Molecularly Imprinted Plasmonic Sensors as Nano-Transducers: An Effective Approach for Environmental Monitoring Applications
Abstract
:1. Introduction
2. History of Molecularly Imprinted Polymers (MIPs)
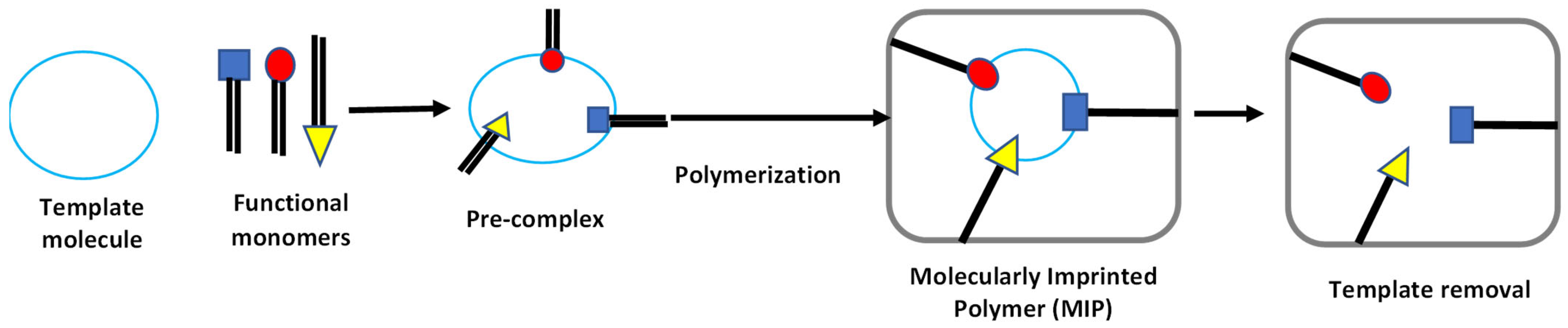
2.1. Molecularly Imprinted Polymers: A General Overview
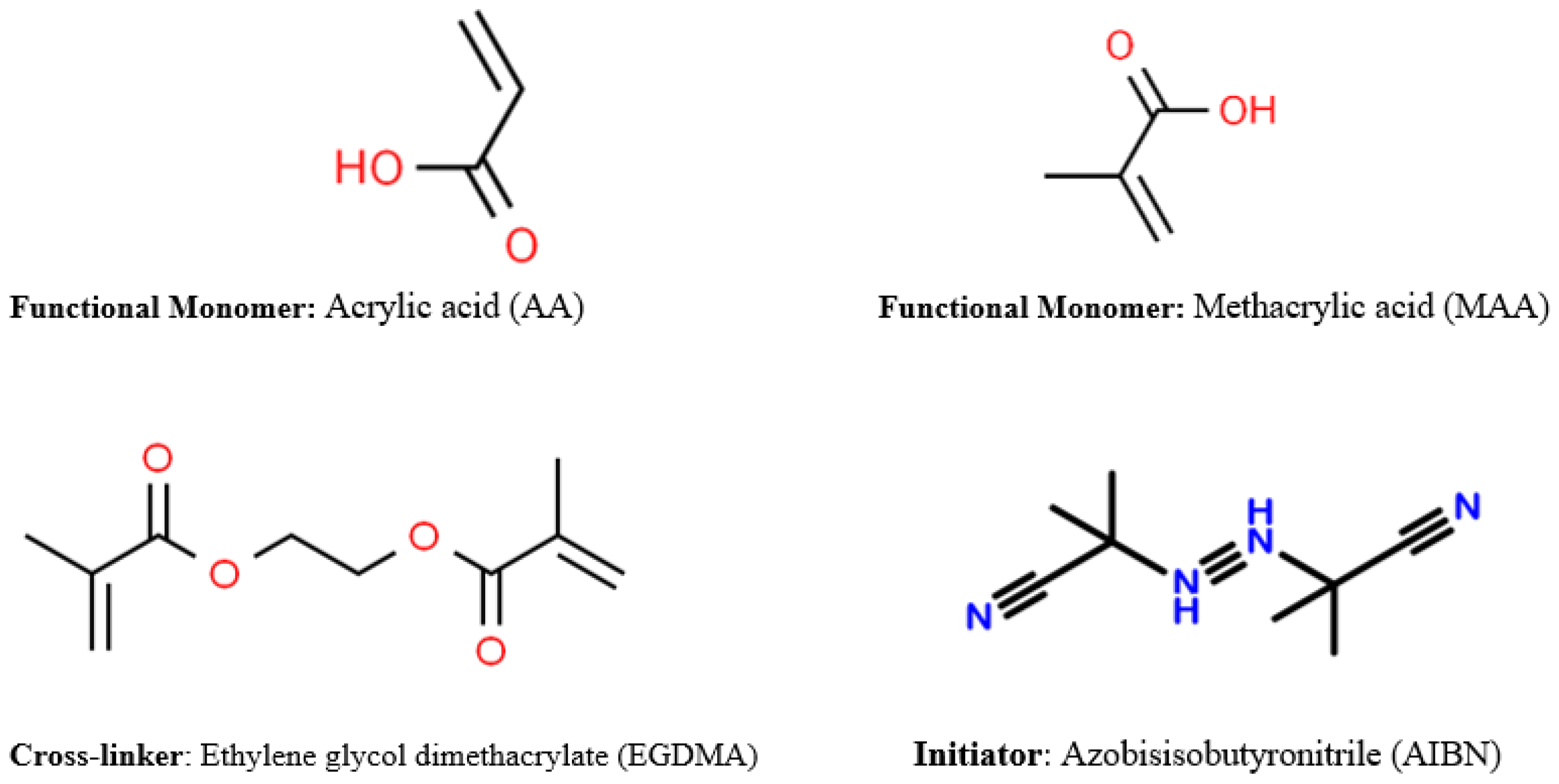
2.2. The Concept of Producing Molecularly Imprinted Polymers
2.3. Organophosphate Pesticides and Environmental Pollution
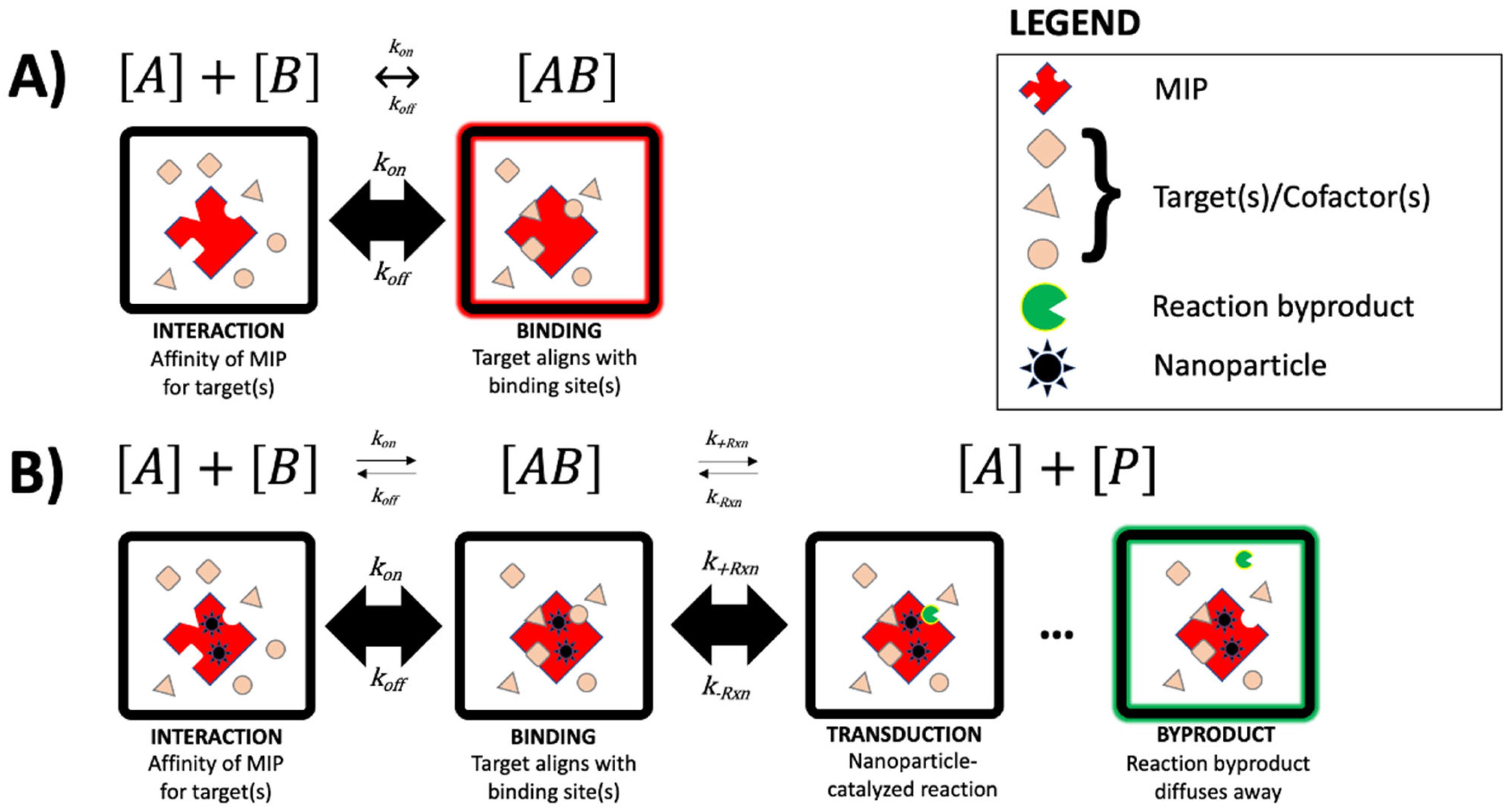
3. Plasmonic Sensing with Molecular Imprinting Technology
3.1. Surface Plasmon Resonance and Surface-Enhanced Raman Spectroscopy MIP Sensors
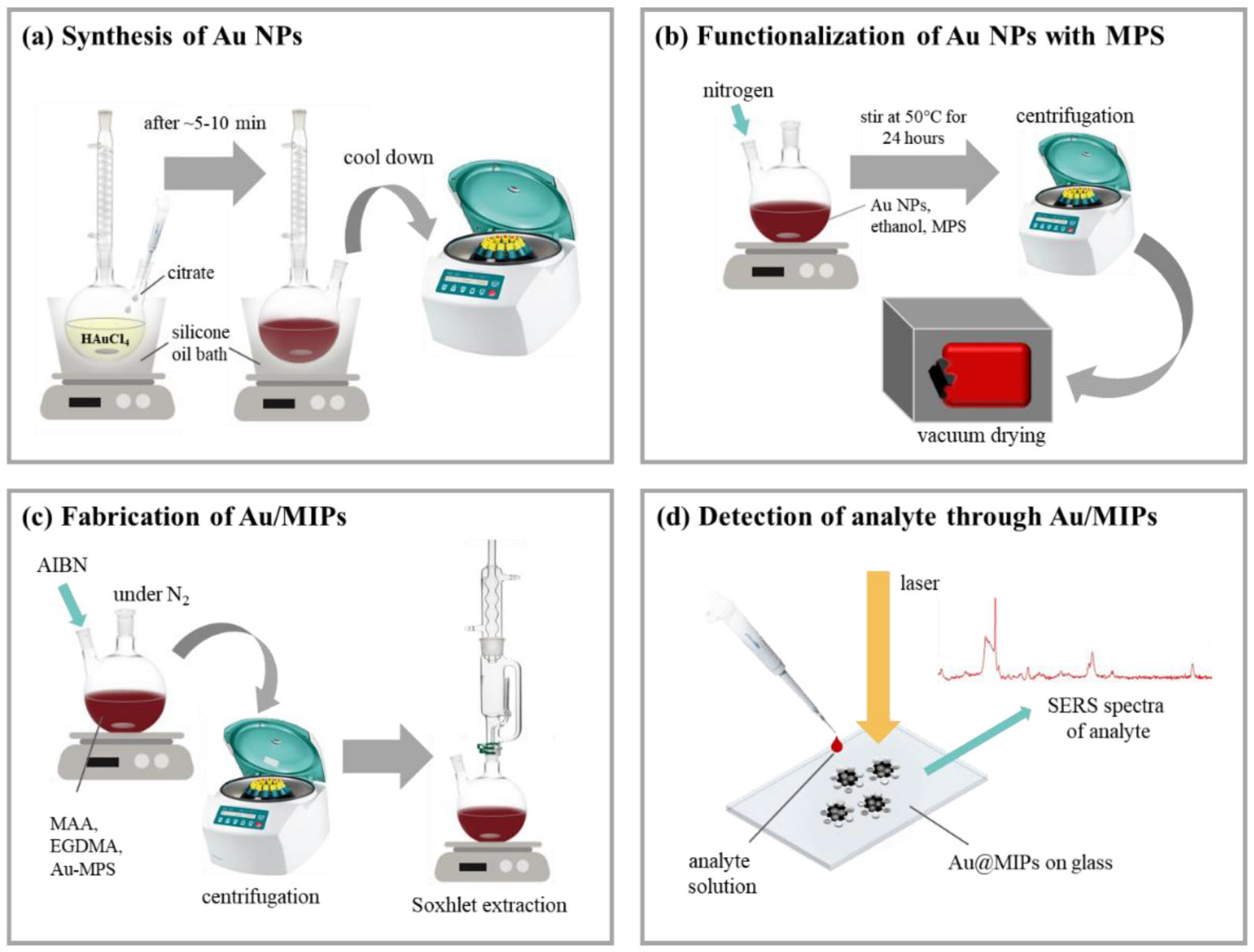
3.2. Nanomaterial-Based Sensors for Environmental Monitoring
3.3. Nanoparticle MIPs (NanoMIPs) for Chemosensing of Environmental Pollutants
- (i)
- High specificity and enhanced affinity that has unparalleled recognition characteristics when coupled with a transducer.
- (ii)
- An intensely sensitive transducer for monitoring and processing the binding potential between the monomer and the imprinted cavities.
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naidu, R.; Biswas, B.; Willett, I.R.; Cribb, J.; Singh, B.K.; Nathanail, C.P.; Coulon, F.; Semple, K.T.; Jones, K.C.; Barclay, A.; et al. Chemical pollution: A growing peril and potential catastrophic risk to humanity. Environ. Int. 2021, 156, 106616. [Google Scholar] [CrossRef]
- Gruber, K. Cleaning up our future health. Nature 2018, 555, S20–S22. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, L.; Li, D.; Zhu, L.; Tang, H.; Ouyang, L.; Li, D.; Zhu, L.; Tang, H. Precision Target Guide Strategy for Applying SERS into Environmental Monitoring. In Raman Spectroscopy and Applications; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Yu, C. Detection of chemical pollutants in water using gold nanoparticles as sensors: A review. Rev. Anal. Chem. 2013, 32, 1–14. [Google Scholar] [CrossRef] [Green Version]
- DLi, W.; Zhai, W.L.; Li, Y.T.; Long, Y.T. Recent progress in surface enhanced Raman spectroscopy for the detection of environmental pollutants. Microchim. Acta 2013, 181, 23–43. [Google Scholar] [CrossRef]
- Condorelli, M.; Litti, L.; Pulvirenti, M.; Scardaci, V.; Meneghetti, M.; Compagnini, G. Silver nanoplates paved PMMA cuvettes as a cheap and re-usable plasmonic sensing device. Appl. Surf. Sci. 2021, 566, 150701. [Google Scholar] [CrossRef]
- Jahn, I.J.; Mühlig, A.; Cialla-May, D. Application of molecular SERS nanosensors: Where we stand and where we are headed towards? Anal. Bioanal. Chem. 2020, 412, 5999. [Google Scholar] [CrossRef]
- Zribi, R.; Fazio, E.; Condorelli, M.; D’Urso, L.; Neri, G.; Corsaro, C.; Neri, F.; Compagnini, G.; Neri, G. H2O2 electrochemical sensing properties of size-tunable triangular Ag nanoplates. In Proceedings of the 2022 IEEE International Symposium on Medical Measurements and Applications, MeMeA 2022-Conference Proceedings, Messina, Italy, 22–24 June 2022. [Google Scholar] [CrossRef]
- Potara, M.; Gabudean, A.M.; Astilean, S. Solution-phase, dual LSPR-SERS plasmonic sensors of high sensitivity and stability based on chitosan-coated anisotropic silver nanoparticles. J. Mater. Chem. 2011, 21, 3625–3633. [Google Scholar] [CrossRef]
- Masson, J.F. Portable and field-deployed surface plasmon resonance and plasmonic sensors. Analyst 2020, 145, 3776–3800. [Google Scholar] [CrossRef] [PubMed]
- Saylan, Y.; Denizli, A. Highly Sensitive and Selective Plasmonic Sensing Platforms. In Plasmonic Sensors and Their Applications; Wiley Online Library: Hoboken, NJ, USA, 2021; pp. 55–69. [Google Scholar] [CrossRef]
- Choi, I. Recent Advances in Nanoplasmonic Sensors for Environmental Detection and Monitoring. J. Nanosci. Nanotechnol. 2016, 16, 4274–4283. [Google Scholar] [CrossRef] [PubMed]
- Esen, C.; Piletsky, S.A. Surface Plasmon Resonance Sensors Based on Molecularly Imprinted Polymers. In Plasmonic Sensors and Their Applications; Wiley Online Library: Hoboken, NJ, USA, 2021; pp. 221–236. [Google Scholar] [CrossRef]
- Rebelo, P.; Costa-Rama, E.; Seguro, I.; Pacheco, J.G.; Nouws, H.P.A.; Cordeiro, M.N.D.S.; Delerue-Matos, C. Molecularly imprinted polymer-based electrochemical sensors for environmental analysis. Biosens. Bioelectron. 2021, 172, 112719. [Google Scholar] [CrossRef]
- Lowdon, J.W.; Diliën, H.; Singla, P.; Peeters, M.; Cleij, T.J.; van Grinsven, B.; Eersels, K. MIPs for commercial application in low-cost sensors and assays—An overview of the current status quo. Sens. Actuators B Chem. 2020, 325, 128973. [Google Scholar] [CrossRef] [PubMed]
- Bowen, J.L.; Manesiotis, P.; Allender, C.J. Twenty years since ‘antibody mimics’ by molecular imprinting were first proposed: A critical perspective. Mol. Impr. 2013, 1, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Rico-Yuste, A.; Carrasco, S. Molecularly Imprinted Polymer-Based Hybrid Materials for the Development of Optical Sensors. Polymers 2019, 11, 1173. [Google Scholar] [CrossRef] [Green Version]
- Obare, S.O.; De, C.; Guo, W.; Haywood, T.L.; Samuels, T.A.; Adams, C.P.; Masika, N.O.; Murray, D.H.; Anderson, G.A.; Campbell, K.; et al. Fluorescent Chemosensors for Toxic Organophosphorus Pesticides: A Review. Sensors 2010, 10, 7018. [Google Scholar] [CrossRef]
- Bagra, B.; Mabe, T.; Tukur, F.; Wei, J. A plasmonic nanoledge array sensor for detection of anti-insulin antibodies of type 1 diabetes biomarker. Nanotechnology 2020, 31, 325503. [Google Scholar] [CrossRef]
- Zeng, Z.; Shi, X.; Mabe, T.; Christie, S.; Gilmore, G.; Smith, A.W.; Wei, J. Protein Trapping in Plasmonic Nanoslit and Nanoledge Cavities: The Behavior and Sensing. Anal. Chem. 2017, 89, 5221–5229. [Google Scholar] [CrossRef]
- Zeng, Z.; Mendis, M.N.; Waldeck, D.H.; Wei, J. A semi-analytical decomposition analysis of surface plasmon generation and the optimal nanoledge plasmonic device. RSC Adv. 2016, 6, 17196–17203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, M.; Lin, Y.; Wei, J.; Bono, T.; Lindquist, R.G. An enhanced LSPR fiber-optic nanoprobe for ultrasensitive detection of protein biomarkers. Biosens. Bioelectron. 2014, 61, 95–101. [Google Scholar] [CrossRef]
- Tukur, F.; Bagra, B.; Jayapalan, A.; Liu, M.; Tukur, P.; Wei, J. Plasmon–Exciton Coupling Effect in Nanostructured Arrays for Optical Signal Amplification and SARS-CoV-2 DNA Sensing. ACS Appl. Nano Mater. 2023, 6, 2071–2082. [Google Scholar] [CrossRef]
- Kaye, S.; Zeng, Z.; Sanders, M.; Chittur, K.; Koelle, P.M.; Lindquist, R.; Manne, U.; Lin, Y.; Wei, J. Label-free detection of DNA hybridization with a compact LSPR-based fiber-optic sensor. Analyst 2017, 142, 1974–1981. [Google Scholar] [CrossRef]
- Sajini, T.; Mathew, B. A brief overview of molecularly imprinted polymers: Highlighting computational design, nano and photo-responsive imprinting. Talanta Open 2021, 4, 100072. [Google Scholar] [CrossRef]
- Lichtenthaler, F.W. 100 Years “Schlüssel-Schloss-Prinzip”: What Made Emil Fischer Use this Analogy? Angew. Chem. Int. Ed. Engl. 1995, 33, 2364–2374. [Google Scholar] [CrossRef]
- Refaat, D.; Aggour, M.G.; Farghali, A.A.; Mahajan, R.; Wiklander, J.G.; Nicholls, I.A.; Piletsky, S.A. Strategies for Molecular Imprinting and the Evolution of MIP Nanoparticles as Plastic Antibodies—Synthesis and Applications. Int. J. Mol. Sci. 2019, 20, 6304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wulff, G.; Sarhan, A. Über die Anwendung von enzymanalog gebauten Polymeren zur Racemattrennung. Angew. Chem. 1972, 84, 364. [Google Scholar] [CrossRef]
- Arshady, R.; Mosbach, K. Synthesis of substrate-selective polymers by host-guest polymerization. Die Makromol. Chem. 1981, 182, 687–692. [Google Scholar] [CrossRef]
- Martínez, I.V.; Ek, J.I.; Ahn, E.C.; Sustaita, A.O. Molecularly imprinted polymers via reversible addition–fragmentation chain-transfer synthesis in sensing and environmental applications. RSC Adv. 2022, 12, 9186–9201. [Google Scholar] [CrossRef]
- Zhang, H. Molecularly Imprinted Nanoparticles for Biomedical Applications. Adv. Mater. 2020, 32, 1806328. [Google Scholar] [CrossRef]
- Canfarotta, F.; Czulak, J.; Guerreiro, A.; Cruz, A.G.; Piletsky, S.; Bergdahl, G.E.; Hedström, M.; Mattiasson, B. A novel capacitive sensor based on molecularly imprinted nanoparticles as recognition elements. Biosens. Bioelectron. 2018, 120, 108–114. [Google Scholar] [CrossRef]
- Hu, T.; Chen, R.; Wang, Q.; He, C.; Liu, S. Recent advances and applications of molecularly imprinted polymers in solid-phase extraction for real sample analysis. J. Sep. Sci. 2021, 44, 274–309. [Google Scholar] [CrossRef]
- Belbruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, X.; Liu, J.; Wang, J.; Liu, J.; Gao, Y.; Ma, N. Chemiluminescence sensors based on molecularly imprinted polymers for the determination of organophosphorus in milk. J. Dairy Sci. 2022, 105, 3019–3031. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.F.; Moreira, G.; Datta, S.P.A.; McLamore, E.; Vanegas, D. An Experimental Framework for Developing Point-of-Need Biosensors: Connecting Bio-Layer Interferometry and Electrochemical Impedance Spectroscopy. Biosensors 2022, 12, 938. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Luo, J.; Li, C.; Ma, M.; Yu, W.; Shen, J.; Wang, Z. Molecularly Imprinted Polymer as an Antibody Substitution in Pseudo-immunoassays for Chemical Contaminants in Food and Environmental Samples. J Agric. Food Chem. 2018, 66, 2561–2571. [Google Scholar] [CrossRef] [PubMed]
- Canfarotta, F.; Cecchini, A.; Piletsky, S. CHAPTER 1 Nano-sized Molecularly Imprinted Polymers as Artificial Antibodies. In Molecularly Imprinted Polymers for Analytical Chemistry Applications; Royal Society of Chemistry: London, UK, 2018; pp. 1–27. [Google Scholar] [CrossRef]
- Parisi, O.I.; Francomano, F.; Dattilo, M.; Patitucci, F.; Prete, S.; Amone, F.; Puoci, F. The Evolution of Molecular Recognition: From Antibodies to Molecularly Imprinted Polymers (MIPs) as Artificial Counterpart. J. Funct. Biomater. 2022, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Haupt, K. Peer Reviewed: Molecularly Imprinted Polymers: The Next Generation. Anal. Chem. 2003, 75, 376A–383A. [Google Scholar] [CrossRef] [Green Version]
- Smolinska-Kempisty, K.; Guerreiro, A.; Canfarotta, F.; Cáceres, C.; Whitcombe, M.J.; Piletsky, S. A comparison of the performance of molecularly imprinted polymer nanoparticles for small molecule targets and antibodies in the ELISA format. Sci. Rep. 2016, 6, 37638. [Google Scholar] [CrossRef] [Green Version]
- Vasapollo, G.; del Sole, R.; Mergola, L.; Lazzoi, M.R.; Scardino, A.; Scorrano, S.; Mele, G. Molecularly Imprinted Polymers: Present and Future Prospective. Int. J. Mol. Sci. 2011, 12, 5908–5945. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; Gao, Y.; Chen, G.; Huang, X.; Xu, X.; Lv, J.; Wang, J.; Xu, D.; Liu, G. Recent Advances and Future Trends in the Detection of Contaminants by Molecularly Imprinted Polymers in Food Samples. Front. Chem. 2020, 8, 1142. [Google Scholar] [CrossRef]
- Leibl, N.; Haupt, K.; Gonzato, C.; Duma, L. Molecularly Imprinted Polymers for Chemical Sensing: A Tutorial Review. Chemosensors 2021, 9, 123. [Google Scholar] [CrossRef]
- Mattiasson, B. MIPs as tools in environmental biotechnology. Adv. Biochem. Eng. Biotechnol. 2015, 150, 183–205. [Google Scholar] [CrossRef]
- Zarejousheghani, M.; Rahimi, P.; Borsdorf, H.; Zimmermann, S.; Joseph, Y. Molecularly imprinted polymer-based sensors for priority pollutants. Sensors 2021, 21, 2406. [Google Scholar] [CrossRef] [PubMed]
- Marć, M.; Wieczorek, P.P. Introduction to MIP synthesis, characteristics and analytical application. Compr. Anal. Chem. 2019, 86, 1–15. [Google Scholar] [CrossRef]
- Fu, X.; Yang, Q.; Zhou, Q.; Lin, Q.; Wang, C. Template-Monomer Interaction in Molecular Imprinting: Is the Strongest the Best? Open J. Org. Polym. Mater. 2015, 5, 58–68. [Google Scholar] [CrossRef] [Green Version]
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Costa, L.G. Organophosphorus Compounds at 80: Some Old and New Issues. Toxicol. Sci. 2018, 162, 24–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolopoulou-Stamati, P.; Hilbeck, A.; Zurich, E.; Alam, M.J.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front. Public Health 2016, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özkara, A.; Akyıl, D.; Konuk, M.; Özkara, A.; Akyıl, D.; Konuk, M. Pesticides, Environmental Pollution, and Health. In Environmental Health Risk-Hazardous Factors to Living Species; Wiley Online Library: Hoboken, NJ, USA, 2016. [Google Scholar] [CrossRef] [Green Version]
- Kaushal, J.; Khatri, M.; Arya, S.K. A treatise on Organophosphate pesticide pollution: Current strategies and advancements in their environmental degradation and elimination. Ecotoxicol. Environ. Saf. 2021, 207, 111483. [Google Scholar] [CrossRef] [PubMed]
- Bhogal, S.; Kaur, K.; Malik, A.K.; Sonne, C.; Lee, S.S.; Kim, K.H. Core-shell structured molecularly imprinted materials for sensing applications. Trends Anal. Chem. 2020, 133, 116043. [Google Scholar] [CrossRef]
- Schasfoort, R.B.M. Chapter 3: Surface Plasmon Resonance Instruments. In Handbook of Surface Plasmon Resonance; RSC Publishing: London, UK, 2017; pp. 60–105. [Google Scholar] [CrossRef]
- Saylan, Y.; Akgönüllü, S.; Denizli, A. Plasmonic Sensors for Monitoring Biological and Chemical Threat Agents. Biosensors 2020, 10, 142. [Google Scholar] [CrossRef]
- Sitjar, J.; Hou, Y.C.; der Liao, J.; Lee, H.; Xu, H.Z.; Fu, W.E.; Chen, G.D. Surface Imprinted Layer of Cypermethrin upon Au Nanoparticle as a Specific and Selective Coating for the Detection of Template Pesticide Molecules. Coatings 2020, 10, 751. [Google Scholar] [CrossRef]
- Stiles, P.L.; Dieringer, J.A.; Shah, N.C.; van Duyne, R.P. Surface-Enhanced Raman Spectroscopy. Annu. Rev. Anal. Chem. 2008, 1, 601–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- RPilot; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A Review on Surface-Enhanced Raman Scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef] [Green Version]
- Vasconcelos, H.; Coelho, L.C.C.; Matias, A.; Saraiva, C.; Jorge, P.A.S.; de Almeida, J.M.M.M. Biosensors for Biogenic Amines: A Review. Biosensors 2021, 11, 82. [Google Scholar] [CrossRef]
- Shrivastav, A.M.; Usha, S.P.; Gupta, B.D. Fiber optic profenofos sensor based on surface plasmon resonance technique and molecular imprinting. Biosens. Bioelectron. 2016, 79, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Saylan, Y.; Akgönüllü, S.; Çimen, D.; Derazshamshir, A.; Bereli, N.; Yılmaz, F.; Denizli, A. Development of surface plasmon resonance sensors based on molecularly imprinted nanofilms for sensitive and selective detection of pesticides. Sens. Actuators B Chem. 2017, 241, 446–454. [Google Scholar] [CrossRef]
- Kamra, T.; Zhou, T.; Montelius, L.; Schnadt, J.; Ye, L. Implementation of molecularly imprinted polymer beads for surface enhanced raman detection. Anal. Chem. 2015, 87, 5056–5061. [Google Scholar] [CrossRef]
- Guo, X.; Li, J.; Arabi, M.; Wang, X.; Wang, Y.; Chen, L. Molecular-Imprinting-Based Surface-Enhanced Raman Scattering Sensors. ACS Sens. 2020, 5, 601–619. [Google Scholar] [CrossRef]
- Oh, B.K.; Lee, W.; Chun, B.S.; Bae, Y.M.; Lee, W.H.; Choi, J.W. The fabrication of protein chip based on surface plasmon resonance for detection of pathogens. Biosens. Bioelectron. 2005, 20, 1847–1850. [Google Scholar] [CrossRef]
- van der Gaag, B.; Spath, S.; Dietrich, H.; Stigter, E.; Boonzaaijer, G.; van Osenbruggen, T.; Koopal, K. Biosensors and multiple mycotoxin analysis. Food Control. 2003, 14, 251–254. [Google Scholar] [CrossRef]
- Decorbie, N.; Tijunelyte, I.; Gam-Derouich, S.; Solard, J.; Lamouri, A.; Decorse, P.; Felidj, N.; Gauchotte-Lindsay, C.; Rinnert, E.; Mangeney, C.; et al. Sensing Polymer/Paracetamol Interaction with an Independent Component Analysis-Based SERS-MIP Nanosensor. Plasmonics 2020, 15, 1533–1539. [Google Scholar] [CrossRef]
- Agrawal, H.; Shrivastav, A.M.; Gupta, B.D. Surface plasmon resonance based optical fiber sensor for atrazine detection using molecular imprinting technique. Sens. Actuators B Chem. 2016, 227, 204–211. [Google Scholar] [CrossRef]
- Lokman, N.F.; Bakar, A.A.A.; Suja, F.; Abdullah, H.; Rahman, W.B.W.A.; Huang, N.M.; Yaacob, M.H. Highly sensitive SPR response of Au/chitosan/graphene oxide nanostructured thin films toward Pb (II) ions. Sens. Actuators B Chem. 2014, 195, 459–466. [Google Scholar] [CrossRef]
- Ren, X.; Feng, X.; Li, X.; Li, X. Preparation of silver with an ultrathin molecular imprinted layer for detection of carbendazim by SERS. Chem. Pap. 2021, 75, 6477–6485. [Google Scholar] [CrossRef]
- Cennamo, N.; D’Agostino, G.; Galatus, R.; Bibbò, L.; Pesavento, M.; Zeni, L. Sensors based on surface plasmon resonance in a plastic optical fiber for the detection of trinitrotoluene. Sens. Actuators B Chem. 2013, 188, 221–226. [Google Scholar] [CrossRef]
- Verma, R.; Gupta, B.D. Optical fiber sensor for the detection of tetracycline using surface plasmon resonance and molecular imprinting. Analyst 2013, 138, 7254–7263. [Google Scholar] [CrossRef]
- Hu, R.; Tang, R.; Xu, J.; Lu, F. Chemical nanosensors based on molecularly-imprinted polymers doped with silver nanoparticles for the rapid detection of caffeine in wastewater. Anal. Chim. Acta 2018, 1034, 176–183. [Google Scholar] [CrossRef]
- Akgönüllü, S.; Armutcu, C.; Denizli, A. Molecularly imprinted polymer film based plasmonic sensors for detection of ochratoxin A in dried Figure. Polym. Bull. 2022, 79, 4049–4067. [Google Scholar] [CrossRef]
- Gupta, B.D.; Shrivastav, A.M.; Usha, S.P. Surface Plasmon Resonance-Based Fiber Optic Sensors Utilizing Molecular Imprinting. Sensors 2016, 16, 1381. [Google Scholar] [CrossRef] [Green Version]
- Verma, R.; Gupta, B.D. Fiber optic SPR sensor for the detection of 3-pyridinecarboxamide (vitamin B3) using molecularly imprinted hydrogel. Sens. Actuators B Chem. 2013, 177, 279–285. [Google Scholar] [CrossRef]
- Castro-Grijalba, A.; Montes-García, V.; Cordero-Ferradás, M.J.; Coronado, E.; Pérez-Juste, J.; Pastoriza-Santos, I. SERS-Based Molecularly Imprinted Plasmonic Sensor for Highly Sensitive PAH Detection. ACS Sens. 2020, 5, 693–702. [Google Scholar] [CrossRef]
- Daniyal, W.M.E.M.M.S.; Sadrolhosseini, A.R.; Saleviter, S.; Omar, N.A.S.; Abdullah, J.; Fen, Y.W. Exploration of surface plasmon resonance for sensing copper ion based on nanocrystalline cellulose-modified thin film. Opt. Express 2018, 26, 34880–34893. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; She, Y.; Cao, X.; Ma, J.; Chen, G.; Hong, S.; Shao, Y.; EI-Aty, A.M.A.; Wang, M.; Wang, J. A molecularly imprinted polymer with integrated gold nanoparticles for surface enhanced Raman scattering based detection of the triazine herbicides, prometryn and simetryn. Mikrochim. Acta 2019, 186, 143. [Google Scholar] [CrossRef]
- Shrivastav, A.M.; Mishra, S.K.; Gupta, B.D. Localized and propagating surface plasmon resonance based fiber optic sensor for the detection of tetracycline using molecular imprinting. Mater. Res. Express 2015, 2, 035007. [Google Scholar] [CrossRef]
- Yu, J.C.C.; Lai, E.P.C. Interaction of ochratoxin A with molecularly imprinted polypyrrole film on surface plasmon resonance sensor. React. Funct. Polym. 2005, 63, 171–176. [Google Scholar] [CrossRef]
- Xue, Y.; Shao, J.; Sui, G.; Ma, Y.; Li, H. Rapid detection of orange II dyes in water with SERS imprinted sensor based on PDA-modified MOFs@Ag. J. Environ. Chem. Eng. 2021, 9, 106317. [Google Scholar] [CrossRef]
- Yao, G.H.; Liang, R.P.; Huang, C.F.; Wang, Y.; Qiu, J.D. Surface plasmon resonance sensor based on magnetic molecularly imprinted polymers amplification for pesticide recognition. Anal. Chem. 2013, 85, 11944–11951. [Google Scholar] [CrossRef]
- Cennamo, N.; D’Agostino, G.; Pesavento, M.; Zeni, L. High selectivity and sensitivity sensor based on MIP and SPR in tapered plastic optical fibers for the detection of l-nicotine. Sens. Actuators B Chem. 2014, 191, 529–536. [Google Scholar] [CrossRef]
- Gao, F.; Grant, E.; Lu, X. Determination of histamine in canned tuna by molecularly imprinted polymers-surface enhanced Raman spectroscopy. Anal. Chim. Acta 2015, 901, 68–75. [Google Scholar] [CrossRef]
- Langer, J.; de Aberasturi, D.J.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and future of surface-enhanced Raman scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef] [Green Version]
- Hua, M.Z.; Feng, S.; Wang, S.; Lu, X. Rapid detection and quantification of 2,4-dichlorophenoxyacetic acid in milk using molecularly imprinted polymers—Surface-enhanced Raman spectroscopy. Food Chem. 2018, 258, 254–259. [Google Scholar] [CrossRef]
- Feng, J.; Hu, Y.; Grant, E.; Lu, X. Determination of thiabendazole in orange juice using an MISPE-SERS chemosensor. Food Chem. 2018, 239, 816–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neng, J.; Liao, C.; Wang, Y.; Wang, Y.; Yang, K. Rapid and Sensitive Detection of Pentachloronitrobenzene by Surface-Enhanced Raman Spectroscopy Combined with Molecularly Imprinted Polymers. Biosensors 2022, 12, 52. [Google Scholar] [CrossRef]
- Sun, J.; Chen, C.; Zhang, Y.; Sun, X. A novel fluorescent molecularly imprinted polymer SiO2 @CdTe QDs@MIP for paraquat detection and adsorption. Luminescence 2021, 36, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Yu, N.; Shi, C.; Wang, X.; Wu, J. Surface Plasmon Resonance Sensor for Antibiotics Detection Based on Photo-Initiated Polymerization Molecularly Imprinted Array. Talanta 2016, 161, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Willner, M.R.; Vikesland, P.J. Nanomaterial enabled sensors for environmental contaminants. J. Nanobiotechnol. 2018, 16, 95. [Google Scholar] [CrossRef]
- Su, S.; Wu, W.; Gao, J.; Lu, J.; Fan, C. Nanomaterials-based sensors for applications in environmental monitoring. J. Mater. Chem. 2012, 22, 18101–18110. [Google Scholar] [CrossRef]
- Swierczewska, M.; Liu, G.; Lee, S.; Chen, X. High-sensitivity nanosensors for biomarker detection. Chem. Soc. Rev. 2012, 41, 2641. [Google Scholar] [CrossRef]
- He, X.; Deng, H.; Hwang, H. Nanosensors for Heavy Metal Detection in Environmental Media: Recent Advances and Future Trends. Nanosensors Environ. Food Agric. 2021, 1, 23–51. [Google Scholar] [CrossRef]
- Adam, T.; Dhahi, T.S. Nanosensors: Recent perspectives on attainments and future promise of downstream applications. Process Biochem. 2022, 117, 153–173. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, J.; Hong, S. Highly selective environmental nanosensors based on anomalous response of carbon nanotube conductance to mercury ions. J. Phys. Chem. C 2009, 113, 19393–19396. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Cheng, J. Molecularly imprinted silica nanospheres embedded Cdse quantum dots for highly selective and sensitive optosensing of pyrethroids. Chem. Mater. 2010, 22, 2451–2457. [Google Scholar] [CrossRef]
- Moraes, F.C.; Mascaro, L.H.; Machado, S.A.S.; Brett, C.M.A. Direct electrochemical determination of carbaryl using a multi-walled carbon nanotube/cobalt phthalocyanine modified electrode. Talanta 2009, 79, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Govindasamy, M.; Mani, V.; Chen, S.M.; Chen, T.W.; Sundramoorthy, A.K. Methyl parathion detection in vegetables and fruits using silver@graphene nanoribbons nanocomposite modified screen printed electrode. Sci. Rep. 2017, 7, 46471. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Cui, X.; Chen, G.; Wang, Y.; Qin, G.; Li, M.; Zhang, X.; Zhang, Y.; Zhang, C.; Du, P.; et al. Simple and sensitive detection of triazophos pesticide by using quantum dots nanobeads based on immunoassay. Food Agric. Immunol. 2019, 30, 522–532. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Kim, G.W.; Park, T.J. A facile and sensitive detection of organophosphorus chemicals by rapid aggregation of gold nanoparticles using organic compounds. Biosens. Bioelectron. 2015, 67, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Altintas, Z. Surface Plasmon Resonance Based Sensor for the Detection of Glycopeptide Antibiotics in Milk Using Rationally Designed NanoMIPs. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Lieberzeit, P.A.; Afzal, A.; Rehman, A.; Dickert, F.L. Nanoparticles for detecting pollutants and degradation processes with mass-sensitive sensors. Sens. Actuators B Chem. 2007, 127, 132–136. [Google Scholar] [CrossRef]
- Poma, A.; Turner, A.P.F.; Piletsky, S.A. Advances in the manufacture of MIP nanoparticles. Trends Biotechnol. 2010, 28, 629–637. [Google Scholar] [CrossRef]
- D’aurelio, R.; Ashley, J.; Rodgers, T.L.; Trinh, L.; Temblay, J.; Pleasants, M.; Tothill, I.E. Development of a NanoMIPs-SPR-Based Sensor for β-Lactoglobulin Detection. Chemosensors 2020, 8, 94. [Google Scholar] [CrossRef]
- Ashley, J.; Shukor, Y.; D’Aurelio, R.; Trinh, L.; Rodgers, T.L.; Temblay, J.; Pleasants, M.; Tothill, I.E. Synthesis of Molecularly Imprinted Polymer Nanoparticles for α-Casein Detection Using Surface Plasmon Resonance as a Milk Allergen Sensor. ACS Sens. 2018, 3, 418–424. [Google Scholar] [CrossRef] [Green Version]
- Garcia, R.; Cabrita, M.J.; Maria, A.; Freitas, C. Application of Molecularly Imprinted Polymers for the Analysis of Pesticide Residues in Food—A Highly Selective and Innovative Approach. Am. J. Analyt. Chem. 2011, 2, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Ghaeni, F.A.; Karimi, G.; Mohsenzadeh, M.S.; Nazarzadeh, M.; Motamedshariaty, V.S.; Mohajeri, S.A. Preparation of dual-template molecularly imprinted nanoparticles for organophosphate pesticides and their application as selective sorbents for water treatment. Sep. Sci. Technol. 2018, 53, 2517–2526. [Google Scholar] [CrossRef]
- Piletska, E.V.; Czulak, J.; Piletsky, S.S.; Guerreiro, A.; Canfarotta, F.; Piletsky, S.A. Novel assay format for proteins based on magnetic molecularly imprinted polymer nanoparticles—Detection of pepsin. J. Chin. Adv. Mater. Soc. 2018, 6, 341–351. [Google Scholar] [CrossRef]
- Motaharian, A.; Motaharian, F.; Abnous, K.; Hosseini, M.R.M.; Hassanzadeh-Khayyat, M. Molecularly imprinted polymer nanoparticles-based electrochemical sensor for determination of diazinon pesticide in well water and apple fruit samples. Anal. Bioanal. Chem. 2016, 408, 6769–6779. [Google Scholar] [CrossRef]
- Wu, M.; Deng, H.; Fan, Y.; Hu, Y.; Guo, Y.; Xie, L. Rapid Colorimetric Detection of Cartap Residues by AgNP Sensor with Magnetic Molecularly Imprinted Microspheres as Recognition Elements. Molecules 2018, 23, 1443. [Google Scholar] [CrossRef] [Green Version]
- Mankar, J.S.; Sharma, M.D.; Krupadam, R.J. Molecularly imprinted nanoparticles (nanoMIPs): An efficient new adsorbent for removal of arsenic from water. J. Mater. Sci. 2020, 55, 6810–6825. [Google Scholar] [CrossRef]
- Guo, W.; Engelman, B.J.; Haywood, T.L.; Blok, N.B.; Beaudoin, D.S.; Obare, S.O. Dual fluorescence and electrochemical detection of the organophosphorus pesticides-ethion, malathion and fenthion. Talanta 2011, 87, 276–283. [Google Scholar] [CrossRef] [PubMed]

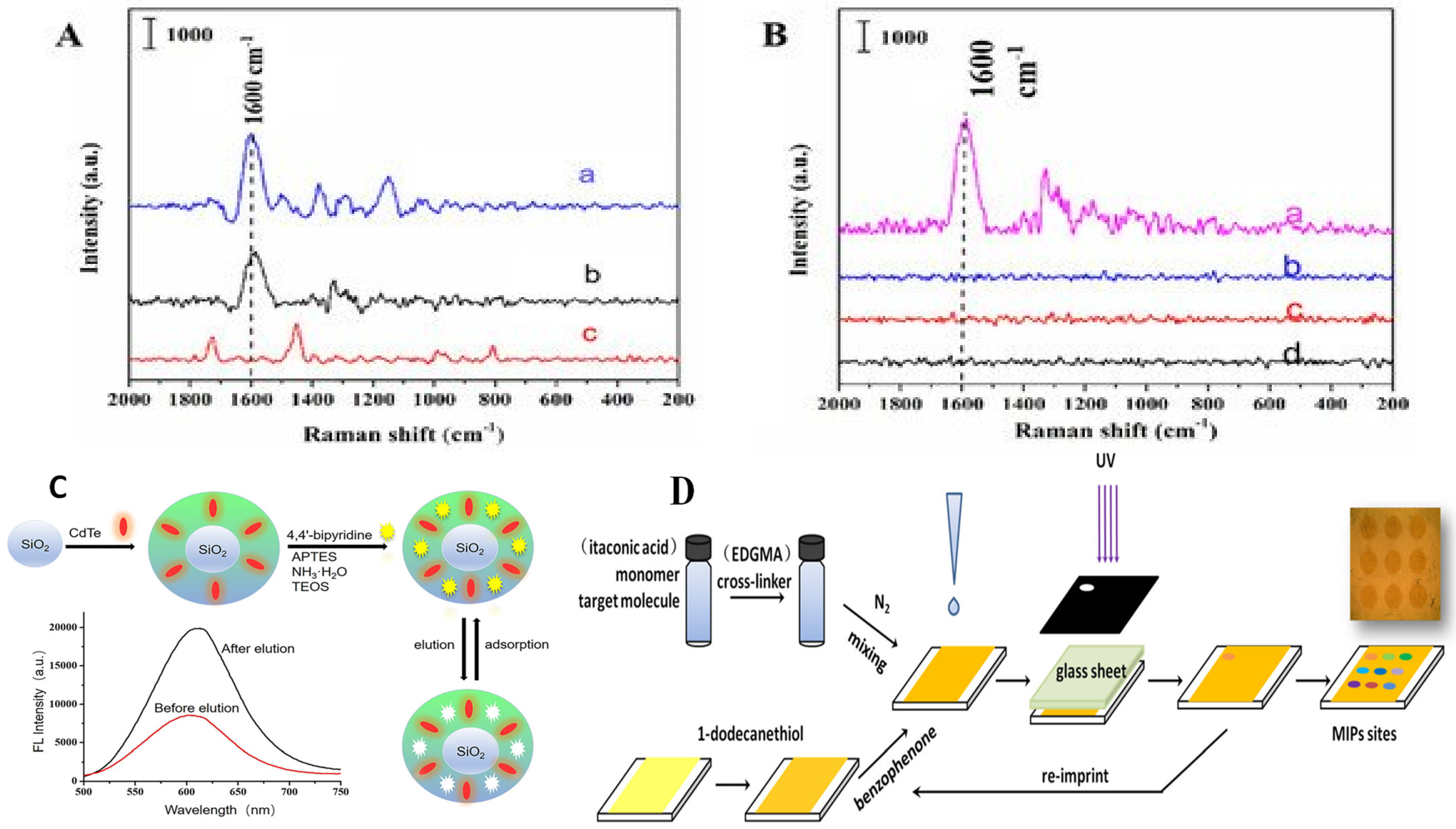
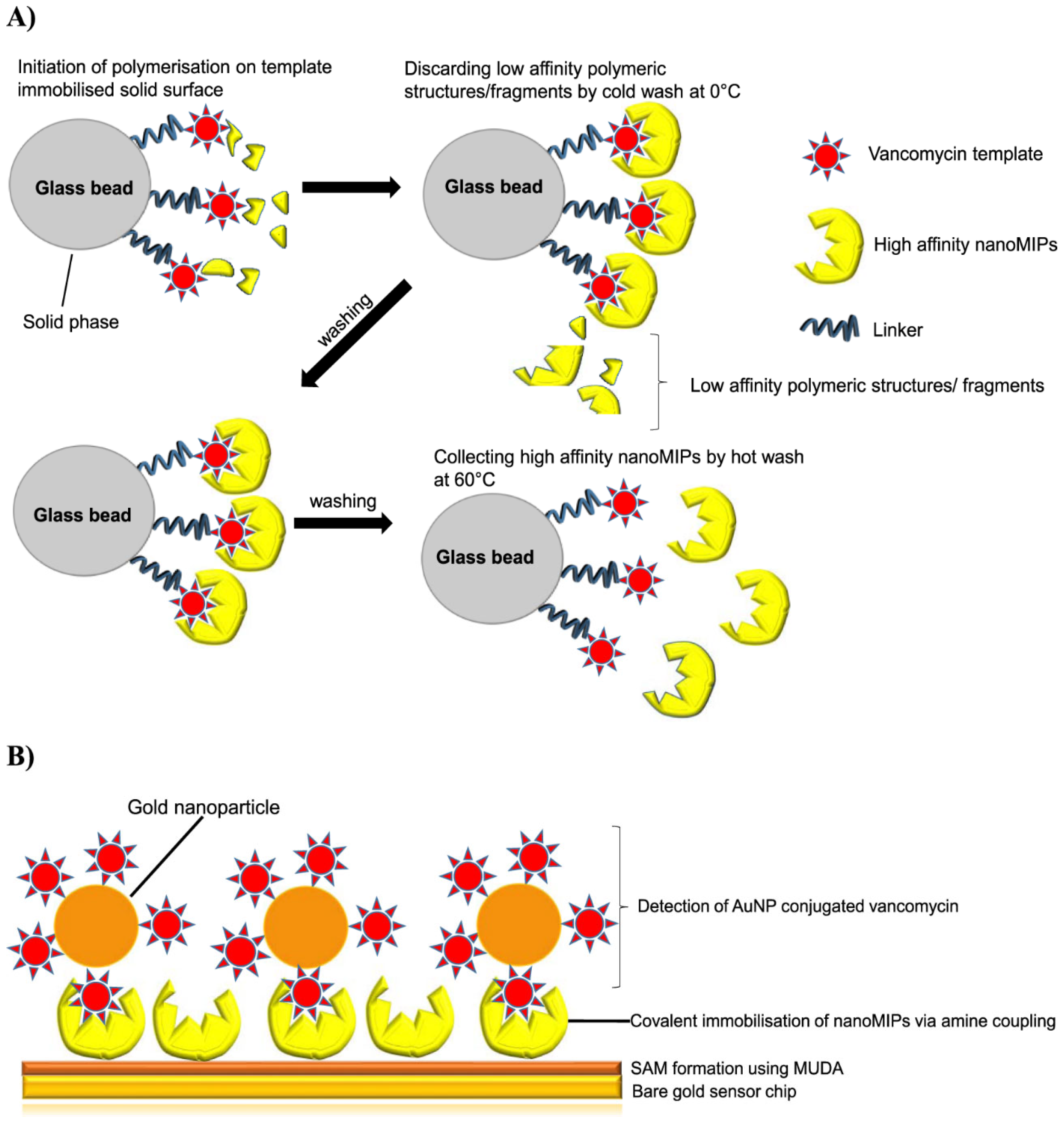
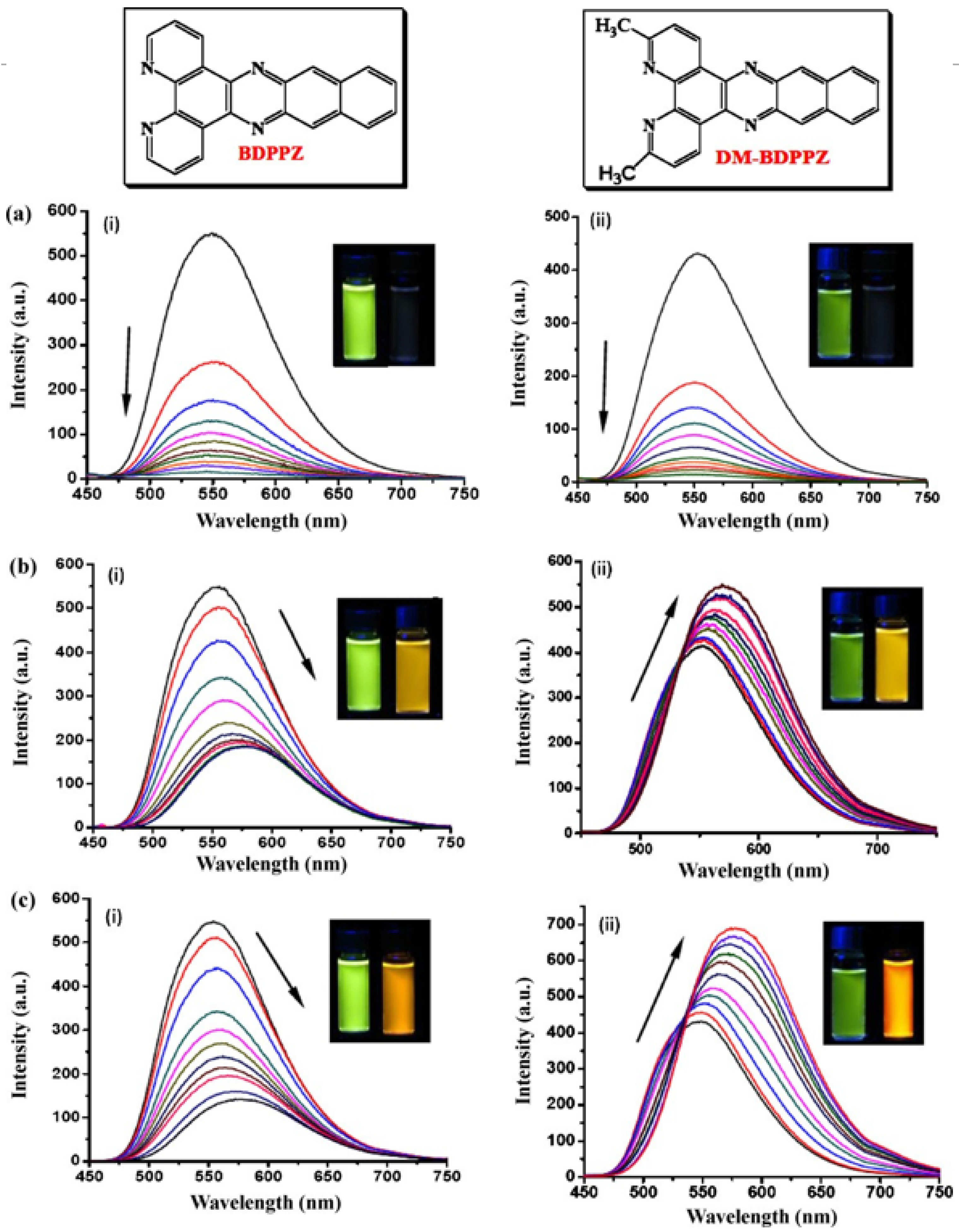
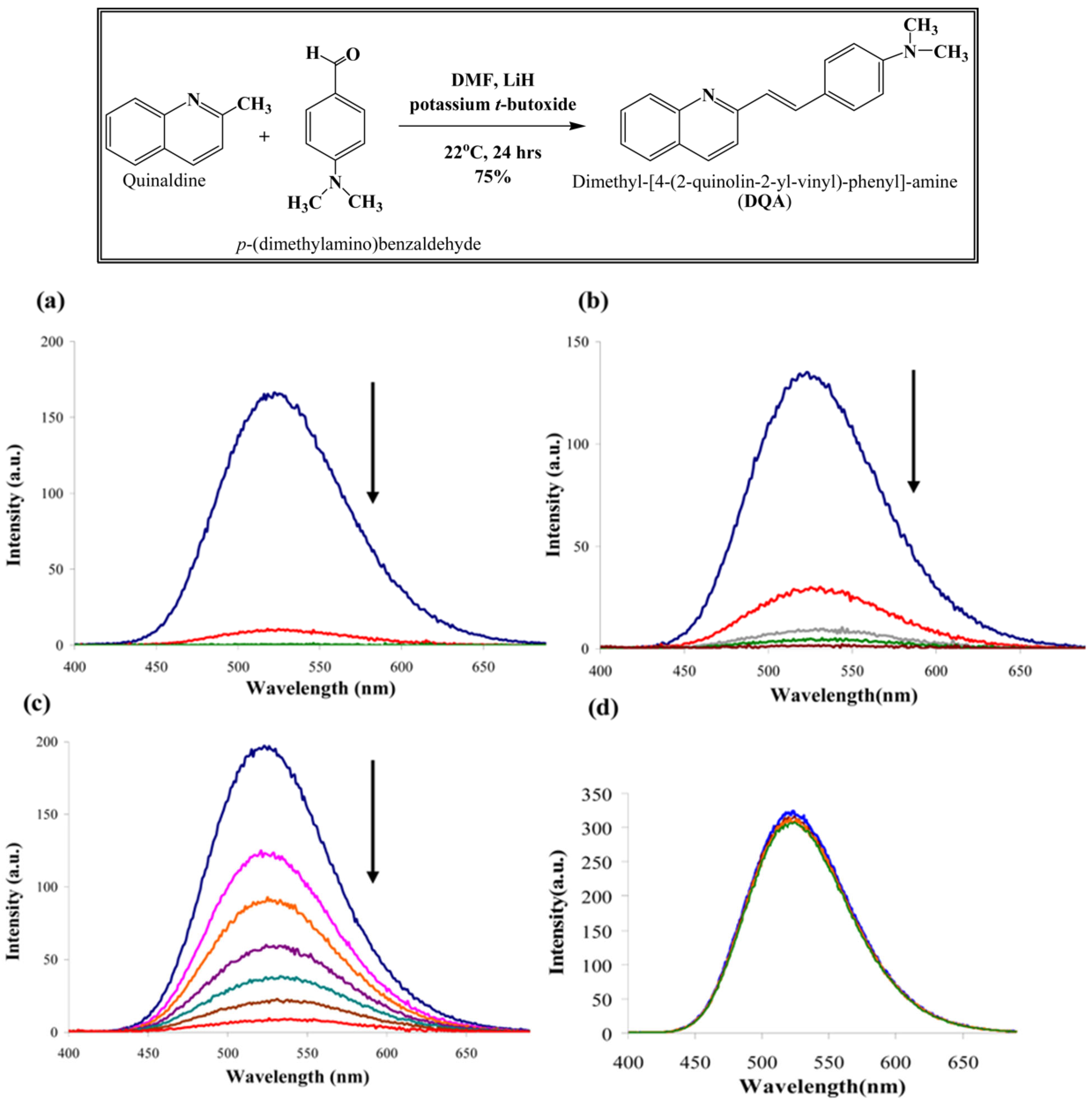
| Analyte | Sample | Method | Sensing Layer/Substrate | LOD | References |
|---|---|---|---|---|---|
| Escherichia coli O157:H7 | Contaminated water | SPR | Antibody/Gold-coated thin glass | 1.0 × 105 CFU/mL | [65] |
| Aflatoxin B1 | Peanuts | SPR | Antigen/Gold-coated thin glass | 0.2 ng/gr | [66] |
| Fenthion | Milk | Chemiluminescence | MIP | 2.36 pg/mL | [35] |
| Diazinon | Milk | Chemiluminescence | MIP | 4.6 pg/mL | [35] |
| Paracetamol | Wastewater | SERS | MIP-AuNPs | 300 nM | [67] |
| Atrazine | Drinking water | SPR | Silver MIP/Fiber optic core | 1.92 × 10−14 | [68] |
| VOCs (1-octanol) | Organic compounds | SPRi | Biomimetic peptides/Gold-coated thin glass | 375 ppb | [43] |
| Lead (II) ions | Wastewater | SPR | Silver-Cesium/Gold-coated thin glass | 30 ppb | [69] |
| Carbendazim | Water | SERS | Ag-MIP | 1.0 × 10−9 M | [70] |
| Parathion | Milk | Chemiluminescence | MIP | 1.14 pg/mL | [35] |
| Fenitrothion | Milk | Chemiluminescence | MIP | 1.1 pg/mL | [35] |
| Trinitrotoluene (TNT) | Soil/water | SPR | Gold MIP/Fiber optic core | 5.1 × 10−5 M | [71] |
| Oxytetracycline | Fruits and vegetables | SPR | Silver MIP/Fiber optic core | 0.01 µM | [72] |
| Caffeine | Wastewater | SERS | Silver MIP | 100 ng L−1 | [73] |
| Ochratoxin A | Dried fig | SPR | MIP | 0.028 ng/mL | [74] |
| Erythromycin | Vegetables | SPR | Silver MIP/Fiber optic core | 6.2 × 10−8 M | [75] |
| Vitamin B3 | Fruits and vegetables | SPR | Silver MIP/Fiber optic core | 0.5 mg/mL | [76] |
| Polycyclic aromatic hydrocarbons (PAH) | Sea water | SERS | MIP/Gold-coated thin glass | 1 nM | [77] |
| Chlorpyrifos | Milk | Chemiluminescence | MIP | 2.14 pg/mL | [35] |
| Copper (II) ions | Drinking water | SPR | CTA-NCC-GO/Gold-coated thin glass | 0.01 ppm | [78] |
| Simetryn | Rice | SERS | GoldNPs-MIP | 0.05 mg/Kg | [79] |
| Fenchlorphos | Milk | Chemiluminescence | MIP | 1.7 pg/mL | [35] |
| Tetracycline | Vegetables | SPR/LSPR | Silver NP/MIP/Fiber optic core | 2.2 × 10−9 M | [80] |
| Prometryn | Wheat | SERS | MIP-AuNPs | 0.05 mg/Kg | [79] |
| Ochratoxin A | Wheat/wine | SPR | Polypyrrole (MIP) film | 0.01 mg/mL | [81] |
| Coumaphos | Milk | Chemiluminescence | MIP | 1.8 pg/mL | [35] |
| Orange II dye | Lake water | SERS | Ag-MIP | 10−10 M | [82] |
| Chlorpyrifos | Apples | SPR | MIP Fe3O4-PDA NPs/Gold-coated thin glass | 0.76 nM | [83] |
| Nicotine | Vegetables | SPR | Gold MIP/Fiber optic core PMMA | 1.86 pM | [84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayivi, R.D.; Adesanmi, B.O.; McLamore, E.S.; Wei, J.; Obare, S.O. Molecularly Imprinted Plasmonic Sensors as Nano-Transducers: An Effective Approach for Environmental Monitoring Applications. Chemosensors 2023, 11, 203. https://doi.org/10.3390/chemosensors11030203
Ayivi RD, Adesanmi BO, McLamore ES, Wei J, Obare SO. Molecularly Imprinted Plasmonic Sensors as Nano-Transducers: An Effective Approach for Environmental Monitoring Applications. Chemosensors. 2023; 11(3):203. https://doi.org/10.3390/chemosensors11030203
Chicago/Turabian StyleAyivi, Raphael D., Bukola O. Adesanmi, Eric S. McLamore, Jianjun Wei, and Sherine O. Obare. 2023. "Molecularly Imprinted Plasmonic Sensors as Nano-Transducers: An Effective Approach for Environmental Monitoring Applications" Chemosensors 11, no. 3: 203. https://doi.org/10.3390/chemosensors11030203
APA StyleAyivi, R. D., Adesanmi, B. O., McLamore, E. S., Wei, J., & Obare, S. O. (2023). Molecularly Imprinted Plasmonic Sensors as Nano-Transducers: An Effective Approach for Environmental Monitoring Applications. Chemosensors, 11(3), 203. https://doi.org/10.3390/chemosensors11030203









