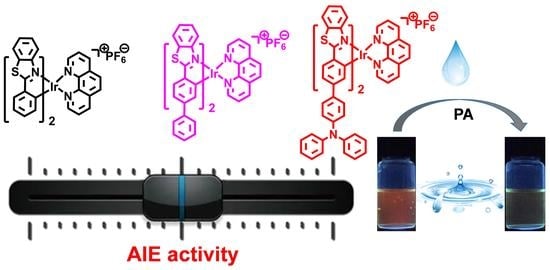
Aggregation-Induced Emission-Active Iridium(III) Complexes for Sensing Picric Acid in Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Measurements
2.2. Synthesis of the Ir(III) Complexes
2.3. Preparation of Stock Solutions for AIE and Detection of PA
3. Results and Discussion
3.1. Photophysical Properties
3.2. AIE Activities
3.3. Sensing of PA
3.4. Mechanism for Sensing PA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, X.; Han, Y.; Shu, Y.; Wang, J.; Qiu, H. Fabrication and application of 2,4,6-trinitrophenol sensors based on fluorescent functional materials. J. Hazard. Mater. 2022, 425, 127987. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Mukherjee, A.; Mahato, A.; Pramanik, A.; Dhak, D. A review on chemoselective reduction of nitroarenes for wastewater remediation using biochar supported metal catalysts: Kinetic and mechanistic studies. Chem. Afr. 2022. [Google Scholar] [CrossRef]
- Ghasemi, F.; Hormozi-Nezhad, M.R. Determination and identification of nitroaromatic explosives by a double-emitter sensor array. Talanta 2019, 201, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Ghorai, P.; Hazra, A.; Mandal, J.; Malik, S.; Brandão, P.; Banerjee, P.; Saha, A. Selective low-level detection of a perilous nitroaromatic compound using tailor-made Cd(II)-based coordination polymers: Study of photophysical properties and effect of functional groups. Inorg. Chem. 2023, 62, 98–113. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, T.; Guo, X.; Cui, Q.; Zhang, W.; Dong, W.; Fei, T. Conjugated polymer nanoparticles based on anthracene and tetraphenylethene for nitroaromatics detection in aqueous phase. Chemosensors 2022, 10, 366. [Google Scholar] [CrossRef]
- Zhu, C.; Huang, H.; Chen, Y. Recent advances in biological removal of nitroaromatics from wastewater. Environ. Pollut. 2022, 307, 119570. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, X.; Zhang, S.; Yang, C.; Ouyang, Z.; Zhang, X. Direct detection of explosives on solid surfaces by low temperature plasma desorption mass spectrometry. Analyst 2009, 134, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Arman, A.; Sağlam, Ş.; Üzer, A.; Apak, R. Electrochemical determination of nitroaromatic explosives using glassy carbon/multi walled carbon nanotube/polyethyleneimine electrode coated with gold nanoparticles. Talanta 2022, 238, 122990. [Google Scholar] [CrossRef]
- Larsson, A.; Angbrant, J.; Ekeroth, J.; Månsson, P.; Liedberg, B. A novel biochip technology for detection of explosives-TNT: Synthesis, characterisation and application. Sens. Actuators B 2006, 113, 730–748. [Google Scholar] [CrossRef]
- Muehlethaler, C.; Leona, M.; Lombardi, J.R. Review of surface enhanced Raman scattering applications in forensic science. Anal. Chem. 2016, 88, 152–169. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Y.; Lei, Y. Fluorescence based explosive detection: From mechanisms to sensory materials. Chem. Soc. Rev. 2015, 44, 8019–8061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Hang, H.; Li, H.; Chen, Y.; Tong, H.; Wang, L. Water-dispersible hyperbranched conjugated polymer nanoparticles with sulfonate terminal groups for amplified fluorescence sensing of trace TNT in aqueous solution. Mater. Chem. Front. 2017, 1, 1875–1880. [Google Scholar] [CrossRef]
- Barata, P.D.; Prata, J.V. Fluorescent calix[4]arene-carbazole-containing polymers as sensors for nitroaromatic explosives. Chemosensors 2020, 8, 128. [Google Scholar] [CrossRef]
- Dhiman, S.; Singla, N.; Ahmad, M.; Singh, P.; Kumar, S. Protonation-and electrostatic-interaction-based fluorescence probes for the selective detection of picric acid (2,4,6-trinitrophenol)—An explosive material. Mater. Adv. 2021, 2, 6466–6498. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Li, D.; Zhang, Y.; Yu, J.; Xu, R. AIE luminogen functionalized mesoporous silica nanoparticles as efficient fluorescent sensor for explosives detection in water. Microporous Mesoporous Mater. 2014, 196, 46–50. [Google Scholar] [CrossRef]
- Li, K.; Yu, R.-H.; Shi, C.-M.; Tao, F.-R.; Li, T.-D.; Cui, Y.-Z. Electrospun nanofibrous membrane based on AIE-active compound for detecting picric acid in aqueous solution. Sens. Actuators B 2018, 262, 637–645. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Y.; Liu, X.; Zhang, Q.; Kong, L.; Tian, Y.; Li, G.; Zhang, X.; Yang, J. AIE-active luminogen for highly sensitive and selective detection of picric acid in water samples: Pyridyl as an effective recognition group. Dyes Pigm. 2019, 163, 1–8. [Google Scholar] [CrossRef]
- Kajjam, A.B.; Didar, S.; Allen, M.J. AIE active triphenylamine-CF3 based α-cyanostilbenes for selective detection of picric acid in aqueous media and solid support. J. Photochem. Photobiol. A 2022, 431, 114036. [Google Scholar] [CrossRef]
- Zan, Y.; Kang, Y.; Wang, B.; Cui, S.; Shen, Z.; Shu, J.; Kong, X.; Chen, L.; Yan, X.; Li, Y. Amphiphilic fluorescent nanospheres for quantitative sensing of trinitrophenol in water system. Dyes Pigm. 2022, 202, 110296. [Google Scholar] [CrossRef]
- Chi, Y.; Chang, T.-K.; Ganesan, P.; Rajakannu, P. Emissive bis-tridentate Ir(III) metal complexes: Tactics, photophysics and applications. Coord. Chem. Rev. 2017, 346, 91–100. [Google Scholar] [CrossRef]
- Aoki, S.; Yokoi, K.; Hisamatsu, Y.; Balachandran, C.; Tamura, Y.; Tanaka, T. Post-complexation functionalization of cyclometalated iridium(III) complexes and applications to biomedical and material sciences. Top. Curr. Chem. 2022, 380, 36. [Google Scholar] [CrossRef] [PubMed]
- Schreier, M.R.; Guo, X.; Pfund, B.; Okamoto, Y.; Ward, T.R.; Kerzig, C.; Wenger, O.S. Water-soluble tris(cyclometalated) iridium(III) complexes for aqueous electron and energy transfer photochemistry. Acc. Chem. Res. 2022, 55, 1290–1300. [Google Scholar] [CrossRef] [PubMed]
- Ramdass, A.; Sathish, V.; Thanasekaran, P. AIE or AIE(P)E-active transition metal complexes for highly sensitive detection of nitroaromatic explosives. Results Chem. 2022, 4, 100337. [Google Scholar] [CrossRef]
- Xie, Z.; Sun, P.; Wang, Z.; Li, H.; Yu, L.; Sun, D.; Chen, M.; Bi, Y.; Xin, X.; Hao, J. Metal–Organic Gels from Silver Nanoclusters with Aggregation-Induced Emission and Fluorescence-to-Phosphorescence Switching. Angew. Chem. Int. Ed. 2020, 59, 9922–9927. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Together we shine, united we soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef]
- Sathiyan, G.; Balasubramaniam, B.; Ranjan, S.; Chatterjee, S.; Sen, P.; Garg, A.; Gupta, R.; Singh, A. A novel star-shaped triazine-triphenylamine–based fluorescent chemosensor for the selective detection of picric acid. Mater. Today Chem. 2019, 12, 178–186. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Tian, Y.; Tao, F.-R.; Yu, W.; You, K.-Y.; Zhou, L.-R.; Su, X.; Li, T.-D.; Cui, Y.-Z. Detection of nitroaromatics based on aggregation induced emission of barbituric acid derivatives. Spectrochim. Acta Part A 2019, 222, 117168. [Google Scholar] [CrossRef]
- Verbitskiy, E.V.; Kvashnin, Y.A.; Baranova, A.A.; Khokhlov, K.O.; Chuvashov, R.D.; Schapov, I.E.; Yakovleva, Y.A.; Zhilina, E.F.; Shchepochkin, A.V.; Makarova, N.I.; et al. Synthesis and characterization of linear 1,4-diazine-triphenylamine–based selective chemosensors for recognition of nitroaromatic compounds and aliphatic amines. Dyes Pigm. 2020, 178, 108344. [Google Scholar] [CrossRef]
- He, P.; Chen, Y.; Li, X.; Yan, Y.; Liu, C. AIPE-active cationic Ir(III) complexes for efficient detection of 2,4,6-trinitrophenol and oxygen. Dalton Trans. 2023, 52, 128–135. [Google Scholar] [CrossRef]
- Rao, X.; Liu, C.; Qiu, J.; Jin, Z. A highly efficient and aerobic protocol for the synthesis of N-heteroaryl substituted 9-arylcarbazolyl derivatives via a palladium-catalyzed ligand-free Suzuki reaction. Org. Biomol. Chem. 2012, 10, 7875–7883. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Gaussian, Inc.: Wallingford, CT, USA, 2016; Available online: https://gaussian.com/gaussian16 (accessed on 5 February 2023).
- Jang, J.-H.; Park, H.J.; Park, J.Y.; Kim, H.U.; Hwang, D.-H. Orange phosphorescent Ir(III) complexes consisting of substituted 2-phenylbenzothiazole for solution-processed organic light-emitting diodes. Org. Electron. 2018, 60, 31–37. [Google Scholar] [CrossRef]
- Lamansky, S.; Djurovich, P.; Murphy, D.; Abdel-Razzaq, F.; Lee, H.-E.; Adachi, C.; Burrows, P.E.; Forrest, S.R.; Thompson, M.E. Highly phosphorescent bis-cyclometalated iridium complexes: synthesis, photophysical characterization, and use in organic light emitting diodes. J. Am. Chem. Soc. 2001, 123, 4304–4312. [Google Scholar] [CrossRef]
- Yu, H.; Liu, C.; Yu, Z.; Zhang, L.; Xiu, J. Effect of ancillary ligands on the properties of diphenylphosphoryl-substituted cationic Ir(III) complexes. J. Mater. Chem. C 2017, 5, 3519–3527. [Google Scholar] [CrossRef]
- Das, P.; Mandal, S.K. Understanding the effect of an amino group on the selective and ultrafast detection of TNP in water using fluorescent organic probes. J. Mater. Chem. C 2018, 6, 3288–3297. [Google Scholar] [CrossRef]
- Hao, H.; Xu, C.; Luo, H.; Yang, J.; Liu, C.; Xu, B.; Shi, G.; Xing, X.; Chi, Z. An AIE luminogen-based electropolymerized film: An ultrasensitive fluorescent probe for TNP and Fe3+ in water. Mater. Chem. Front. 2021, 5, 492–499. [Google Scholar] [CrossRef]
- Cui, Y.; Wen, L.-L.; Shan, G.-G.; Sun, H.-Z.; Mao, H.-T.; Zhang, M.; Su, Z.-M. Di-/trinuclear cationic Ir(III) complexes: Design, synthesis and application for highly sensitive and selective detection of TNP in aqueous solution. Sens. Actuators B 2017, 244, 314–322. [Google Scholar] [CrossRef]
- Yi, S.; Lu, Z.; Xie, Z.; Hou, L. Amphiphilic gemini-iridium (III) complex for rapid and selective detection of picric acid in water and intracellular. Talanta 2020, 208, 120372. [Google Scholar] [CrossRef]
- Alam, P.; Kaur, G.; Kachwal, V.; Gupta, A.; Roy Choudhury, A.; Laskar, I.R. Highly sensitive explosive sensing by “aggregation induced phosphorescence” active cyclometalated iridium(III) complexes. J. Mater. Chem. C 2015, 3, 5450–5456. [Google Scholar] [CrossRef]
- Wen, L.-L.; Hou, X.-G.; Shan, G.-G.; Song, W.-L.; Zhang, S.-R.; Sun, H.-Z.; Su, Z.-M. Rational molecular design of aggregation-induced emission cationic Ir(III) phosphors achieving supersensitive and selective detection of nitroaromatic explosives. J. Mater. Chem. C 2017, 5, 10847–10854. [Google Scholar] [CrossRef]
- Hou, X.-G.; Wu, Y.; Cao, H.-T.; Sun, H.-Z.; Li, H.-B.; Shan, G.-G.; Su, Z.-M. A cationic iridium(III) complex with aggregation-induced emission (AIE) properties for highly selective detection of explosives. Chem. Commun. 2014, 50, 6031–6034. [Google Scholar] [CrossRef] [PubMed]
- Che, W.; Li, G.; Liu, X.; Shao, K.; Zhu, D.; Su, Z.; Bryce, M.R. Selective sensing of 2,4,6-trinitrophenol (TNP) in aqueous media with “aggregation-induced emission enhancement” (AIEE)-active iridium(III) complexes. Chem. Commun. 2018, 4, 1730–1733. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Complex | λabsa (nm) | λemb (nm) | ΦPLc | τ d (μs) | kre (106 s−1) | knre (106 s−1) |
|---|---|---|---|---|---|---|
| Ir1 | 274 (5.17) 315 (3.21) 322 (3.34) 410 (0.81) | 522, 559 | 0.55 | 3.73 | 0.148 | 0.120 |
| Ir2 | 273 (3.61) 336 (4.15) 423 (0.88) | 556, 595 | 0.20 | 4.81 | 0.042 | 0.166 |
| Ir3 | 275 (3.91) 297 (4.00) 423 (3.55) | 596 | 0.05 | 10.36 | 0.005 | 0.092 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, P.; Chen, Y.; Li, X.-N.; Yan, Y.-Y.; Liu, C. Aggregation-Induced Emission-Active Iridium(III) Complexes for Sensing Picric Acid in Water. Chemosensors 2023, 11, 177. https://doi.org/10.3390/chemosensors11030177
He P, Chen Y, Li X-N, Yan Y-Y, Liu C. Aggregation-Induced Emission-Active Iridium(III) Complexes for Sensing Picric Acid in Water. Chemosensors. 2023; 11(3):177. https://doi.org/10.3390/chemosensors11030177
Chicago/Turabian StyleHe, Ping, Yan Chen, Xiao-Na Li, Ying-Ying Yan, and Chun Liu. 2023. "Aggregation-Induced Emission-Active Iridium(III) Complexes for Sensing Picric Acid in Water" Chemosensors 11, no. 3: 177. https://doi.org/10.3390/chemosensors11030177
APA StyleHe, P., Chen, Y., Li, X.-N., Yan, Y.-Y., & Liu, C. (2023). Aggregation-Induced Emission-Active Iridium(III) Complexes for Sensing Picric Acid in Water. Chemosensors, 11(3), 177. https://doi.org/10.3390/chemosensors11030177