Effect of UV Irradiation on the Growth of ZnO:Er Nanorods and Their Intrinsic Defects
Abstract
1. Introduction
2. Experimental Section
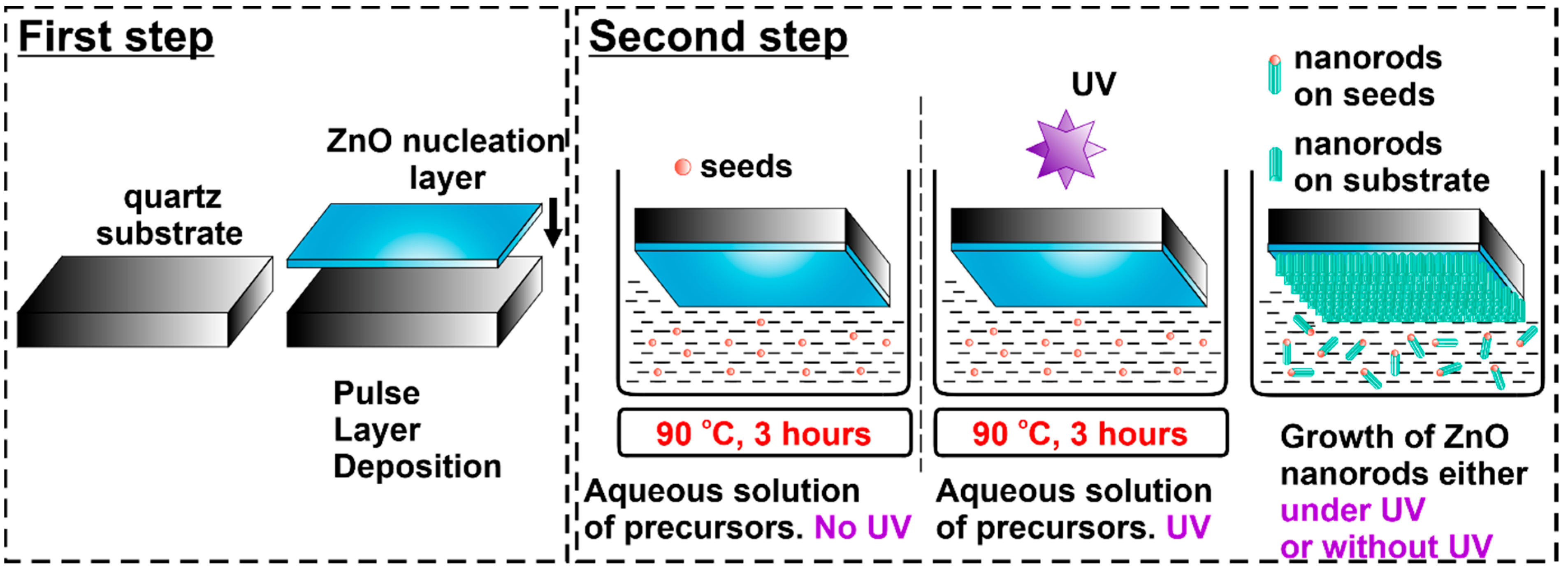
2.1. Sample Preparation: ZnO:Er Nanorods Deposited on a Quartz Substrate
2.2. Sample Preparation: Free-Standing ZnO:Er Nanorods
2.3. Experimental Techniques
3. Results and Discussion
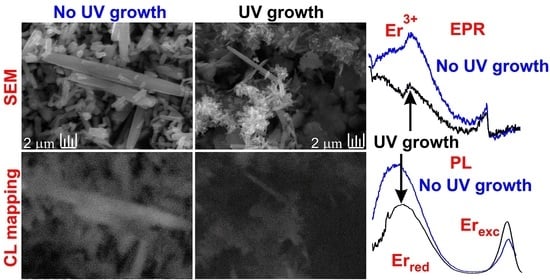
3.1. Structural Analysis
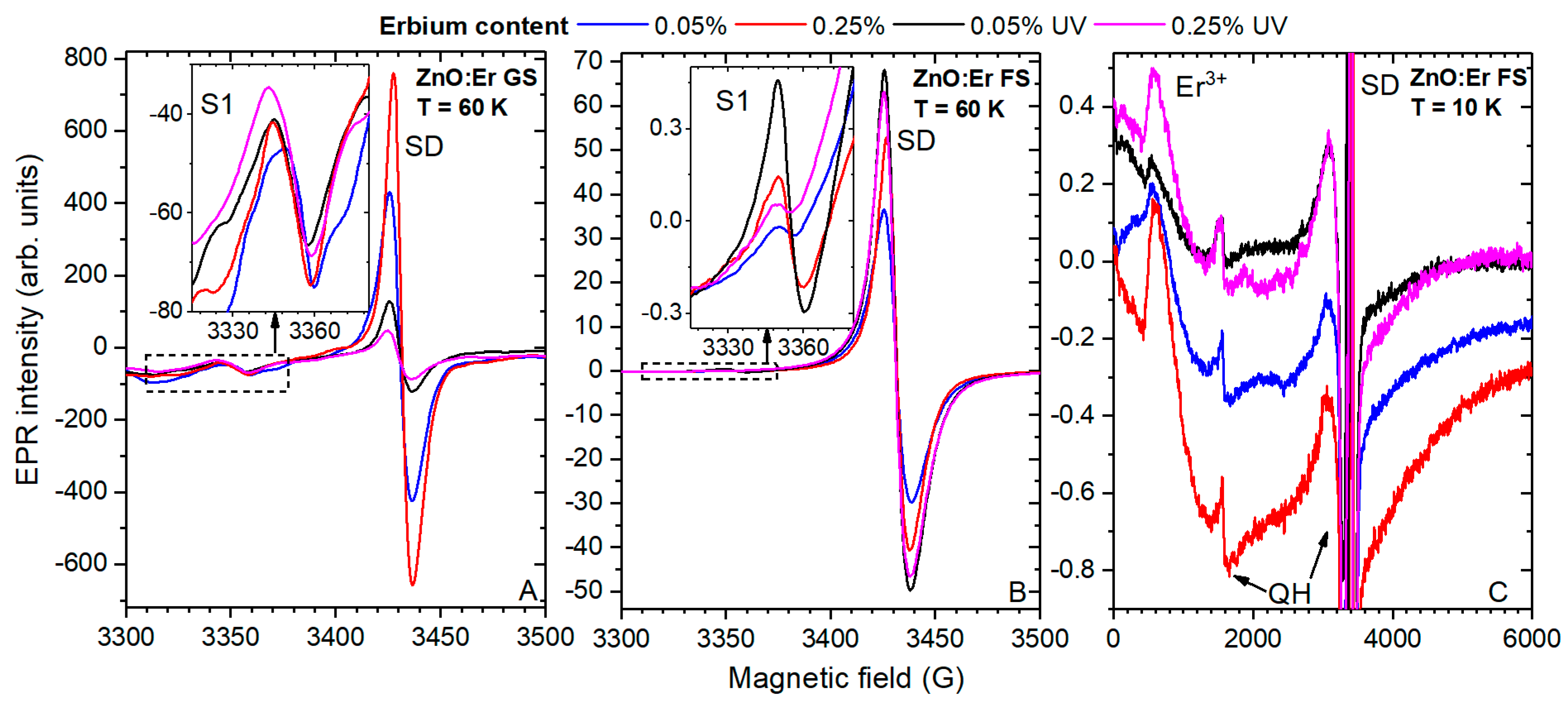
3.2. Paramagnetic States by EPR
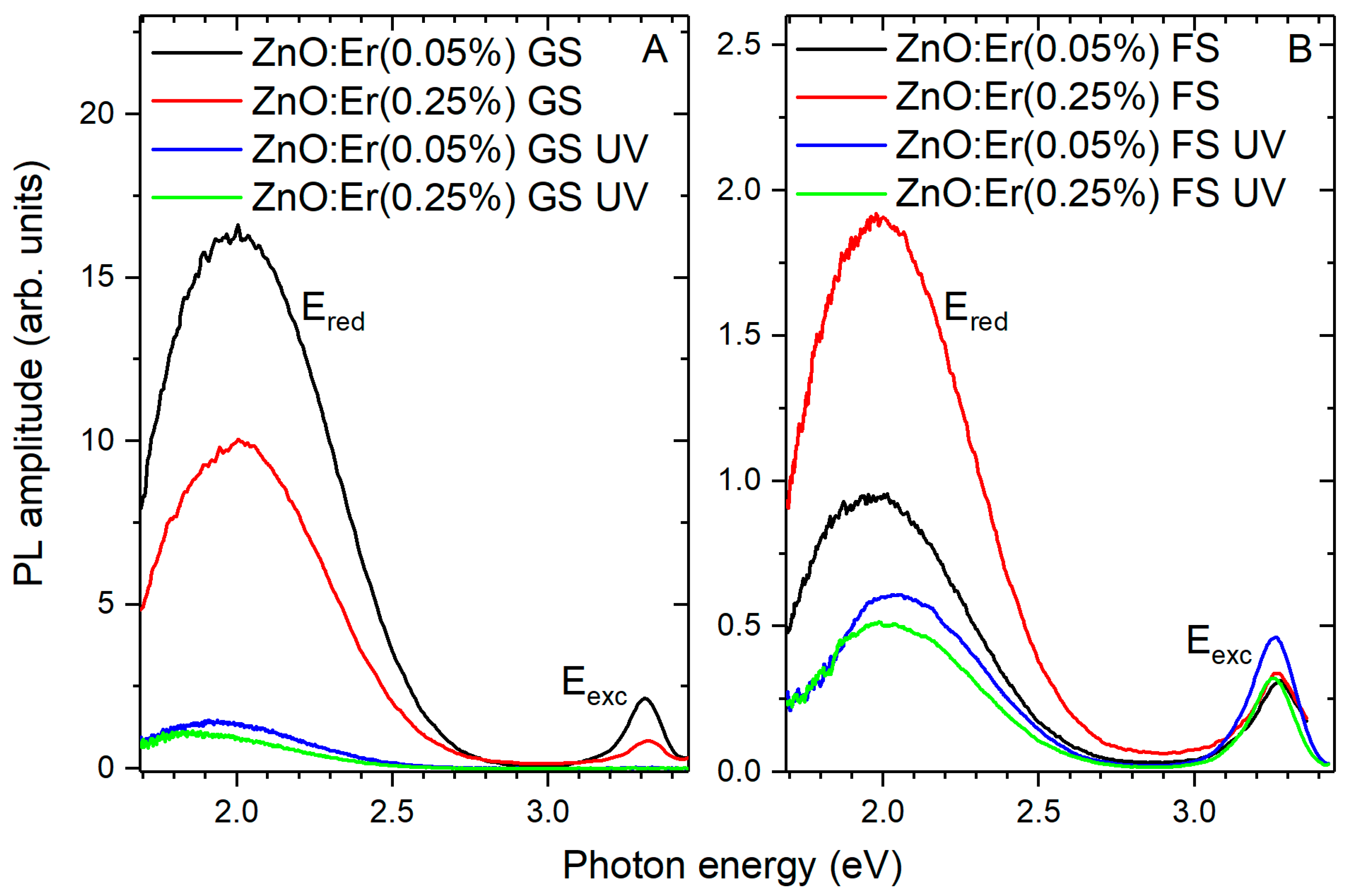
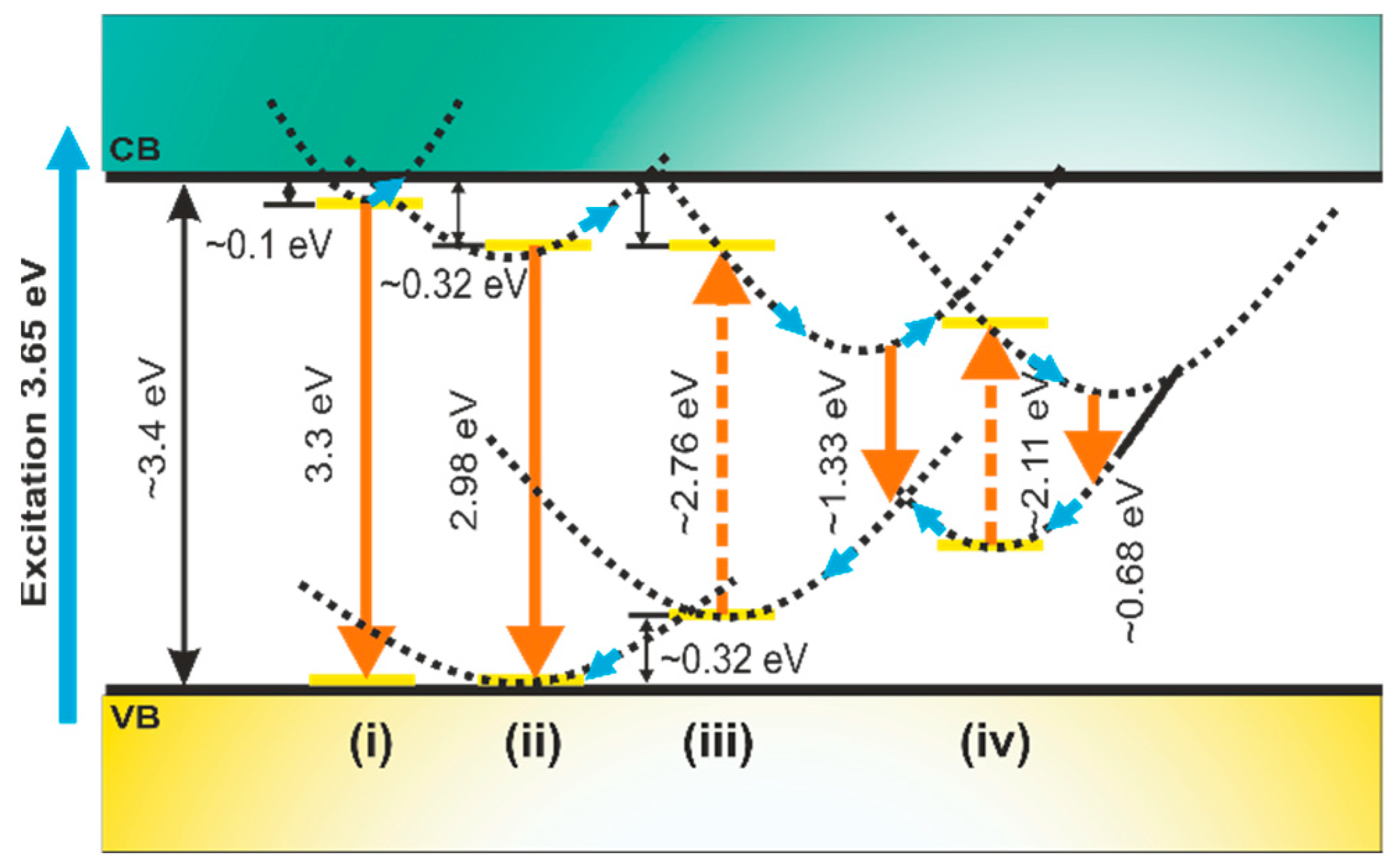
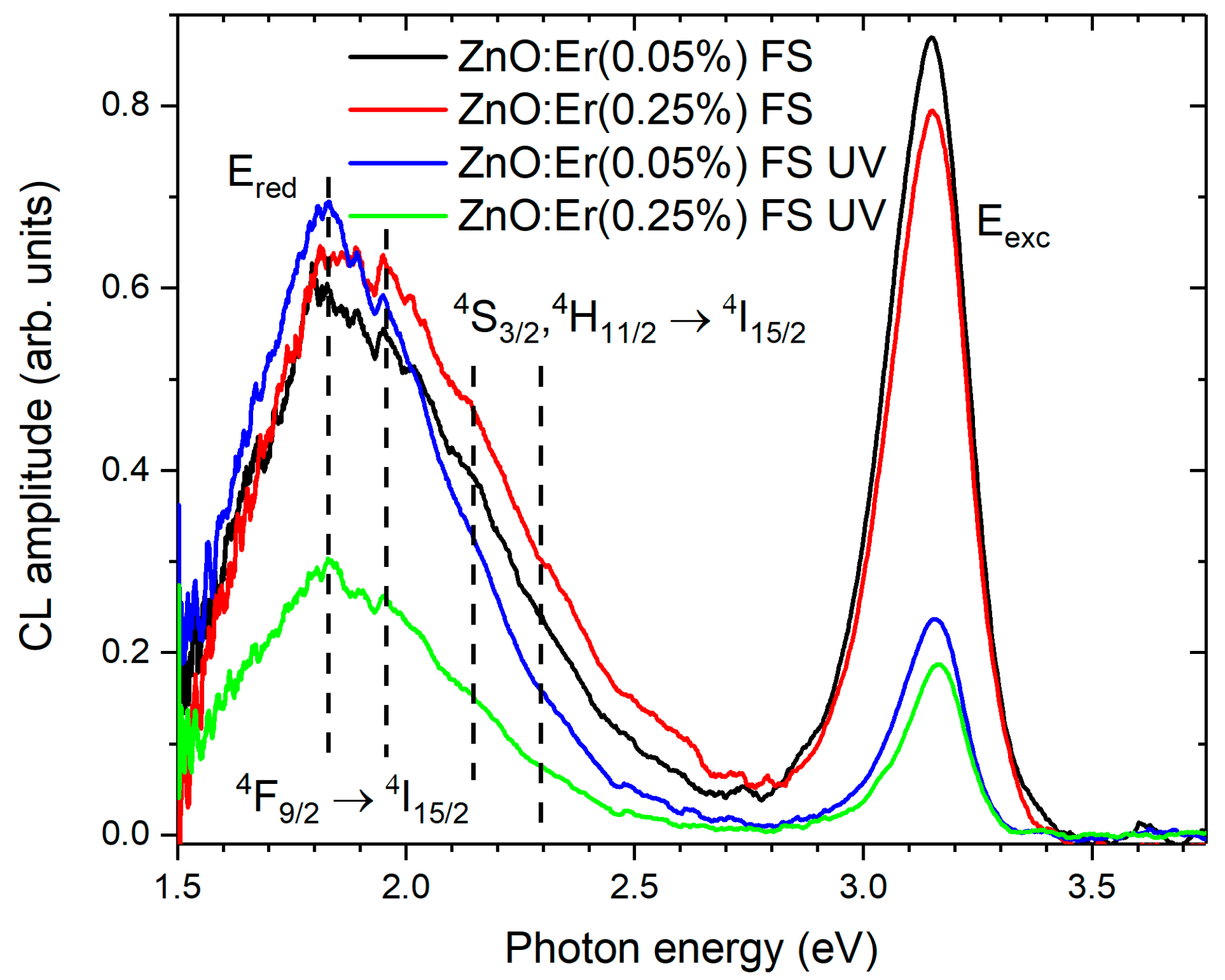
3.3. Photoluminescence Properties
3.4. Morphology and Spatial Correlation of Luminescence and Elemental Composition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Awasthi, K. (Ed.) Nanostructured Zinc Oxide: Synthesis; Properties and Applications, 1st ed.; Elsevier: Cambridge, UK, 2021. [Google Scholar]
- Fedotov, A.; Pashkevich, A.; Fedotova, J.; Fedotov, A.; Kołtunowicz, T.; Zukowski, P.; Ronassi, A.; Fedotova, V.; Svito, I.; Budzyński, M. Electron transport and thermoelectric properties of ZnO ceramics doped with Fe. J. Alloys Compd. 2021, 854, 156169. [Google Scholar] [CrossRef]
- Pashkevich, A.; Fedotov, A.; Poddenezhny, E.; Bliznyuk, L.; Fedotova, J.; Basov, N.; Kharchanka, A.; Zukowski, P.; Koltunowicz, T.; Korolik, O.; et al. Structure, electric and thermoelectric properties of binary ZnO-based ceramics doped with Fe and Co. J. Alloys Compd. 2022, 895, 162621. [Google Scholar] [CrossRef]
- Selim, M.; El-Safty, S.; Abbas, M.; Shenashen, M. Facile design of graphene oxide-ZnO nanorod-based ternary nanocomposite as a superhydrophobic and corrosion-barrier coating. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125793. [Google Scholar] [CrossRef]
- Selim, M.; Shenashen, M.; Elmarakbi, A.; EL-Saeed, A.; Selim, M.; El-Safty, S. Sunflower oil-based hyperbranched alkyd/spherical ZnO nanocomposite modeling for mechanical and anticorrosive applications. RSC Adv. 2017, 7, 21796–21808. [Google Scholar] [CrossRef]
- Sánchez-López, A.; Perfecto-Avalos, Y.; Sanchez-Martinez, A.; Ceballos-Sanchez, O.; Sepulveda-Villegas, M.; Rincón-Enríquez, G.; Rodríguez-González, V.; Garcia-Varela, R.; Lozano, L.; Navarro-López, D.E.; et al. Influence of erbium doping on zinc oxide nanoparticles: Structural, optical and antimicrobial activity. Appl. Surf. Sci. 2022, 575, 151764. [Google Scholar] [CrossRef]
- Askar, A.; Selim, M.; El-Safty, S.; Hashem, A.; Selim, M.; Shenashen, M. Antimicrobial and immunomodulatory potential of nanoscale hierarchical one-dimensional zinc oxide and silicon carbide materials. Mater. Chem. Phys. 2021, 263, 124376. [Google Scholar] [CrossRef]
- Neykova, N.; Brož, A.; Remeš, Z.; Hruška, K.; Kalbáčová, M.; Kromka, A.; Vaněček, M. ZnO hedgehog-like structures for control cell cultivation. Appl. Surf. Sci. 2012, 258, 3485–3489. [Google Scholar] [CrossRef]
- Yang, C.; Zhan, S.; Li, Q.; Wu, Y.; Jia, X.; Li, C.; Liu, K.; Qu, S.; Wang, Z.; Wang, Z. Systematic investigation on stability influence factors for organic solar cells. Nano Energy 2022, 98, 107299. [Google Scholar] [CrossRef]
- Pezhooli, N.; Rahimi, J.; Hasti, F.; Maleki, A. Synthesis and evaluation of composite TiO2@ZnO quantum dots on hybrid nanostructure perovskite solar cell. Sci. Rep. 2022, 12, 9885. [Google Scholar] [CrossRef]
- Neykova, N.; Hruska, K.; Holovsky, J.; Remes, Z.; Vanecek, M. Arrays of ZnO nanocolumns for 3-dimensional very thin amorphous and microcrystalline silicon solar cells. Thin Solid Films 2013, 543, 110–113. [Google Scholar] [CrossRef]
- Laokae, D.; Phuruangrat, A.; Wannapop, S.; Dumrongrojthanath, P.; Thongtem, T.; Thongtem, S. Preparation, characterization and photocatalytic properties of Er-doped ZnO nanoparticles synthesized by combustion method. Int. J. Mater. Res. 2023, 114, 34–42. [Google Scholar] [CrossRef]
- Gomaa, H.; El-Safty, S.; Shenashen, M.; Kawada, S.; Yamaguchi, H.; Abdelmottaleb, M.; Cheira, M. Three-Dimensional, Vertical Platelets of ZnO Carriers for Selective Extraction of Cobalt Ions from Waste Printed Circuit Boards. ACS Sustain. Chem. Eng. 2018, 6, 13813–13825. [Google Scholar] [CrossRef]
- D’Agostino, D.; Di Giorgio, C.; Bobba, F.; Di Trolio, A.; Alippi, P.; Cucolo, A.; Bonapasta, A.A. Effects of cobalt substitution on ZnO surface reactivity and electronic structure. J. Mater. Chem. C 2019, 7, 8364–8373. [Google Scholar] [CrossRef]
- Olejnik, M.; Kersting, M.; Rosenkranz, N.; Loza, K.; Breisch, M.; Rostek, A.; Prymak, O.; Schürmeyer, L.; Westphal, G.; Köller, M.; et al. Cell-biological effects of zinc oxide spheres and rods from the nano- to the microscale at sub-toxic levels. Cell Biol. Toxicol. 2021, 37, 573–593. [Google Scholar] [CrossRef]
- Kapat, K.; Shubhra, Q.; Zhou, M.; Leeuwenburgh, S. Piezoelectric Nano-Biomaterials for Biomedicine and Tissue Regeneration. Adv. Funct. Mater. 2020, 30, 1909045. [Google Scholar] [CrossRef]
- Siebert, L.; Luna-Cerón, E.; García-Rivera, L.; Oh, J.; Jang, J.; Rosas-Gómez, D.; Pérez-Gómez, M.; Maschkowitz, G.; Fickenscher, H.; Oceguera-Cuevas, D.; et al. Light-Controlled Growth Factors Release on Tetrapodal ZnO-Incorporated 3D-Printed Hydrogels for Developing Smart Wound Scaffold. Adv. Funct. Mater. 2021, 31, 2007555. [Google Scholar] [CrossRef]
- Janotti, A.; Van de Walle, C. Native point defects in ZnO. Phys. Rev. B 2007, 76, 165202. [Google Scholar] [CrossRef]
- Janotti, A.; Van de Walle, C. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef]
- Morazzoni, F.; Scotti, R.; Volontè, S. Electron paramagnetic resonance investigation of paramagnetic point defects in ZnO and ZnO-supported ruthenium. J. Chem. Soc. Faraday Trans. 1990, 86, 1587–1591. [Google Scholar] [CrossRef]
- Van de Walle, C. Hydrogen as a Cause of Doping in Zinc Oxide. Phys. Rev. Lett. 2000, 85, 1012–1015. [Google Scholar] [CrossRef]
- Frodason, Y.; Johansen, K.; Bjørheim, T.; Svensson, B.; Alkauskas, A. Zn vacancy as a polaronic hole trap in ZnO. Phys. Rev. B 2017, 95, 094105. [Google Scholar] [CrossRef]
- Mackova, A.; Malinsky, P.; Pupikova, H.; Nekvindova, P.; Cajzl, J.; Svecova, B.; Oswald, J.; Wilhelm, R.; Kolitsch, A. A comparison of the structural changes and optical properties of LiNbO3, Al2O3 and ZnO after Er+ ion implantation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2014, 331, 182–186. [Google Scholar] [CrossRef]
- Anjana, R.; Jayaraj, M.; Yadav, A.; Jha, S.; Bhattacharyya, D. Investigating the evolution of local structure around Er and Yb in ZnO:Er and ZnO:Er, Yb on annealing using X-ray absorption spectroscopy. J. Appl. Phys. 2018, 123, 153102. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, M.; Hark, S.; Li, Q.; Tang, D.; Chu, M.; Chen, C. Local electronic structure and luminescence properties of Er doped ZnO nanowires. Appl. Phys. Lett. 2006, 89, 221917. [Google Scholar] [CrossRef]
- Honglin, L.; Yingbo, L.; Jinzhu, L.; Ke, Y. Experimental and first-principles studies of structural and optical properties of rare earth (RE = La, Er, Nd) doped ZnO. J. Alloys Compd. 2014, 617, 102–107. [Google Scholar] [CrossRef]
- Zhang, M.; Averseng, F.; Krafft, J.-M.; Borghetti, P.; Costentin, G.; Stankic, S. Controlled Formation of Native Defects in Ultrapure ZnO for the Assignment of Green Emissions to Oxygen Vacancies. J. Phys. Chem. C 2020, 124, 12696–12704. [Google Scholar] [CrossRef]
- Jakes, P.; Erdem, E. Finite size effects in ZnO nanoparticles: An electron paramagnetic resonance (EPR) analysis: Finite size effects in ZnO nanoparticles: An electron paramagnetic resonance (EPR) analysis. Phys. Status Solidi RRL 2011, 5, 56–58. [Google Scholar] [CrossRef]
- Kaftelen, H.; Ocakoglu, K.; Thomann, R.; Tu, S.; Weber, S.; Erdem, E. EPR and photoluminescence spectroscopy studies on the defect structure of ZnO nanocrystals. Phys. Rev. B 2012, 86, 014113. [Google Scholar] [CrossRef]
- Parashar, S.; Murty, B.; Repp, S.; Weber, S.; Erdem, E. Investigation of intrinsic defects in core-shell structured ZnO nanocrystals. J. Appl. Phys. 2012, 111, 113712. [Google Scholar] [CrossRef]
- Nadupalli, S.; Repp, S.; Weber, S.; Erdem, E. About defect phenomena in ZnO nanocrystals. Nanoscale 2021, 13, 9160–9171. [Google Scholar] [CrossRef]
- Buryi, M.; Remeš, Z.; Babin, V.; Artemenko, A.; Vaněček, V.; Dragounová, K.A.; Landová, L.; Kučerková, R.; Mičová, J. Transformation of free-standing ZnO nanorods upon Er doping. Appl. Surf. Sci. 2021, 562, 150217. [Google Scholar] [CrossRef]
- Buryi, M.; Remeš, Z.; Babin, V.; Novotný, M.; Vaněček, V.; Dragounová, K.A.; Mičová, J.; Landová, L.; Kučerková, R.; More-Chevalier, J.; et al. Influence of Mo doping on the luminescence properties and defect states in ZnO nanorods. Comparison with ZnO:Mo thin films. Appl. Surf. Sci. 2021, 555, 149679. [Google Scholar] [CrossRef]
- Erdem, E. Microwave power; temperature, atmospheric and light dependence of intrinsic defects in ZnO nanoparticles: A study of electron paramagnetic resonance (EPR) spectroscopy. J. Alloys Compd. 2014, 605, 34–44. [Google Scholar] [CrossRef]
- Yamazoe, N. New approaches for improving semiconductor gas sensors. Sens. Actuators B Chem. 1991, 5, 7–19. [Google Scholar] [CrossRef]
- Nikolic, M.; Milovanovic, V.; Vasiljevic, Z.; Stamenkovic, Z.; Sensors, S.G. Semiconductor gas sensors: Materials, technology, design, and application. Sensors 2020, 20, 6694. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Ho, H.-P. Light-Activated Metal Oxide Gas Sensors: A Review. Micromachines 2017, 8, 333. [Google Scholar] [CrossRef]
- Peng, X.; Wang, Z.; Huang, P.; Chen, X.; Fu, X.; Dai, W. Comparative Study of Two Different TiO2 Film Sensors on Response to H2 under UV Light and Room Temperature. Sensors 2016, 16, 1249. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.-W.; Srivastava, A.; Dravid, V. UV-activated room-temperature gas sensing mechanism of polycrystalline ZnO. Appl. Phys. Lett. 2009, 95, 142106. [Google Scholar] [CrossRef]
- Yu, K.; Shi, J.; Zhang, Z.; Liang, Y.; Liu, W. Synthesis, Characterization, and Photocatalysis of ZnO and Er-Doped ZnO. J. Nanomater. 2013, 2013, 372951. [Google Scholar] [CrossRef]
- Buryi, M.; Remeš, Z.; Babin, V.; Artemenko, A.; Chertopalov, S.; Mičová, J. Cold plasma treatment of ZnO:Er nano- and microrods: The effect on luminescence and defects creation. J. Alloys Compd. 2022, 895, 162671. [Google Scholar] [CrossRef]
- Lyons, J.; Varley, J.; Steiauf, D.; Janotti, A.; Van de Walle, C. First-principles characterization of native-defect-related optical transitions in ZnO. J. Appl. Phys. 2017, 122, 035704. [Google Scholar] [CrossRef]
- Stehr, J.; Chen, S.; Chen, W.; Cai, L.; Shen, S.; Buyanova, I. Effects of N implantation on defect formation in ZnO nanowires. Thin Solid Films 2019, 687, 137449. [Google Scholar] [CrossRef]
- Vlasenko, L. Magnetic Resonance Studies of Intrinsic Defects in ZnO: Oxygen Vacancy. Appl. Magn. Reson. 2010, 39, 103–111. [Google Scholar] [CrossRef]
- Vlasenko, L.; Watkins, G. Optical detection of electron paramagnetic resonance for intrinsic defects produced in ZnO by 2.5-MeV electron irradiation in situ at 4.2 K. Phys. Rev. B 2005, 72, 035203. [Google Scholar] [CrossRef]
- Gurylev, V.; Perng, T. Defect engineering of ZnO: Review on oxygen and zinc vacancies. J. Eur. Ceram. Soc. 2021, 41, 4977–4996. [Google Scholar] [CrossRef]
- Villafuerte, J.; Donatini, F.; Kioseoglou, J.; Sarigiannidou, E.; Chaix-Pluchery, O.; Pernot, J.; Consonni, V. Zinc Vacancy–Hydrogen Complexes as Major Defects in ZnO Nanowires Grown by Chemical Bath Deposition. J. Phys. Chem. C 2020, 124, 16652–16662. [Google Scholar] [CrossRef]
- Sun, Y.-F.; Liu, S.-B.; Meng, F.-L.; Liu, J.-Y.; Jin, Z.; Kong, L.-T.; Liu, J.-H. Metal Oxide Nanostructures and Their Gas Sensing Properties: A Review. Sensors 2012, 12, 2610–2631. [Google Scholar] [CrossRef]
- Moseley, P. Progress in the development of semiconducting metal oxide gas sensors: A review. Meas. Sci. Technol. 2017, 28, 082001. [Google Scholar] [CrossRef]
- Korotcenkov, G. Metal oxides for solid-state gas sensors: What determines our choice? Mater. Sci. Eng. B 2007, 139, 1–23. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Neykova, N.; Stuchlik, J.; Hruska, K.; Poruba, A.; Remes, Z.; Pop-Georgievski, O. Study of the surface properties of ZnO nanocolumns used for thin-film solar cells. Beilstein J. Nanotechnol. 2017, 8, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Greene, L.; Law, M.; Tan, D.; Montano, M.; Goldberger, J.; Somorjai, G.; Yang, P. General Route to Vertical ZnO Nanowire Arrays Using Textured ZnO Seeds. Nano Lett. 2005, 5, 1231–1236. [Google Scholar] [CrossRef] [PubMed]
- Baruah, S.; Dutta, J. Hydrothermal growth of ZnO nanostructures. Sci. Technol. Adv. Mater. 2009, 10, 013001. [Google Scholar] [CrossRef]
- Kumar, V.; Gupta, R.; Bansal, A. Hydrothermal Growth of ZnO Nanorods for Use in Dye-Sensitized Solar Cells. ACS Appl. Nano Mater. 2021, 4, 6212–6222. [Google Scholar] [CrossRef]
- Demes, T.; Ternon, C.; Morisot, F.; Riassetto, D.; Legallais, M.; Roussel, H.; Langlet, M. Mechanisms involved in the hydrothermal growth of ultra-thin and high aspect ratio ZnO nanowires. Appl. Surf. Sci. 2017, 410, 423–431. [Google Scholar] [CrossRef]
- Buryi, M.; Remeš, Z.; Babin, V.; Chertopalov, S.; Děcká, K.; Dominec, F.; Mičová, J.; Neykova, N. Free-Standing ZnO:Mo Nanorods Exposed to Hydrogen or Oxygen Plasma: Influence on the Intrinsic and Extrinsic Defect States. Materials 2022, 15, 2261. [Google Scholar] [CrossRef] [PubMed]
- Buryi, M.; Babin, V.; Chang, Y.-Y.; Remeš, Z.; Mičová, J.; Šimek, D. Influence of precursor age on defect states in ZnO nanorods. Appl. Surf. Sci. 2020, 525, 146448. [Google Scholar] [CrossRef]
- Davydova, M.; Laposa, A.; Smarhak, J.; Kromka, A.; Neykova, N.; Nahlik, J.; Kroutil, J.; Drahokoupil, J.; Voves, J. Gas-sensing behaviour of ZnO/diamond nanostructures. Beilstein J. Nanotechnol. 2018, 9, 22–29. [Google Scholar] [CrossRef]
- Neykova, N.; Chang, Y.-Y.; Buryi, M.; Davydova, M.; Kucerkova, R.; Simek, D.; Remes, Z.; Pop-Georgievski, O. Study of ZnO nanorods grown under UV irradiation. Appl. Surf. Sci. 2019, 472, 105–111. [Google Scholar] [CrossRef]
- Achehboune, M.; Khenfouch, M.; Boukhoubza, I.; Derkaoui, I.; Leontie, L.; Carlescu, A.; Mothudi, B.; Zorkani, I.; Jorio, A. Optimization of the luminescence and structural properties of Er-doped ZnO nanostructures: Effect of dopant concentration and excitation wavelength. J. Lumin. 2022, 246, 118843. [Google Scholar] [CrossRef]
- Li, Q.; Liu, X.; Gu, M.; Hu, Y.; Li, F.; Liu, S.; Wu, Q.; Sun, Z.; Zhang, J.; Huang, S.; et al. Development of ZnO-based nanorod arrays as scintillator layer for ultrafast and high-spatial-resolution X-ray imaging system. Opt. Express 2018, 26, 31290. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, D.; Hao, S.; An, R.; Yuan, R.; Yang, Y.; Yang, X.; Huang, M.; Zhang, Z.; Liu, X.; et al. Development of the ZnO:Ga microrods—Epoxy composite as a scintillation screen for ultrafast X-ray detection. Opt. Mater. 2020, 102, 109805. [Google Scholar] [CrossRef]
- Liao, J.; Wang, M.; Lin, F.; Han, Z.; Fu, B.; Tu, D.; Chen, X.; Qiu, B.; Wen, H.-R. Thermally boosted upconversion and downshifting luminescence in Sc2(MoO4)3:Yb/Er with two-dimensional negative thermal expansion. Nat. Commun. 2022, 13, 2090. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Y.; Pang, T.; Chen, B.; Xin, F.; Xing, M.; Tian, M.; Fu, Y.; Luo, X.; Tian, Y. Engineering Er3+-sensitized nanocrystals to enhance NIR II-responsive upconversion luminescence. Nanoscale 2022, 14, 962–968. [Google Scholar] [CrossRef]
- Huang, H.; Yuan, M.; Hu, S.; Zhong, Y.; Cui, W.; Guo, C.; Song, C.; Zhao, G.; Han, K. Nanosecond kinetics of multiphoton upconversion in an optically trapped single microcrystal. J. Mater. Chem. C 2022, 10, 9208–9215. [Google Scholar] [CrossRef]
- Elfeky, S.; Reda, S. MOF/Up-converting combination for photovoltaic application. J. Electroanal. Chem. 2021, 895, 115485. [Google Scholar] [CrossRef]
- Hu, J.; Yin, C.; Xu, S.; Cheng, M.; Wei, T.; Liu, Q.; Li, W.; Ling, Y.; Zhang, Y.; Liu, B. Enhanced room temperature NO2 sensing performance based on N-doped carbon nanosheets@ZnO nanoplates by morphology transition and white light illumination. Appl. Surf. Sci. 2022, 599, 153980. [Google Scholar] [CrossRef]
- Photaram, W.; Liangruksa, M.; Aiempanakit, M.; Suwanchawalit, C.; Wisitsoraat, A.; Sukunta, J.; Laksee, S.; Siriwong, C. Design and fabrication of zinc oxide-graphene nanocomposite for gas sensing applications. Appl. Surf. Sci. 2022, 595, 153510. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, H.; Li, L.; Tuokedaerhan, K.; Jia, Z. Er-enhanced humidity sensing performance in black ZnO-based sensor. J. Alloys Compd. 2018, 744, 364–369. [Google Scholar] [CrossRef]
- D’Arienzo, M.; Redaelli, M.; Di Credico, B.; Polizzi, S.; Scotti, R.; Morazzoni, F. New insights into the sensing mechanism of shape controlled ZnO particles. RSC Adv. 2016, 6, 52987–52997. [Google Scholar] [CrossRef]
- Chang, Y.-Y.; Remes, Z.; Micova, J. Mass production of hydrogenated ZnO nanorods. In Proceedings of the NANOCON 2019—Conference Proceedings, Brno, Czech Republic, 16–18 October 2020; pp. 221–225. [Google Scholar] [CrossRef]
- Chang, Y.-Y.; Stuchlík, J.; Neykova, N.; Souček, J.; Remeš, Z. Optical properties of the plasma hydrogenated ZnO thin films. J. Electr. Eng. 2017, 68, 70–73. [Google Scholar] [CrossRef]
- Mooney, J.; Kambhampati, P. Get the Basics Right: Jacobian Conversion of Wavelength and Energy Scales for Quantitative Analysis of Emission Spectra. J. Phys. Chem. Lett. 2013, 4, 3316–3318. [Google Scholar] [CrossRef]
- Buryi, M.; Neykova, N.; Ridzoňová, K.; Remeš, Z.; Děcká, K.; Hájek, F.; Artemenko, A.; Mičová, J.; Landová, L.; Jakubec, I. Peculiarities of erbium incorporation into ZnO microrods at high doping level leading to upconversion and the morphology change. Influence on excitonic as well as shallow donor states. Appl. Surf. Sci. 2023, 611, 155651. [Google Scholar] [CrossRef]
- Buryi, M.; Babin, V.; Remeš, Z.; Mičová, J. Charge trapping processes in hydrothermally grown Er-doped ZnO. Radiat. Meas. 2022, 150, 106700. [Google Scholar] [CrossRef]
- Klyui, N.; Bratus, V.; Temchenko, V.; Lozinskii, V.; Yukhymchuk, V.; Han, W.; Liu, B. EPR and Raman study of silicon layers obtained by gas detonation spraying. Mater. Sci. Semicond. Process. 2017, 71, 232–239. [Google Scholar] [CrossRef]
- Feher, G. Electron Spin Resonance Experiments on Donors in Silicon. I. Electronic Structure of Donors by the Electron Nuclear Double Resonance Technique. Phys. Rev. 1959, 114, 1219–1244. [Google Scholar] [CrossRef]
- Abragam, A.; Bleaney, B. Electron Paramagnetic Resonance of Transition Ions; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Buryi, M.; Havlák, L.; Jarý, V.; Bárta, J.; Laguta, V.; Beitlerová, A.; Li, J.; Chen, X.; Yuan, Y.; Liu, Q.; et al. Specific absorption in Y3Al5O12:Eu ceramics and the role of stable Eu2+ in energy transfer processes. J. Mater. Chem. C 2020, 8, 8823–8839. [Google Scholar] [CrossRef]
- Shimada, R.; Urban, B.; Sharma, M.; Singh, A.; Avrutin, V.; Morkoç, H.; Neogi, A. Energy transfer in ZnO-anthracene hybrid structure. Opt. Mater. Express 2012, 2, 526. [Google Scholar] [CrossRef]
- Buryi, M.; Babin, V.; Hubáček, T.; Jarý, V.; Hájek, F.; Kuldová, K.; Artemenko, A.; Hospodková, A. The influence of Si on the properties of MOVPE grown GaN thin films: Optical and EPR study. Radiat. Meas. 2022, 157, 106842. [Google Scholar] [CrossRef]
- Vaněk, T.; Jarý, V.; Hubáček, T.; Hájek, F.; Kuldová, K.; Gedeonová, Z.; Babin, V.; Remeš, Z.; Buryi, M. Acceleration of the yellow band luminescence in GaN layers via Si and Ge doping. J. Alloys Compd. 2022, 914, 165255. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buryi, M.; Ridzoňová, K.; Neykova, N.; Landová, L.; Hájek, F.; Babin, V.; Děcká, K.; Sharma, R.K.; Pop-Georgievski, O. Effect of UV Irradiation on the Growth of ZnO:Er Nanorods and Their Intrinsic Defects. Chemosensors 2023, 11, 156. https://doi.org/10.3390/chemosensors11030156
Buryi M, Ridzoňová K, Neykova N, Landová L, Hájek F, Babin V, Děcká K, Sharma RK, Pop-Georgievski O. Effect of UV Irradiation on the Growth of ZnO:Er Nanorods and Their Intrinsic Defects. Chemosensors. 2023; 11(3):156. https://doi.org/10.3390/chemosensors11030156
Chicago/Turabian StyleBuryi, Maksym, Katarína Ridzoňová, Neda Neykova, Lucie Landová, František Hájek, Vladimir Babin, Kateřina Děcká, Rupendra Kumar Sharma, and Ognen Pop-Georgievski. 2023. "Effect of UV Irradiation on the Growth of ZnO:Er Nanorods and Their Intrinsic Defects" Chemosensors 11, no. 3: 156. https://doi.org/10.3390/chemosensors11030156
APA StyleBuryi, M., Ridzoňová, K., Neykova, N., Landová, L., Hájek, F., Babin, V., Děcká, K., Sharma, R. K., & Pop-Georgievski, O. (2023). Effect of UV Irradiation on the Growth of ZnO:Er Nanorods and Their Intrinsic Defects. Chemosensors, 11(3), 156. https://doi.org/10.3390/chemosensors11030156