Recent Progress of Molecularly Imprinted Optical Sensors
Abstract
:1. Introduction
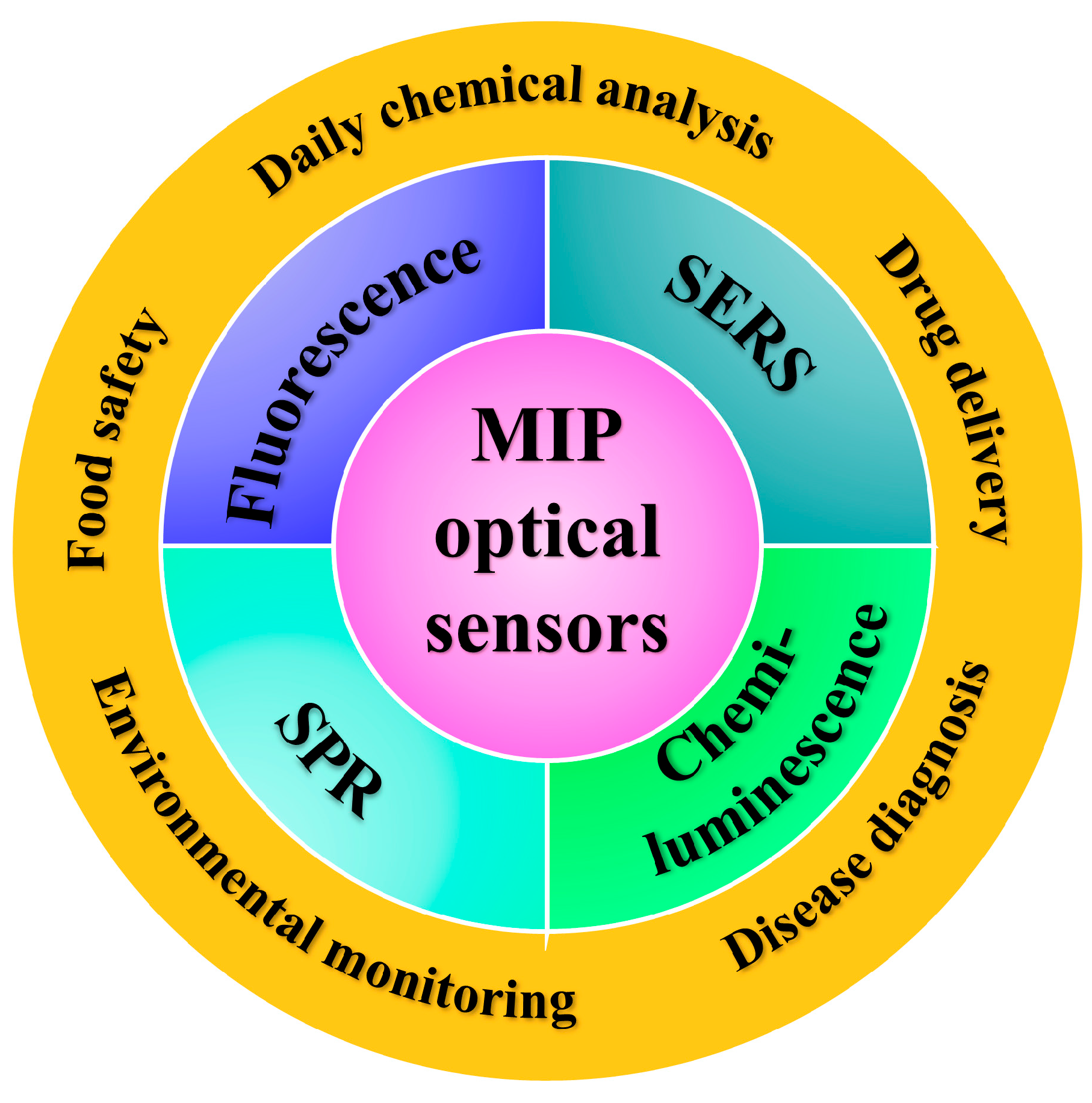
2. Molecularly Imprinted Optical Sensors
2.1. Construction of MIP Optical Sensors
2.1.1. Molecularly Imprinted Fluorescence Sensors
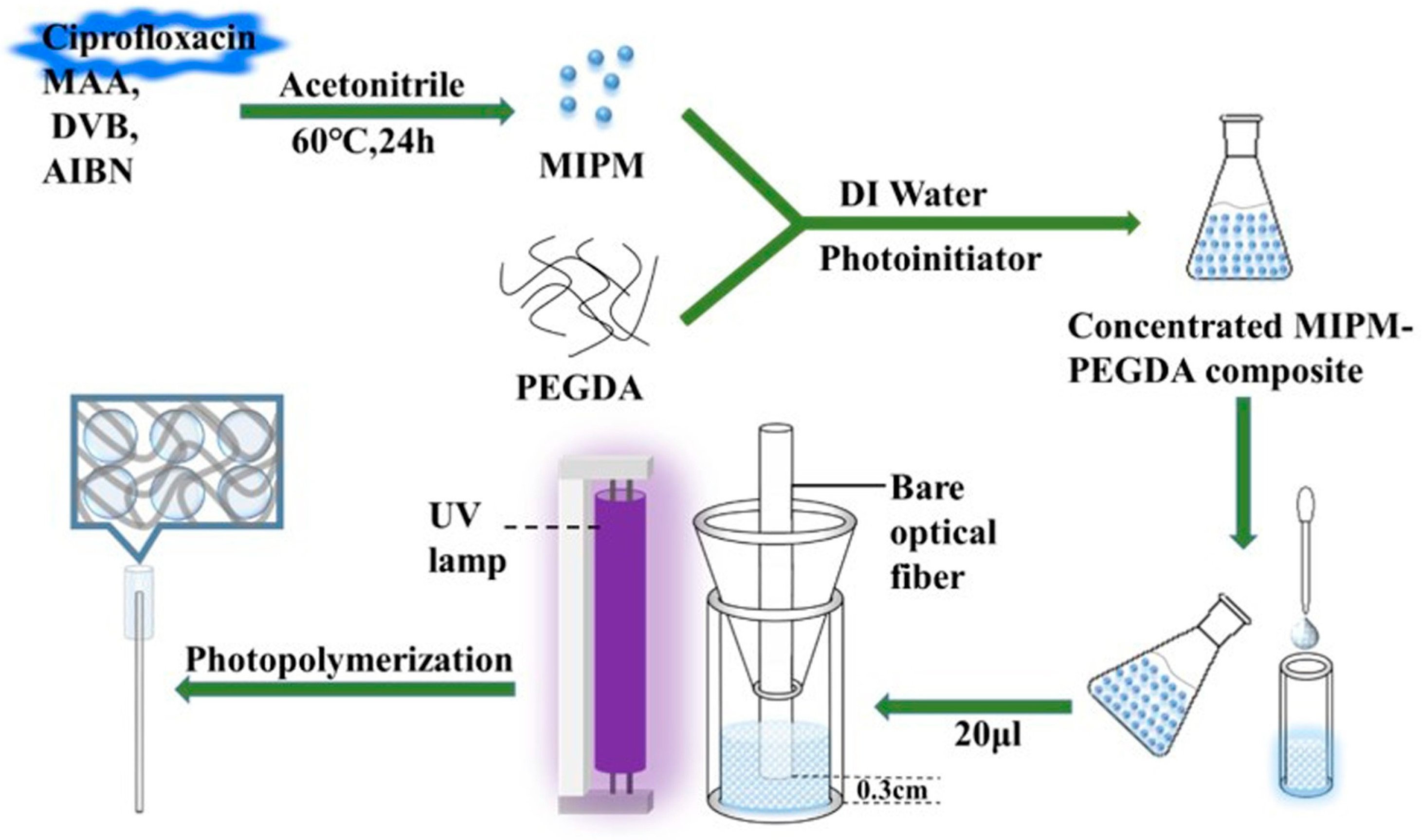
2.1.2. Molecularly Imprinted SPR Sensors
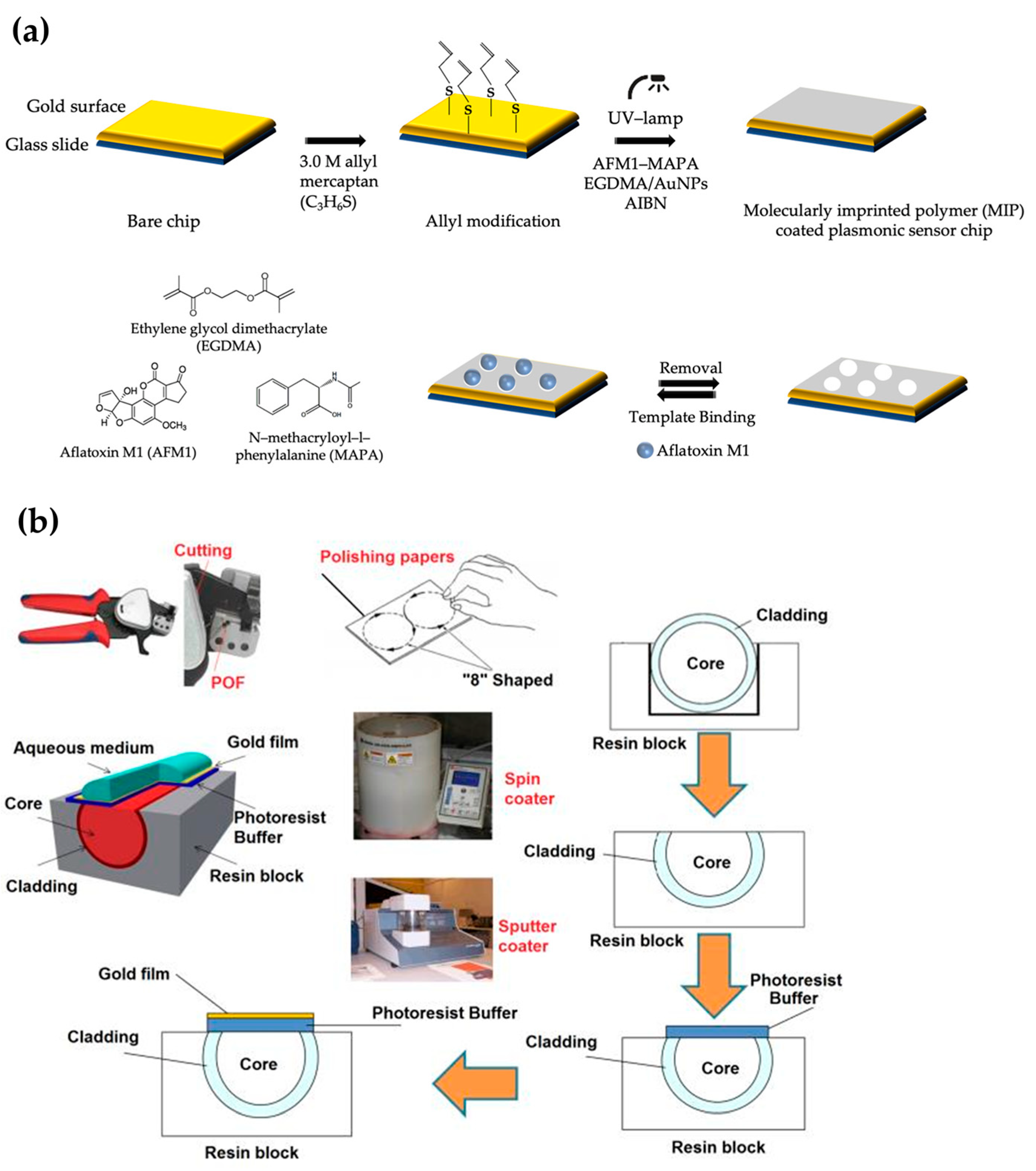
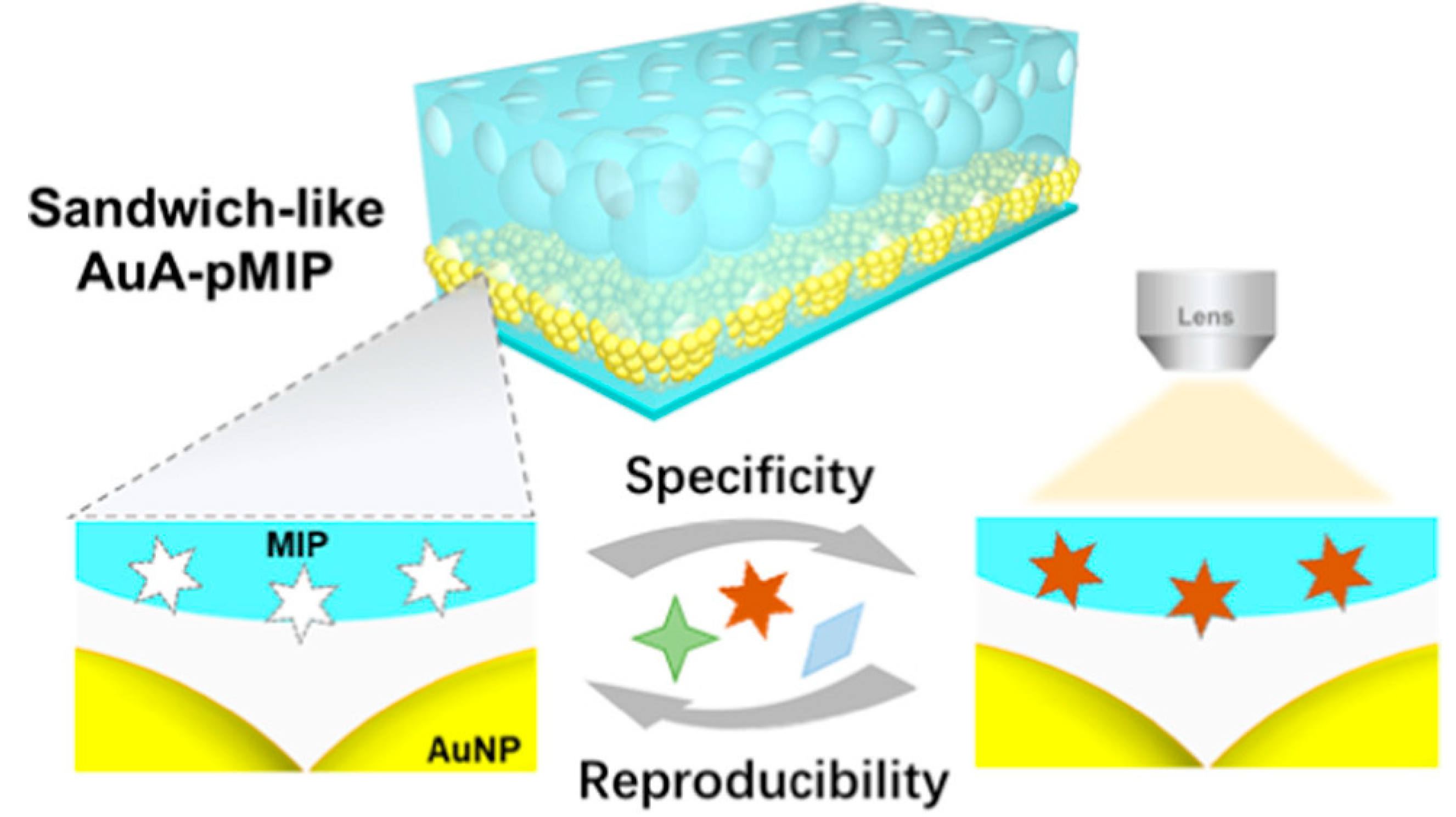
2.1.3. Molecularly Imprinted SERS Sensors
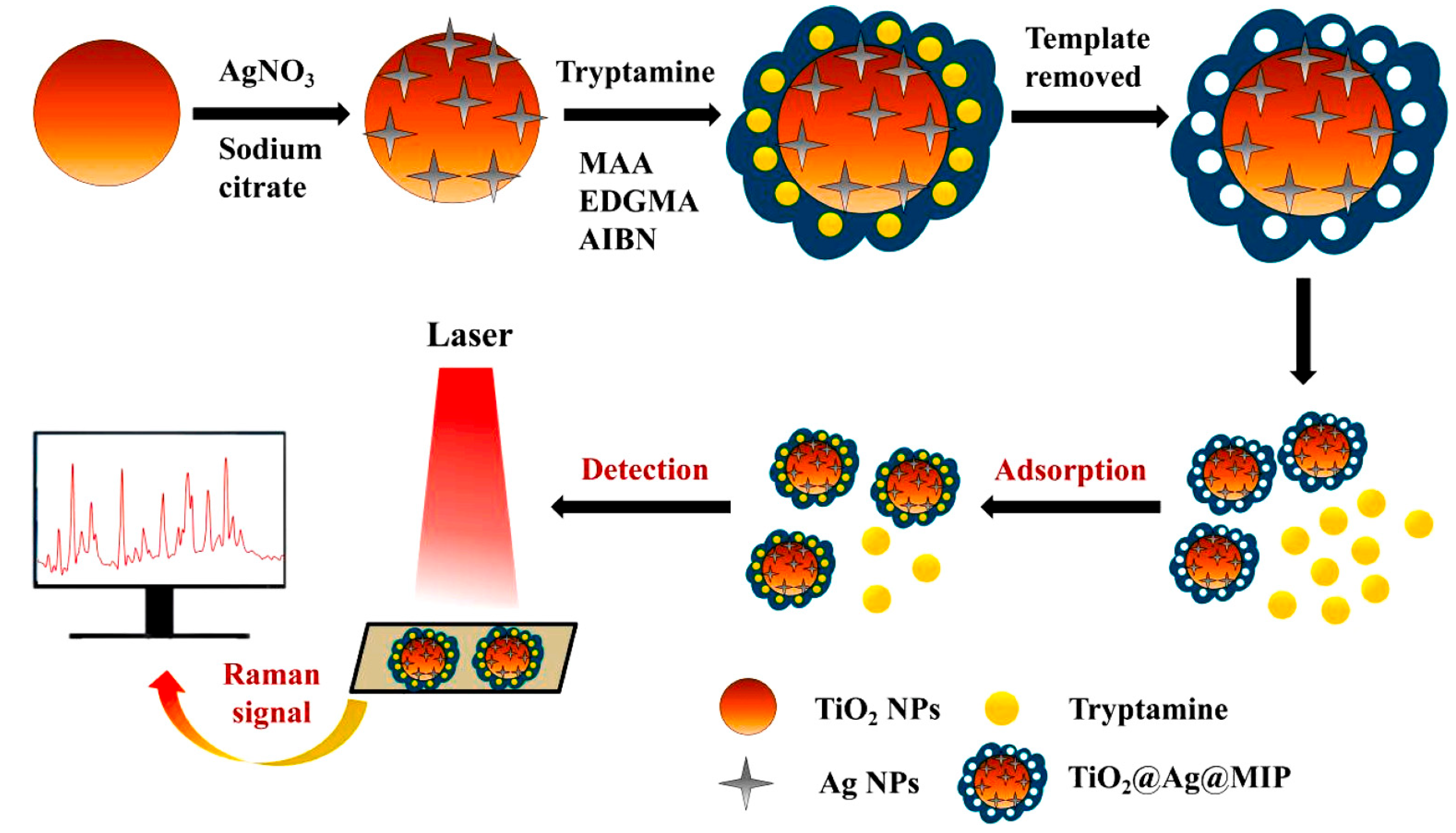
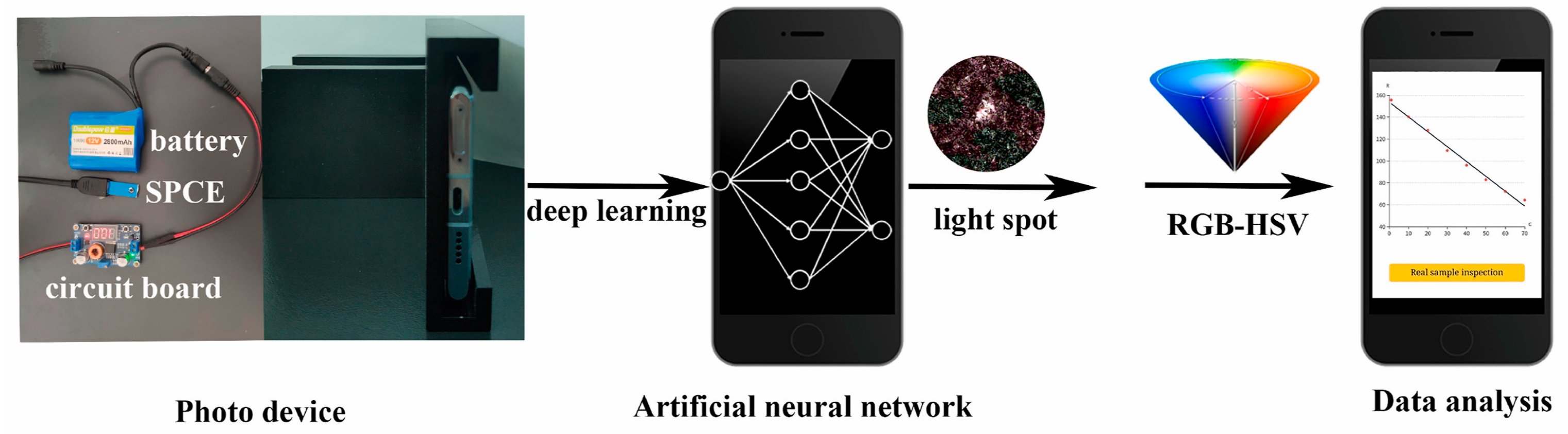
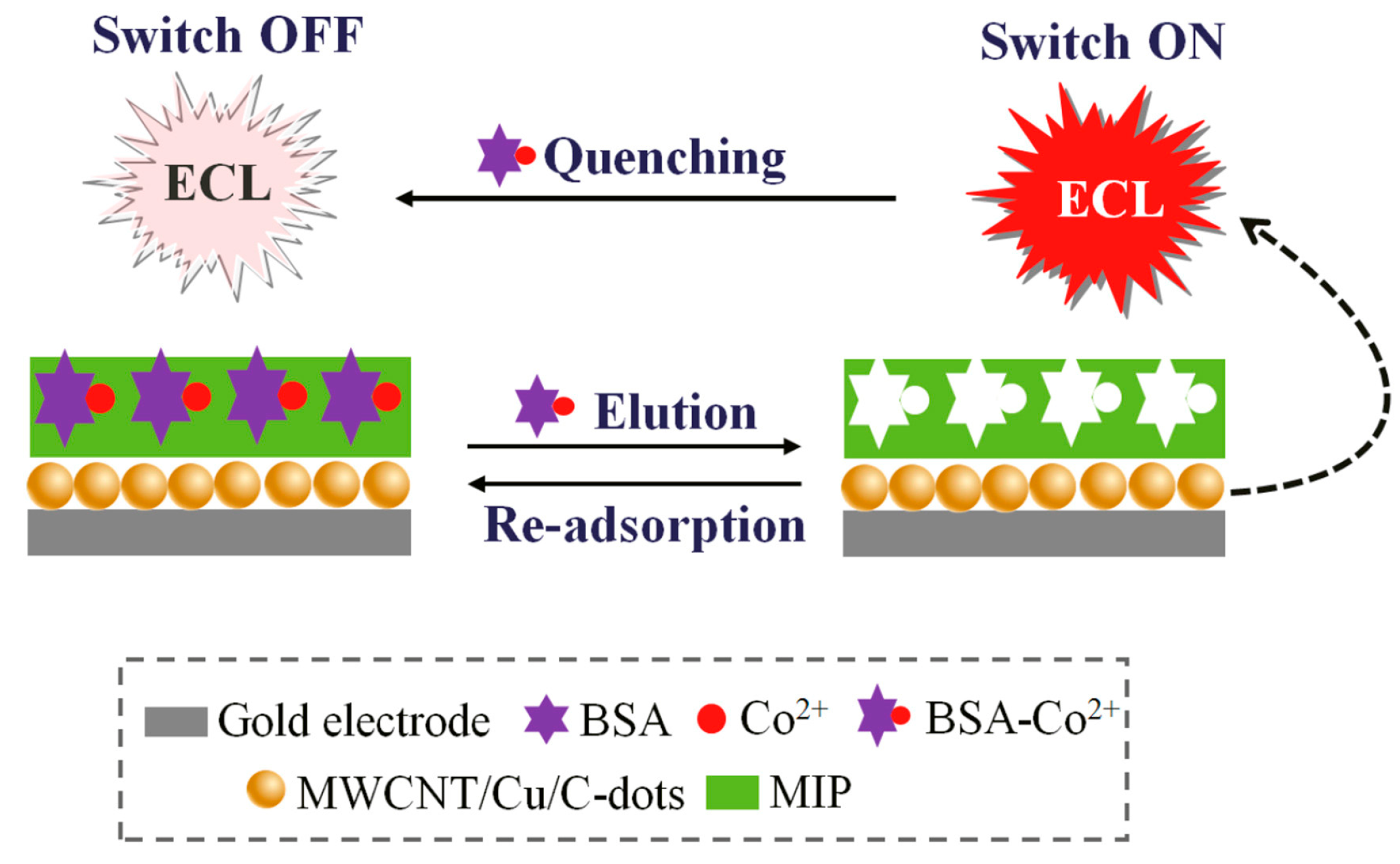
2.1.4. Molecularly Imprinted CL Sensors
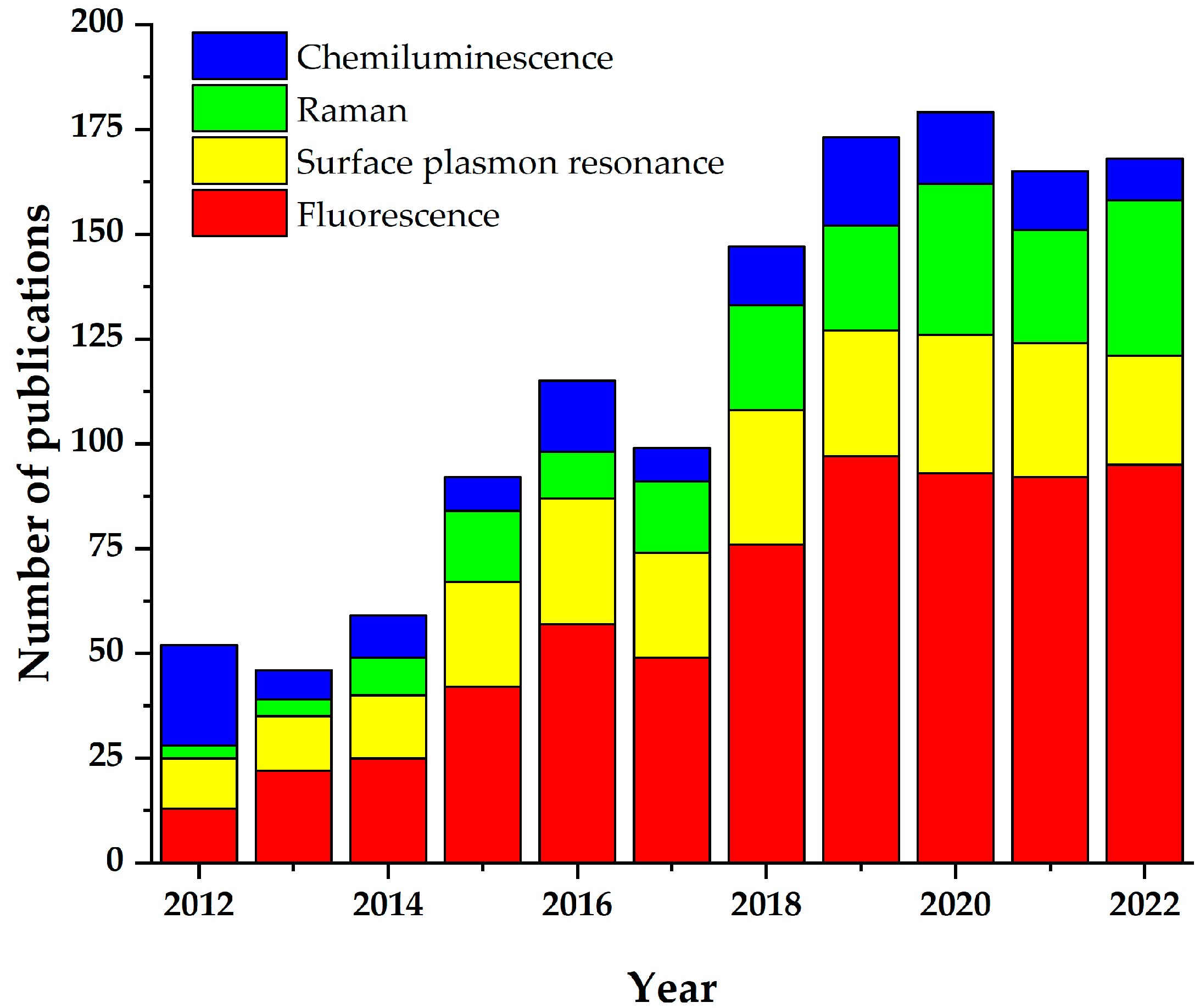
2.2. Applications of MIP Optical Sensors
2.2.1. Food Analysis
2.2.2. Healthcare
2.2.3. Environmental Safety
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arshady, R.; Mosbach, K. Synthesis of substrate-selective polymers by host-guest polymerization. Die Makromol. Chem. 1981, 182, 687–692. [Google Scholar] [CrossRef]
- Schirhagl, R. Bioapplications for Molecularly Imprinted Polymers. Anal. Chem. 2014, 86, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xia, L.; Liang, R.; Lu, Z.; Li, L.; Huo, B.; Li, G.; Hu, Y. Advanced materials for sample preparation in recent decade. TrAC Trends Anal. Chem. 2019, 120, 115652. [Google Scholar] [CrossRef]
- Turiel, E.; Martín-Esteban, A. Molecularly imprinted polymers-based microextraction techniques. TrAC Trends Anal. Chem. 2019, 118, 574–586. [Google Scholar] [CrossRef]
- Wackerlig, J.; Lieberzeit, P.A. Molecularly imprinted polymer nanoparticles in chemical sensing–Synthesis, characterisation and application. Sens. Actuators B Chem. 2015, 207, 144–157. [Google Scholar] [CrossRef]
- Ahmad, O.S.; Bedwell, T.S.; Esen, C.; Garcia-Cruz, A.; Piletsky, S.A. Molecularly Imprinted Polymers in Electrochemical and Optical Sensors. Trends Biotechnol. 2019, 37, 294–309. [Google Scholar] [CrossRef]
- Rachkov, A.; McNiven, S.; El’skaya, A.; Yano, K.; Karube, I. Fluorescence detection of β-estradiol using a molecularly imprinted polymer. Anal. Chim. Acta 2000, 405, 23–29. [Google Scholar] [CrossRef]
- Chen, C.; Wang, X.; Zhang, Y.; Li, X.; Gao, H.; Waterhouse, G.I.N.; Qiao, X.; Xu, Z. A molecularly-imprinted SERS sensor based on a TiO2@Ag substrate for the selective capture and sensitive detection of tryptamine in foods. Food Chem. 2022, 394, 133536. [Google Scholar] [CrossRef]
- Chen, C.; Wang, X.; Waterhouse, G.I.N.; Qiao, X.; Xu, Z. A surface-imprinted surface-enhanced Raman scattering sensor for histamine detection based on dual semiconductors and Ag nanoparticles. Food Chem. 2022, 369, 130971. [Google Scholar] [CrossRef]
- Akgönüllü, S.; Armutcu, C.; Denizli, A. Molecularly imprinted polymer film based plasmonic sensors for detection of ochratoxin A in dried Figure. Polym. Bull. 2022, 79, 4049–4067. [Google Scholar] [CrossRef]
- Castro-Grijalba, A.; Montes-García, V.; Cordero-Ferradás, M.J.; Coronado, E.; Pérez-Juste, J.; Pastoriza-Santos, I. SERS-Based Molecularly Imprinted Plasmonic Sensor for Highly Sensitive PAH Detection. ACS Sens. 2020, 5, 693–702. [Google Scholar] [CrossRef]
- Huang, Q.-D.; Lv, C.-H.; Yuan, X.-L.; He, M.; Lai, J.-P.; Sun, H. A novel fluorescent optical fiber sensor for highly selective detection of antibiotic ciprofloxacin based on replaceable molecularly imprinted nanoparticles composite hydrogel detector. Sens. Actuators B Chem. 2021, 328, 129000. [Google Scholar] [CrossRef]
- Bakhshpour, M.; Göktürk, I.; Bereli, N.; Yılmaz, F.; Denizli, A. Selective Detection of Penicillin G Antibiotic in Milk by Molecularly Imprinted Polymer-Based Plasmonic SPR Sensor. Biomimetics 2021, 6, 72. [Google Scholar] [CrossRef]
- Decorbie, N.; Tijunelyte, I.; Gam-Derouich, S.; Solard, J.; Lamouri, A.; Decorse, P.; Felidj, N.; Gauchotte-Lindsay, C.; Rinnert, E.; Mangeney, C.; et al. Sensing Polymer/Paracetamol Interaction with an Independent Component Analysis-Based SERS-MIP Nanosensor. Plasmonics 2020, 15, 1533–1539. [Google Scholar] [CrossRef]
- Göktürk, I.; Bakhshpour, M.; Çimen, D.; Yılmaz, F.; Bereli, N.; Denizli, A. SPR Signal Enhancement with Silver Nanoparticle-Assisted Plasmonic Sensor for Selective Adenosine Detection. IEEE Sens. J. 2022, 22, 14862–14869. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, X.; Liu, J.; Wang, J.; Liu, J.; Gao, Y.; Ma, N. Chemiluminescence sensors based on molecularly imprinted polymers for the determination of organophosphorus in milk. J. Dairy Sci. 2022, 105, 3019–3031. [Google Scholar] [CrossRef]
- He, H.; Muhammad, P.; Guo, Z.; Peng, Q.; Lu, H.; Liu, Z. Controllably prepared molecularly imprinted core-shell plasmonic nanostructure for plasmon-enhanced fluorescence assay. Biosens. Bioelectron. 2019, 146, 111733. [Google Scholar] [CrossRef]
- Yang, Y.-Y.; Li, Y.-T.; Li, X.-J.; Zhang, L.; Kouadio Fodjo, E.; Han, S. Controllable in situ fabrication of portable AuNP/mussel-inspired polydopamine molecularly imprinted SERS substrate for selective enrichment and recognition of phthalate plasticizers. Chem. Eng. J. 2020, 402, 125179. [Google Scholar] [CrossRef]
- Gui, W.; Wang, H.; Liu, Y.; Ma, Q. Ratiometric fluorescent sensor with molecularly imprinted mesoporous microspheres for malachite green detection. Sens. Actuators B Chem. 2018, 266, 685–691. [Google Scholar] [CrossRef]
- Jia, B.J.; He, X.; Cui, P.L.; Liu, J.X.; Wang, J.P. Detection of chloramphenicol in meat with a chemiluminescence resonance energy transfer platform based on molecularly imprinted graphene. Anal. Chim. Acta 2019, 1063, 136–143. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Li, Y.; Zhang, J.; Qiao, Y.; Wang, Q.; Che, G. Fabrication of pollutant-resistance SERS imprinted sensors based on SiO2@TiO2@Ag composites for selective detection of pyrethroids in water. J. Phys. Chem. Solids 2020, 138, 109254. [Google Scholar] [CrossRef]
- Tian, H.; Li, Z.; Wang, C.; Xu, P.; Xu, S. Construction and Application of Molecularly Imprinted Fluorescence Sensor. Prog. Chem. 2022, 34, 593–608. [Google Scholar]
- Shu, J.; Tang, D. Current Advances in Quantum-Dots-Based Photoelectrochemical Immunoassays. Chem. Asian J. 2017, 12, 2780–2789. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, B.; Li, Y.; Liu, C.; Jiao, P.; Wei, Y. Molecularly Imprinted Magnetic Fluorescent Nanocomposite-Based Sensor for Selective Detection of Lysozyme. Nanomaterials 2021, 11, 1575. [Google Scholar] [CrossRef] [PubMed]
- Bahari, D.; Babamiri, B.; Salimi, A. Ultrasensitive molecularly imprinted fluorescence sensor for simultaneous determination of CA125 and CA15–3 in human serum and OVCAR-3 and MCF-7 cells lines using Cd and Ni nanoclusters as new emitters. Anal. Bioanal. Chem. 2021, 413, 4049–4061. [Google Scholar] [CrossRef]
- Lu, H.; Xu, S. Ultrasensitive turn on molecularly imprinted fluorescence sensor for glycoprotein detection based on nanoparticles signal amplification. Sens. Actuators B Chem. 2020, 306, 127566. [Google Scholar] [CrossRef]
- Liu, G.; Huang, X.; Li, L.; Xu, X.; Zhang, Y.; Lv, J.; Xu, D. Recent Advances and Perspectives of Molecularly Imprinted Polymer-Based Fluorescent Sensors in Food and Environment Analysis. Nanomaterials 2019, 9, 1030. [Google Scholar] [CrossRef] [Green Version]
- Homola, J.; Yee, S.S.; Gauglitz, G. Surface plasmon resonance sensors: Review. Sens. Actuators B Chem. 1999, 54, 3–15. [Google Scholar] [CrossRef]
- Wang, J.; Lin, W.; Cao, E.; Xu, X.; Liang, W.; Zhang, X. Surface Plasmon Resonance Sensors on Raman and Fluorescence Spectroscopy. Sensors 2017, 17, 2719. [Google Scholar] [CrossRef] [Green Version]
- Akgönüllü, S.; Yavuz, H.; Denizli, A. Development of Gold Nanoparticles Decorated Molecularly Imprinted-Based Plasmonic Sensor for the Detection of Aflatoxin M1 in Milk Samples. Chemosensors 2021, 9, 363. [Google Scholar] [CrossRef]
- Arcadio, F.; Seggio, M.; Del Prete, D.; Buonanno, G.; Mendes, J.; Coelho, L.C.C.; Jorge, P.A.S.; Zeni, L.; Bossi, A.M.; Cennamo, N. A Plasmonic Biosensor Based on Light-Diffusing Fibers Functionalized with Molecularly Imprinted Nanoparticles for Ultralow Sensing of Proteins. Nanomaterials 2022, 12, 1400. [Google Scholar] [CrossRef]
- Nivedha, S.; Babu, P.R.; Senthilnathan, K. Surface plasmon resonance physics and technology. Curr. Sci. 2018, 115, 56–63. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, W.; Liu, X.; Li, M.; Lang, X.; Singh, R.; Marques, C.; Zhang, B.; Kumar, S. Novel Optical Fiber-Based Structures for Plasmonics Sensors. Biosensors 2022, 12, 1016. [Google Scholar] [CrossRef]
- Cennamo, N.; Maniglio, D.; Tatti, R.; Zeni, L.; Bossi, A.M. Deformable molecularly imprinted nanogels permit sensitivity-gain in plasmonic sensing. Biosens. Bioelectron. 2020, 156, 112126. [Google Scholar] [CrossRef]
- Cennamo, N.; Arcadio, F.; Seggio, M.; Maniglio, D.; Zeni, L.; Bossi, A.M. Spoon-shaped polymer waveguides to excite multiple plasmonic phenomena: A multisensor based on antibody and molecularly imprinted nanoparticles to detect albumin concentrations over eight orders of magnitude. Biosens. Bioelectron. 2022, 217, 114707. [Google Scholar] [CrossRef]
- Arcadio, F.; Zeni, L.; Minardo, A.; Eramo, C.; Di Ronza, S.; Perri, C.; D’Agostino, G.; Chiaretti, G.; Porto, G.; Cennamo, N. A Nanoplasmonic-Based Biosensing Approach for Wide-Range and Highly Sensitive Detection of Chemicals. Nanomaterials 2021, 11, 1961. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S. Molecularly imprinted polymers-surface-enhanced Raman spectroscopy: State of the art and prospects. Int. J. Environ. Anal. Chem. 2022, 102, 1385–1415. [Google Scholar] [CrossRef]
- Raman, C.V.; Krishnan, K.S. A New Type of Secondary Radiation. Nature 1928, 121, 501–502. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, Y.; Hao, R.; Shi, Y.; You, H.; Nan, H.; Dai, Y.; Liu, D.; Lei, D.; Fang, J. Ultra-rapid and highly efficient enrichment of organic pollutants via magnetic mesoporous nanosponge for ultrasensitive nanosensors. Nat. Commun. 2021, 12, 6849. [Google Scholar] [CrossRef]
- Guo, X.; Li, J.; Arabi, M.; Wang, X.; Wang, Y.; Chen, L. Molecular-Imprinting-Based Surface-Enhanced Raman Scattering Sensors. ACS Sens. 2020, 5, 601–619. [Google Scholar] [CrossRef] [PubMed]
- Kantarovich, K.; Tsarfati, I.; Gheber, L.A.; Haupt, K.; Bar, I. Writing Droplets of Molecularly Imprinted Polymers by Nano Fountain Pen and Detecting Their Molecular Interactions by Surface-Enhanced Raman Scattering. Anal. Chem. 2009, 81, 5686–5690. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Gao, F.; Chen, Z.; Grant, E.; Kitts, D.D.; Wang, S.; Lu, X. Determination of α-Tocopherol in Vegetable Oils Using a Molecularly Imprinted Polymers–Surface-Enhanced Raman Spectroscopic Biosensor. J. Agric. Food Chem. 2013, 61, 10467–10475. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yan, R.; Liao, S.; Miao, Y.; Zhang, B.; Wang, F.; Yang, H. In situ reduced silver nanoparticles embedded molecularly imprinted reusable sensor for selective and sensitive SERS detection of Bisphenol A. Appl. Surf. Sci. 2018, 457, 323–331. [Google Scholar] [CrossRef]
- Sun, M.; Su, Y.; Lv, Y. Advances in chemiluminescence and electrogenerated chemiluminescence based on silicon nanomaterials. Luminescence 2020, 35, 978–988. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, H.; Zhao, S. Fabrication of chemiluminescence resonance energy transfer platform based on nanomaterial and its application in optical sensing, biological imaging and photodynamic therapy. TrAC Trends Anal. Chem. 2020, 122, 115747. [Google Scholar] [CrossRef]
- He, T.; Wang, G.N.; Liu, J.X.; Zhao, W.L.; Huang, J.J.; Xu, M.X.; Wang, J.P.; Liu, J. Dummy molecularly imprinted polymer based microplate chemiluminescence sensor for one-step detection of Sudan dyes in egg. Food Chem. 2019, 288, 347–353. [Google Scholar] [CrossRef]
- Zhang, R.-R.; Gan, X.-T.; Xu, J.-J.; Pan, Q.-F.; Liu, H.; Sun, A.-L.; Shi, X.-Z.; Zhang, Z.-M. Ultrasensitive electrochemiluminescence sensor based on perovskite quantum dots coated with molecularly imprinted polymer for prometryn determination. Food Chem. 2022, 370, 131353. [Google Scholar] [CrossRef]
- Zou, L.; Ding, R.; Li, X.; Miao, H.; Xu, J.; Pan, G. Typical Fluorescent Sensors Exploiting Molecularly Imprinted Hydrogels for Environmentally and Medicinally Important Analytes Detection. Gels 2021, 7, 67. [Google Scholar] [CrossRef]
- Akgönüllü, S.; Yavuz, H.; Denizli, A. SPR nanosensor based on molecularly imprinted polymer film with gold nanoparticles for sensitive detection of aflatoxin B1. Talanta 2020, 219, 121219. [Google Scholar] [CrossRef]
- Erdem, Ö.; Cihangir, N.; Saylan, Y.; Denizli, A. Comparison of molecularly imprinted plasmonic nanosensor performances for bacteriophage detection. New J. Chem. 2020, 44, 17654–17663. [Google Scholar] [CrossRef]
- Perçin, I.; Idil, N.; Bakhshpour, M.; Yılmaz, E.; Mattiasson, B.; Denizli, A. Microcontact Imprinted Plasmonic Nanosensors: Powerful Tools in the Detection of Salmonella paratyphi. Sensors 2017, 17, 1375. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Li, J.; Zeng, C.; Qu, Q.; Wang, M.; Qi, W.; Su, R.; He, Z. Sandwich-Like Sensor for the Highly Specific and Reproducible Detection of Rhodamine 6G on a Surface-Enhanced Raman Scattering Platform. ACS Appl. Mater. Interfaces 2020, 12, 4699–4706. [Google Scholar] [CrossRef]
- Cai, Y.; He, X.; Cui, P.L.; Liu, J.; Li, Z.B.; Jia, B.J.; Zhang, T.; Wang, J.P.; Yuan, W.Z. Preparation of a chemiluminescence sensor for multi-detection of benzimidazoles in meat based on molecularly imprinted polymer. Food Chem. 2019, 280, 103–109. [Google Scholar] [CrossRef]
- Babamiri, B.; Salimi, A.; Hallaj, R. A molecularly imprinted electrochemiluminescence sensor for ultrasensitive HIV-1 gene detection using EuS nanocrystals as luminophore. Biosens. Bioelectron. 2018, 117, 332–339. [Google Scholar] [CrossRef]
- Babamiri, B.; Salimi, A.; Hallaj, R.; Hasanzadeh, M. Nickel nanoclusters as a novel emitter for molecularly imprinted electrochemiluminescence based sensor toward nanomolar detection of creatinine. Biosens. Bioelectron. 2018, 107, 272–279. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, Y.; Sun, M.; Wang, T.; Liu, T.; Dai, X.; Zou, P.; Zhao, Y.; Wang, X.; Wang, Y.; et al. Deep learning-assisted smartphone-based molecularly imprinted electrochemiluminescence detection sensing platform: Protable device and visual monitoring furosemide. Biosens. Bioelectron. 2022, 209, 114262. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Chen, S.; Liu, Y.; Zhuang, J.; Lei, B. Synthesis of molecularly imprinted carbon dot grafted YVO4:Eu3+ for the ratiometric fluorescent determination of paranitrophenol. Biosens. Bioelectron. 2016, 86, 706–713. [Google Scholar] [CrossRef]
- Saylan, Y.; Denizli, A. Molecular Fingerprints of Hemoglobin on a Nanofilm Chip. Sensors 2018, 18, 3016. [Google Scholar] [CrossRef] [Green Version]
- Arabi, M.; Ostovan, A.; Zhang, Z.; Wang, Y.; Mei, R.; Fu, L.; Wang, X.; Ma, J.; Chen, L. Label-free SERS detection of Raman-Inactive protein biomarkers by Raman reporter indicator: Toward ultrasensitivity and universality. Biosens. Bioelectron. 2021, 174, 112825. [Google Scholar] [CrossRef]
- Feng, J.; Tao, Y.; Shen, X.; Jin, H.; Zhou, T.; Zhou, Y.; Hu, L.; Luo, D.; Mei, S.; Lee, Y.-I. Highly sensitive and selective fluorescent sensor for tetrabromobisphenol-A in electronic waste samples using molecularly imprinted polymer coated quantum dots. Microchem. J. 2019, 144, 93–101. [Google Scholar] [CrossRef]
- Xue, Y.; Shao, J.; Sui, G.; Ma, Y.; Li, H. Rapid detection of orange II dyes in water with SERS imprinted sensor based on PDA-modified MOFs@Ag. J. Environ. Chem. Eng. 2021, 9, 106317. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Ma, X.; Pang, C.; Yin, G.; Luo, J. Molecularly imprinted electroluminescence switch sensor with a dual recognition effect for determination of ultra-trace levels of cobalt (II). Biosens. Bioelectron. 2019, 139, 111321. [Google Scholar] [CrossRef]
- Hou, J.; Li, H.; Wang, L.; Zhang, P.; Zhou, T.; Ding, H.; Ding, L. Rapid microwave-assisted synthesis of molecularly imprinted polymers on carbon quantum dots for fluorescent sensing of tetracycline in milk. Talanta 2016, 146, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Ren, Y.; Li, G. Detection of naringin by fluorescent polarization molecularly imprinted polymer. Polym. Bull. 2022, 80, 1411–1424. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Cheng, R.; Sun, L.; Dai, X.; Yan, Y. Synthesis of molecularly imprinted dye-silica nanocomposites with high selectivity and sensitivity: Fluorescent imprinted sensor for rapid and efficient detection of τ-fluvalinate in vodka. J. Sep. Sci. 2018, 41, 1880–1887. [Google Scholar] [CrossRef]
- Li, M.; Shen, F.; Zhang, Z.; Ren, X. A Novel 2-Acrylamide-6-Methoxybenzothiazole Fabricated Molecularly Imprinted Polymers for Direct Fluorescent Sensing of Alachlor. Chromatographia 2016, 79, 71–78. [Google Scholar] [CrossRef]
- Lu, H.; Xu, S. Visualizing BPA by molecularly imprinted ratiometric fluorescence sensor based on dual emission nanoparticles. Biosens. Bioelectron. 2017, 92, 147–153. [Google Scholar] [CrossRef]
- Zeni, L.; Pesavento, M.; Marchetti, S.; Cennamo, N. [INVITED] Slab plasmonic platforms combined with Plastic Optical Fibers and Molecularly Imprinted Polymers for chemical sensing. Opt. Laser Technol. 2018, 107, 484–490. [Google Scholar] [CrossRef]
- Neng, J.; Xu, K.; Wang, Y.; Jia, K.; Zhang, Q.; Sun, P. Sensitive and Selective Detection of New Red Colorant Based on Surface-Enhanced Raman Spectroscopy Using Molecularly Imprinted Hydrogels. Appl. Sci. 2019, 9, 2672. [Google Scholar] [CrossRef] [Green Version]
- Mugo, S.M.; Lu, W. Determination of β-Estradiol by Surface-Enhance Raman Spectroscopy (SERS) Using a Surface Imprinted Methacrylate Polymer on Nanoporous Biogenic Silica. Anal. Lett. 2022, 55, 378–387. [Google Scholar] [CrossRef]
- Yin, W.; Wu, L.; Ding, F.; Li, Q.; Wang, P.; Li, J.; Lu, Z.; Han, H. Surface-imprinted SiO2@Ag nanoparticles for the selective detection of BPA using surface enhanced Raman scattering. Sens. Actuators B Chem. 2018, 258, 566–573. [Google Scholar] [CrossRef]
- Hua, M.Z.; Feng, S.; Wang, S.; Lu, X. Rapid detection and quantification of 2,4-dichlorophenoxyacetic acid in milk using molecularly imprinted polymers–surface-enhanced Raman spectroscopy. Food Chem. 2018, 258, 254–259. [Google Scholar] [CrossRef]
- Xu, Y.; Hassan, M.M.; Ali, S.; Li, H.; Chen, Q. SERS-based rapid detection of 2,4-dichlorophenoxyacetic acid in food matrices using molecularly imprinted magnetic polymers. Microchim. Acta 2020, 187, 454. [Google Scholar] [CrossRef]
- Jiang, Z.Q.; Cai Zhang, H.; Zhang, X.Y.; Ping Wang, J. Determination of Tetracyclines in Milk with a Molecularly Imprinted Polymer-Based Microtiter Chemiluminescence Sensor. Anal. Lett. 2019, 52, 1315–1327. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, R.; Liu, C.; Sun, A.; Chen, J.; Zhang, Z.; Shi, X. Highly Selective Electrochemiluminescence Sensor Based on Molecularly Imprinted-quantum Dots for the Sensitive Detection of Cyfluthrin. Sensors 2020, 20, 884. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.B.; Liu, J.; Liu, J.X.; Wang, Z.H.; Wang, J.P. Determination of sulfonamides in meat with dummy-template molecularly imprinted polymer-based chemiluminescence sensor. Anal. Bioanal. Chem. 2019, 411, 3179–3189. [Google Scholar] [CrossRef]
- Huang, J.J.; Liu, J.; Liu, J.X.; Wang, J.P. A microtitre chemiluminescence sensor for detection of pyrethroids based on dual-dummy-template molecularly imprinted polymer and computational simulation. Luminescence 2020, 35, 120–128. [Google Scholar] [CrossRef]
- Jia, B.-J.; Huang, J.; Liu, J.-X.; Wang, J.-P. Detection of chloramphenicol in chicken, pork and fish with a molecularly imprinted polymer-based microtiter chemiluminescence method. Food Addit. Contam. Part A 2019, 36, 74–83. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, P.; Li, N.; Qiao, X.; Xu, Z. Development of a chemiluminescence sensor based on molecular imprinting technology for the determination of trace monocrotophos in vegetables. Adv. Polym. Technol. 2018, 37, 1401–1409. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Liu, J.; Wang, J.P. Preparation of a molecularly imprinted polymer based chemiluminescence sensor for the determination of amantadine and rimantadine in meat. Anal. Methods 2018, 10, 5025–5031. [Google Scholar] [CrossRef]
- Wang, Q.; Li, S.; Li, J. A molecularly imprinted sensor with enzymatic enhancement of electrochemiluminescence of quantum dots for ultratrace clopyralid determination. Anal. Bioanal. Chem. 2018, 410, 5165–5172. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, Y.; Fang, M.; Tian, Y.; Bai, G.; Zhuo, K. Silanized carbon dot-based thermo-sensitive molecularly imprinted fluorescent sensor for bovine hemoglobin detection. Anal. Bioanal. Chem. 2020, 412, 5811–5817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, S.; Jiang, R.; Sun, L.; Pang, S.; Luo, A. Fluorescent molecularly imprinted membranes as biosensor for the detection of target protein. Sens. Actuators B Chem. 2018, 254, 1078–1086. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, H.; Guo, M. Preparation of ECB Molecularly Imprinted Film Fluorescent Sensor for Detection of Traditional Chinese Herbal Medicine. Chem. J. Chin. Univ. 2019, 40, 1381. [Google Scholar]
- Hariati, R.; Rezaei, B.; Jamei, H.R.; Ensafi, A.A. Manufacturing of a Sensitive and Selective Optical Sensor Based on Molecularly Imprinted Polymers and Green Carbon Dots Synthesized from Cedrus Plant for Trace Analysis of Propranolol. Anal. Sci. 2019, 35, 1083–1088. [Google Scholar] [CrossRef] [Green Version]
- Lv, P.; Xie, D.; Zhang, Z. Magnetic carbon dots based molecularly imprinted polymers for fluorescent detection of bovine hemoglobin. Talanta 2018, 188, 145–151. [Google Scholar] [CrossRef]
- Peng, L.; Xu, Y.; Huang, T.; Liu, X.; Yang, X.; Meng, M.; Yan, Y. Molecularly Imprinted Fluorescent Sensors Based on Nitrogen-Doped CDs for Highly Selective Detection of Aspirin. Nano 2020, 16, 2150019. [Google Scholar] [CrossRef]
- Hu, R.; Yan, Y.; Jiang, L.; Huang, C.; Shen, X. Determination of total cathinones with a single molecularly imprinted fluorescent sensor assisted by electromembrane microextraction. Microchim. Acta 2022, 189, 324. [Google Scholar] [CrossRef]
- Safran, V.; Göktürk, I.; Derazshamshir, A.; Yılmaz, F.; Sağlam, N.; Denizli, A. Rapid sensing of Cu+2 in water and biological samples by sensitive molecularly imprinted based plasmonic biosensor. Microchem. J. 2019, 148, 141–150. [Google Scholar] [CrossRef]
- Fan, L.; Wang, Z.; Zhang, Y.; Song, Y.; Yang, H.; Wang, F. Molecularly imprinted Monolithic column-based SERS sensor for selective detection of cortisol in dog saliva. Talanta 2022, 249, 123609. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.X.; Li Zhao, W.; Liu, J.; He, T.; Huang, J.J.; Ping Wang, J. Determination of β-Agonists in Porcine Urine by Molecularly Imprinted Polymer Based Chemiluminescence. Anal. Lett. 2019, 52, 1771–1787. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, S.; Liang, Y.; Wang, X.; Zhuang, X.; Tian, C.; Luan, F.; Chen, L. Selective detection of enrofloxacin in biological and environmental samples using a molecularly imprinted electrochemiluminescence sensor based on functionalized copper nanoclusters. Talanta 2022, 236, 122835. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Zhao, F.; Zeng, B. A novel ratiometric molecularly imprinted electrochemiluminescence sensor for sensitive and selective detection of sialic acid based on PEI-CdS quantum dots as anodic coreactant and cathodic luminophore. Sens. Actuators B Chem. 2020, 313, 128042. [Google Scholar] [CrossRef]
- Liu, G.; Chen, Z.; Jiang, X.; Feng, D.-Q.; Zhao, J.; Fan, D.; Wang, W. In-situ hydrothermal synthesis of molecularly imprinted polymers coated carbon dots for fluorescent detection of bisphenol A. Sens. Actuators B Chem. 2016, 228, 302–307. [Google Scholar] [CrossRef]
- Limaee, N.Y.; Rouhani, S.; Olya, M.E.; Najafi, F. Selective Recognition of Herbicides in Water Using a Fluorescent Molecularly Imprinted Polymer Sensor. J. Fluoresc. 2020, 30, 375–387. [Google Scholar] [CrossRef]
- Wang, M.; Gao, M.; Deng, L.; Kang, X.; Zhang, K.; Fu, Q.; Xia, Z.; Gao, D. A sensitive and selective fluorescent sensor for 2,4,6-trinitrophenol detection based on the composite material of magnetic covalent organic frameworks, molecularly imprinted polymers and carbon dots. Microchem. J. 2020, 154, 104590. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, H.; Shen, H.; Pan, J.; Dai, X.; Yan, Y.; Pan, G.; Sellergren, B. Molecularly imprinted fluorescent hollow nanoparticles as sensors for rapid and efficient detection λ-cyhalothrin in environmental water. Biosens. Bioelectron. 2016, 85, 387–394. [Google Scholar] [CrossRef]
- Aznar-Gadea, E.; Rodríguez-Canto, P.J.; Martínez-Pastor, J.P.; Lopatynskyi, A.; Chegel, V.; Abargues, R. Molecularly Imprinted Silver Nanocomposites for Explosive Taggant Sensing. ACS Appl. Polym. Mater. 2021, 3, 2960–2970. [Google Scholar] [CrossRef]
- Erdem, Ö.; Saylan, Y.; Cihangir, N.; Denizli, A. Molecularly imprinted nanoparticles based plasmonic sensors for real-time Enterococcus faecalis detection. Biosens. Bioelectron. 2019, 126, 608–614. [Google Scholar] [CrossRef]
- Kou, Y.; Wu, T.; Zheng, H.; Kadasala, N.R.; Yang, S.; Guo, C.; Chen, L.; Liu, Y.; Yang, J. Recyclable Magnetic MIP-Based SERS Sensors for Selective, Sensitive, and Reliable Detection of Paclobutrazol Residues in Complex Environments. ACS Sustain. Chem. Eng. 2020, 8, 14549–14556. [Google Scholar] [CrossRef]
- Zhang, R.-R.; Zhan, J.; Xu, J.-J.; Chai, J.-Y.; Zhang, Z.-M.; Sun, A.-L.; Chen, J.; Shi, X.-Z. Application of a novel electrochemiluminescence sensor based on magnetic glassy carbon electrode modified with molecularly imprinted polymers for sensitive monitoring of bisphenol A in seawater and fish samples. Sens. Actuators B Chem. 2020, 317, 128237. [Google Scholar] [CrossRef]

| Application | Sensor Type | Sensing System | Sample | Analytes | LOD | Ref. |
|---|---|---|---|---|---|---|
| Food analysis | Fluorescent | MIP hydrogel | corn juice | ZON | 1.6 µmol/L | [49] |
| CDs@MIPs | milk | tetracycline | 5.48 nmol/L | [64] | ||
| fluorescent MIP-Fe3O4@SiO2 | ketchup | naringin | 100 pmol/L | [65] | ||
| MIPs-dye@SiO2 | vodka | tau-fluvalinate | 13.251 nmol/L | [66] | ||
| F-MIP | corn seed | alachlor | 0.5 μmol/L | [67] | ||
| M-R-MIPs@D-NPs | water | bisphenol A | 29 nmol/L | [68] | ||
| SPR | MIP coated Au chip | dried fig | ochratoxin A | 0.028 ng/mL | [10] | |
| AgNPs based MIP chip | milk | penicillin G | 1.2 fmol/L | [13] | ||
| MIP coated Au chip | milk | aflatoxin M1 | 0.4 pg/mL | [30] | ||
| MIP coated Au chip | corn and peanuts | aflatoxin B1 | 1.04 pg/mL | [50] | ||
| MINPs-Au | water | T4 bacteriophages | 1.4 × 106 CFU/mL | [51] | ||
| AuNPs–MIP chip | apple juice | salmonella paratyphi | 0.49 ng/mL | [52] | ||
| MIP-Au-POF | drinking water | 2-FAL, MW = 96.4 | 0.03 mg/L | [69] | ||
| SERS | TiO2@Ag@MIP | spiked white vinegar | tryptamine | 4.85 × 10−7 mol/L | [8] | |
| ZnO@TiO2@Ag@MIP | vinegar and prawn | histamine | 3.088 × 10−9 mol/L | [9] | ||
| AuNP/PDA-MIP | water, wine | phthalate plasticizer | 1.0 × 10−10 mol/L | [18] | ||
| Au Array-MIP | orange juice | R6G | 10−10 mol/L | [53] | ||
| AuNPs-MIPs | sports drink | new red | 1.64 × 10−7 mol/L | [70] | ||
| MIP@BS (biogenic silica) | milk | β-estradiol | 0.073 ng/mL | [71] | ||
| MIP-SiO2@Ag | water | bisphenol A | 1.46 × 10−11 mol/L | [72] | ||
| MIPs-AgNPs | milk | 2,4-D | 0.008 mg/kg | [73] | ||
| Mag@MIP/Au | milk and tap water | 2,4-D | 0.00147 ng/mL | [74] | ||
| CL | 4IP-luminol-H2O2 | milk | organophosphorus | 0.001 ng/mL | [16] | |
| 4IP-luminol-H2O2 | meat | chloramphenicol | 2.0 pg/g | [20] | ||
| TCPO-IMZ-H2O2 | egg | Sudan dyes | 1.5 pg/mL | [47] | ||
| 4IP-luminol-H2O2 | meat | benzimidazoles | 1.5 pg/mL | [54] | ||
| TCPO-IMZ-H2O2 | milk | tetracyclines | 1 pg/mL | [75] | ||
| MWCNT/MIP-QD | fish and seawater | cyfluthrin | 0.05 µg/L | [76] | ||
| 4IP-luminol-H2O2 | meat | sulfonamides | 1.0 pg/mL | [77] | ||
| TCPO-IMZ-H2O2 | blank chicken | pyrethroids | 0.010 μg/kg | [78] | ||
| 4IP-luminol-H2O2 | chicken, pork and fish | chloramphenicol | - | [79] | ||
| luminol-H2O2 | spinach | monocrotophos | 0.001 mg/L | [80] | ||
| 4IP-luminol-H2O2 | chicken and pork | amantadine and rimantadine | 1.0 pg/mL | [81] | ||
| CdTe QDs, H2O2 | vegetable | clopyralid | 4.1 × 10−12 mol/L | [82] | ||
| Healthcare | Fluorescent | MNP/QD@MIPs | urine and egg white | lysozyme | 4.53 × 10−3 mol/L | [24] |
| FL-MMIPs | cell | CA 125 and CA 15-3 | 50 μU/mL | [25] | ||
| C-Y@MIPs | water and urine | 4-nitrophenol | 0.15 μmol/L | [58] | ||
| CD@SiO2@MIP | urine | bovine hemoglobin | 0.155 μmol/L | [83] | ||
| QDs embedded MIM | human serum and saliva | lysozyme | 10.2 nmol/L | [84] | ||
| FL-MIF | Euphorbia fischeriana Steud | Ebracteolata compound B | 0.1 mg/L | [85] | ||
| MIP-CDs | human blood | propranolol | - | [86] | ||
| M-CDs@MIPs | bovine urine | bovine hemoglobin | 17.3 nmol/L | [87] | ||
| N-CDs@SiO2@MIPs | human urine and saliva | Asp | 0.198 mg/L | [88] | ||
| AIE-MIPs | urine | cathinone | 0.3 μmol/L | [89] | ||
| SPR | SPR-LDF-nanoMIP | human serum | HTR | 4 fmol/L | [31] | |
| nanoMIP-Au | human blood | hemoglobin | 3.5 × 10−4 mg/mL | [59] | ||
| nanoMIP-Au | urine | copper(II) ion | - | [90] | ||
| SERS | AuNCs@MIP | tablet | paracetamol | 300 nmol/L | [14] | |
| AuNSs-MIP | biological fluid | trypsin enzyme | 4.1 × 10−3 μg/L | [60] | ||
| MIMC@Ag | dog saliva | cortisol | 10−7 mol/L | [91] | ||
| CL | EuS NCs, K2S2O8 | human serum | HIV-1 DNA | 0.3 fmol/L | [55] | |
| NiNCs-MIP@GO-Fe3O4, TPrA | human serum and urine | creatinine | 0.5 nmol/L | [56] | ||
| CdS QDs, Luc | human urine | furosemide | 4 nmol/L | [57] | ||
| TCPO-IMZ-H2O2 | porcine urine | beta-agonists | 0.3 pg/mL | [92] | ||
| MPA-Cu NCs | biological samples | enrofloxacin | 27 pmol/L | [93] | ||
| GO/TiO2-Ru(bpy)32+@PEI-CdS | human serum, bird’s nest | sialic acid | 0.017 nmol/L | [94] | ||
| Environmental safety | Fluorescent | MINs@PEGDA | environmental water | antibiotics | 6.86 μmol/L | [12] |
| QD@SiO2@mSiO2 | environmental water | malachite green | 17.0 nmol/L | [19] | ||
| MIP-QDs | electronic waste | TBBPA | 3.6 ng/g | [61] | ||
| MIP-CDs | river water | bisphenol A | 30 nmol/L | [95] | ||
| fluorescent MIP | environmental water | 2,4-D | 16.8 nmol/L | [96] | ||
| MCOFs@MIPs@CDs | environmental water | 2,4,6-trinitrophenol | 100 pmol/L | [97] | ||
| SiO2-APTES-FITC@MIPs | environmental water | lambda-cyhalothrin | 10.26 nmol/L | [98] | ||
| SPR | Ag-PEI MIP | air | 3-nitrotoluene | 1.37 ng/mL | [99] | |
| MIP-Au film | seawater | Enterococcus faecalis | 100 bacteria/mL | [100] | ||
| SERS | SiO2@TiO2@Ag@MIPs | river water | pyrethroid | 0.2 nmol/L | [21] | |
| Ag@MOF/PDA-MIPs | river water | orange II | 10-10 mol/L | [62] | ||
| Fe3O4@SiO2−Au@Ag | soil | paclobutrazol | 0.075 μg/g | [101] | ||
| CL | MIP/CsPbBr3-QDs | aquaculture products | prometryn | 5.0 pg/g | [48] | |
| MWCNT/Cu/CDs | water | Co2+ | 3.07 × 10−10 mol/L | [63] | ||
| MIP-Fe3O4-NCs | seawater and fish | bisphenol A | 2.0 × 10−4 μg/L | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Xia, L.; Li, G. Recent Progress of Molecularly Imprinted Optical Sensors. Chemosensors 2023, 11, 168. https://doi.org/10.3390/chemosensors11030168
Huang X, Xia L, Li G. Recent Progress of Molecularly Imprinted Optical Sensors. Chemosensors. 2023; 11(3):168. https://doi.org/10.3390/chemosensors11030168
Chicago/Turabian StyleHuang, Xianzhi, Ling Xia, and Gongke Li. 2023. "Recent Progress of Molecularly Imprinted Optical Sensors" Chemosensors 11, no. 3: 168. https://doi.org/10.3390/chemosensors11030168
APA StyleHuang, X., Xia, L., & Li, G. (2023). Recent Progress of Molecularly Imprinted Optical Sensors. Chemosensors, 11(3), 168. https://doi.org/10.3390/chemosensors11030168






