM | Test strips | [39] |
| Quinoline-based | Colorimetric | Cu2+ and CN− | 5.0 × 10−5 M and 4.3 µM | Test strips | [40] |
| Azo-based | Colorimetric | Cu2+ and CN− | 2.39 × 10−6 M and 2.7 × 10−8 M | Test strips | [41] |
| Quinoline-based | Colorimetric | Cu2+ and CN− | 0.9 μM and 210 μM | NA | [42] |
| Benzil dihydrazone derivative | Fluorescent | Cu2+ and CN− | 7.3 × 10−10 M and 1.5 × 10−7 M | NA | [43] |
| Quinoline derivative | Colorimetric | Cu2+ and CN− | 7.3 μM and 20 μM | Test strips | [44] |
| 4-(diethylamino)-2-hydroxybenzaldehyde | Colorimetric | Cu2+ and CN− | 0.88 μM and 27.21 μM | NA | [45] |
| Chromen-based | Fluorescent | Cu2+ and CN− | 120 nM and 5.77 µM | Cell imaging | [46] |
| Naphthalene derivative | Colorimetric and Fluorescent | Cu2+ and CN− | 1.4 × 10−5 M and 5.7 × 10−8 M | NA | [47] |
| Naphthalimide derivative | Fluorescent | Cu2+ and CN− | 0.53 μM and 1.34 μM | Cell imaging | [48] |
| Pyrene-appended Schiff base L | Fluorescent | Cu2+ and CN− | 219 nM and 580 nM | Test strips, cell imaging, polymer membrane sensing applications | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shellaiah, M.; Venkatesan, P.; Thirumalaivasan, N.; Wu, S.-P.; Sun, K.-W. Pyrene-Based Fluorescent Probe for “Off-on-Off” Sequential Detection of Cu2+ and CN− with HeLa Cells Imaging. Chemosensors 2023, 11, 115. https://doi.org/10.3390/chemosensors11020115
Shellaiah M, Venkatesan P, Thirumalaivasan N, Wu S-P, Sun K-W. Pyrene-Based Fluorescent Probe for “Off-on-Off” Sequential Detection of Cu2+ and CN− with HeLa Cells Imaging. Chemosensors. 2023; 11(2):115. https://doi.org/10.3390/chemosensors11020115
Chicago/Turabian StyleShellaiah, Muthaiah, Parthiban Venkatesan, Natesan Thirumalaivasan, Shu-Pao Wu, and Kien-Wen Sun. 2023. "Pyrene-Based Fluorescent Probe for “Off-on-Off” Sequential Detection of Cu2+ and CN− with HeLa Cells Imaging" Chemosensors 11, no. 2: 115. https://doi.org/10.3390/chemosensors11020115
APA StyleShellaiah, M., Venkatesan, P., Thirumalaivasan, N., Wu, S.-P., & Sun, K.-W. (2023). Pyrene-Based Fluorescent Probe for “Off-on-Off” Sequential Detection of Cu2+ and CN− with HeLa Cells Imaging. Chemosensors, 11(2), 115. https://doi.org/10.3390/chemosensors11020115










