An Aptamer Biosensing Strategy for Label-Free Assay of Dual Acute Myocardial Infarction Biomarkers Built upon AuNPs/Ti3C2-MXenes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
2.3. Fabrication of Aptamer/AuNPs/Ti3C2-MXenes Aptasensors
2.4. cTnI and Myo Detection
3. Results and Discussion
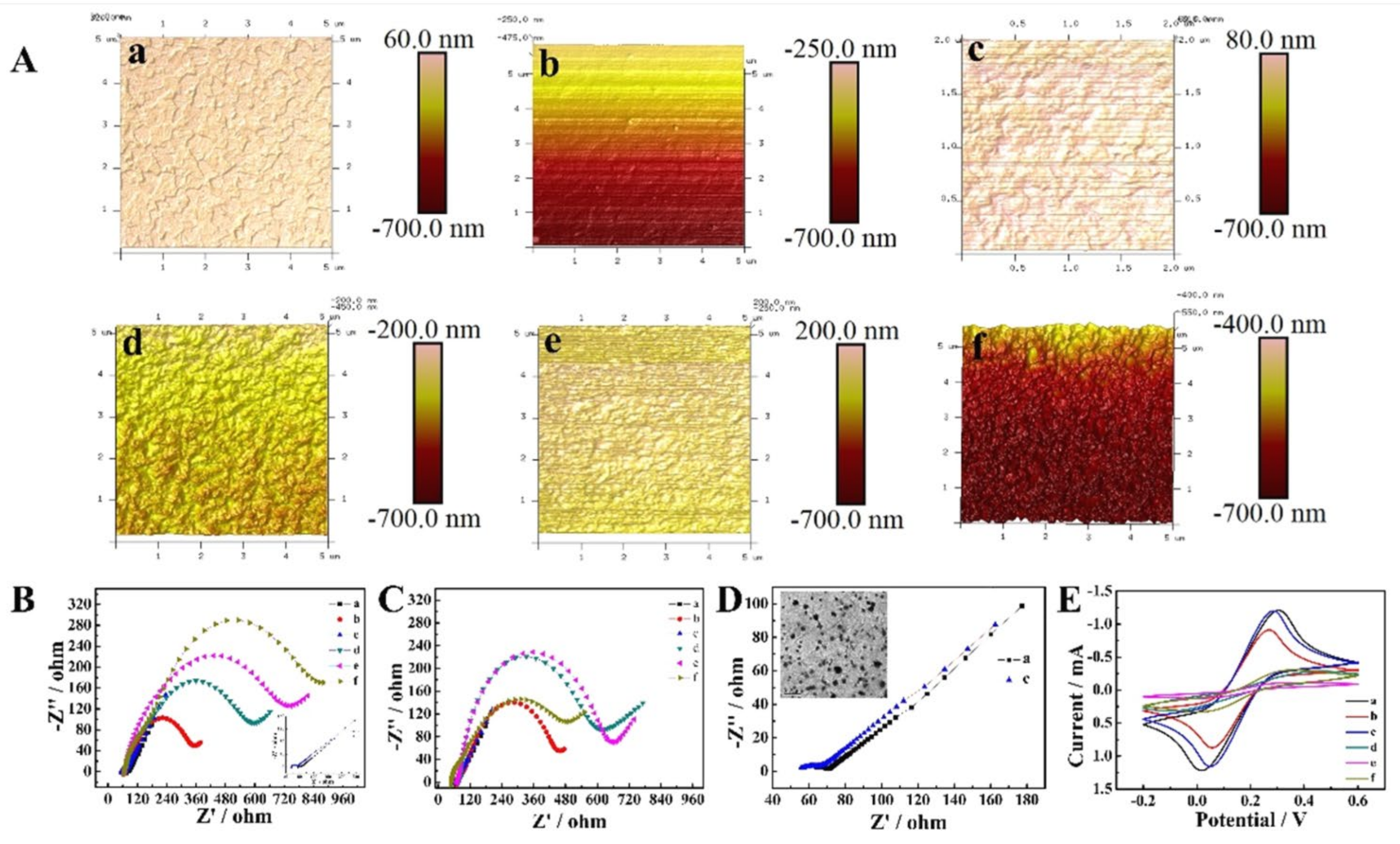
3.1. Monitoring the Preparation of Aptasensors
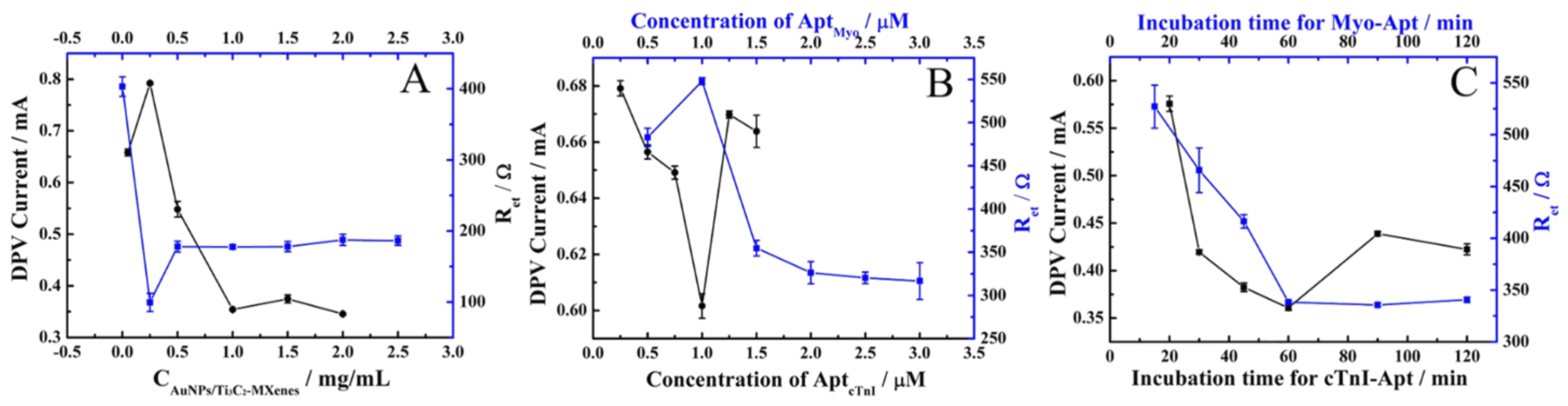
3.2. Optimization of the Conditions for Sensor Preparation and Operation
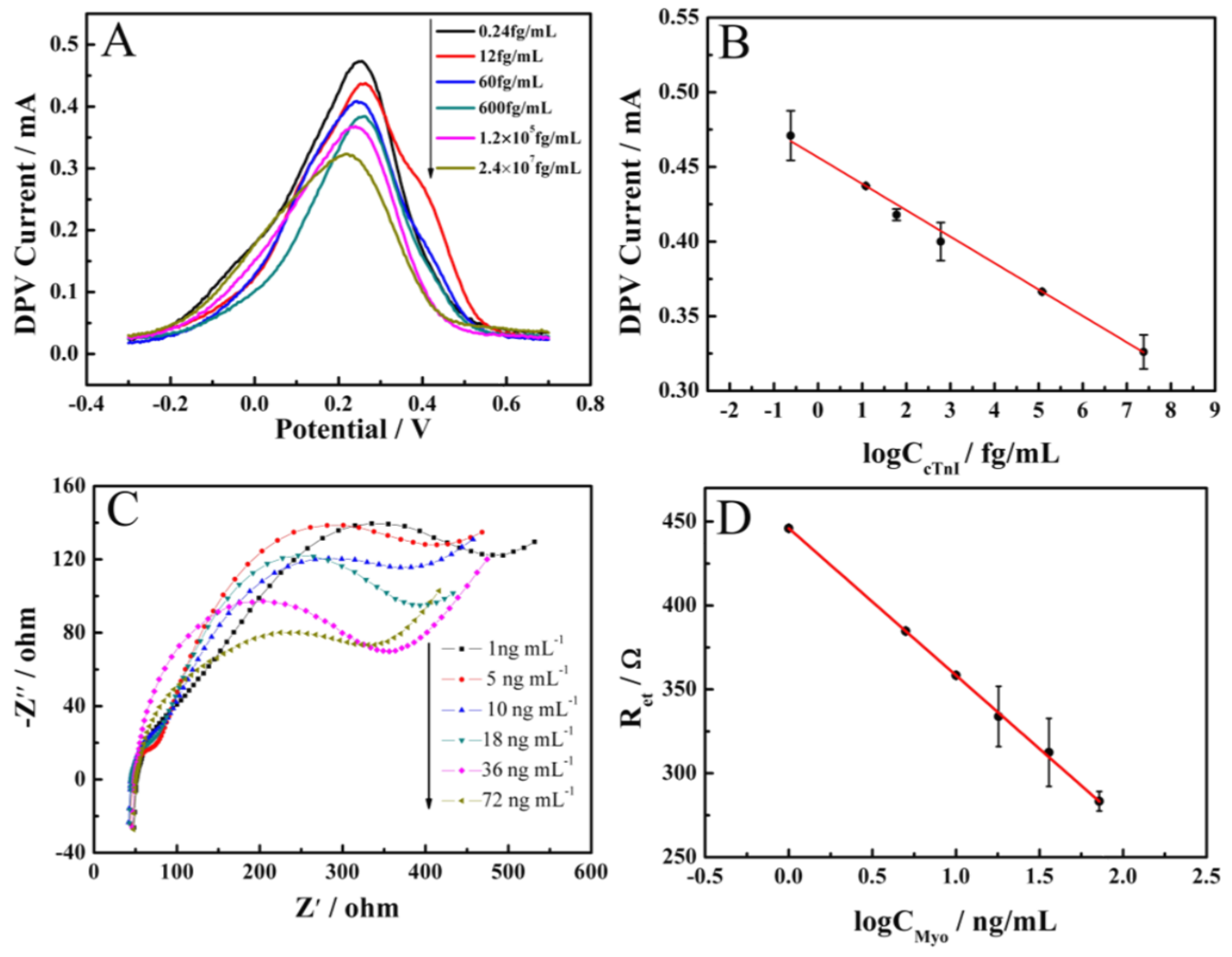
3.3. The Analytical Performance of Prepared Aptasensors
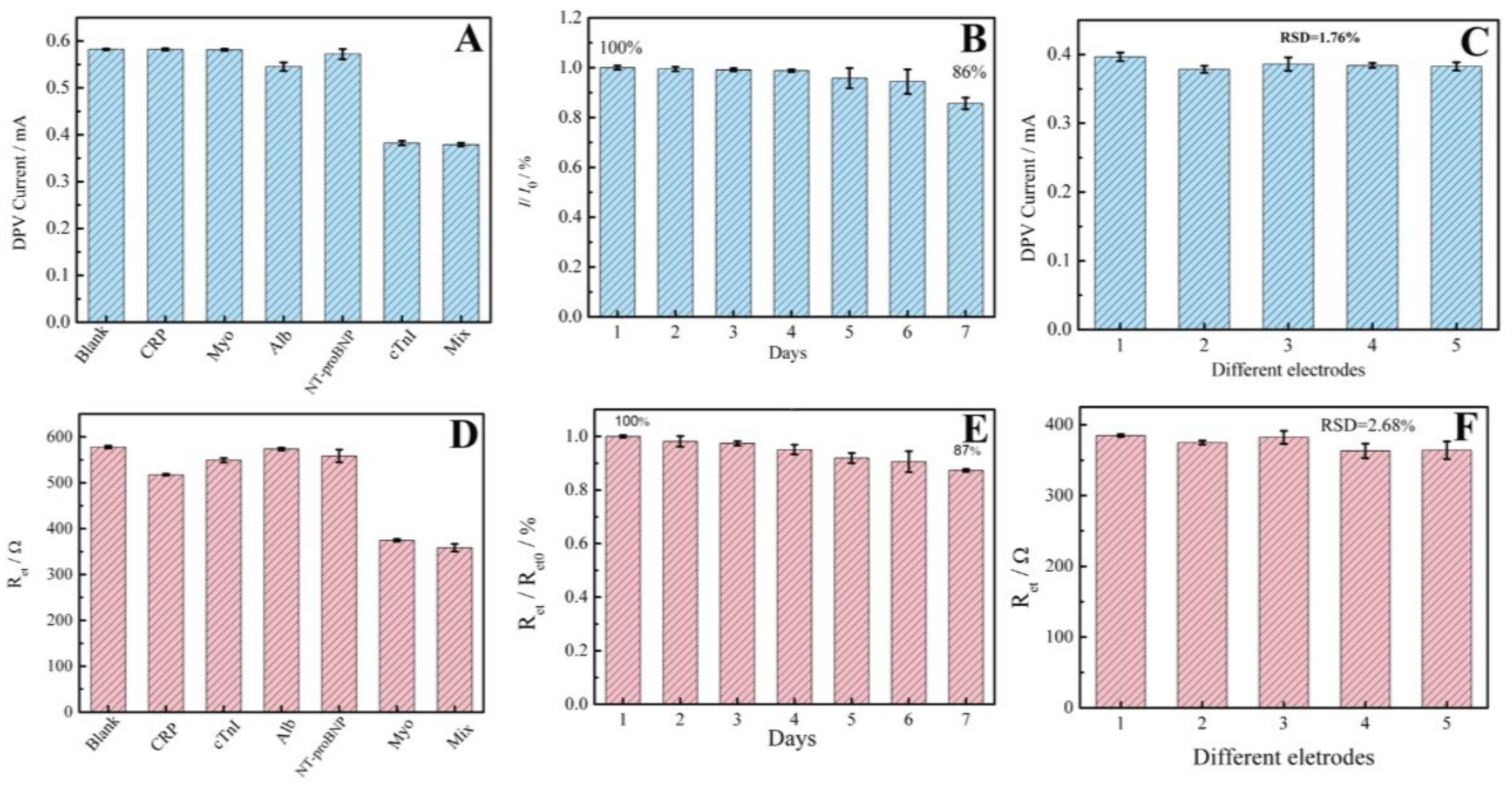
3.4. Specificity, Stability and Reproducibility of the Proposed Aptasensors
3.5. Detection of cTnI or Myo in Human Serum Sample
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ji, J.; Lu, W.; Zhu, Y.; Jin, H.; Yao, Y.; Zhang, H.; Zhao, Y. Porous Hydrogel-Encapsulated Photonic Barcodes for Multiplex Detection of Cardiovascular Biomarkers. ACS Sens. 2019, 4, 1384–1390. [Google Scholar] [CrossRef] [PubMed]
- Khushaim, W.; Peramaiah, K.; Beduk, T.; Vijjapu, M.T.; Filho, J.I.D.O.; Huang, K.-W.; Mani, V.; Salama, K.N. Porous graphitic carbon nitrides integrated biosensor for sensitive detection of cardiac troponin I. Biosens. Bioelectron. X 2022, 12, 100234. [Google Scholar] [CrossRef]
- Mi, X.; Li, H.; Tan, R.; Tu, Y. Dual-Modular Aptasensor for Detection of Cardiac Troponin I Based on Mesoporous Silica Films by Electrochemiluminescence/Electrochemical Impedance Spectroscopy. Anal. Chem. 2020, 92, 14640–14647. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Wang, L.; Chen, J.; Su, W.; Li, A.; Su, G.; Liu, P.; Zhou, X. Electrochemical strategies for the detection of cTnI. Anal. 2021, 146, 5474–5495. [Google Scholar] [CrossRef]
- Maisel, A.; Mueller, C.; Neath, S.-X.; Christenson, R.H.; Morgenthaler, N.G.; McCord, J.; Nowak, R.M.; Vilke, G.; Daniels, L.B.; Hollander, J.E.; et al. Copeptin Helps in the Early Detection of Patients with Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2013, 62, 150–160. [Google Scholar] [CrossRef]
- Yoo, S.S.; Kim, S.Y.; Kim, K.S.; Hong, S.; Oh, M.J.; Nam, M.G.; Kim, W.-J.; Park, J.; Chung, C.-H.; Choe, W.-S.; et al. Controlling inter-sheet-distance in reduced graphene oxide electrodes for highly sensitive electrochemical impedimetric sensing of myoglobin. Sens. Actuators B Chem. 2020, 305, 127477. [Google Scholar] [CrossRef]
- Zhang, J.X.; Tang, C.; Chen, D.N.; Jiang, L.Y.; Wang, A.J.; Feng, J.J. Ultrasensitive Label-Free Sandwich Immunoassay of Cardiac Biomarker Myoglobin Using Meso-SiO2@Ploydapamine@PtPd Nanocrystals and PtNi Nanodendrites for Effective Signal Amplification. Appl. Surf. Sci. 2023, 608, 155216. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Kang, M.; Huang, X.; Liu, Y.; Zhou, N.; Zhang, Z. Nanodiamonds and hydrogen-substituted graphdiyne heteronanostructure for the sensitive impedimetric aptasensing of myocardial infarction and cardiac troponin I. Anal. Chim. Acta 2021, 1141, 110–119. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, L.; Liu, B.; Su, E.B.; Chen, H.Y.; Gu, Z.Z.; Zhao, X.W. Quantitative Detection of Multiplex Cardiac Bi-omarkers with Encoded SERS Nanotags on a Single T Line in Lateral Flow Assay. Sens. Actuators B 2018, 277, 502–509. [Google Scholar] [CrossRef]
- Guo, X.; Zong, L.; Jiao, Y.; Han, Y.; Zhang, X.; Xu, J.; Li, L.; Zhang, C.-W.; Liu, Z.; Ju, Q.; et al. Signal-Enhanced Detection of Multiplexed Cardiac Biomarkers by a Paper-Based Fluorogenic Immunodevice Integrated with Zinc Oxide Nanowires. Anal. Chem. 2019, 91, 9300–9307. [Google Scholar] [CrossRef]
- Wang, B.; Shi, S.W.; Yang, X.L.; Wang, Y.; Qi, H.L.; Gao, Q.; Zhang, C.X. Separation-Free Electrogenerated Chemilumi-nescence Immunoassay Incorporating Target Assistant Proximity Hybridization and Dynamically Competitive Hybridization of a DNA Signal Probe. Anal. Chem. 2020, 92, 884–891. [Google Scholar] [CrossRef]
- Alwarappan, S.; Nesakumar, N.; Sun, D.; Hu, T.Y.; Li, C.-Z. 2D metal carbides and nitrides (MXenes) for sensors and biosensors. Biosens. Bioelectron. 2022, 205, 113943. [Google Scholar] [CrossRef] [PubMed]
- Lorencova, L.; Gajdosova, V.; Hroncekova, S.; Bertok, T.; Blahutova, J.; Vikartovska, A.; Parrakova, L.; Gemeiner, P.; Kasak, P.; Tkac, J. 2D MXenes as Perspective Immobilization Platforms for Design of Electrochemical Nanobiosensors. Electroanalysis 2019, 31, 1833–1844. [Google Scholar] [CrossRef]
- Aguedo, J.; Lorencova, L.; Barath, M.; Farkas, P.; Tkac, J. Electrochemical Impedance Spectroscopy on 2D Nanomaterial MXene Modified Interfaces: Application as a Characterization and Transducing Tool. Chemosensors 2020, 8, 127–148. [Google Scholar] [CrossRef]
- Zhang, H.X.; Zhuang, T.T.; Wang, L.; Du, L.; Xia, J.F.; Wang, Z.H. Efficient Au Nanocluster@Ti3C2 Heterostructure Lumi-nophore Combined with Cas12a for Electrochemiluminescence Detection of miRNA. Sens. Actuators B 2022, 370, 132428. [Google Scholar] [CrossRef]
- Wei, Z.H.; Zhang, H.X.; Wang, Z.H. High-Intensity Focused Ultrasound Combined with Ti3C2–TiO2 to Enhance Electro-chemiluminescence of Luminol for the Sensitive Detection of Polynucleotide Kinase. ACS Appl. Mater. Interfaces 2023, 15, 3804–3811. [Google Scholar] [CrossRef]
- Wu, L.; Lu, X.; Dhanjai; Wu, Z.-S.; Dong, Y.; Wang, X.; Zheng, S.; Chen, J. 2D transition metal carbide MXene as a robust biosensing platform for enzyme immobilization and ultrasensitive detection of phenol. Biosens. Bioelectron. 2018, 107, 69–75. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, F.; Zhang, H.; Zhang, Y.; Liu, M.; Liu, Y. Universal Ti3C2 MXenes Based Self-Standard Ratiometric Fluorescence Resonance Energy Transfer Platform for Highly Sensitive Detection of Exosomes. Anal. Chem. 2018, 90, 12737–12744. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Huang, Y.; Xiong, M.H.; Wang, F.; Li, C. A Label-Free Electrochemical Biosensor for Highly Sensitive De-tection of Gliotoxin based on DNA Nanostructure/MXene Nanocomplexes. Biosens. Bioelectron. 2019, 142, 111531. [Google Scholar] [CrossRef]
- Mi, X.N.; Li, H.; Tan, R.; Feng, B.N.; Tu, Y.F. The TDs/Aptamer cTnI Biosensors based on HCR and Au/Ti3C2-MXene Amplification for Screening Serious Patient in COVID-19 Pandemic. Biosens. Bioelectron. 2021, 192, 113482. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Zhuang, T.; Liu, J.; Sojic, N.; Wang, Z. Sensitive electrochemiluminescence biosensing of polynucleotide kinase using the versatility of two-dimensional Ti3C2TX MXene nanomaterials. Anal. Chim. Acta 2022, 1191, 339346. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, W.; Xing, Y.; Yang, X.; Wang, K.; Jiang, R.; Wang, P.; Zhao, Q. Screening of DNA Aptamers against Myoglobin Using a Positive and Negative Selection Units Integrated Microfluidic Chip and Its Biosensing Application. Anal. Chem. 2014, 86, 6572–6579. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Gu, H.; Jeon, W.; Youn, H.; Her, J.; Kim, S.-K.; Lee, J.; Shin, J.H.; Ban, C. Electrochemical Aptasensor of Cardiac Troponin I for the Early Diagnosis of Acute Myocardial Infarction. Anal. Chem. 2015, 87, 9869–9875. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-J.; Pan, J.-B.; Wang, Y.-Z.; Ji, S.-Y.; Zhao, W.; Luo, X.-L.; Xu, J.-J.; Chen, H.-Y. Electrochemiluminescence Energy Resonance Transfer System between RuSi Nanoparticles and Hollow Au Nanocages for Nucleic Acid Detection. Anal. Chem. 2018, 90, 10434–10441. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Xie, Q.; Xu, M.; Wang, S.; Zhou, J.; Liu, F. RNA aptamer based electrochemical biosensor for sensitive and selective detection of cAMP. Biosens. Bioelectron. 2015, 66, 238–243. [Google Scholar] [CrossRef]
- Mishra, S.K.; Kumar, D.; Biradar, A.M.; Rajesh. Electrochemical Impedance Spectroscopy Characterization of Mercapto-propionic Acid Capped ZnS Nanocrystal Based Bioelectrode for the Detection of the Cardiac Biomarker—Myoglobin. Bioelectrochemistry 2012, 88, 118–126. [Google Scholar] [CrossRef]
- Li, D.; Tan, R.; Mi, X.; Fang, C.; Tu, Y. An electrochemiluminescent biosensor for noninvasive glucose detection based on cluster-like AuAg hollowed-nanoparticles. Microchem. J. 2021, 167, 106271. [Google Scholar] [CrossRef]
- Ge, X.-Y.; Zhang, J.-X.; Feng, Y.-G.; Wang, A.-J.; Mei, L.-P.; Feng, J.-J. Label-free electrochemical biosensor for determination of procalcitonin based on graphene-wrapped Co nanoparticles encapsulated in carbon nanobrushes coupled with AuPtCu nanodendrites. Microchim. Acta 2022, 189, 110. [Google Scholar] [CrossRef]
- Lv, H.; Li, Y.; Zhang, X.; Li, X.; Xu, Z.; Chen, L.; Li, D.; Dong, Y. Thionin functionalized signal amplification label derived dual-mode electrochemical immunoassay for sensitive detection of cardiac troponin I. Biosens. Bioelectron. 2019, 133, 72–78. [Google Scholar] [CrossRef]
- Rauf, S.; Mani, V.; Lahcen, A.A.; Yuvaraja, S.; Beduk, T.; Salama, K.N. Binary transition metal oxide modified laser-scribed graphene electrochemical aptasensor for the accurate and sensitive screening of acute myocardial infarction. Electrochim. Acta 2021, 386, 138489. [Google Scholar] [CrossRef]
- Dong, L.; Yin, L.; Tian, G.; Wang, Y.; Pei, H.; Wu, Q.; Cheng, W.; Ding, S.; Xia, Q. An enzyme-free ultrasensitive electrochemical immunosensor for calprotectin detection based on PtNi nanoparticles functionalized 2D Cu-metal organic framework nanosheets. Sens. Actuators B 2020, 308, 127687. [Google Scholar] [CrossRef]
- Lopa, N.S.; Rahman, M.; Ahmed, F.; Ryu, T.; Sutradhar, S.C.; Lei, J.; Kim, J.; Kim, D.H.; Lee, Y.H.; Kim, W. Simple, low-cost, sensitive and label-free aptasensor for the detection of cardiac troponin I based on a gold nanoparticles modified titanium foil. Biosens. Bioelectron. 2019, 126, 381–388. [Google Scholar] [CrossRef]
- Song, Z.; Song, J.; Gao, F.; Chen, X.; Wang, Q.; Zhao, Y.; Huang, X.; Yang, C.; Wang, Q. Novel electroactive ferrocene-based covalent organic frameworks towards electrochemical label-free aptasensors for the detection of Cardiac Troponin I. Sens. Actuators B 2022, 368, 132205. [Google Scholar] [CrossRef]
- Chen, K.; Zhao, H.; Wang, Z.; Zhou, F.; Shi, Z.; Cao, S.; Lan, M. Sandwich-type electrochemical aptasensor based on Au-modified conductive octahedral carbon architecture and snowflake-like PtCuNi for the sensitive detection of cardiac troponin I. Biosens. Bioelectron. 2022, 212, 114431. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, H.; Chen, K.; Li, H.; Lan, M. Sandwich-type electrochemical aptasensor based on hollow mesoporous carbon spheres loaded with porous dendritic Pd@Pt nanoparticles as signal amplifier for ultrasensitive detection of cardiac troponin I. Anal. Chim. Acta 2021, 1188, 339202. [Google Scholar] [CrossRef]
- Adeel, M.; Rahman, M.; Lee, J.-J. Label-free aptasensor for the detection of cardiac biomarker myoglobin based on gold nanoparticles decorated boron nitride nanosheets. Biosens. Bioelectron. 2019, 126, 143–150. [Google Scholar] [CrossRef]
- Taghdisi, S.M.; Danesh, N.M.; Ramezani, M.; Emrani, A.S.; Abnous, K. A novel electrochemical aptasensor based on Y-shape structure of dual-aptamer-complementary strand conjugate for ultrasensitive detection of myoglobin. Biosens. Bioelectron. 2016, 80, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Shumyantseva, V.V.; Bulko, T.V.; Sigolaeva, L.V.; Kuzikov, A.V.; Archakov, A.I. Electrosynthesis and binding properties of molecularly imprinted poly-o-phenylenediamine for selective recognition and direct electrochemical detection of myoglobin. Biosens. Bioelectron. 2016, 86, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Zapp, E.; Westphal, E.; Gallardo, H.; de Souza, B.; Vieira, I.C. Liquid crystal and gold nanoparticles applied to electrochemical immunosensor for cardiac biomarker. Biosens. Bioelectron. 2014, 59, 127–133. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, H.; Dong, X.; Yan, H.; Lei, C.; Luo, Y. Giant magnetoimpedance based immunoassay for cardiac biomarker myoglobin. Anal. Methods 2017, 9, 3636–3642. [Google Scholar] [CrossRef]
- Singh, S.; Tuteja, S.K.; Sillu, D.; Deep, A.; Suri, C.R. Gold nanoparticles-reduced graphene oxide based electrochemical immunosensor for the cardiac biomarker myoglobin. Microchim. Acta 2016, 183, 1729–1738. [Google Scholar] [CrossRef]

| Electrode Materials | Target | Method | Detection Range | LOD | Refs. |
|---|---|---|---|---|---|
| HsGDY@NDs | cTnI | EIS | 0.01 pg/mL–100 ng/mL | 6.29 fg/mL | [8] |
| Fc-SiNP | SWV | 0.024 ng/mL–240 ng/mL | 24 pg/mL | [23] | |
| Ti/AuNPs | DPV | 0.024 ng/mL–26.4 ng/mL | 4.32 pg/mL | [32] | |
| Fc-COFNs | DPV | 10 fg/mL–10 ng/mL | 2.6 fg/mL | [33] | |
| PCN-AuNPs | SWV | 0.1 pg/mL–103 ng/mL | 0.01 pg/mL | [2] | |
| Au/Zr–C | i-t | 0.01 pg/mL-100 ng/mL | 1.24 fg/mL | [34] | |
| Pt@Pd DNs/NH2-HMCS | i-t | 100 fg/mL–100 ng/mL | 15.4 fg/mL | [35] | |
| AuNPs/Ti3C2-MXenes | DPV | 0.24 fg/mL–24 ng/mL | 0.14 fg/mL | This work | |
| AuNPs/BNNSs | Myo | DPV | 0.1 μg/mL–100 μg/mL | 34.6 ng/mL | [36] |
| DApt-CS conjugate | DPV | 1.67 ng/mL–666.67 ng/mL | 0.45 ng/mL | [37] | |
| Poly-o-phenylenediamine | DPV | 10 ng/mL–1780 ng/ml | 10 ng/mL | [38] | |
| AuNP-PEI | SWV | 9.96 ng/mL–72.8 ng/mL | 6.29 ng/mL | [39] | |
| magnetic beads | GMI | 1 ng/mL–10 ng/mL | 0.5 ng/mL | [40] | |
| AuNPs@rGO | DPV | 1 ng/mL–1400 ng/mL | 0.67 ng/mL | [41] | |
| AuNPs/Ti3C2-MXenes | EIS | 1 ng/mL–70 ng/mL | 0.2 ng/mL | This work |
| Sample | Found cTnI (ng/mL) | Added cTnI (ng/mL) | Total Detected cTnI (ng/mL) | Recovery (%) | ELISA (ng/mL) | RSD (%) |
| 1 | 0.0667 ± 0.0028 | 0.01 | 0.0773 ± 0.0015 | 106 | 0.07 | 1.98 |
| 2 | 0.110 ± 0.007 | 0.01 | 0.120 ± 0.001 | 100 | 0.09 | 0.61 |
| 3 | 0.0121 ± 0.0010 | 0.01 | 0.0220 ± 0.0020 | 99.0 | <0.029 | 0.91 |
| 4 | 0.0243 ± 0.0021 | 0.01 | 0.0344 ± 0.0020 | 101 | <0.029 | 0.60 |
| Sample | Found Myo (ng/mL) | Added Myo (ng/mL) | Total Detected Myo (ng/mL) | Recovery (%) | ELISA (ng/mL) | RSD (%) |
| 1 | 15.5 ± 0.39 | 5.0 | 20.7 ± 1.13 | 104 | 15.7 | 5.50 |
| 2 | 24.9 ± 0.63 | 5.0 | 29.5 ± 0.75 | 92.0 | 25.0 | 2.54 |
| 3 | 69.6 ± 0.60 | 0.5 | 70.1 ± 0.98 | 100 | 70.1 | 1.40 |
| 4 | 14.1 ± 0.10 | 5.0 | 19.5 ± 1.06 | 108 | 14.1 | 5.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mi, X.; Li, H.; Tu, Y. An Aptamer Biosensing Strategy for Label-Free Assay of Dual Acute Myocardial Infarction Biomarkers Built upon AuNPs/Ti3C2-MXenes. Chemosensors 2023, 11, 157. https://doi.org/10.3390/chemosensors11030157
Mi X, Li H, Tu Y. An Aptamer Biosensing Strategy for Label-Free Assay of Dual Acute Myocardial Infarction Biomarkers Built upon AuNPs/Ti3C2-MXenes. Chemosensors. 2023; 11(3):157. https://doi.org/10.3390/chemosensors11030157
Chicago/Turabian StyleMi, Xiaona, Huiling Li, and Yifeng Tu. 2023. "An Aptamer Biosensing Strategy for Label-Free Assay of Dual Acute Myocardial Infarction Biomarkers Built upon AuNPs/Ti3C2-MXenes" Chemosensors 11, no. 3: 157. https://doi.org/10.3390/chemosensors11030157
APA StyleMi, X., Li, H., & Tu, Y. (2023). An Aptamer Biosensing Strategy for Label-Free Assay of Dual Acute Myocardial Infarction Biomarkers Built upon AuNPs/Ti3C2-MXenes. Chemosensors, 11(3), 157. https://doi.org/10.3390/chemosensors11030157





