Chiral Recognition of Phenylglycinamide Enantiomer Based on Electrode Modified by Silver-Ammonia Ion-Functionalized Carbon Nanotubes Complex
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Apparatus
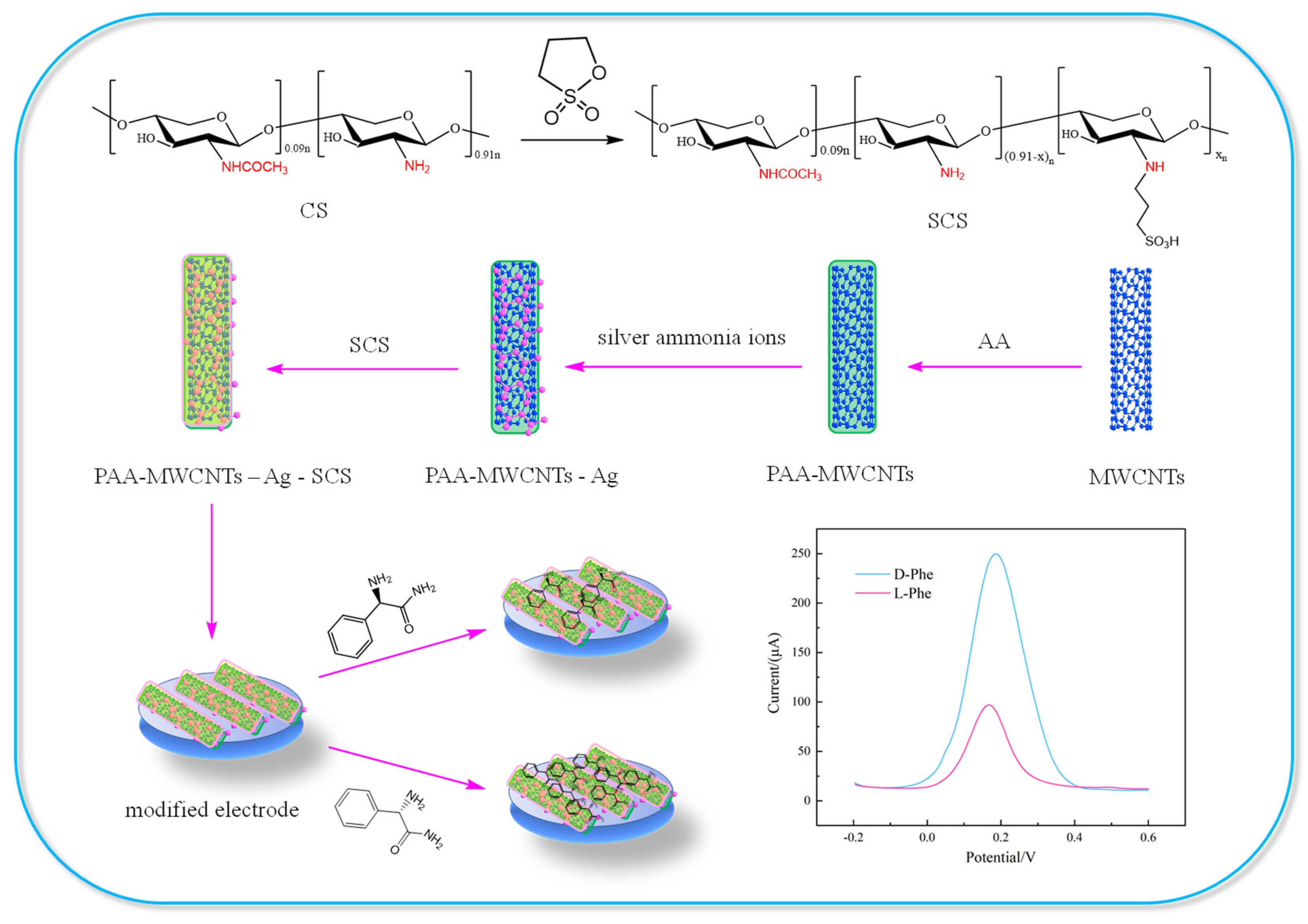
2.2. Preparation of SCS
2.3. Preparation of PAA-MWCNTs
2.4. Preparation of PAA-MWCNTs-Ag-SCS
2.5. Preparation of PAA-MWCNTs-Ag-SCS/GCE
2.6. Electrochemical Chiral Discrimination of Pen Enantiomers
3. Results and Discussion
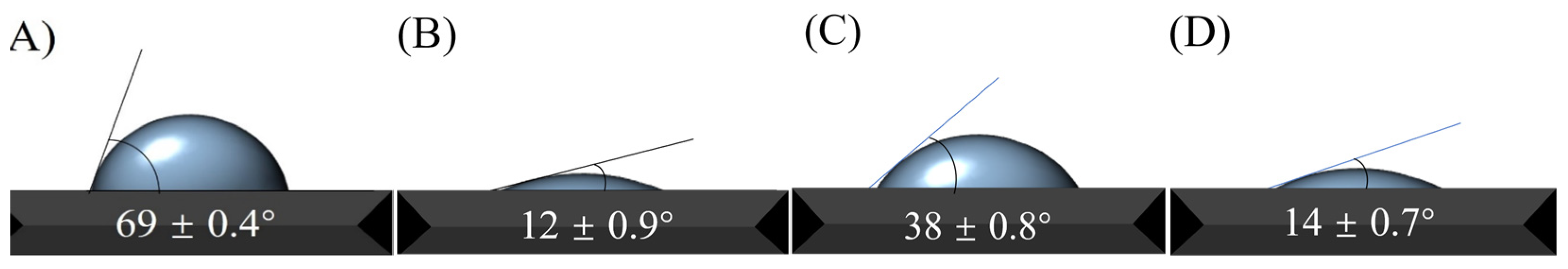
3.1. Characterization of Chiral Materials
3.2. Electrochemical Properties of Chiral-Sensing Platform
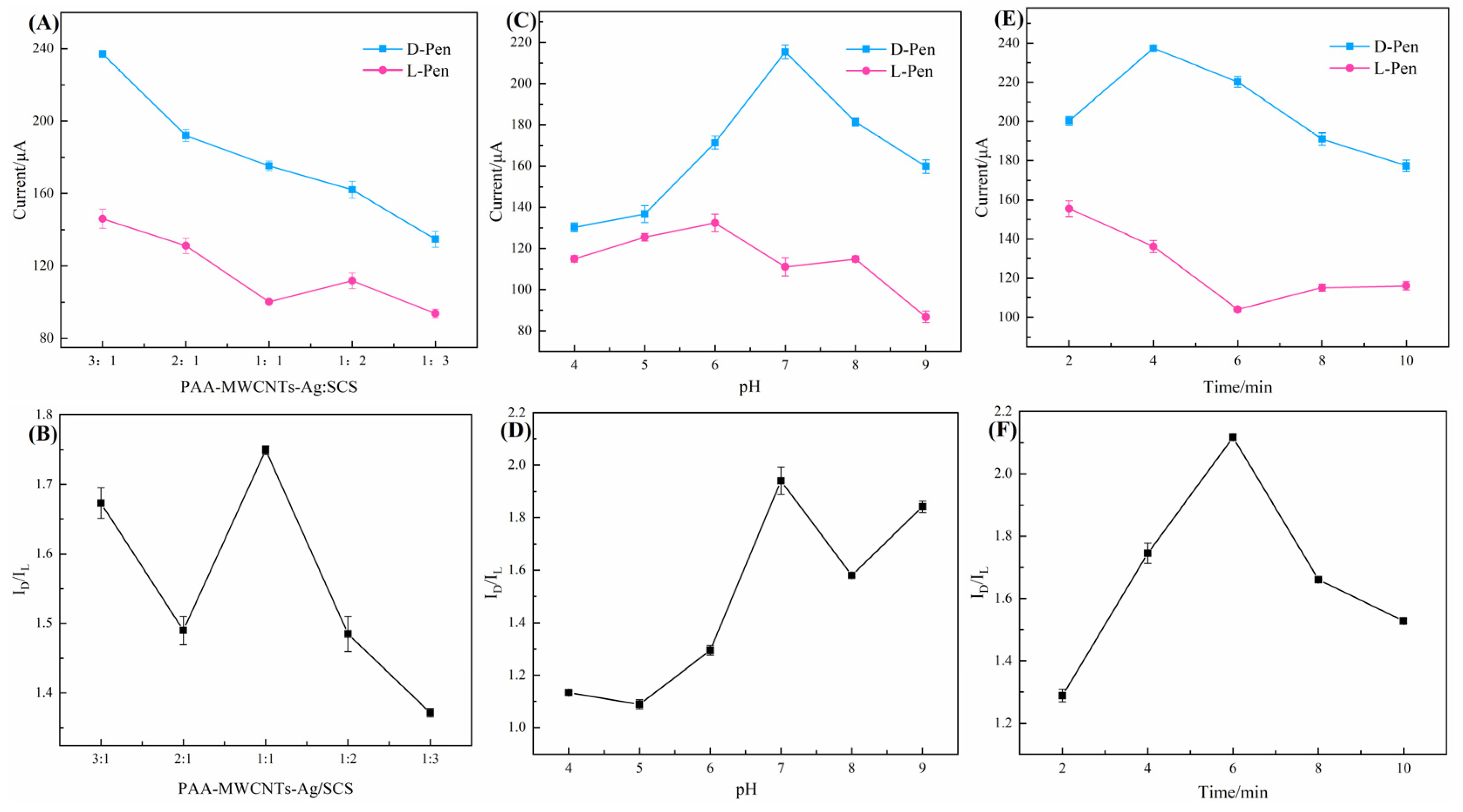
3.3. Optimization of Chiral Recognition Conditions
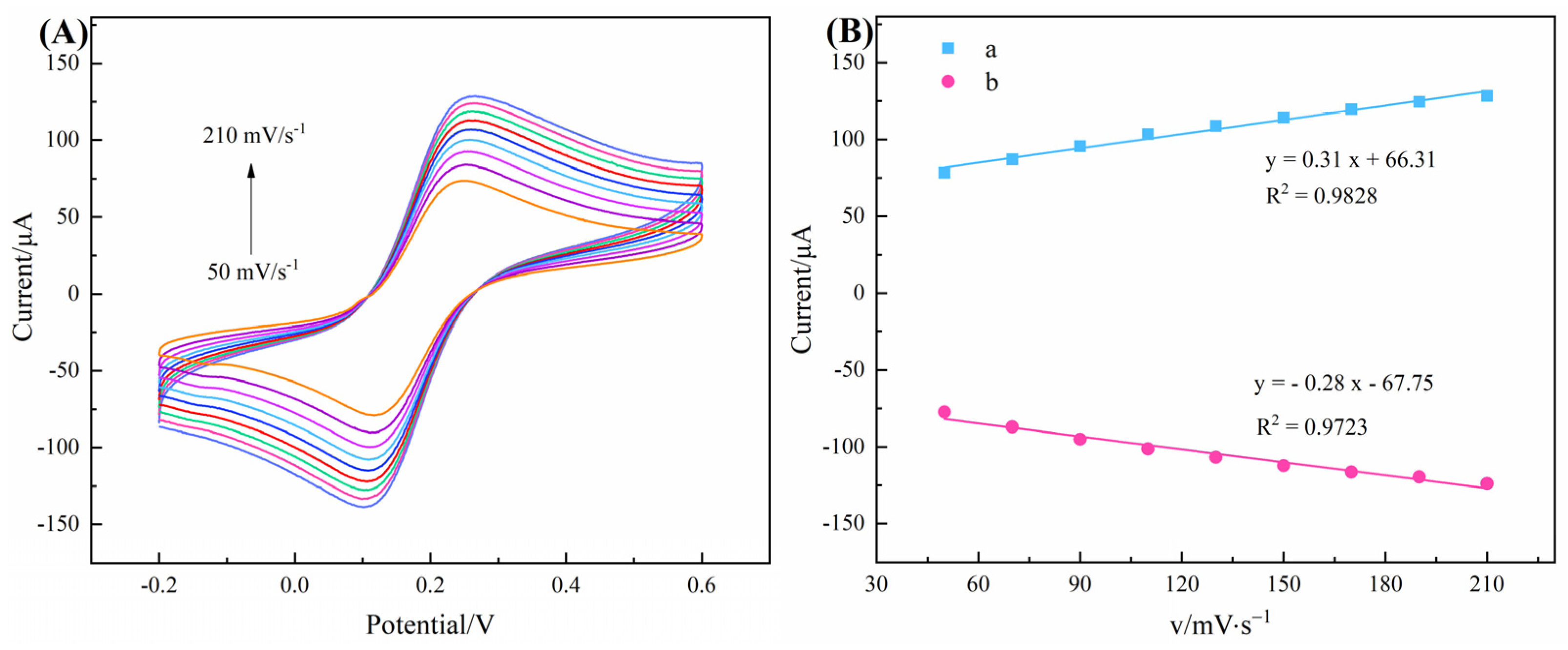
3.4. Thermodynamic Characterization of Chiral Recognition Process
3.5. Practical Application of Chiral-Sensor Platform
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Globus, N.; Blandford, R.D. The chiral puzzle of life. Astrophys. J. 2020, 895, L11. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kang, B.; Seo, J. Metalloporphyrin dimers bridged by a peptoid helix: Host-guest interaction and chiral recognition. Molecules 2018, 23, 2741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, B.; de La Cotte, A.; Grelet, E. Chirality-controlled crystallization via screw dislocations. Nat. Commun. 2018, 9, 1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Yang, X.; Gao, J. 3D Janus plasmonic helical nanoapertures for polarization-encrypted data storage. Light Sci. Appl. 2019, 8, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhou, X.; Xu, C.; Jin, Y.; Li, B. Gold nanorods as visual sensing platform for chiral recognition with naked eyes. Sci. Rep. 2018, 8, 5296. [Google Scholar] [CrossRef] [Green Version]
- Managadze, G.G.; Engel, M.H.; Getty, S.; Wurz, P.; Brinckerhoff, W.B.; Shokolov, A.G.; Sholin, G.V.; Terent’ev, S.A.; Chumikov, A.E.; Skalkin, A.S.; et al. Excess of L-alanine in amino acids synthesized in a plasma torch generated by a hypervelocity meteorite impact reproduced in the laboratory. Planet Space Sci. 2016, 131, 70–78. [Google Scholar] [CrossRef] [Green Version]
- Hu, M.; Yuan, Y.X.; Wang, W.; Li, D.M.; Zhang, H.C.; Wu, B.X.; Liu, M.; Zheng, Y.S. Chiral recognition and enantiomer excess determination based on emission wavelength change of AIEgen rotor. Nat. Commun. 2020, 11, 2274. [Google Scholar] [CrossRef]
- Bicchi, C.; Amato, A.D.; Rubiolo, P. Cyclodextrin derivatives as chiral selectors for direct gas chromatographic separation of enantiomers in the essential oil aroma and flavour fields. J. Chromatogr. A 1999, 843, 99–121. [Google Scholar] [CrossRef]
- Evans, A.C.; Meinert, C.; Giri, C.; Goesmann, F.; Meierhenrich, U.J. Chirality photochemistry and the detection of amino acids in interstellar ice analogues and comets. Chem. Soc. Rev. 2012, 41, 5447–5458. [Google Scholar] [CrossRef]
- Sloboda, R.D. Separation of tubulin and microtubule-associated proteins by ion exchange chromatography. Cold Spring Harb. Protoc. 2015, 1, 78–81. [Google Scholar] [CrossRef]
- Zhang, Q.; Xue, S.; Li, A.; Ren, S. Functional materials in chiral capillary electrophoresis. Coord. Chem. Rev. 2021, 445, 214108. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, J.; Yoon, J. Recent advances in development of chiral fluorescent and colorimetric sensors. Chem. Rev. 2014, 114, 4918–4959. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Tao, Y.; Xue, J.; Kong, Y.; Dai, J.; Deng, L. Electrochemical enantiorecognition of tryptophan enantiomers based on graphene quantum dots-chitosan composite film. Electrochem. Commun. 2015, 57, 5–9. [Google Scholar] [CrossRef]
- Rahman, M.M.; Karim, M.R.; Alam, M.M.; Zaman, M.B.; Alharthi, N.; Alharbi, H.; Asiri, A.M. Facile and efficient 3-chloro phenol sensor development based on photolumenescent core-shell CdSe/ZnS quantum dots. Sci. Rep. 2020, 10, 557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathew, G.; Narayanan, N.; Abraham, D.A.; De, M.; Neppolian, B. Facile green approach for developing electrochemically reduced graphene oxide-embedded platinum nanoparticles for ultrasensitive detection of nitric oxide. ACS Omega 2021, 6, 8068–8080. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, R.; Thangaraj, P.R.; Sen, A.K. Direct and rapid measurement of hydrogen peroxide in human blood using a microfluidic device. Sci. Rep. 2021, 11, 2960. [Google Scholar] [CrossRef]
- Li, Z.; Mo, Z.; Meng, S.; Gao, H.; Niu, X.; Guo, R.; Wei, T. The construction of electrochemical chiral interfaces using hydrox ypropyl chitosan. RSC Adv. 2017, 7, 8542–8549. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Tang, S.; Huang, B.; Shen, X.; Hong, F. Preparation and characterization of bacterial cellulose/hydroxypropyl chitosan blend as-spun fibers. Fiber Polym. 2013, 14, 935–940. [Google Scholar] [CrossRef]
- Yeh, Y.T.; Lin, Z.; Zheng, S.Y.; Terrones, M. A carbon nanotube integrated microfluidic device for blood plasma extraction. Sci Rep. 2018, 8, 13623. [Google Scholar] [CrossRef] [Green Version]
- Corletto, A.; Shapter, J.G. Nanoscale patterning of carbon nanotubes: Techniques, applications, and future. Adv. Sci. 2021, 8, 2001778. [Google Scholar] [CrossRef]
- Zhang, A.; Pan, S.; Zhang, Y.; Chang, J.; Cheng, J.; Huang, Z.; Li, T.; Zhang, C.; de La Fuentea, J.M.; Zhang, Q.; et al. Carbon-gold hybrid nanoprobes for real-time imaging, photothermal/photodynamic and nanozyme oxidative therapy. Theranostics 2019, 9, 3443–3458. [Google Scholar] [CrossRef] [PubMed]
- Barsan, M.M.; Ghica, M.E.; Brett, C.M.A. Electrochemical sensors and biosensors based on redox polymer/carbon nanotube modified electrodes: A review. Anal. Chim. Acta. 2015, 881, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Yi, Y.; Zhang, D.; Zhu, G. Electrochemical recognition of tryptophan enantiomers using a multi-walled carbon nanotube@polydopamine composite loaded with copper (II). Microchim. Acta 2019, 186, 358. [Google Scholar] [CrossRef] [PubMed]
- Goulart, L.A.; Gonçalves, R.; Correa, A.A.; Pereira, E.C.; Mascaro, L.H. Synergic effect of silver nanoparticles and carbon nanotubes on the simultaneous voltammetric determination of hydroquinone, catechol, bisphenol a and phenol. Microchim. Acta 2018, 185, 12. [Google Scholar] [CrossRef]
- Yu, F.; Ma, J.; Han, S. Adsorption of tetracycline from aqueous solutions onto multi-walled carbon nanotubes with different oxygen contents. Sci. Rep. 2014, 4, 5326. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, A.-E.; Tsai, S.-Y.; Hsu, M.W.; Chang, S.J. Decoration of multi-walled carbon nanotubes by polymer wrapping and its application in MWCNT/polyethylene composites. Nanoscale Res. Lett. 2012, 7, 240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldalbahi, A.; Rahaman, M.; Almoiqli, M. Performance enhancement of modified 3D SWCNT/RVC electrodes using microwave-irradiated graphene oxide. Nanoscale Res Lett. 2019, 14, 351. [Google Scholar] [CrossRef]
- Sun, Z.; Shi, C.; Wang, X.; Fang, Q.; Huang, J. Synthesis, characterization and antimicrobial activities of sulfonated chitosan. Carbohydr. Polym. 2017, 155, 321–328. [Google Scholar] [CrossRef] [Green Version]
- Fu, H.; Du, Z.J.; Zou, W.; Li, H.Q.; Zhang, C. Enhanced electrical conductivity of sodium polyacrylate encapsulated multi-walled carbon nanotubes. Mater. Lett. 2012, 78, 54–57. [Google Scholar] [CrossRef]
- Al-Mutabagani, L.A.; Alshabanah, L.A.; Ahmed, H.A.; Hagar, M.; Abu Al-Ola, K.A. New symmetrical U-and wavy-shaped supramolecular H-bonded systems; geometrical and mesomorphic approaches. Molecules 2020, 25, 1420. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.S.; Srivastava, A.K. Hydroxypropyl β-cyclodextrin cross-linked multi-walled carbon nanotube-based chiral nanocomposite electrochemical sensors for the discrimination of multi-chiral drug atorvastatin isomers. New J. Chem. 2019, 43, 11178–11188. [Google Scholar] [CrossRef]
- Niu, X.; Yang, X.; Li, H.; Shi, Q.; Wang, K. Chiral voltammetric sensor for tryptophan enantiomers by using a self-assembled multiwalled carbon nanotubes/polyaniline/sodium alginate composite. Chirality 2021, 33, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Chen, X.; Yang, B.; Tao, Y.; Kong, Y. Construction of electrochemical chiral interfaces with integrated polysaccharides via amidation. ACS Appl. Mater. Interfaces 2016, 8, 21710–21720. [Google Scholar] [CrossRef]
- Maachou, H.; Genet, M.J.; Aliouche, D.; Dupont-Gillain, C.C.; Rouxhet, P.G. XPS Analysis of chitosan-hydroxyapatite biomaterials: From elements to compounds. Surf. Interface Anal. 2013, 45, 1088–1097. [Google Scholar] [CrossRef]
- Amaral, I.F.; Granja, P.L.; Barbosa, M.A. Chemical modification of chitosan by phosphorylation: An XPS, FT-IR and SEM study. J. Biomater. Sci. Polym. Ed. 2005, 16, 1575–1593. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Mo, Z.; Wang, J.; Shuai, C.; Pei, H.; Chen, Y.; Yue, R. A novel chiral carbon nanocomposite based on cellulose gum modifying chiral tri-electrode system for the enantio-recognition of tryptophan. Electroanal. Chem. 2021, 895, 115390. [Google Scholar] [CrossRef]
- Zomorrodian, A.; Mesarwi, A.; Wu, N.J.; Ignatiev, A. XPS oxygen line broadening in lead zirconium titanate and related materials. Appl Surf Sci. 1995, 90, 343–348. [Google Scholar] [CrossRef]
- Mikhlin, Y.L.; Pal’yanova, G.A.; Tomashevich, Y.V.; Vishnyakova, E.A.; Vorobyev, S.A.; Kokh, K.A. XPS and Ag L3-Edge XANES Characterization of silver and silver–gold sulfoselenides. J. Phys. Chem. Solids 2018, 116, 292–298. [Google Scholar] [CrossRef]
- Berthod, A. Chiral recognition mechanism. Anal. Chem. 2006, 78, 2093–2099. [Google Scholar] [CrossRef]
- Niu, X.; Yang, X.; Li, H.; Liu, J.; Liu, Z.; Wang, K. Application of chiral materials in electrochemical sensors. Microchim. Acta 2020, 187, 676. [Google Scholar] [CrossRef]
- Gong, T.; Zhu, S.; Huang, S.; Gu, P.; Xiong, Y.; Zhang, J.; Jiang, X. A renewable electrochemical sensor based on a self-assembled framework of chiral molecules for efficient identification of tryptophan isomers. Anal. Chim. Acta 2022, 1191, 339276. [Google Scholar] [CrossRef] [PubMed]
- Zagitova, L.; Yarkaeva, Y.; Zagitov, V.; Nazyrov, M.; Gainanova, S.; Maistrenko, V. Voltammetric chiral recognition of naproxen enantiomers by N-tosylproline functionalized chitosan and reduced graphene oxide based sensor. Electroanal. Chem. 2022, 922, 116744. [Google Scholar] [CrossRef]
- Wang, L.; Gao, W.; Ng, S.; Pumera, M. Chiral protein-covalent organic framework 3D-printed structures as chiral biosensors. Anal. Chem. 2021, 93, 5277–5283. [Google Scholar] [CrossRef]
- Luo, H.; Li, H.; Ge, Q.; Cong, H.; Tao, Z.; Liu, M. An electrochemical sensor for enantio-recognition of tyrosine based on a chiral macrocycle functionalized rGO. Microchem. J. 2021, 164, 105949. [Google Scholar] [CrossRef]
- Zou, J.; Yu, J. Nafion-stabilized black phosphorus nanosheets-maltosyl-β-cyclodextrin as a chiral sensor for tryptophan enantiomers. Mat. Sci. Eng. R 2020, 112, 110910. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Lan, X.; Zhao, G.; Huang, Z.; Liu, Y.; Yu, J. Immobilization of 6-O-α-maltosyl-β-cyclodextrin on the surface of black phosphorus nanosheets for selective chiral recognition of tyrosine enantiomers. Microchim. Acta 2020, 187, 636. [Google Scholar] [CrossRef] [PubMed]
- Jing, P.; Yin, Z.; Cai, W.; Li, J.; Wu, D.; Kong, Y. The hybrids of perylene tetracarboxylic acid functionalized multi-walled carbon nanotubes and chitosan for electrochemical chiral sensing of tryptophan enantiomers. Bioelectrochemistry 2022, 146, 108110. [Google Scholar] [CrossRef]
- Sun, X.; Fu, Z.; Zhang, M.; Fu, H.; Lin, C.; Kuang, J.; Zhang, H.; Hu, P. An electrochemical sensor employing β-cyclodextrin chiral cross-linked metal organic framework and graphene oxide for chiral enantiomer recognition. Microchem. J. 2022, 183, 108074. [Google Scholar] [CrossRef]
- Pei, H.; Wang, J.; Jin, X.; Zhang, X.; Liu, W.; Guo, R.; Liu, N.; Mo, Z. An electrochemical chiral sensor based on glutamic acid functionalized graphene-gold nanocomposites for chiral recognition of tryptophan enantiomers. Electroanal. Chem. 2022, 913, 116283. [Google Scholar] [CrossRef]
- Li, Z.; Xu, H.; Wu, D.; Zhang, J.; Liu, X.; Gao, S.; Kong, Y. Electrochemical chiral recognition of tryptophan isomers based on nonionic surfactant-assisted molecular imprinting sol-gel silica. ACS Appl. Mater. Interfaces 2019, 11, 2840–2848. [Google Scholar] [CrossRef]
- Yao, W.; Li, S.; Xie, L.; Jiang, Y. Chiral recognition of tryptophan enantiomer based on the electrode modified by polyaniline adsorption bovine serum albumin complex. Chirality 2022, 35, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Zhang, Y.; Duan, X.; Zhu, X.; Sun, H.; Xu, J. Chiral PEDOT-based enantioselective electrode modification material for chiral electrochemical sensing: Mechanism and model of chiral recognition. Anal. Chem. 2017, 89, 9695–9702. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Niu, X.; Mo, Z.; Guo, R.; Liu, N.; Zhao, P.; Liu, Z. Electrochemical chiral interface based on the Michael addition/Schiff base reaction of polydopamine functionalized reduced graphene oxide. Electrochim. Acta 2019, 319, 705–715. [Google Scholar] [CrossRef]





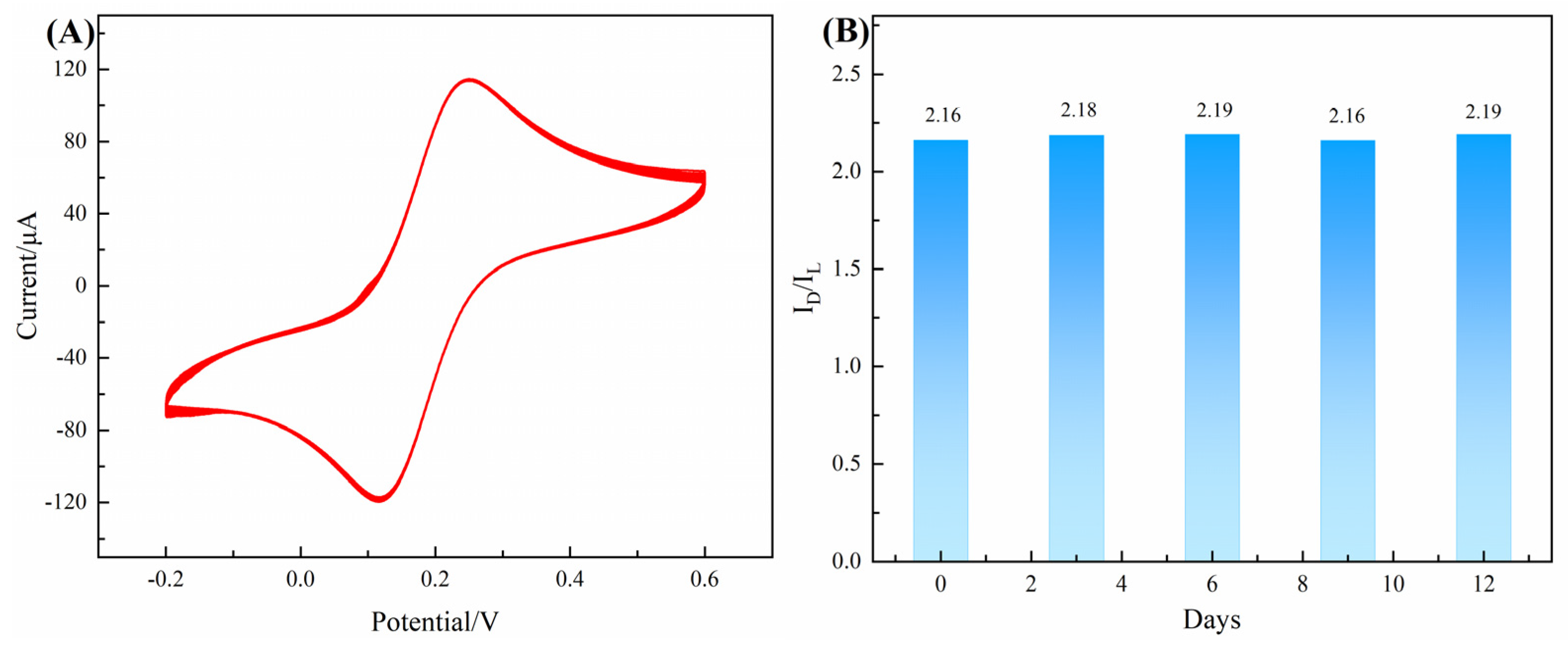
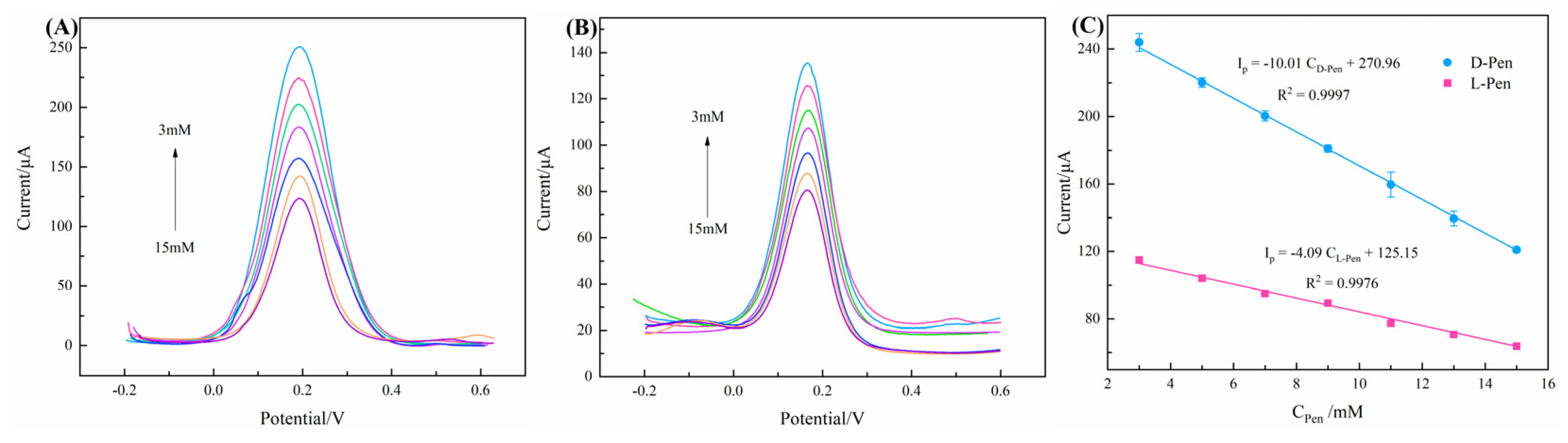
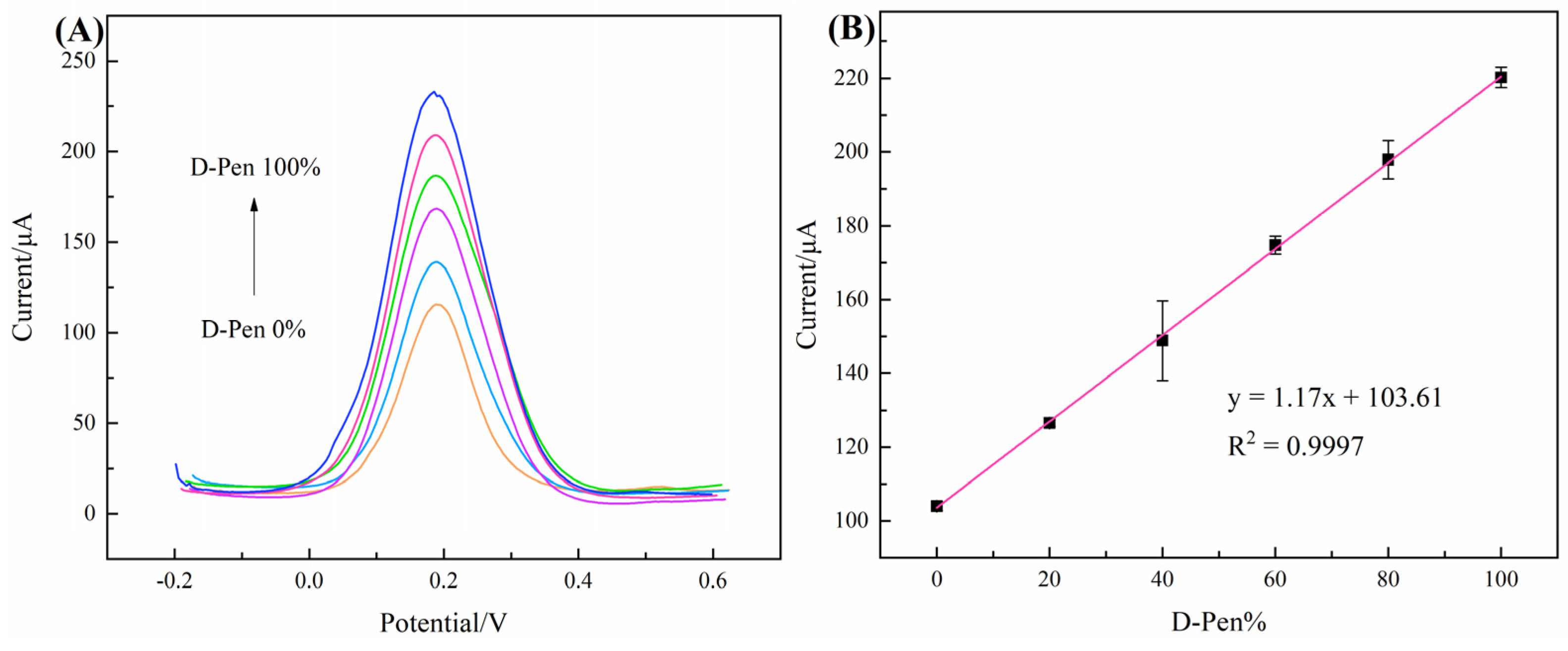
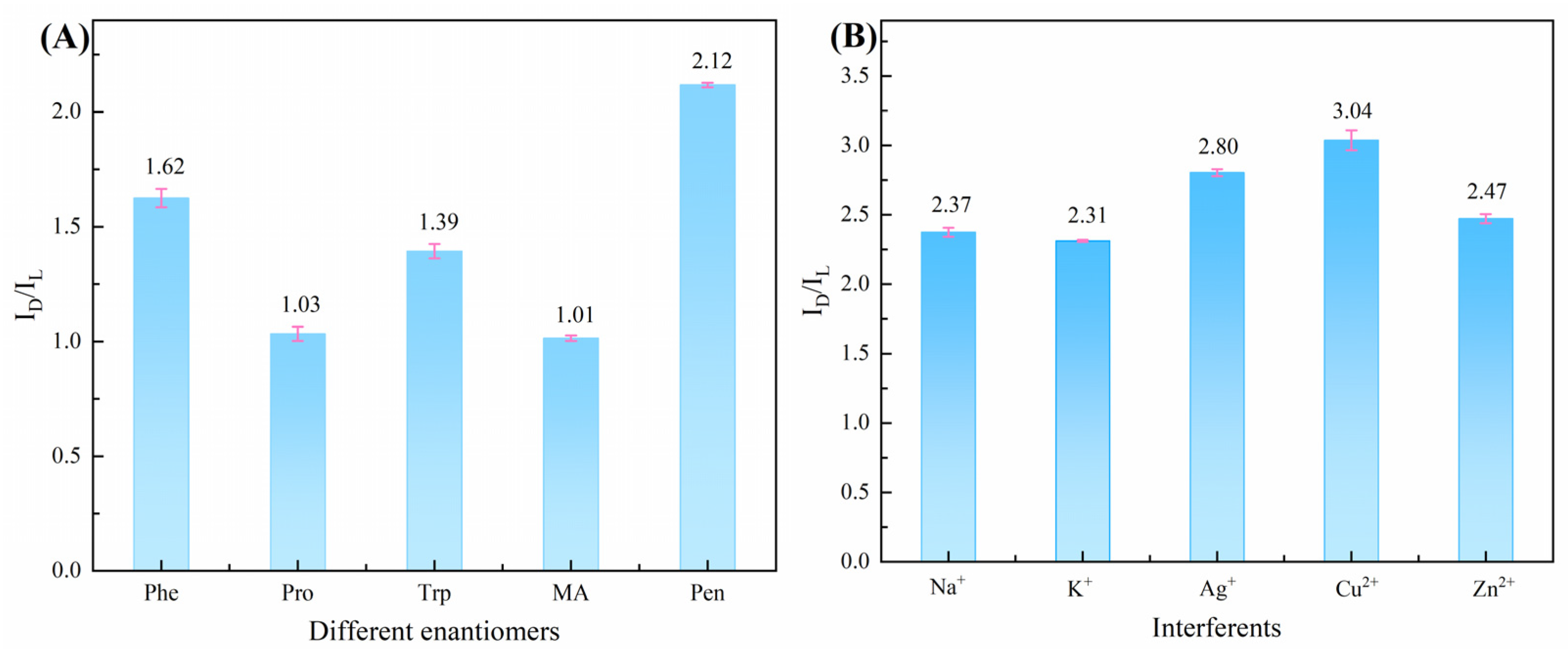
| Chiral Material | Enantiomer | Peak Current Interval/μA | Peak Current Ratio | References |
|---|---|---|---|---|
| Cu2-β-CD/NH2-CS-MWCNTs | tryptophan | 5.32–6.34 | 1.64 | [41] |
| rGO-TsPro-CS | naproxen | 7.34–10.83 | 1.60 | [42] |
| Fe3O4@COF@BSA | tryptophan | 12.53–17.52 | 1.45 | [43] |
| rGO-CHMF | tyrosine | 12–18.73 | 1.58 | [44] |
| NF/BPNSs-G2-β-CD | tryptophan | 14–21.63 | 1.49 | [45] |
| Mal-βCD/BP NSs | tryptophan | 9.51–13.25 | 1.51 | [46] |
| CS/MWCNTs–PTCA | tryptophan | 19–32.68 | 1.72 | [47] |
| PAA-MWCNTs-Ag-SCS | phenylglycinamide | 222.42–102.91 | 2.16 | This work |
| Chiral Material | Enantiomer | Limit of Detection (mM) | References | |
|---|---|---|---|---|
| GO-CLMOF | mandelic acid (MA) | 0.091 (L-MA) | 0.15 (D-MA) | [48] |
| RGO-Au/L-Glu | Tryptophan (Trp) | 0.28 (L-Trp) | 0.86 (D-Trp) | [49] |
| MISiO2/ITO | Tryptophan (Trp) | 0.11 (L-Trp) | 0.13 (D-Trp) | [50] |
| APS-DPANI-BSA | Tryptophan (Trp) | 0.071 (L-Trp) | 0.048 (D-Trp) | [51] |
| CMC-CS | Tryptophan (Trp) | 0.041 (L-Trp) | 0.052 (D-Trp) | [33] |
| PAA-MWCNTs-Ag-SCS | Phenylglycinamide (Pen) | 0.015 (L-Trp) | 0.036 (D-Trp) | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, W.; Li, S.; Kong, Y.; Xie, L.; Jiang, Y. Chiral Recognition of Phenylglycinamide Enantiomer Based on Electrode Modified by Silver-Ammonia Ion-Functionalized Carbon Nanotubes Complex. Chemosensors 2023, 11, 86. https://doi.org/10.3390/chemosensors11020086
Yao W, Li S, Kong Y, Xie L, Jiang Y. Chiral Recognition of Phenylglycinamide Enantiomer Based on Electrode Modified by Silver-Ammonia Ion-Functionalized Carbon Nanotubes Complex. Chemosensors. 2023; 11(2):86. https://doi.org/10.3390/chemosensors11020086
Chicago/Turabian StyleYao, Wenyan, Sha Li, Yong Kong, Licheng Xie, and Yan Jiang. 2023. "Chiral Recognition of Phenylglycinamide Enantiomer Based on Electrode Modified by Silver-Ammonia Ion-Functionalized Carbon Nanotubes Complex" Chemosensors 11, no. 2: 86. https://doi.org/10.3390/chemosensors11020086




