A Facile Surface-Imprinting Strategy for Trypsin-Imprinted Polymeric Chemosensors Using Two-Step Spin-Coating
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Molecularly Imprinted Films
2.3. Characteristics
3. Results
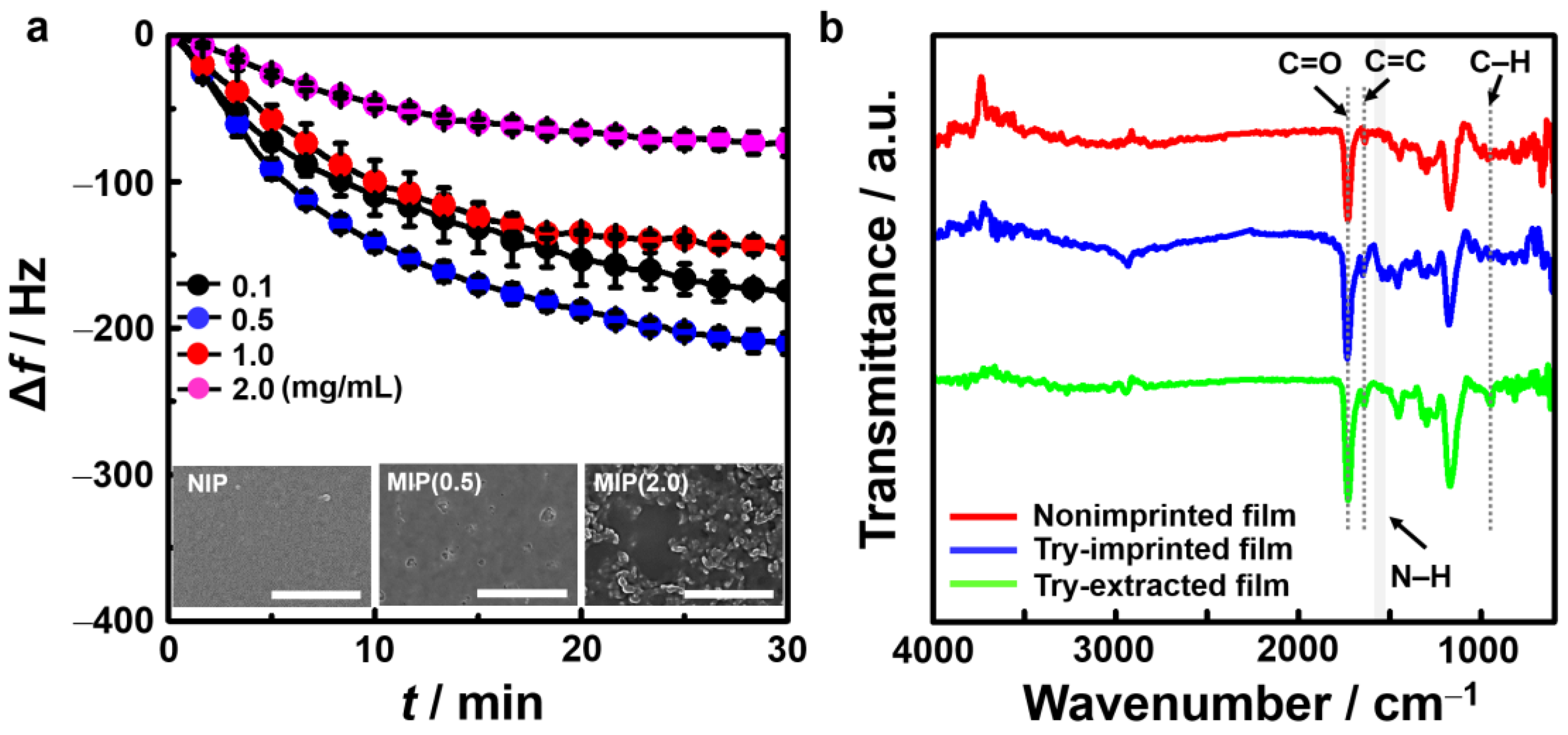
3.1. Optimized MIP Films
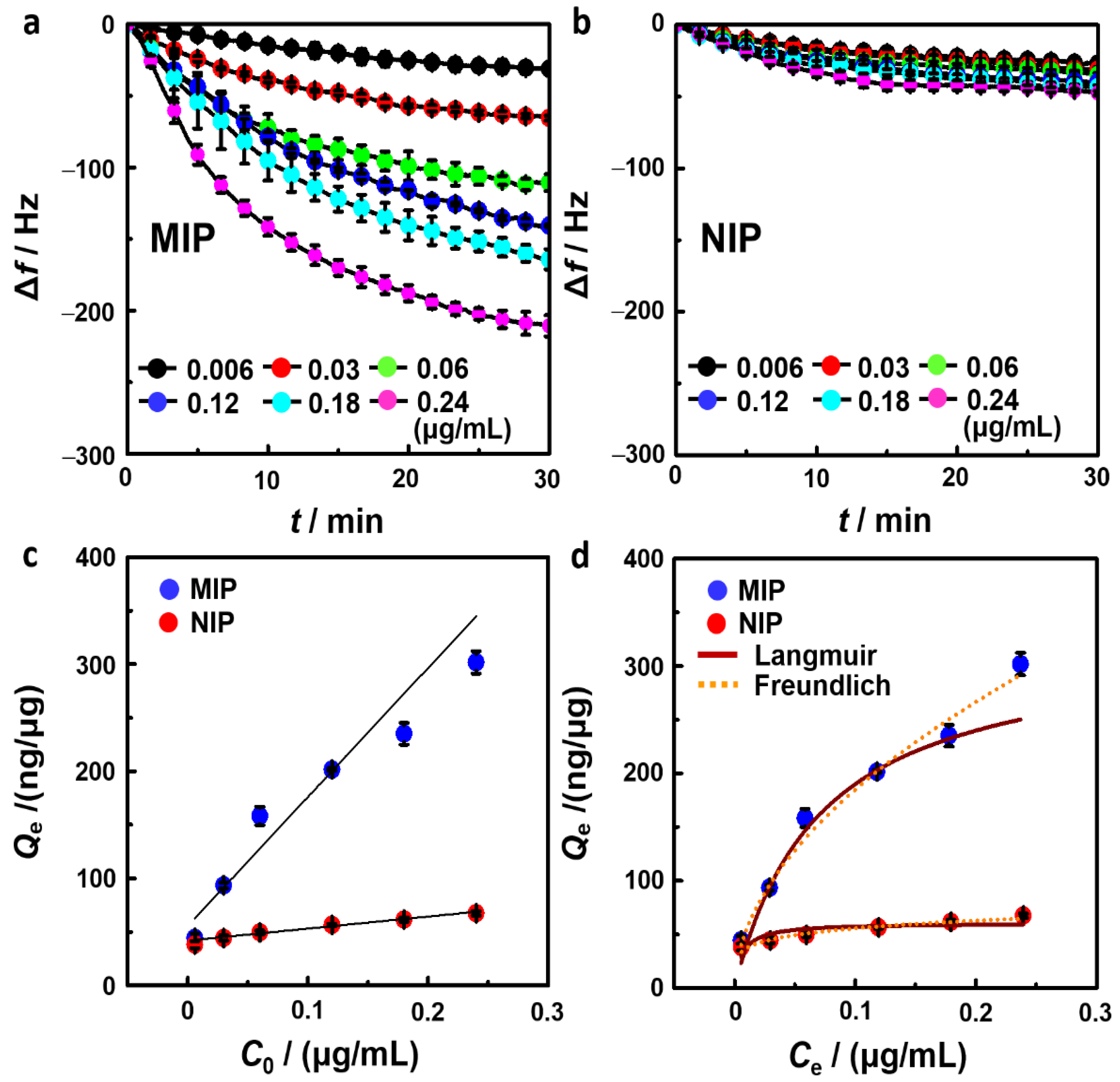
3.2. Sensing Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mótyán, J.A.; Tóth, F.; Tőzsér, J. Research applications of proteolytic enzymes in molecular biology. Biomolecules 2013, 3, 923–942. [Google Scholar] [CrossRef]
- Shah, D.; Mital, K. The role of trypsin: Chymotrypsin in tissue repair. Adv. Ther. 2018, 35, 31–42. [Google Scholar] [CrossRef]
- Logsdon, C. Phosphatidylinositol 3-kinase and trypsin activation in pancreatitis. J. Clin. Investig. 2001, 108, 1267–1268. [Google Scholar] [CrossRef]
- Ko, J.; Cho, J.; Petrov, M.S. Low serum amylase, lipase, and trypsin as biomarkers of metabolic disorders: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2020, 159, 107974. [Google Scholar] [CrossRef]
- Yi, Q.; Liu, Q.; Gao, F.; Chen, Q.; Wang, G. Application of an electrochemical immunosensor with a MWCNT/PDAA modified electrode for detection of serum trypsin. Sensors 2014, 14, 10203–10212. [Google Scholar] [CrossRef]
- Weiss, F.U. Pancreatic cancer risk in hereditary pancreatitis. Front. Physiol. 2014, 5, 70. [Google Scholar] [CrossRef]
- Fonseca, V.; Epstein, O.; Katrak, A.; Junglee, D.; Mikhailidis, D.P.; McIntyre, N.; Dandona, P. Serum immunoreactive trypsin and pancreatic lipase in primary biliary cirrhosis. J. Clin. Pathol. 1986, 39, 638–640. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Li, Q.; Xia, L.; Li, X.; Sun, H.; Kong, R.M.; Qu, F. Photoelectrochemical determination of trypsin by using an indium tin oxide electrode modified with a composite prepared from MoS2 nanosheets and TiO2 nanorods. Microchim. Acta 2019, 186, 490. [Google Scholar] [CrossRef]
- Zhang, L.; Du, J. A sensitive and label-free trypsin colorimetric sensor with cytochrome c as a substrate. Biosens. Bioelectron. 2016, 79, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Karaseva, N.A.; Pluhar, B.; Beliaeva, E.A.; Ermolaeva, T.N.; Mizaikoff, B. Synthesis and application of molecularly imprinted polymers for trypsin piezoelectric sensors. Sens. Actuators B Chem. 2019, 280, 272–279. [Google Scholar] [CrossRef]
- Braatz, J.A.; Elias, C.; Finny, J.G.; Tran, H.; McCaman, M. Quantitation of residual trypsin in cell-based therapeutics using immobilized α-1-antitrypsin or SBTI in an ELISA format. J. Immunol. Methods 2015, 417, 131–133. [Google Scholar] [CrossRef]
- BelBruno, J.J. Molecularly imprinted polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef]
- Bossi, A.; Bonini, F.; Turner, A.P.F.; Piletsky, S.A. Molecularly imprinted polymers for the recognition of proteins: The state of the art. Biosens. Bioelectron. 2007, 22, 1131–1137. [Google Scholar] [CrossRef]
- Bognár, Z.; Supala, E.; Yarman, A.; Zhang, X.; Bier, F.F.; Scheller, F.W.; Gyurcsányi, R.E. Peptide epitope-imprinted polymer microarrays for selective protein recognition. Application for SARS-CoV-2 RBD protein. Chem. Sci. 2022, 13, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Bartold, K.; Iskierko, Z.; Borowicz, P.; Noworyta, K.; Lin, C.Y.; Kalecki, J.; Sharma, P.S.; Lin, H.Y.; Kutner, W. Molecularly imprinted polymer-based extended-gate field-effect transistor (EG-FET) chemosensor for selective determination of matrix metalloproteinase-1 (MMP-1) protein. Biosens. Bioelectron. 2022, 208, 114203. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Sun, Y.; Zhang, L.; Ding, Q.; Chen, W.; Yu, H.; Liu, Q.; Fu, M. Surface imprinted core-shell nanorod for selective extraction of glycoprotein. J. Colloid Interface Sci. 2022, 615, 597–605. [Google Scholar] [CrossRef]
- Luo, Y.; Bai, C.C.; Liu, M.X.; Wang, D.; Chen, M.Y.; Yu, S.S.; Bu, X.Y.; Wang, X.H. PEGylation of boronate-affinity-oriented surface imprinting magnetic nanoparticles with improved performance. Talanta 2022, 238, 122992. [Google Scholar] [CrossRef]
- Liu, Z.; Yin, Z.Z.; Cai, W.R.; Wu, D.; Li, J.; Kong, Y. A surface protein-imprinted biosensor based on boronate affinity for the detection of anti-human immunoglobulin G. Microchim. Acta 2022, 189, 106. [Google Scholar] [CrossRef]
- Kanubaddi, K.R.; Huang, P.Y.; Chang, Y.L.; Wu, C.H.; Li, W.; Kankala, R.K.; Tai, D.F.; Lee, C.H. Deviation of Trypsin Activity Using Peptide Conformational Imprints. Nanomaterials 2021, 11, 334. [Google Scholar] [CrossRef]
- Hayden, O.; Haderspöck, C.; Krassnig, S.; Chen, X.; Dickert, F.L. Surface imprinting strategies for the detection of Trypsin. Analyst 2006, 131, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.Y.; Yang, J.C.; Park, J. Optimization and characterization of electrochemical protein imprinting on hemispherical porous gold patterns for the detection of trypsin. Sens. Actuators B Chem. 2022, 350, 130855. [Google Scholar] [CrossRef]
- Kalecki, J.; Iskierko, Z.; Cieplak, M.; Sharma, P.S. Oriented immobilization of protein templates: A new trend in surface imprinting. ACS Sens. 2020, 5, 3710–3720. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.J.; Tong, Y.W. Molecularly imprinted beads by surface imprinting. Anal. Bioanal. Chem. 2007, 389, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.H.; Mosbach, K.; Haupt, K. A simple method for spin-coating molecularly imprinted polymer films of controlled thickness and porosity. Adv. Mater. 2004, 16, 719–722. [Google Scholar] [CrossRef]
- Toolan, D.T.W.; Howse, J.R. Development of in situ studies of spin coated polymer films. J. Mater. Chem. C 2013, 1, 603–616. [Google Scholar] [CrossRef]
- Hawkins, D.M.; Stevenson, D.; Reddy, S.M. Investigation of protein imprinting in hydrogel-based molecularly imprinted polymers (HydroMIPs). Anal. Chim. Acta 2005, 542, 61–65. [Google Scholar] [CrossRef]
- Lowdon, J.W.; Diliën, H.; Singla, P.; Peeters, M.; Cleij, T.J.; van Grinsven, V.; Eersels, K. MIPs for commercial application in low-cost sensors and assays—An overview of the current status quo. Sens. Actuators B Chem. 2020, 325, 128973. [Google Scholar] [CrossRef]
- Akgönüllü, S.; Kılıç, S.; Esen, C.; Denizli, A. Molecularly imprinted polymer-based sensors for protein detection. Polymers 2023, 15, 629. [Google Scholar] [CrossRef]
- Bunina, Z.Y.; Bryleva, K.; Yurchenko, O.; Belikov, K. Sorption materials based on ethylene glycol dimethacrylate and methacrylic acid copolymers for rare earth elements extraction from aqueous solutions. Adsorp. Sci. Technol. 2017, 35, 545–559. [Google Scholar] [CrossRef]
- Qin, D.; Wang, L.; Wang, Y.; Du, X.; Zhang, L.; Zhang, Q.; He, B. Shape-controlled synthesis of protein-conjugated CdS nanocrystals (NCs) and study on the binding of Cd2+/CdS to trypsin. J. Nanopart. Res. 2017, 19, 252. [Google Scholar] [CrossRef]
- Gryshchenko, A.O.; Bottaro, C.S. Development of molecularly imprinted polymer in porous film format for binding of phenol and alkylphenols from water. Int. J. Mol. Sci. 2014, 15, 1338–1357. [Google Scholar] [CrossRef]
- Poole, S.; West, S.I.; Walters, C.L. Protein–protein interactions: Their importance in the foaming of heterogeneous protein systems. J. Sci. Food Agric. 1984, 35, 701–711. [Google Scholar] [CrossRef]
- Tyona, M.D. A theoritical study on spin coating technique. Adv. Mater. Res. 2013, 2, 195–208. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Zhang, Q.D.; Liu, G.O.; Zhao, R. A continuous-flow mass biosensor for the real-time dynamic analysis of protease inhibition. Chem. Commun. 2015, 51, 6601–6604. [Google Scholar] [CrossRef]
- Dong, Z.M.; Cheng, L.; Zhang, P.; Zhao, G.C. Label-free analytical performances of a peptide-based QCM biosensor for trypsin. Analyst 2020, 145, 3329–3338. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, Y.; Jauregui-Haza, U.; Guo, Z.; Campos, L.C. Investigation of metaldehyde removal by powdered activated carbon from different water samples. Environ. Sci. Water Res. Technol. 2020, 6, 1432–1444. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Deng, Z.; Chen, W.; Li, T.; Zhang, Y.; Zhang, Z.; He, Y.; Tan, Z.; Zhong, S. PEGylated thermo-sensitive bionic magnetic core-shell structure molecularly imprinted polymers based on halloysite nanotubes for specific adsorption and separation of bovine serum albumin. Polymers 2020, 12, 536. [Google Scholar] [CrossRef]


| Sensor Configuration | Preparation Method | Concentration Range (μg/mL) | LOD (ng/mL) | IF | Reference |
|---|---|---|---|---|---|
| Poly(MAA-co-EGDMA) MIP particles | Mini emulsion polymerization | 0.125–2 | 70 | <3.5 | [10] |
| Poly(AA-co-EGDMA) MIP film | Thermal polymerization | 10–100 | 100 | – | [20] |
| Poly(o-PD) MIP film | Electropolymerization | 0.24–48 | 70.9 | 3.51 | [21] |
| TI-Cys-GA | Self-assembly | 25–125 | 3800 | – | [34] |
| Au NP-MCA-Peptide | Self-assembly | 0–0.75 | 8.6 | – | [35] |
| Poly(MAA-co-EGDMA) MIP film | Photopolymerization and spin-coating | 0.006–0.24 | 25.33 | <4.5 | This work |
| Sensors | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| KL (mL/μg) | Qm (ng/μg) | R2 | KF | 1/n | R2 | |
| MIP | 14.01 | 325.42 | 0.976 | 623.51 | 0.528 | 0.995 |
| NIP | 163.51 | 60.97 | 0.494 | 83.22 | 0.174 | 0.942 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byeon, J.W.; Yang, J.C.; Cho, C.H.; Lim, S.J.; Park, J.P.; Park, J. A Facile Surface-Imprinting Strategy for Trypsin-Imprinted Polymeric Chemosensors Using Two-Step Spin-Coating. Chemosensors 2023, 11, 189. https://doi.org/10.3390/chemosensors11030189
Byeon JW, Yang JC, Cho CH, Lim SJ, Park JP, Park J. A Facile Surface-Imprinting Strategy for Trypsin-Imprinted Polymeric Chemosensors Using Two-Step Spin-Coating. Chemosensors. 2023; 11(3):189. https://doi.org/10.3390/chemosensors11030189
Chicago/Turabian StyleByeon, Je Wook, Jin Chul Yang, Chae Hwan Cho, Seok Jin Lim, Jong Pil Park, and Jinyoung Park. 2023. "A Facile Surface-Imprinting Strategy for Trypsin-Imprinted Polymeric Chemosensors Using Two-Step Spin-Coating" Chemosensors 11, no. 3: 189. https://doi.org/10.3390/chemosensors11030189
APA StyleByeon, J. W., Yang, J. C., Cho, C. H., Lim, S. J., Park, J. P., & Park, J. (2023). A Facile Surface-Imprinting Strategy for Trypsin-Imprinted Polymeric Chemosensors Using Two-Step Spin-Coating. Chemosensors, 11(3), 189. https://doi.org/10.3390/chemosensors11030189








