Surface Plasmon Electrochemistry: Tutorial and Review
Abstract
:1. Introduction
2. Surface Plasmons
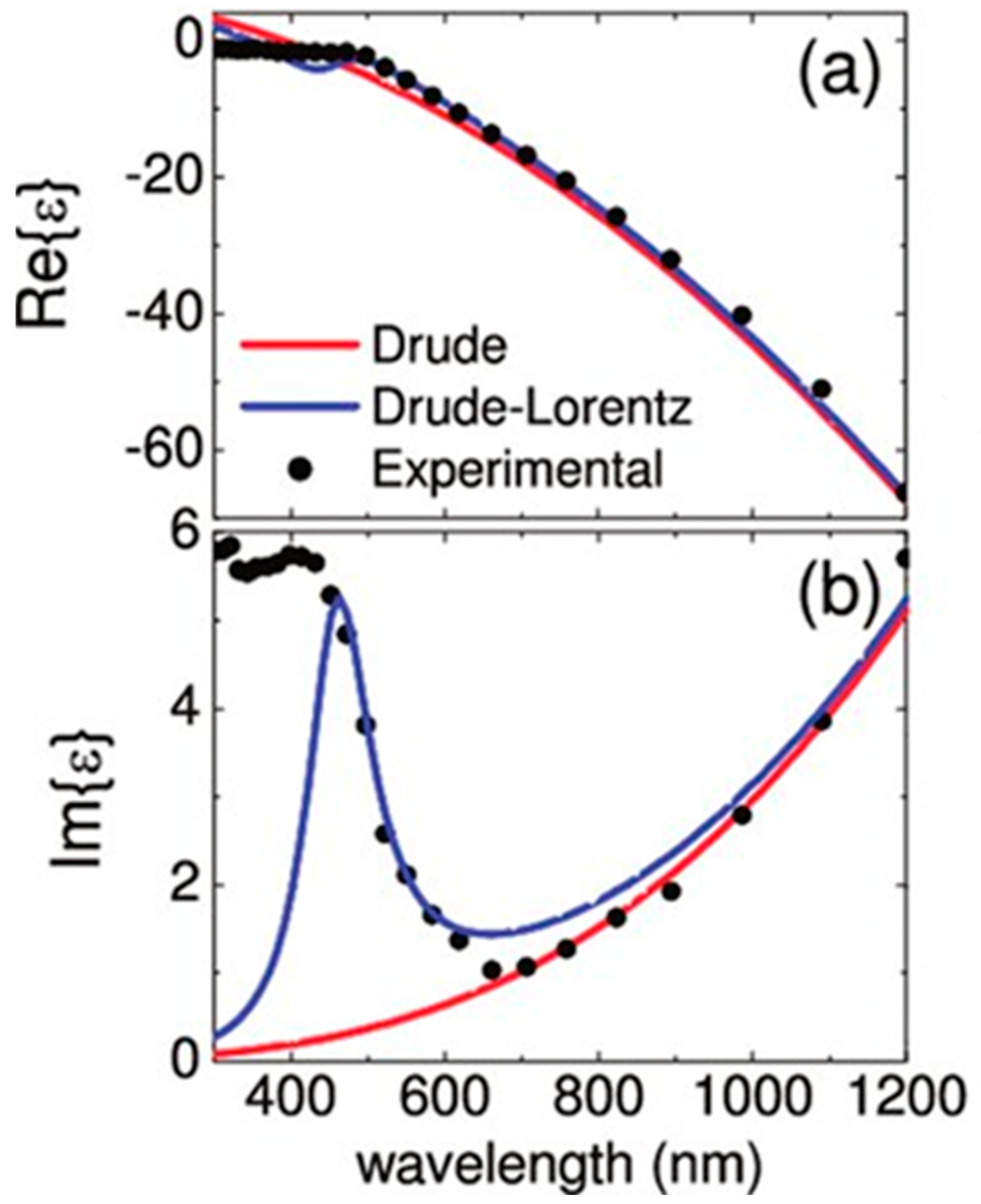
2.1. Optics of Metals
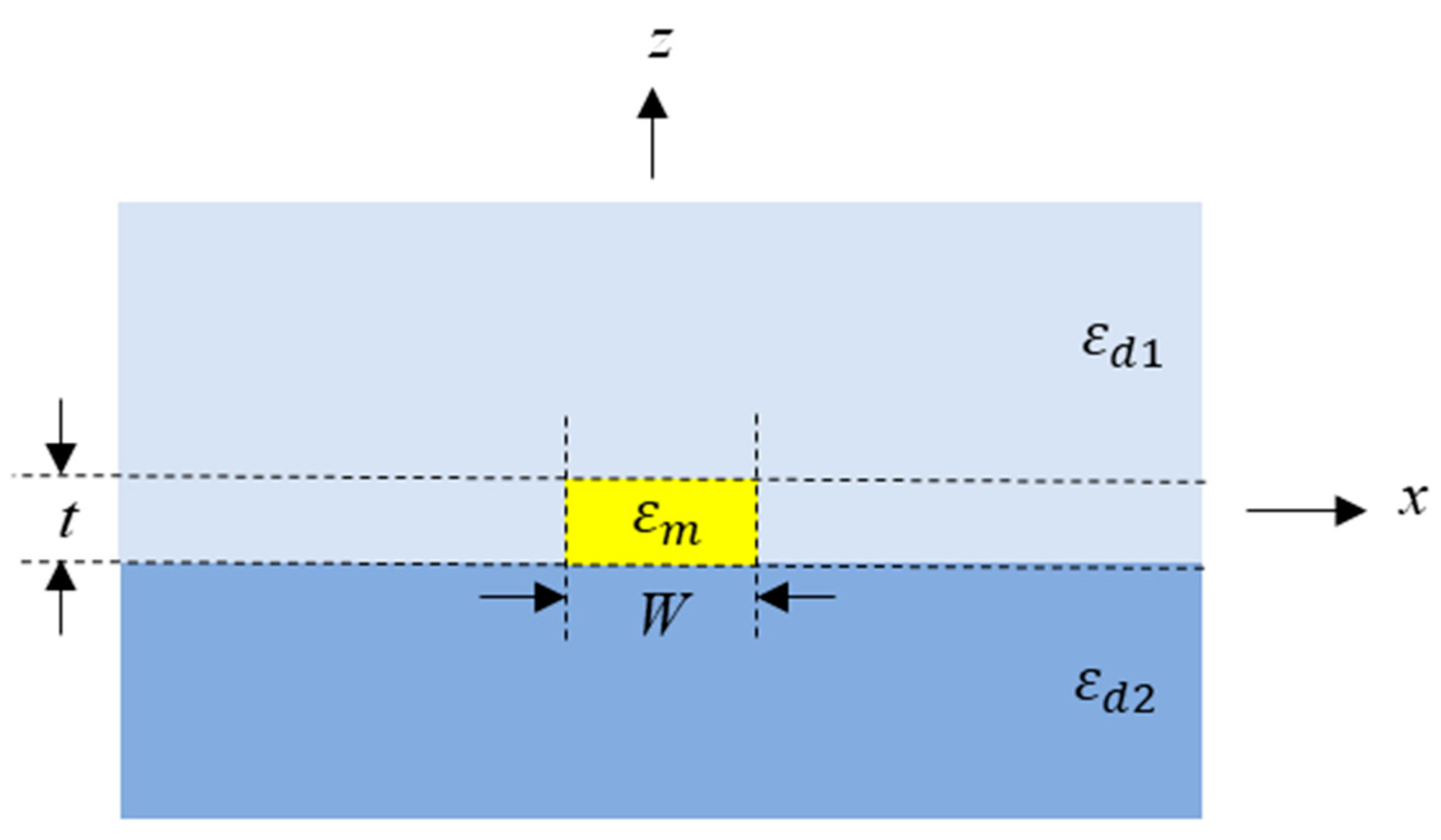
2.2. Surface Plasmon Polaritons (SPPs)
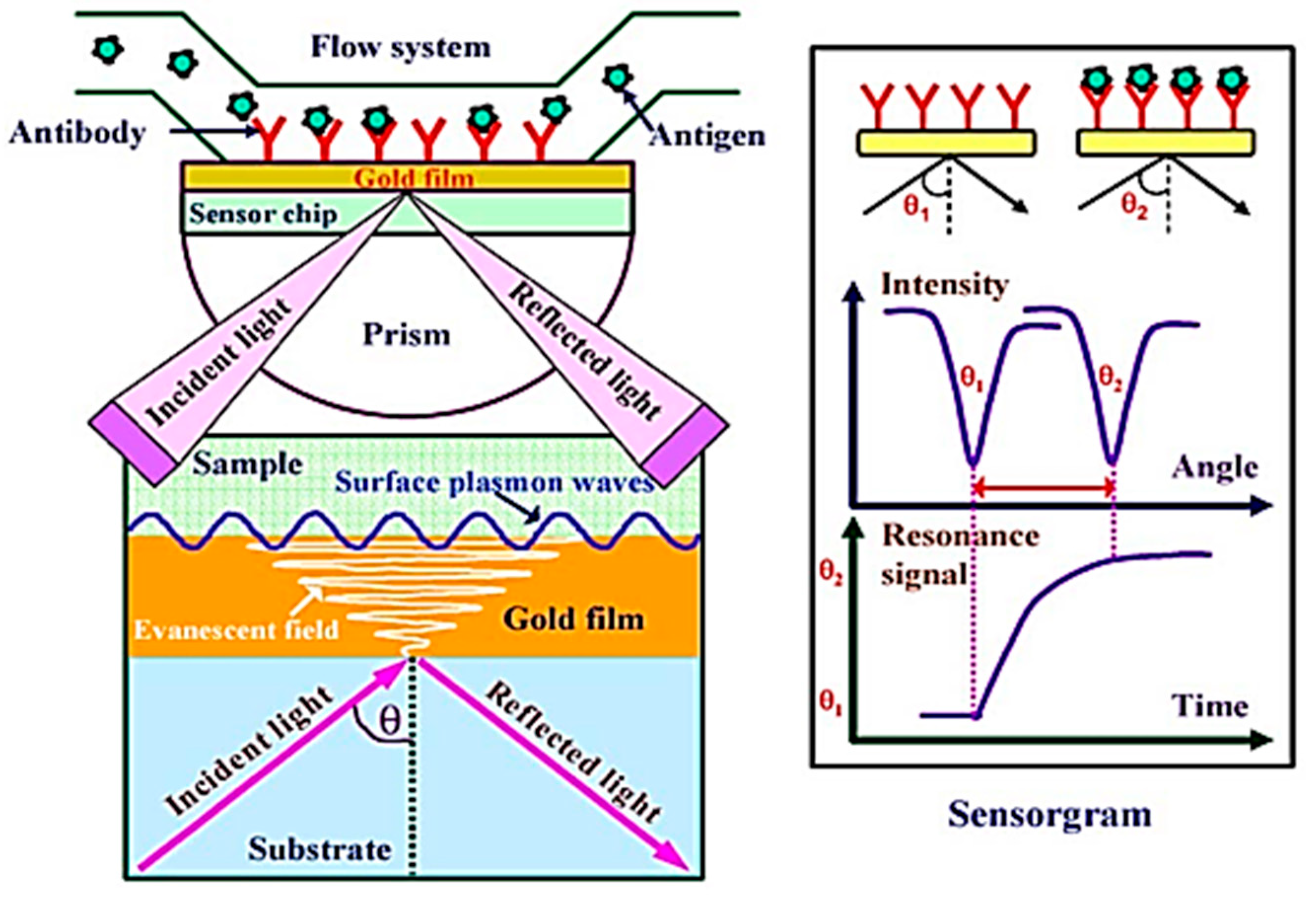
2.3. Excitation of Surface Plasmon Polaritons
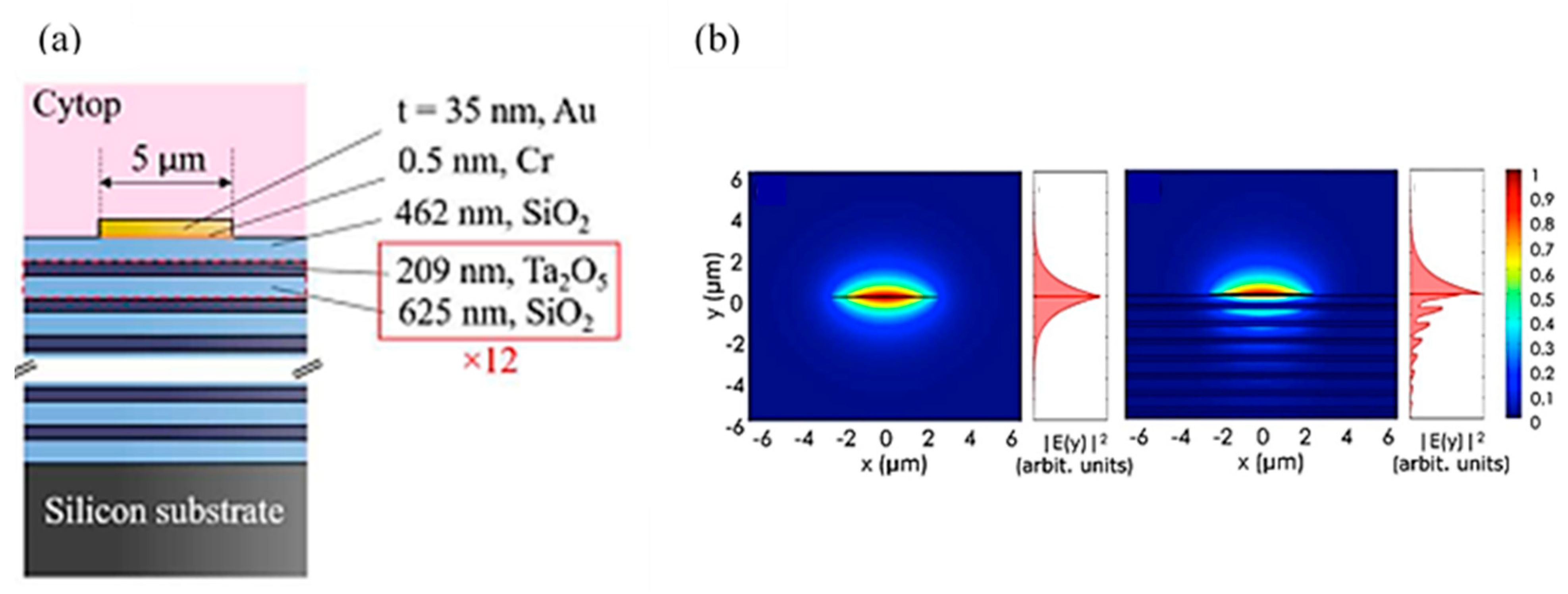
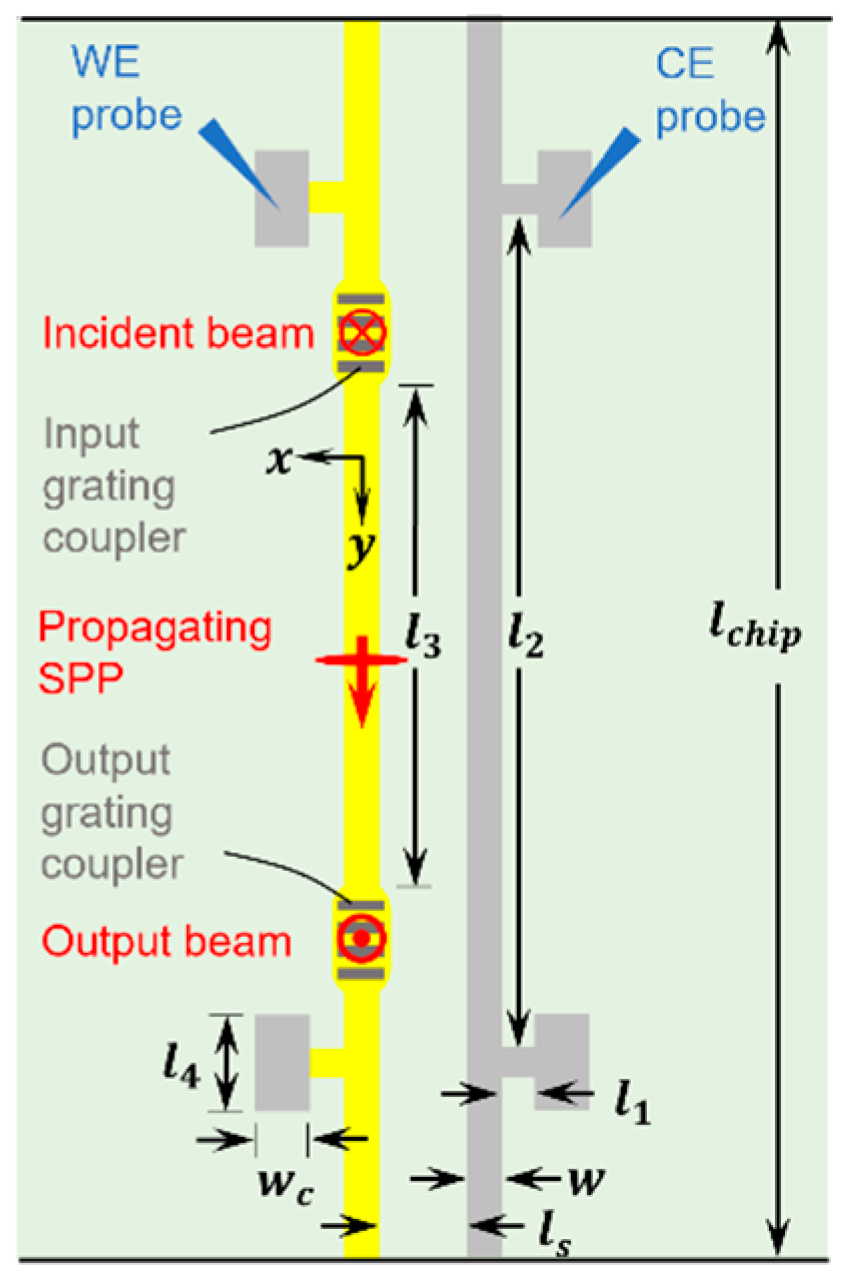
2.4. Bloch Long-Range Surface Plasmon Polaritons (Bloch LRSPPs)
2.5. Optical Interrogation of Surface Plasmon Biosensors
3. Electrochemical Surface Plasmon Sensors
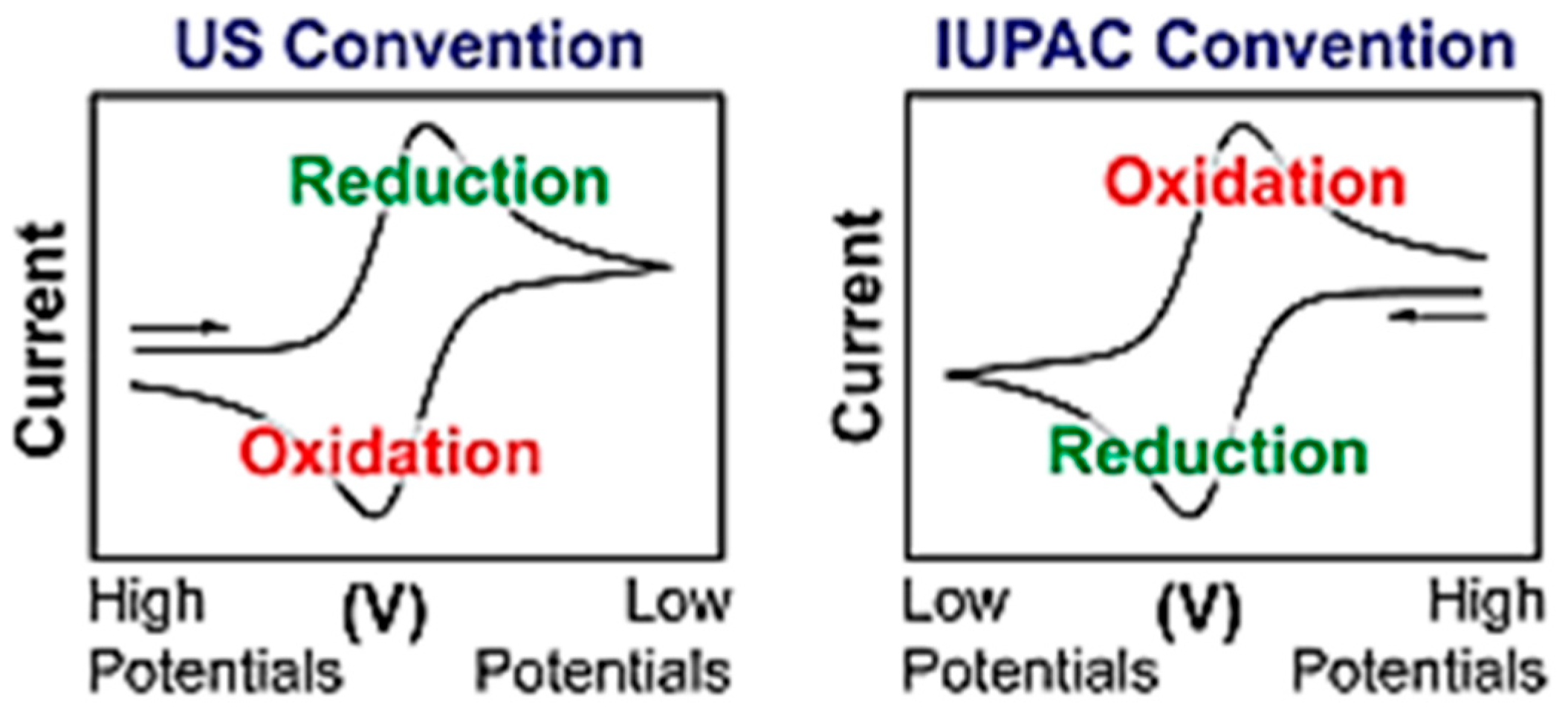
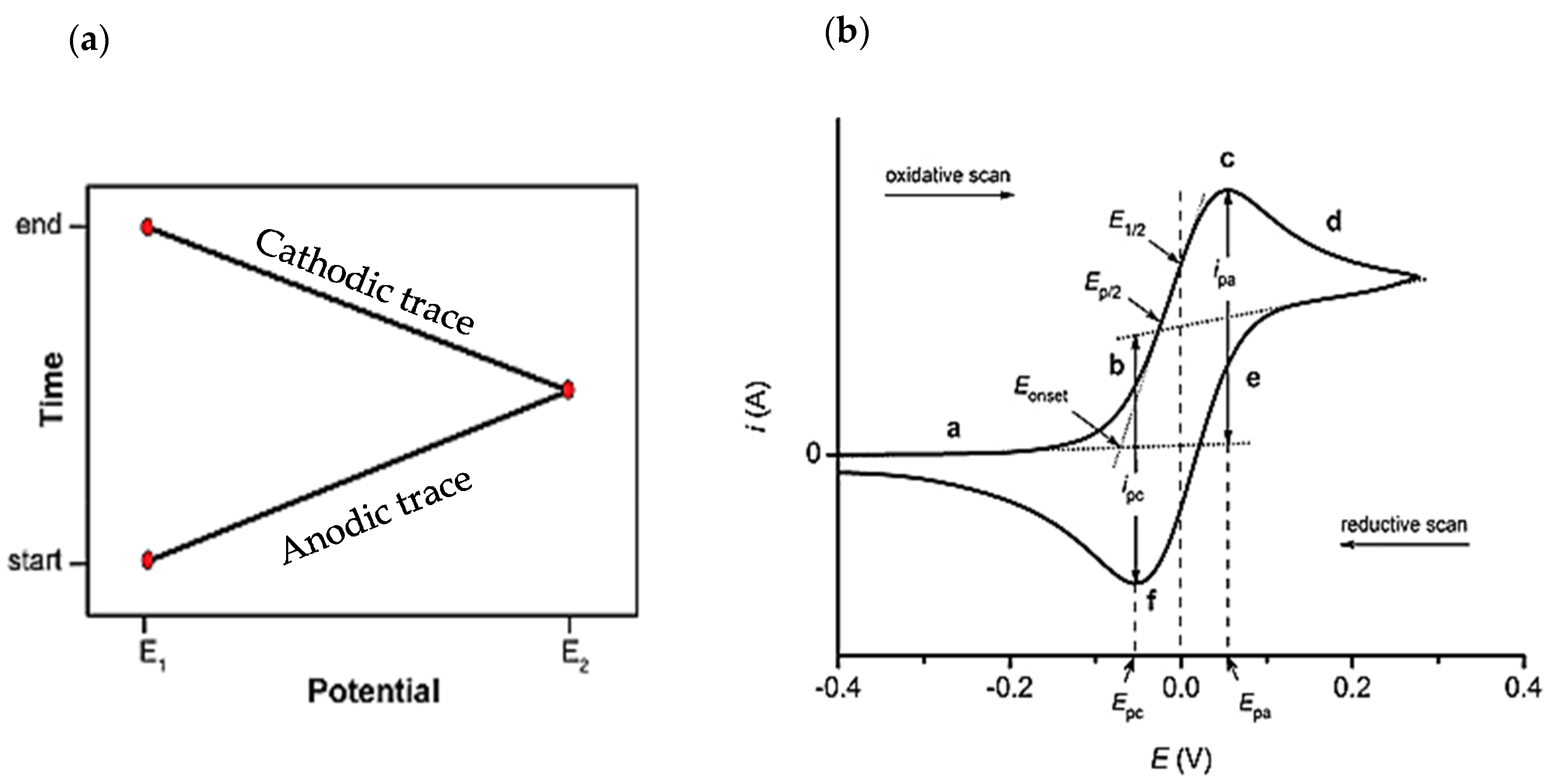
3.1. Cyclic Voltammetry
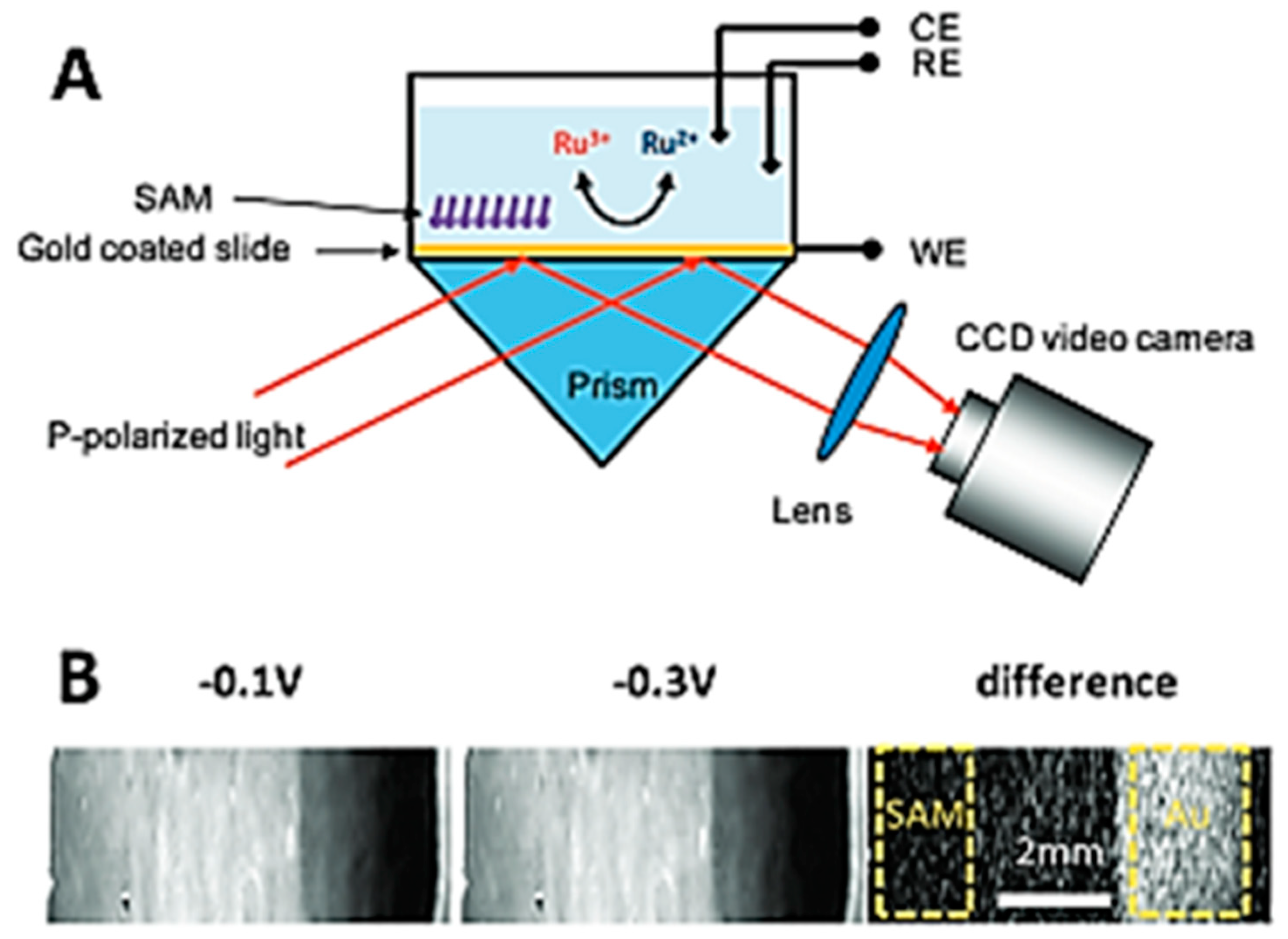
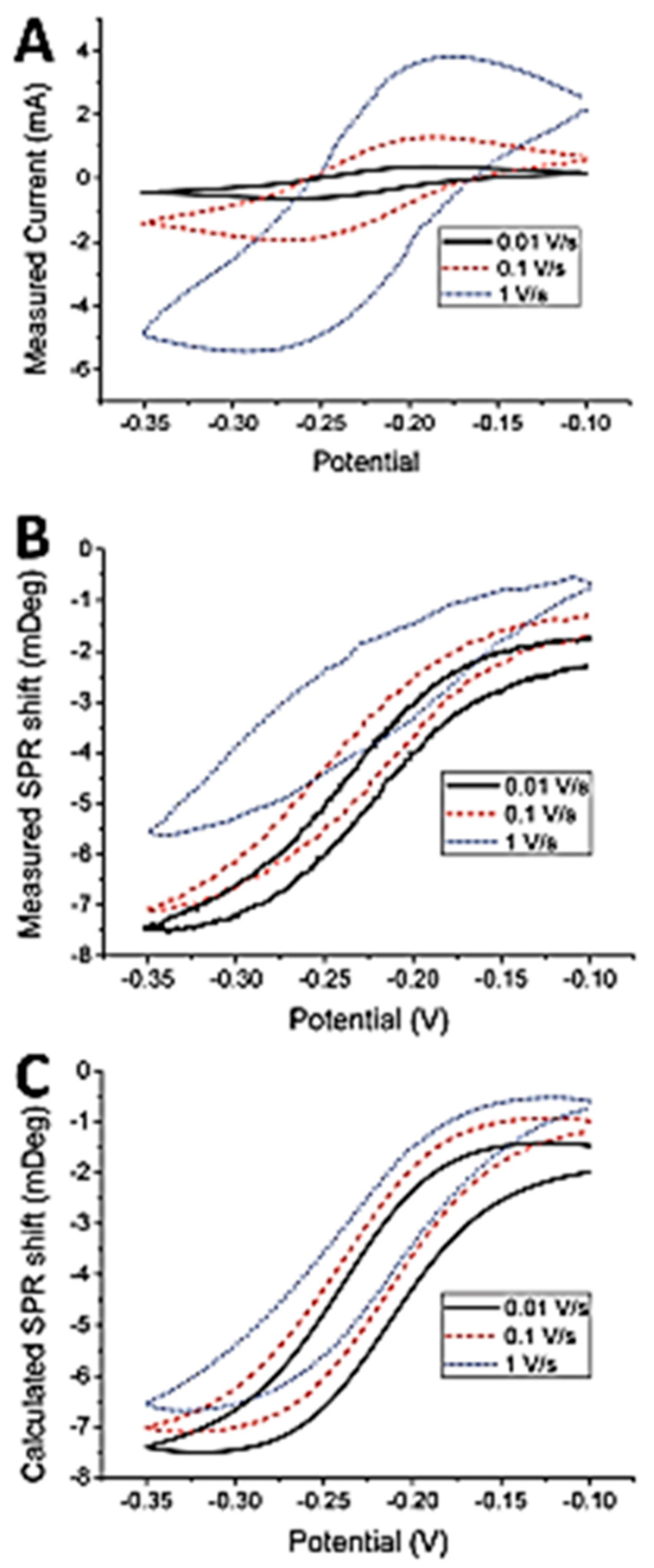
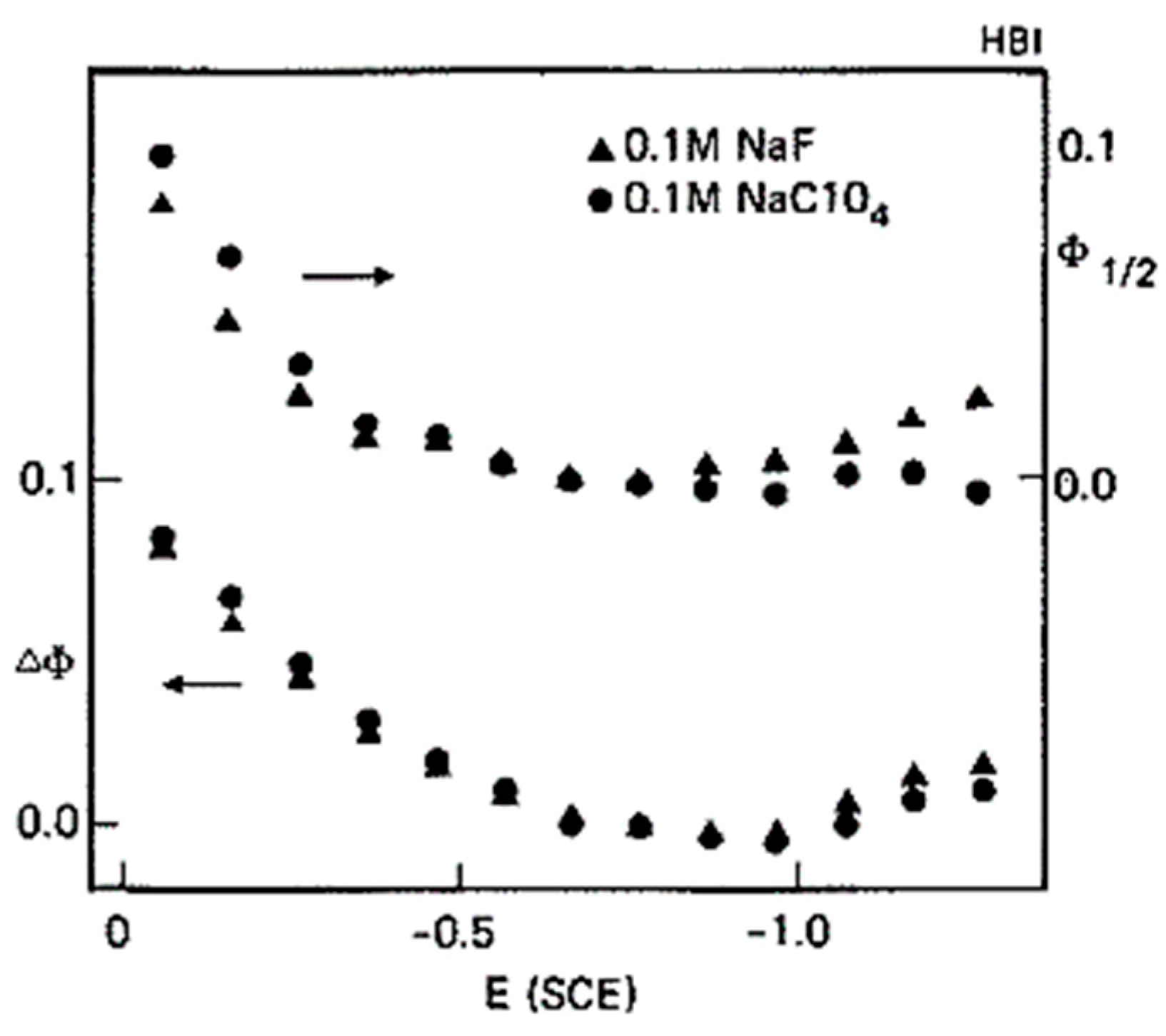
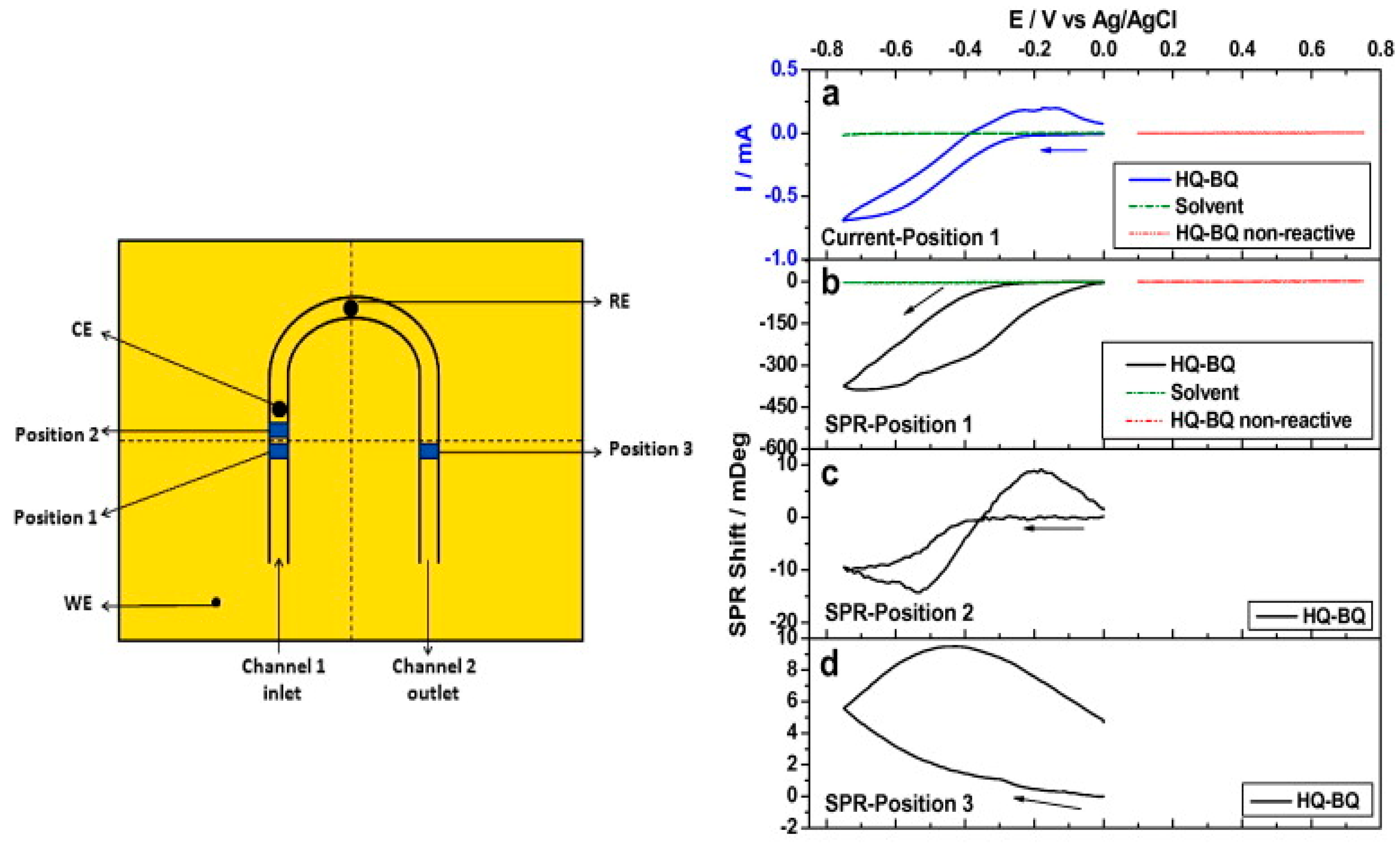
3.2. Electrochemical Surface Plasmon Resonance (EC-SPR)
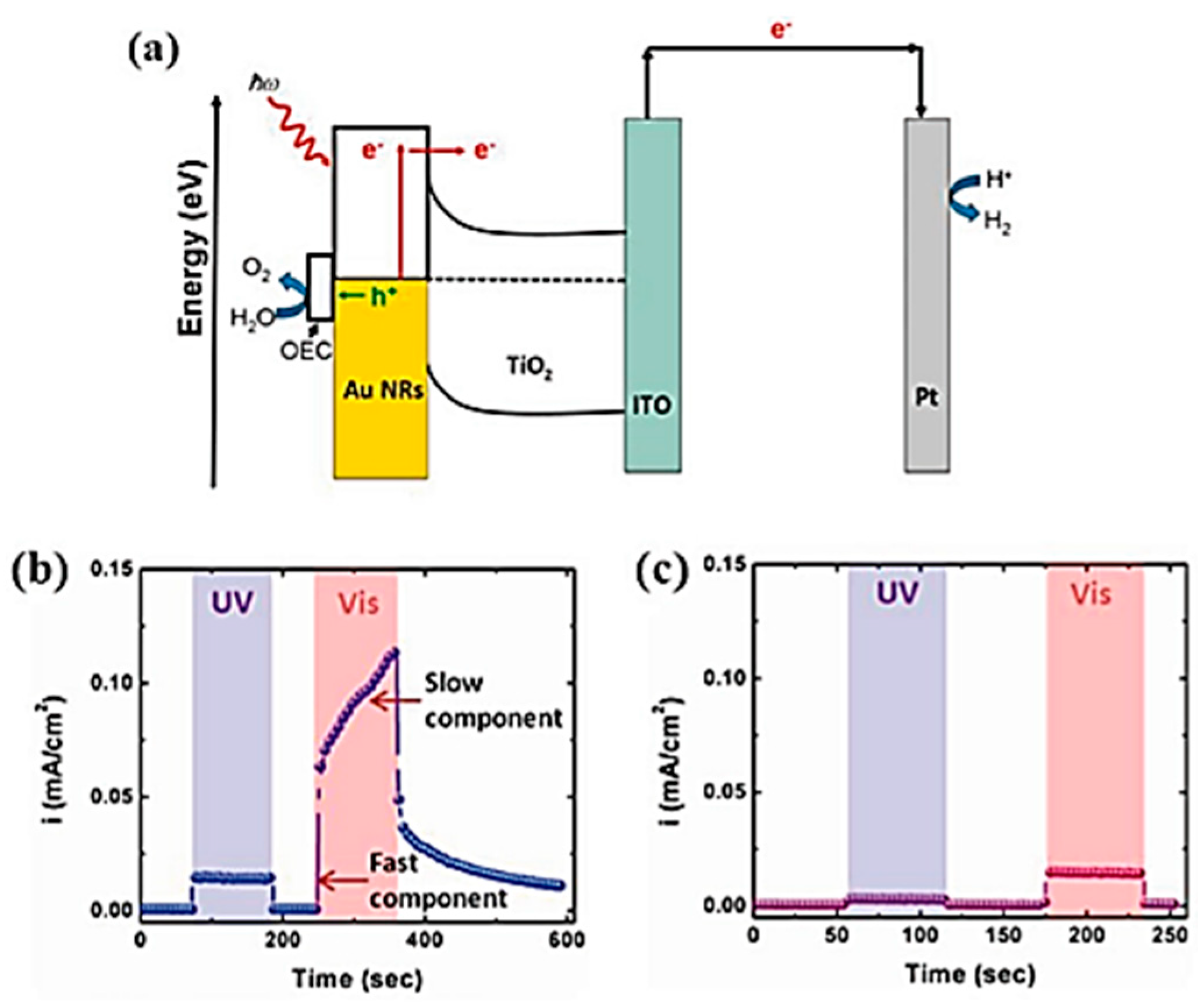
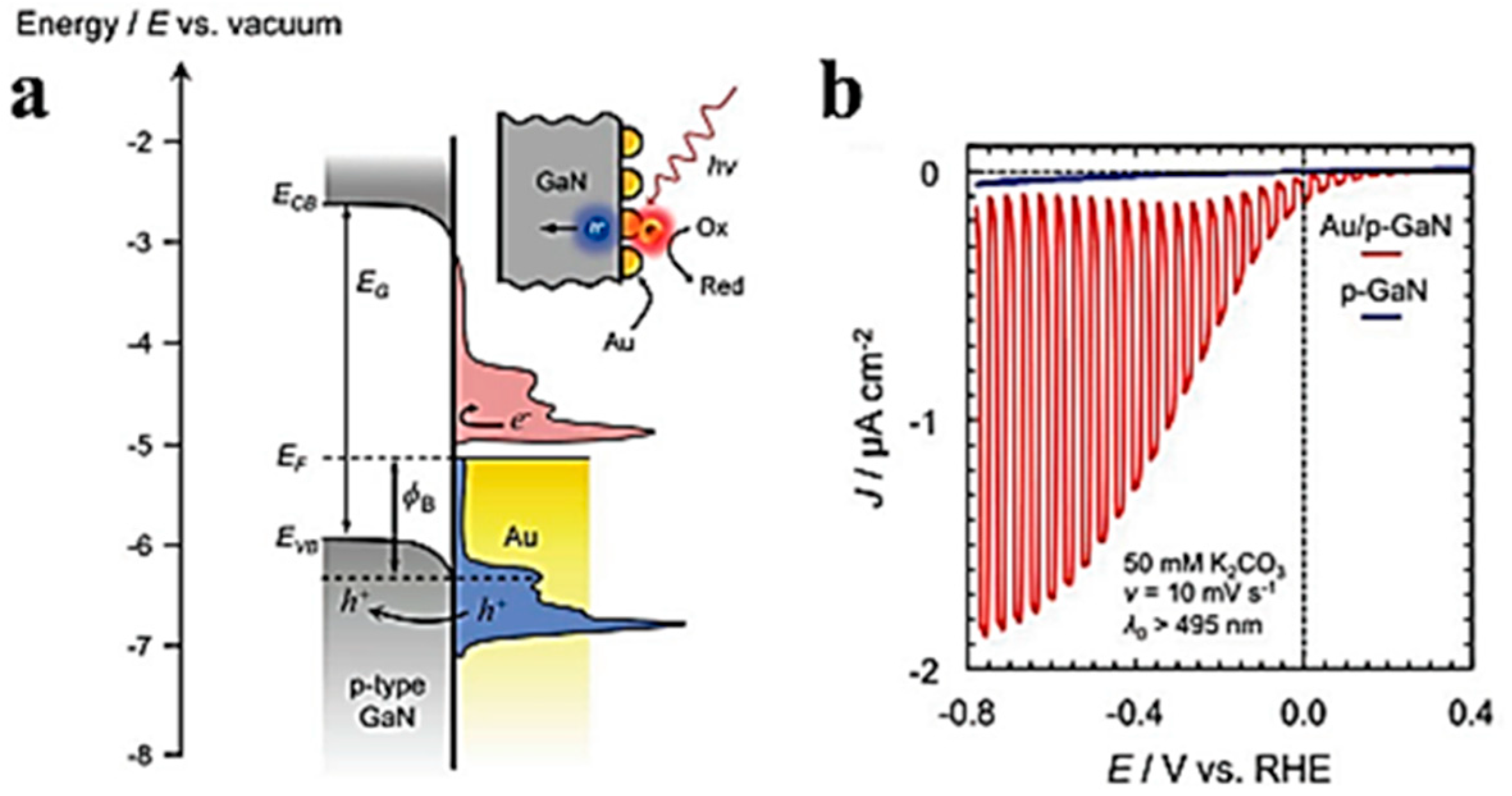
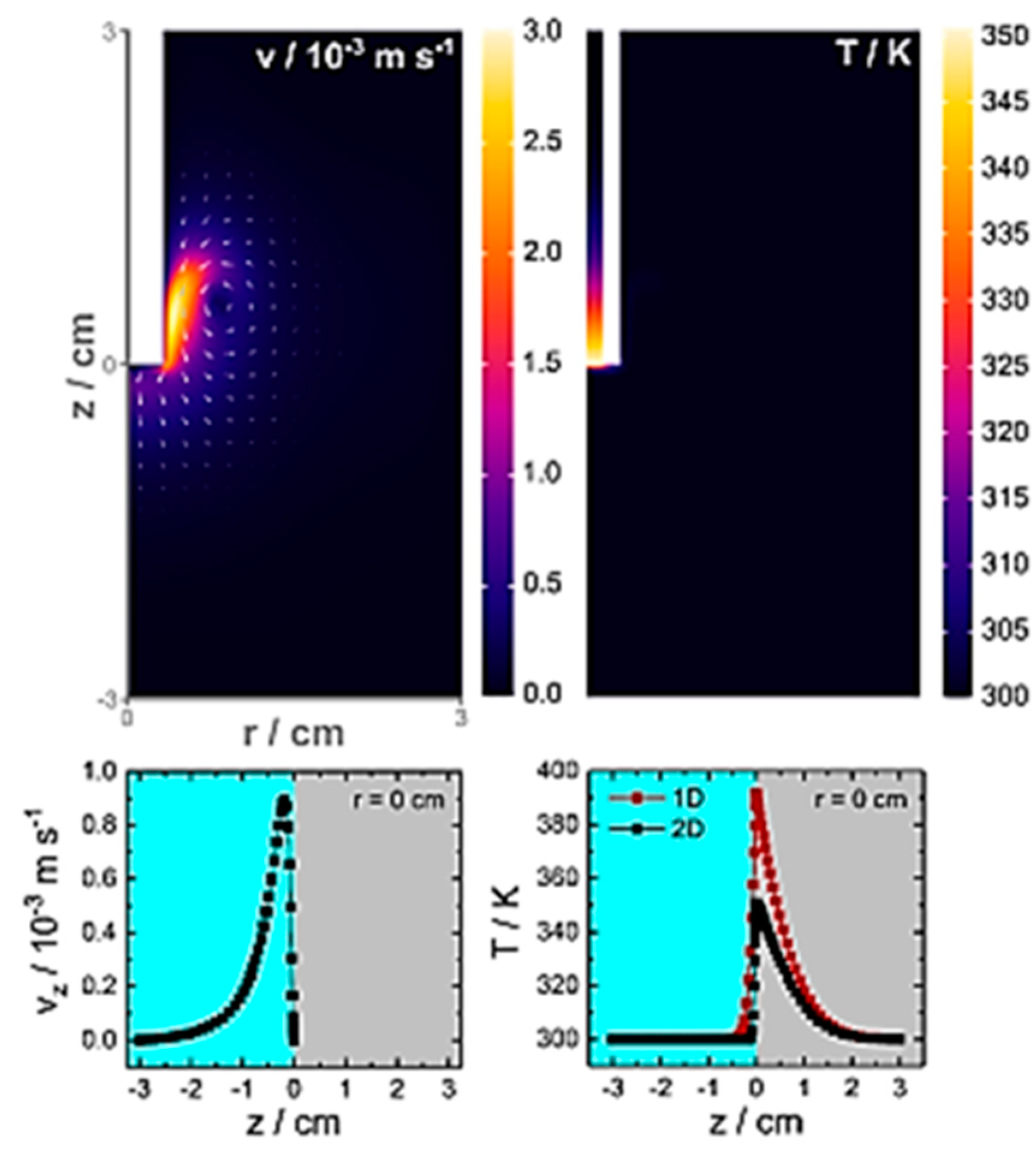
3.3. Energetic (Hot) Charge Carriers in EC-SPR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jia, M.; Li, S.; Zang, L.; Lu, X.; Zhang, H. Analysis of Biomolecules Based on the Surface Enhanced Raman Spectroscopy. J. Nanomater. 2018, 8, 730. [Google Scholar] [CrossRef] [Green Version]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, N.P. Introduction to biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar] [PubMed] [Green Version]
- Cooper, M.A. Optical biosensors in drug discovery. Nat. Rev. Drug Discov. 2002, 1, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Lequin, R.M. Enzyme Immunoassay (EIA)/Enzyme-Linked Immunosorbent Assay (ELISA). Clin. Chem. 2005, 51, 2415–2418. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Lv, J.; Liu, X.; Tang, Z.; Wang, X.; Xu, Y.; Hammock, B.D. Development of a Nanobody-Avi Tag Fusion Protein and Its Application in a Streptavidin-Biotin-Amplified Enzyme-Linked Immunosorbent Assay for Ochratoxin A in Cereal. Anal. Chem. 2018, 90, 10628–10634. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Okuyama, A.; Matsubara, Y.; Nishi, K.; Kobayashi, M.; Yamamura, S.; Morita, Y.; Takamura, Y.; Mizukami, H.; Tamiya, E. Fluorescence-based assay with enzyme amplification on a micro-flow immunosensor chip for monitoring coplanar polychlorinated biphenyls. Anal. Chim. Acta 2005, 531, 7–13. [Google Scholar] [CrossRef]
- Yuan, F.; Chen, M.; Leng, B.Y.; Wang, B.S. An efficient autofluorescence method for screening limonium bicolor mutants for abnormal salt gland density and salt secretion. S. Afr. J. Bot. 2013, 88, 110–117. [Google Scholar] [CrossRef]
- Deng, Y.; Feng, Z.; Yuan, F.; Guo, J.; Suo, S.; Wang, B. Identification and functional analysis of the autofluorescent substance in limonium bicolor salt glands. J. Plant Physiol. 2015, 97, 20–27. [Google Scholar] [CrossRef]
- Harris, E. A Low-Cost Approach to PCR: Appropriate Transfer of Biomolecular Techniques; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Chang, L.; Li, J.; Wang, L. Immuno-PCR: An ultrasensitive immunoassay for biomolecular Detection. Anal. Chem. Acta 2016, 910, 12–24. [Google Scholar] [CrossRef]
- Chard, T. Introduction to Radioimmunoassay and Related Techniques, 5th ed.; Elsevier: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Liu, R.; Xiong, Y.; Tang, W.; Guo, Y.; Yan, X.; Si, M. Near-infrared surface-enhanced Raman spectroscopy (NIR-SERS) studies on oxyheamoglobin (OxyHb) of liver cancer based on PVA-Ag nanofilm. J. Raman Spectrosc. 2013, 44, 362–369. [Google Scholar] [CrossRef]
- Munch, M.; Nielsen, L.P.; Handberg, K.J.; Ørgensen, P.H. Detection and subtyping (H5 and H7) of avian type A influenza virus by reverse transcription-PCR and PCR-ELISA. Arch. Virol. 2001, 146, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Daly, P.; Collier, T.; Doyle, S. PCR-ELISA detection of Escherichia coli in milk. Lett. Appl. Microbiol. 2002, 34, 222–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medintz, I.L.; Clapp, A.R.; Melinger, J.S.; Deschamps, J.R.; Mattoussi, H. A reagentless biosensing assembly based on quantum dot-donor Forster resonance energy transfer. Adv. Mater. 2005, 17, 2450–2455. [Google Scholar] [CrossRef]
- Nedelkov, D.; Nelson, R.W. Practical considerations in BIA/MS: Optimizing the biosensor-mass spectrometry interface. J. Mol. Recognit. 2000, 13, 140–145. [Google Scholar] [CrossRef]
- Yao, C.; Zhu, T.; Qi, Y.; Zhao, Y.; Xia, H.; Fu, W. Development of a quartz crystal microbalance biosensor with aptamers as bio-recognition element. Sensors 2010, 10, 5859–5871. [Google Scholar] [CrossRef] [Green Version]
- Dostálek, J.; Čtyroký, J.; Homola, J.; Brynda, E.; Skalský, M.; Nekvindová, P.; Špirková, J.; Škvor, J.; Schröfel, J. Surface plasmon resonance biosensor based on integrated optical waveguide. Sens. Actuators B Chem. 2001, 76, 8–12. [Google Scholar] [CrossRef]
- Haes, A.J.; Van Duyne, R.P. A unified view of propagating and localized surface plasmon resonance biosensors. Anal. Bioanal. Chem. 2004, 379, 920–930. [Google Scholar] [CrossRef]
- Krupin, O.; Asiri, H.; Wang, C.; Tait, R.N.; Berini, P. Biosensing using straight long-range surface plasmon waveguides. Opt. Express 2013, 21, 698–709. [Google Scholar] [CrossRef]
- Watanabe, M.; Kajikawa, K. An optical fiber biosensor based on anomalous reflection of gold. Sens. Actuators B Chem. 2003, 89, 126–130. [Google Scholar] [CrossRef]
- Yoon, J.; Shin, M.; Lee, T.; Choi, J.W. Highly sensitive biosensors based on biomolecules and functional nanomaterials depending on the types of nanomaterials: A perspective review. Materials 2020, 13, 299. [Google Scholar] [CrossRef] [Green Version]
- Homola, J. Surface plasmon resonance sensors for detection of chemical and biological Species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Yuan, R.; Chai, Y.; Liu, Y.; Dai, J.J.; Zhong, X. Novel potentiometric immunosensor for determination of diphtheria antigen based on compound nanoparticles and bilayer two-dimensional sol-gel as matrices. Anal. Bioanal. Chem. 2005, 381, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Darain, F.; Park, S.U.; Shim, Y.B. Disposable amperometric immunosensor system for rabbit IgG using a conducting polymer modified screen-printed electrode. Biosens. Bioelectr. 2003, 18, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Miura, N.; Higobashi, H.; Sakai, G.; Takeyasu, A.; Uda, T.; Yamazoe, N. Piezoelectric crystal immunosensor for sensitive detection of methamphetamine (stimulant drug) in human urine. Sens. Actuators B Chem. 1993, 13, 188–191. [Google Scholar] [CrossRef]
- Zhang, B.; Mao, Q.; Zhang, X.; Jiang, T.; Chen, M.; Yu, F.; Fu, W. A novel piezoelectric quartz micro-array immunosensor based on self-assembled monolayer for determination of human chorionic gonadotropin. Biosens. Bioelectr. 2004, 19, 711–720. [Google Scholar] [CrossRef]
- Ramanathan, K.; Danielsson, B. Principles and applications of thermal biosensors. Biosens. Bioelectr. 2001, 16, 417–423. [Google Scholar] [CrossRef]
- Kumbhat, S.; Sharma, K.; Gehlot, R.; Solanki, A.; Joshi, V. Surface plasmon resonance based immunosensor for serological diagnosis of dengue virus infection. J. Pharm. Biomed. Anal. 2010, 52, 255–259. [Google Scholar] [CrossRef]
- Wang, J. Towards Genoelectronics: Electrochemical Biosensing of DNA Hybridization. Chem-Eur. J. 1999, 5, 1681–1685. [Google Scholar] [CrossRef]
- Cho, I.H.; Kim, D.H.; Park, S. Electrochemical biosensors: Perspective on functional nanomaterials for on-site analysis. Biomater. Res. 2020, 24, 6. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors—Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef] [PubMed]
- Dertien, E.; Regtien, P.P. Sensors for Mechatronics; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Yao, C.; Qi, Y.; Zhao, Y.; Xiang, Y.; Chen, Q.; Fu, W. Aptamer-based piezoelectric quartz crystal microbalance biosensor array for the quantification of IgE. Biosens. Bioelectr. 2009, 24, 2499–2503. [Google Scholar] [CrossRef]
- Danielsson, B.; Mattiasson, B.; Mosbach, K. Enzyme thermistor devices and their analytical applications. Appl. Biochem. 1981, 3, 97–143. [Google Scholar]
- Mattiasson, B.; Borrebaeck, C.; Sanfridson, B.; Mosbach, K. Thermometric enzyme linked immunosorbent assay: TELISA. Biochim. Biophys. Acta 1977, 483, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Kazura, E.; Mernaugh, R.; Baudenbacher, F. A capillary-perfused, nanocalorimeter platform for thermometric enzyme-linked immunosorbent assay with femtomole sensitivity. Biosensors 2020, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Sawant, S.N. Development of biosensors from biopolymer composites. In Biopolymer Composites in Electronics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 353–383. [Google Scholar]
- Long, F.; Zhu, A.; Shi, H. Recent advances in optical biosensors for environmental monitoring and early warning. Sensors 2013, 13, 13928–13948. [Google Scholar] [CrossRef] [Green Version]
- Mol, N.J.; Fischer, M.J.E. Surface Plasmon Resonance: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Damborský, P.; Švitel, J.; Katrlík, J. Optical biosensors. Essays Biochem. 2016, 60, 91–100. [Google Scholar]
- Fong, N.R.; Menotti, M.; Lisicka-Skrzek, E.; Northfield, H.; Olivieri, A.; Tait, N.; Liscidini, M.; Berini, P. Bloch long range surface plasmon polaritons on metal stripe waveguides on a multilayer substrate. ACS Photonics 2017, 4, 593–599. [Google Scholar] [CrossRef]
- Berini, P. Long-range surface plasmon polaritons. Adv. Opt. Photonics 2009, 1, 484–588. [Google Scholar] [CrossRef]
- Maier, S.A. Plasmonics: Fundamentals and Applications; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Berini, P. Plasmon polariton waves guided by thin lossy metal films of finite width: Bound modes of symmetric structures. Phys. Rev. B 2000, 61, 10484–10503. [Google Scholar] [CrossRef]
- Vargas, W.E. Optical and electrical properties of hydrided palladium thin films studied by an inversion approach from transmittance measurements. Thin Solid Films 2006, 496, 189–196. [Google Scholar] [CrossRef]
- Rakić, A.D. Optical properties of metallic films for vertical-cavity optoelectronic devices. Appl. Opt. 1998, 37, 5271–5283. [Google Scholar] [CrossRef]
- Johnson, P.B.; Christy, R.W. Optical constants of the noble metals. Phys. Rev. B 1972, 6, 4370. [Google Scholar] [CrossRef]
- Giannini, V.; Fernández-Domínguez, A.I.; Heck, S.C.; Maier, S.A. Plasmonic nanoantennas: Fundamentals and their use in controlling the radiative properties of nanoemitters. Chem. Rev. 2011, 111, 3888–3912. [Google Scholar] [CrossRef]
- Boardman, A.D.; Paranjape, B.V. The optical surface modes of metal spheres. J. Phys. F 1977, 7, 1935–1945. [Google Scholar] [CrossRef]
- Schmidt, F.P.; Ditlbacher, H.; Hohenester, U.; Hohenau, A.; Hofer, F.; Krenn, J.R. Universal dispersion of surface plasmons in flat nanostructures. Nat. Commun. 2014, 5, 3604. [Google Scholar] [CrossRef] [Green Version]
- Benson, O. Assembly of hybrid photonic architectures from nanophotonic Constituents. Nature 2011, 480, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Homola, J. Electromagnetic Theory of Surface Plasmons. Springer Ser. Chem. Sens. Biosens. 2006, 4, 3–44. [Google Scholar]
- McPeak, K.M.; Jayanti, S.V.; Kress, S.J.P.; Meyer, S.; Iotti, S.; Rossinelli, A.; Norris, D.J. Plasmonic Films Can Easily Be Better: Rules and Recipes. ACS Photonics 2015, 2, 326–333. [Google Scholar] [CrossRef]
- West, P.R.; Ishii, S.; Naik, G.V.; Emani, N.K.; Shalaev, V.M.; Boltasseva, A. Searching for better plasmonic materials. Lasers Phot. Rev. 2010, 4, 795–808. [Google Scholar] [CrossRef] [Green Version]
- Berini, P. Plasmon-polariton waves guided by thin lossy metal films of finite width: Bound modes of asymmetric structures. Phys. Rev. B 2001, 63, 125417. [Google Scholar] [CrossRef]
- Boltasseva, A.; Nikolajsen, T.; Leosson, K.; Kjaer, K.; Larsen, M.S.; Bozhevolnyi, S.I. Integrated optical components utilizing long-range surface plasmon polaritons. J. Light. Technol. 2005, 23, 413–422. [Google Scholar] [CrossRef] [Green Version]
- Charbonneau, R.; Scales, C.; Breukelaar, I.; Fafard, S.; Lahoud, N.; Mattiussi, G.; Berini, P. Passive integrated optics elements based on long-range surface plasmon polaritons. J. Light. Technol. 2006, 24, 477–494. [Google Scholar] [CrossRef]
- Ebbesen, T.W.; Genet, C.; Bozhevolnyi, S.I. Surface plasmon circuitry. Phys. Today 2008, 61, 44. [Google Scholar] [CrossRef] [Green Version]
- Minh, T.; Tanaka, K.; Tanaka, M. Complex propagation constants of surface plasmon polariton rectangular waveguide by method of lines. Opt. Express 2008, 16, 9378–9390. [Google Scholar] [CrossRef]
- Berini, P.; Buckley, R. On the convergence and accuracy of numerical mode computations of surface plasmon waveguides. J. Comput. Theor. Nanosci. 2009, 6, 2040–2053. [Google Scholar] [CrossRef]
- Veronis, G.; Kocaba, S.; Miller, D.A.B.; Fan, S. Modeling of Plasmonic Waveguide Components and Networks. J. Comput. Theor. Nanosci. 2009, 6, 1808–1826. [Google Scholar] [CrossRef]
- Otto, A. Excitation of nonradiative surface plasma waves in silver by the method of frustrated total reflection. Z. Für Phys. A 1968, 216, 398–410. [Google Scholar] [CrossRef]
- Otto, A. Excitation by light of ω+ and ω- surface plasma waves in thin metal layers. Z. Für Phys. A 1969, 219, 227–233. [Google Scholar] [CrossRef]
- Kretschmann, E. Die Bestimmung optischer Konstanten von Metallen durch Anregung von Oberfl¨achenplasmaschwingungen. Z. Für Phys. A 1971, 241, 313–324. [Google Scholar] [CrossRef]
- Novotny, L.; Hecht, B. Principles of Nano-Optics; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Fischer, M.J.E. Surface Plasmon Resonance; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Shankaran, D.R.; Gobi, V.; Miura, N. Recent advancements in surface plasmon resonance immunosensors for detection of small molecules of biomedical, food and environmental interest. Sens. Actuators B Chem. 2007, 121, 158–177. [Google Scholar] [CrossRef]
- Cooper, M.A. Label-Free Biosensors: Techniques and Applications; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Slavik, R.; Homola, J. Ultrahigh resolution long range surface plasmon-based sensor. Sens. Actuators B Chem. 2007, 123, 10–12. [Google Scholar] [CrossRef]
- Mauriz, E.; Calle, A.; Manclus, J.J.; Montoya, A.; Lechuga, L.M. Multi-analyte SPR immunoassays for environmental biosensing of pesticides. Anal. Bioanal. Chem. 2007, 387, 1449–1458. [Google Scholar] [CrossRef]
- Chabot, V.; Miron, Y.; Grandbois, M.; Charette, P.G. Long range surface plasmon resonance for increased sensitivity in living cell biosensing through greater probing depth. Sens. Actuators B Chem. 2012, 174, 94–101. [Google Scholar] [CrossRef]
- Monzon Hernandez, D.; Velazquez-Gonzalez, J.S.; Luna-Moreno, D.; Torres-Cisneros, M.; Hernandez-Romano, I. Prism-Based Surface Plasmon Resonance for Dual-Parameter Sensing. IEEE Sens. J. 2018, 18, 4030–4037. [Google Scholar] [CrossRef]
- Boruah, R.; Mohanta, D.; Choudhury, A.; Nath, P.; Ahmed, G.A. Surface Plasmon Resonance-Based Protein Bio-Sensing Using a Kretschmann Configured Double Prism Arrangement. IEEE Sens. J. 2015, 15, 6791–6796. [Google Scholar] [CrossRef]
- Yang, C.-T.; Mejard, R.; Griesser, H.J.; Bagnaninchi, P.O.; Thierry, B. Cellular Micromotion Monitored by Long-Range Surface Plasmon Resonance with Optical Fluctuation Analysis. Anal. Chem. 2015, 87, 1456–1461. [Google Scholar] [CrossRef]
- Vala, M.; Etheridge, S.; Roach, J.; Homola, J. Long-range surface plasmons for sensitive detection of bacterial analytes. Sens. Actuators B Chem. 2009, 139, 59–63. [Google Scholar] [CrossRef]
- Koubova, A.V.; Brynda, E.; Karasova, L.; Homola, J.; Dostalek, J.; Tobiska, P.; Rosicky, J. Detection of foodborne pathogens using surface plasmon resonance biosensors. Sens. Actuators B Chem. 2001, 74, 100–105. [Google Scholar] [CrossRef]
- Lee, W.; Lee, D.-B.; Oh, B.-K.; Lee, W.H.; Choi, J.-W. Nanoscale fabrication of protein A on self-assembled monolayer and its application to surface plasmon resonance immunosensor. Enzyme Microb. Technol. 2004, 35, 678–682. [Google Scholar] [CrossRef]
- Wei, J.; Mu, Y.; Song, D.; Fang, X.; Liu, X.; Bu, L.; Zhang, H.; Zhang, G.; Ding, J.; Wang, W.; et al. A novel sandwich immunosensing method for measuring cardiac troponin I in sera. Anal. Biochem. 2003, 321, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Gobi, K.V.; Kataoka, C.; Miura, N. Surface plasmon resonance detection of endocrine disruptors using immunoprobes based on self assembled monolayers. Sens. Actuators B Chem. 2005, 108, 784–790. [Google Scholar] [CrossRef]
- Trevino, J.; Calle, A.; Rodriguez-Frade, J.M.; Mellado, M.; Lechuga, L.M. Surface plasmon resonance immunoassay analysis of pituitary hormones in urine and serum samples. Clin. Chim. Acta 2009, 403, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Dostalek, J.; Kasry, A.; Knoll, W. Long range surface plasmons for observation of biomolecular binding events at metallic surfaces. Plasmonics 2007, 2, 97–106. [Google Scholar] [CrossRef]
- Degiron, A.; Cho, S.Y.; Tyler, T.; Jokerst, N.; Smith, D.R. Directional coupling between dielectric and long-range plasmon waveguides. New J. Phys. 2009, 11, 015002. [Google Scholar] [CrossRef]
- Hirbodvash, Z.; Khodami, M.; Fong, N.R.; Lisicka-Skrzek, E.; Olivieri, A.; Northfield, H.; Tait, R.N.; Berini, P. Grating couplers fabricated by e-beam lithography for long range surface plasmon waveguides embedded in a fluoropolymer. Appl. Opt. 2019, 58, 2994–3002. [Google Scholar] [CrossRef]
- Khodami, M.; Hirbodvash, Z.; Krupin, O.; Wong, W.R.; Lisicka-Skrzek, E.; Northfield, H.; Hahn, C.; Berini, P. Fabrication of Bloch Long Range Surface Plasmon Waveguides Integrating Counter Electrodes and Microfluidic Channels for Multimodal Biosensing. J. Microelectromech. Syst. 2021, 30, 686–695. [Google Scholar] [CrossRef]
- Tellez, G.A.C.; Hassan, S.; Tait, R.N.; Berini, P.; Gordon, R. Atomically flat symmetric elliptical nanohole arrays in a gold film for ultrasensitive refractive index sensing. Lab A Chip 2013, 13, 2541–2546. [Google Scholar] [CrossRef]
- Hajebifard, A.; Berini, P. Fano resonances in plasmonic heptamer nano-hole arrays. Opt. Express 2017, 25, 18566–18580. [Google Scholar] [CrossRef] [Green Version]
- Asahi Glass Company. Cytop Technical Brochure. 2009. Available online: http://www.agc.com (accessed on 8 February 2023).
- Dupont. “Teflon AF Properties”. Available online: www.dupont.com (accessed on 8 February 2023).
- Berini, P. Bulk and surface sensitivities of surface plasmon waveguides. New J. Phys. 2008, 10, 105010. [Google Scholar] [CrossRef]
- Krupin, O.; Wong, W.R.; Béland, P.; Adikan, F.R.M.; Berini, P. Long-Range Surface Plasmon-Polariton Waveguide Biosensors for Disease Detection. J. Light. Technol. 2016, 34, 4673–4681. [Google Scholar] [CrossRef]
- Chen, C.; Berini, P. Grating couplers for broadside input and output coupling of long-range surface plasmons. Opt. Express 2010, 18, 8006–8018. [Google Scholar] [CrossRef] [PubMed]
- Homola, J. Surface Plasmon Resonance Based Sensors; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Golden, J.; Yates, M.D.; Halsted, M.; Tender, L. Application of electrochemical surface plasmon resonance (ESPR) to the study of electroactive microbial biofilms. Phys. Chem. Chem. Phys. 2018, 20, 25648–25656. [Google Scholar] [CrossRef] [PubMed]
- Rusling, J.F. Biomolecular Films: Design, Function, and Applications; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A practical beginner’s guide to cyclic voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Wang, S.; Huang, X.; Shan, X.; Foley, K.J.; Tao, N. Electrochemical surface plasmon resonance: Basic formalism and experimental validation. Anal. Chem. 2010, 82, 935–941. [Google Scholar] [CrossRef]
- Gordon, J.G.; Ernest, S. Surface plasmons as a probe of the electrochemical interface. Surf. Sci. 1980, 101, 499–506. [Google Scholar] [CrossRef]
- Huang, X.; Wang, S.; Shan, X.; Chang, X.; Tao, N. Flow-through electrochemical surface plasmon resonance: Detection of intermediate reaction products. J. Electroanal. Chem. 2010, 649, 37–41. [Google Scholar] [CrossRef]
- Patskovsky, S.; Dallaire, A.M.; Meunier, M. Electrochemical surface plasmon resonance sensing with absorptive redox mediator film. Sens. Actuators B Chem. 2016, 222, 71–77. [Google Scholar] [CrossRef]
- Sannomia, T.; Dermutz, H.; Hafner, C.; Voros, J.; Dahilin, A.B. Electrochemistry on a localized surface plasmon sensor. Langmuir 2010, 26, 7619–7626. [Google Scholar] [CrossRef]
- Ahmed, I.; Shi, L.; Pasanen, H.; Vivo, P.; Maity, P.; Hatamvand, M.; Zhan, Y. There is plenty of room at the top: Generation of hot charge carriers and their applications in perovskite and other semiconductor-based optoelectronic devices. Light Sci. Appl. 2021, 10, 174. [Google Scholar] [CrossRef]
- Reddy, H.; Shalaev, V.M. Plasmonic hot-carriers and their applications: Opinion. Opt. Mater. Express 2021, 11, 3827–3832. [Google Scholar] [CrossRef]
- Tagliabue, G.; Jermyn, A.S.; Sundararaman, R.; Welch, A.J.; DuChene, J.S.; Pala, R.; Davoyan, A.R.; Narang, P.; Atwater, H.A. Quantifying the role of surface plasmon excitation and hot carrier transport in plasmonic devices. Nat. Commun. 2018, 9, 3394. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Mubeen, S.; Ji, X.; Stucky, G.D.; Moskovits, M. Plasmonic Photoanodes for Solar Water Splitting with Visible Light. Nano Lett. 2012, 12, 5014–5019. [Google Scholar] [CrossRef]
- DuChene, J.S.; Tagliabue, G.; Welch, A.J.; Cheng, W.; Atwater, H.A. Hot hole collection and photoelectrochemical CO2 reduction with plasmonic Au/p-GaN photocathodes. Nano Lett. 2018, 18, 2545–2550. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Swearer, D.L.; Zhang, C.; Robatjazi, H.; Zhao, H.; Henderson, L.; Dong, L.; Christopher, P.; Carter, E.A.; Nordlander, P.; et al. Quantifying hot carrier and thermal contributions in plasmonic photocatalysis. Science 2018, 362, 69–72. [Google Scholar] [CrossRef] [Green Version]
- Jain, P.K. Taking the Heat Off of Plasmonic Chemistry. J. Phys. Chem. C 2019, 123, 24347–24351. [Google Scholar] [CrossRef] [Green Version]
- Baffou, G.; Bordacchini, I.; Baldi, A.; Quidant, R. Simple experimental procedures to distinguish photothermal from hot-carrier processes in plasmonics. Light Sci. Appl. 2020, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Cortés, E.; Besteiro, L.V.; Alabastri, A.; Baldi, A.; Tagliabue, A.; Demetriadou, A.; Narang, P. Challenges in Plasmonic Catalysis. ACS Nano 2020, 14, 16202–16219. [Google Scholar] [CrossRef] [PubMed]
- Dubi, Y.; Un, I.W.; Sivan, Y. Thermal effects—An alternative mechanism for plasmon-assisted photocatalysis. Chem. Sci. 2020, 11, 5017–5027. [Google Scholar] [CrossRef] [Green Version]
- Maley, M.; Hill, J.W.; Saha, P.; Walmsley, J.D.; Hill, C.M. The role of heating in the electrochemical response of plasmonic nanostructures under illumination. J. Phys. Chem. C 2019, 123, 12390–12399. [Google Scholar] [CrossRef]
- Bauer, M.; Marienfeld, A.; Aeschlimann, M. Hot electron lifetimes in metals probed by time-resolved two-photon photoemission. Prog. Surf. Sci. 2015, 90, 319–376. [Google Scholar] [CrossRef]
- Kravets, V.G.; Kabashin, A.V.; Barnes, W.L.; Grigorenko, A.N. Plasmonic surface lattice resonances: A review of properties and applications. Chem. Rev. 2018, 118, 5912–5951. [Google Scholar] [CrossRef]
- Deng, S.; Zhang, B.; Choo, P.; Smeets, P.J.M.; Odom, T.W. Plasmonic Photoelectrocatalysis in Copper–Platinum Core–Shell Nanoparticle Lattices. Nano Lett. 2021, 21, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Hirbodvash, Z.; Houache, M.S.E.; Krupin, O.; Khodami, M.; Northfield, H.; Olivieri, A.; Baranova, E.A.; Berini, P. Electrochemical Performance of Lithographically-Defined Micro-Electrodes for Integration and Device Applications. Chemosensors 2021, 9, 277. [Google Scholar] [CrossRef]
- Hirbodvash, Z.; Krupin, O.; Northfield, H.; Olivieri, A.; Baranova, E.A.; Berini, P. Infrared surface plasmons on a Au waveguide electrode open new redox channels associated with the transfer of energetic carriers. Sci. Adv. 2022, 8, eabm9303. [Google Scholar] [CrossRef] [PubMed]
- Hirbodvash, Z.; Baranova, E.A.; Berini, P. Real-time convolutional voltammetry enhanced by energetic (hot) electrons and holes on a surface plasmon waveguide electrode. Anal. Chem. 2022, 94, 13145–13152. [Google Scholar] [CrossRef] [PubMed]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirbodvash, Z.; Berini, P. Surface Plasmon Electrochemistry: Tutorial and Review. Chemosensors 2023, 11, 196. https://doi.org/10.3390/chemosensors11030196
Hirbodvash Z, Berini P. Surface Plasmon Electrochemistry: Tutorial and Review. Chemosensors. 2023; 11(3):196. https://doi.org/10.3390/chemosensors11030196
Chicago/Turabian StyleHirbodvash, Zohreh, and Pierre Berini. 2023. "Surface Plasmon Electrochemistry: Tutorial and Review" Chemosensors 11, no. 3: 196. https://doi.org/10.3390/chemosensors11030196




