Electrospun Nanofibers as Chemosensors for Detecting Environmental Pollutants: A Review
Abstract
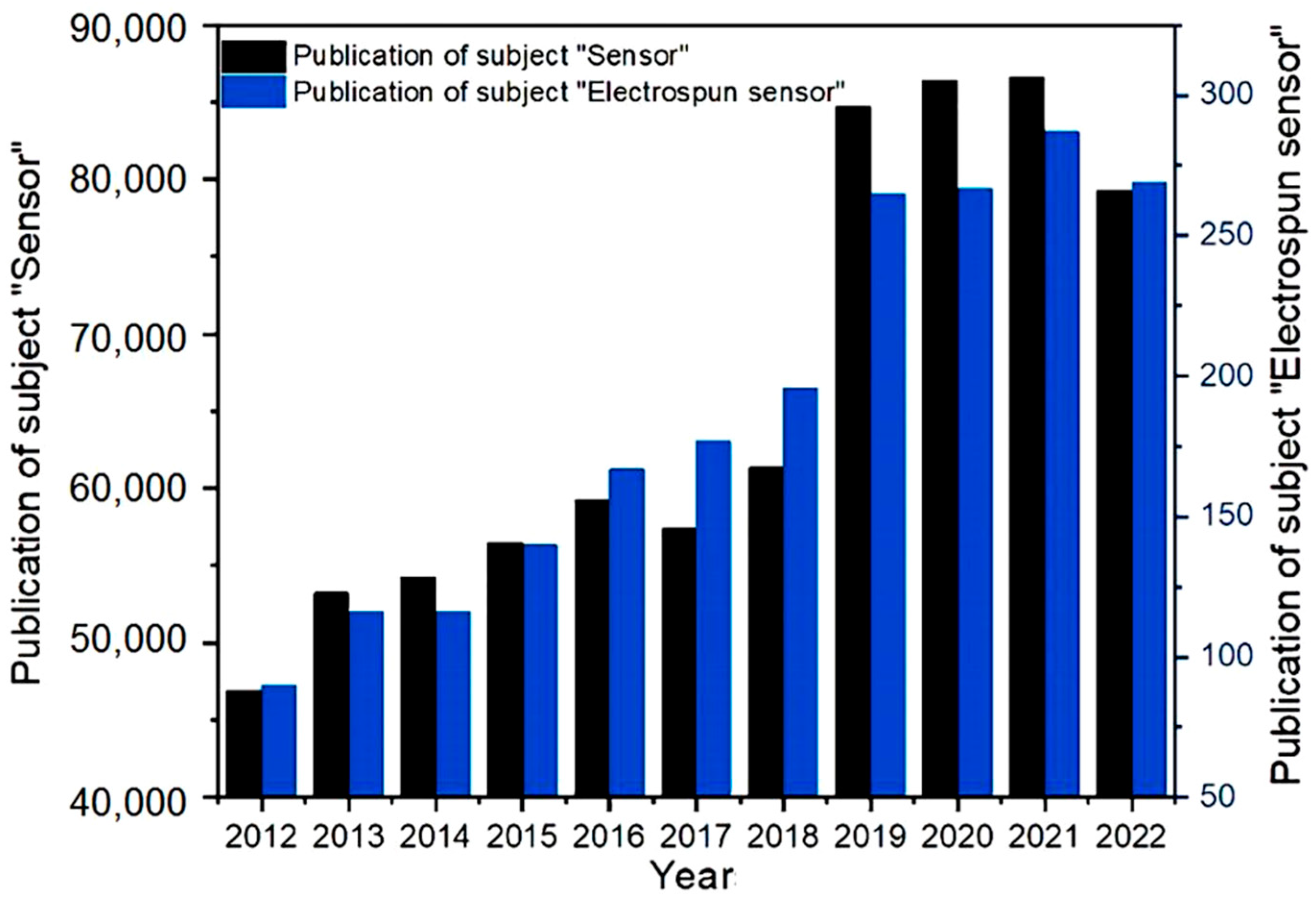
1. Introduction
2. Electrospinning
3. Type of Detection Method for the Electrospun-Nanofibers-Based Chemosensors
3.1. Electrochemical Sensor
3.2. Optical Sensor
4. Application Area for the Electrospun-Nanofibers-Based Chemosensors
4.1. Water Pollutant
4.2. Gas Pollutant
4.3. Soil Pollutant
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dey, T.K.; Uddin, M.E.; Jamal, M. Detection and Removal of Microplastics in Wastewater: Evolution and Impact. Environ. Sci. Pollut. Res. 2021, 28, 16925–16947. [Google Scholar] [CrossRef]
- Wayman, C.; Niemann, H. The Fate of Plastic in the Ocean Environment—A Minireview. Environ. Sci.-Process. Impacts 2021, 23, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Tullo, E.; Finzi, A.; Guarino, M. Review: Environmental Impact of Livestock Farming and Precision Livestock Farming as a Mitigation Strategy. Sci. Total Environ. 2019, 650, 2751–2760. [Google Scholar] [CrossRef]
- Mai, L.; Bao, L.-J.; Shi, L.; Wong, C.S.; Zeng, E.Y. A Review of Methods for Measuring Microplastics in Aquatic Environments. Environ. Sci. Pollut. Res. 2018, 25, 11319–11332. [Google Scholar] [CrossRef]
- Yuan, Z.; Nag, R.; Cummins, E. Human Health Concerns Regarding Microplastics in the Aquatic Environment—From Marine to Food Systems. Sci. Total Environ. 2022, 823, 153730. [Google Scholar] [CrossRef]
- Smith, D.M.; Scaife, A.A.; Eade, R.; Athanasiadis, P.; Bellucci, A.; Bethke, I.; Bilbao, R.; Borchert, L.F.; Caron, L.-P.; Counillon, F.; et al. North Atlantic Climate Far More Predictable than Models Imply. Nature 2020, 583, 796–800. [Google Scholar] [CrossRef]
- Liu, X.; Reddi, K.; Elgowainy, A.; Lohse-Busch, H.; Wang, M.; Rustagi, N. Comparison of Well-to-Wheels Energy Use and Emissions of a Hydrogen Fuel Cell Electric Vehicle Relative to a Conventional Gasoline-Powered Internal Combustion Engine Vehicle. Int. J. Hydrogen Energy 2020, 45, 972–983. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Chauhan, A.; Datta, S.; Wani, A.B.; Singh, N.; Singh, J. Toxicity, Degradation and Analysis of the Herbicide Atrazine. Environ. Chem. Lett. 2018, 16, 211–237. [Google Scholar] [CrossRef]
- Li, H.-Y.; Zhao, S.-N.; Zang, S.-Q.; Li, J. Functional Metal-Organic Frameworks as Effective Sensors of Gases and Volatile Compounds. Chem. Soc. Rev. 2020, 49, 6364–6401. [Google Scholar] [CrossRef] [PubMed]
- Le, V.T.; Vasseghian, Y.; Doan, V.D.; Nguyen, T.T.T.; Vo, T.-T.T.; Do, H.H.; Vu, K.B.; Vu, Q.H.; Lam, T.D.; Tran, V.A. Flexible and High-Sensitivity Sensor Based on Ti3C2-MoS2 MXene Composite for the Detection of Toxic Gases. Chemosphere 2022, 291, 133025. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Cheng, B.; Fan, J.; Yu, J. A High-Response Formaldehyde Sensor Based on Fibrous Ag-ZnO/In2O3 with Multi-Level Heterojunctions. J. Hazard. Mater. 2021, 413, 125352. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhu, B.; Jiang, X.; Han, G.; Li, S.; Lau, C.H.; Wu, Y.; Zhang, Y.; Shao, L. Symbiosis-Inspired de Novo Synthesis of Ultrahigh MOF Growth Mixed Matrix Membranes for Sustainable Carbon Capture. Proc. Natl. Acad. Sci. USA 2022, 119, e2114964119. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Meng, W.-K.; Li, L.; Xu, G.-J.; Wang, X.; Chen, L.-Z.; Wang, M.-L.; Lin, J.-M.; Zhao, R.-S. Facile Room-Temperature Synthesis of a Spherical Mesoporous Covalent Organic Framework for Ultrasensitive Solid-Phase Microextraction of Phenols Prior to Gas Chromatography-Tandem Mass Spectrometry. Chem. Eng. J. 2019, 369, 920–927. [Google Scholar] [CrossRef]
- Liu, L.; Meng, W.-K.; Zhou, Y.-S.; Wang, X.; Xu, G.-J.; Wang, M.-L.; Lin, J.-M.; Zhao, R.-S. Beta-Ketoenamine-Linked Covalent Organic Framework Coating for Ultra-High-Performance Solid-Phase Microextraction of Polybrominated Diphenyl Ethers from Environmental Samples. Chem. Eng. J. 2019, 356, 926–933. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Zhang, J.-W.; Cao, C.-F.; Guo, K.-Y.; Zhao, L.; Zhang, G.-D.; Gao, J.-F.; Tang, L.-C. Temperature-Responsive Resistance Sensitivity Controlled by L-Ascorbic Acid and Silane Co-Functionalization in Flame-Retardant GO Network for Efficient Fire Early-Warning Response. Chem. Eng. J. 2020, 386, 123894. [Google Scholar] [CrossRef]
- Tang, W.; Wang, D.; Wang, J.; Wu, Z.; Li, L.; Huang, M.; Xu, S.; Yan, D. Pyrethroid Pesticide Residues in the Global Environment: An Overview. Chemosphere 2018, 191, 990–1007. [Google Scholar] [CrossRef]
- van den Berg, N.J.; van Soest, H.L.; Hof, A.F.; den Elzen, M.G.J.; van Vuuren, D.P.; Chen, W.; Drouet, L.; Emmerling, J.; Fujimori, S.; Hoehne, N.; et al. Implications of Various Effort-Sharing Approaches for National Carbon Budgets and Emission Pathways. Clim. Chang. 2020, 162, 1805–1822. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, P.; Manna, C.; Jain, M. Abundance, Interaction, Ingestion, Ecological Concerns, and Mitigation Policies of Microplastic Pollution in Riverine Ecosystem: A Review. Sci. Total Environ. 2021, 782, 146695. [Google Scholar] [CrossRef]
- Kubra, K.T.; Salman, M.S.; Znad, H.; Hasan, M.N. Efficient Encapsulation of Toxic Dye from Wastewater Using Biodegradable Polymeric Adsorbent. J. Mol. Liq. 2021, 329, 115541. [Google Scholar] [CrossRef]
- Pang, X.; Skillen, N.; Gunaratne, N.; Rooney, D.W.; Robertson, P.K.J. Removal of Phthalates from Aqueous Solution by Semiconductor Photocatalysis: A Review. J. Hazard. Mater. 2021, 402, 123461. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, G.; Li, M.; Singh, R.; Marques, C.; Min, R.; Kaushik, B.K.; Zhang, B.; Jha, R.; Kumar, S. Water Pollutants P-Cresol Detection Based on Au-ZnO Nanoparticles Modified Tapered Optical Fiber. Ieee Trans. Nanobiosci. 2021, 20, 377–384. [Google Scholar] [CrossRef]
- Fan, L.; Zhao, D.; Li, B.; Wang, F.; Deng, Y.; Peng, Y.; Wang, X.; Zhang, X. Luminescent Binuclear Zinc(II) Organic Framework as Bifunctional Water-Stable Chemosensor for Efficient Detection of Antibiotics and Cr(VI) Anions in Water. Spectrochim. Acta Part-Mol. Biomol. Spectrosc. 2022, 264, 120232. [Google Scholar] [CrossRef]
- Wagner, M.; Lin, K.-Y.A.; Oh, W.-D.; Lisak, G. Metal-Organic Frameworks for Pesticidal Persistent Organic Pollutants Detection and Adsorption—A Mini Review. J. Hazard. Mater. 2021, 413, 125325. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Han, X.; Zhang, L.; Wang, X.; Chen, L. Fluorescent Probe for Mercury Ion Imaging Analysis: Strategies and Applications. Chem. Eng. J. 2021, 406, 127166. [Google Scholar] [CrossRef]
- Qian, L.; Durairaj, S.; Prins, S.; Chen, A. Nanomaterial-Based Electrochemical Sensors and Biosensors for the Detection of Pharmaceutical Compounds. Biosens. Bioelectron. 2021, 175, 112836. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, A.; Xiong, M.; Macharia, D.K.; Liu, J.; Chen, Z.; Li, M.; Zhang, L. TiO2/BiOI p-n Junction-Decorated Carbon Fibers as Weavable Photocatalyst with UV-Vis Photoresponsive for Efficiently Degrading Various Pollutants. Chem. Eng. J. 2021, 415, 129019. [Google Scholar] [CrossRef]
- Balram, D.; Lian, K.-Y.; Sebastian, N.; Al-Mubaddel, F.S.; Noman, M.T. Bi-Functional Renewable Biopolymer Wrapped CNFs/Ag Doped Spinel Cobalt Oxide as a Sensitive Platform for Highly Toxic Nitroaromatic Compound Detection and Degradation. Chemosphere 2022, 291, 132998. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-J.; Huang, X.-P.; Xiang, L.; Wang, Y.-Z.; Li, Y.-W.; Li, H.; Cai, Q.-Y.; Mo, C.-H.; Wong, M.-H. Source, Migration and Toxicology of Microplastics in Soil. Environ. Int. 2020, 137, 105263. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, Z.M. The next Generation of Soil and Water Bodies Heavy Metals Prediction and Detection: New Expert System Based Edge Cloud Server and Federated Learning Technology. Environ. Pollut. 2022, 313, 120081. [Google Scholar] [CrossRef]
- Lu, Y.; Liang, X.; Niyungeko, C.; Zhou, J.; Xu, J.; Tian, G. A Review of the Identification and Detection of Heavy Metal Ions in the Environment by Voltammetry. Talanta 2018, 178, 324–338. [Google Scholar] [CrossRef]
- He, S.; Wei, Y.; Yang, C.; He, Z. Interactions of Microplastics and Soil Pollutants in Soil-Plant Systems. Environ. Pollut. 2022, 315, 120357. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Nejad, F.G.; Dourandish, Z.; Khalilzadeh, M.A.; Jang, H.W.; Venditti, R.A.; Varma, R.S.; Shokouhimehr, M. Recent Developments in Polymer Nanocomposite-Based Electrochemical Sensors for Detecting Environmental Pollutants. Ind. Eng. Chem. Res. 2021, 60, 1112–1136. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Zhao, H.; Ye, H.; Zhao, L. Be-Original Break New Ground: Fluorescence Sensing of Humic Acid in Natural Water and Soil by Pitaya Seed Carbon Dots. Spectrochim. Acta Part-Mol. Biomol. Spectrosc. 2023, 286, 121950. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, A.K.; Tripathy, S.K.; Sundaray, M. An Ultra-Sensitive Optical Sensor Based on Agarose Coated Microscopic Sphere to Detect Cu2+ ion in Soil. Comput. Electron. Agric. 2022, 202, 107424. [Google Scholar] [CrossRef]
- Huang, D.; Gao, L.; Zheng, M.; Qiao, L.; Xu, C.; Wang, K.; Wang, S. Screening Organic Contaminants in Soil by Two-Dimensional Gas Chromatography High-Resolution Time-of-Flight Mass Spectrometry: A Non-Target Analysis Strategy and Contaminated Area Case Study. Environ. Res. 2022, 205, 112420. [Google Scholar] [CrossRef]
- Shi, T.; Chen, Y.; Liu, Y.; Wu, G. Visible and Near-Infrared Reflectance Spectroscopy-An Alternative for Monitoring Soil Contamination by Heavy Metals. J. Hazard. Mater. 2014, 265, 166–176. [Google Scholar] [CrossRef]
- Ivleva, N.P. Chemical Analysis of Microplastics and Nanoplastics: Challenges, Advanced Methods, and Perspectives. Chem. Rev. 2021, 121, 11886–11936. [Google Scholar] [CrossRef]
- Kaushal, J.; Khatri, M.; Arya, S.K. A Treatise on Organophosphate Pesticide Pollution: Current Strategies and Advancements in Their Environmental Degradation and Elimination. Ecotoxicol. Environ. Saf. 2021, 207, 111483. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Huang, X.; Chen, L.; Yan, L.; Zhu, F.; Li, H. Fabrication of Molecularly Imprinted Nanochannel Membrane for Ultrasensitive Electrochemical Detection of Triphenyl Phosphate. Anal. Chim. Acta 2022, 1192, 339374. [Google Scholar] [CrossRef]
- Rebelo, P.; Costa-Rama, E.; Seguro, I.; Pacheco, J.G.; Nouws, H.P.A.; Cordeiro, M.N.D.S.; Delerue-Matos, C. Molecularly Imprinted Polymer-Based Electrochemical Sensors for Environmental Analysis. Biosens. Bioelectron. 2021, 172, 112719. [Google Scholar] [CrossRef]
- Song, J.; Huang, M.; Lin, X.; Li, S.F.Y.; Jiang, N.; Liu, Y.; Guo, H.; Li, Y. Novel Fe-Based Metal-Organic Framework (MOF) Modified Carbon Nanofiber as a Highly Selective and Sensitive Electrochemical Sensor for Tetracycline Detection. Chem. Eng. J. 2022, 427, 130913. [Google Scholar] [CrossRef]
- Chen, G.; Bai, W.; Jin, Y.; Zheng, J. Fluorescence and Electrochemical Assay for Bimodal Detection of Lead Ions Based on Metal-Organic Framework Nanosheets. Talanta 2021, 232, 122405. [Google Scholar] [CrossRef]
- Tang, X.; Su, R.; Luo, H.; Zhao, Y.; Feng, L.; Chen, J. Colorimetric Detection of Aflatoxin B-1 by Using Smartphone-Assisted Microfluidic Paper-Based Analytical Devices. Food Control. 2022, 132, 108497. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Z.; Qi, J.; You, J.; Ma, J.; Chen, L. Colorimetric Detection of Heavy Metal Ions with Various Chromogenic Materials: Strategies and Applications. J. Hazard. Mater. 2023, 441, 129889. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Qu, K. Binary Organic-Inorganic Nanocomposite of Polyaniline-MnO2 for Non-Enzymatic Electrochemical Detection of Environmental Pollutant Nitrite. Environ. Res. 2022, 214, 114066. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Abd El-Aty, A.M.; Eun, J.-B.; Shim, J.-H.; Zhao, J.; Lei, X.; Gao, S.; She, Y.; Jin, F.; Wang, J.; et al. Recent Advances in Rapid Detection Techniques for Pesticide Residue: A Review. J. Agric. Food Chem. 2022, 70, 13093–13117. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Gao, N.; Zhu, L.; Hunter, M.; Chen, S.; Zang, L. PEDOT:PSS/PEDOT Film Chemiresistive Sensors for Hydrogen Peroxide Vapor Detection under Ambient Conditions. Chemosensors 2023, 11, 124. [Google Scholar] [CrossRef]
- Berhanu, A.L.; Gaurav; Mohiuddin, I.; Malik, A.K.; Aulakh, J.S.; Kumar, V.; Kim, K.-H. A Review of the Applications of Schiff Bases as Optical Chemical Sensors. Trac-Trends Anal. Chem. 2019, 116, 74–91. [Google Scholar] [CrossRef]
- Kulandaivel, S.; Lo, W.-C.; Lin, C.-H.; Yeh, Y.-C. Cu-PyC MOF with Oxidoreductase-like Catalytic Activity Boosting Colorimetric Detection of Cr(VI) on Paper. Anal. Chim. Acta 2022, 1227, 340335. [Google Scholar] [CrossRef]
- Aijaz, A.; Raja, D.A.; Khan, F.-A.; Barek, J.; Malik, M.I. A Silver Nanoparticles-Based Selective and Sensitive Colorimetric Assay for Ciprofloxacin in Biological, Environmental, and Commercial Samples. Chemosensors 2023, 11, 91. [Google Scholar] [CrossRef]
- Rahman, S.; Bozal-Palabiyik, B.; Unal, D.N.; Erkmen, C.; Siddiq, M.; Shah, A.; Uslu, B. Molecularly Imprinted Polymers (MIPs) Combined with Nanomaterials as Electrochemical Sensing Applications for Environmental Pollutants. Trends Environ. Anal. Chem. 2022, 36, e00176. [Google Scholar] [CrossRef]
- Zhang, X.; Hao, X.; Zhai, Z.; Wang, J.; Li, H.; Sun, Y.; Qin, Y.; Niu, B.; Li, C. Flexible H2S Sensors: Fabricated by Growing NO2-UiO-66 on Electrospun Nanofibers for Detecting Ultralow Concentration H2S. Appl. Surf. Sci. 2022, 573, 151446. [Google Scholar] [CrossRef]
- Lazarević-Pašti, T.; Tasić, T.; Milanković, V.; Potkonjak, N. Molecularly Imprinted Plasmonic-Based Sensors for Environmental Contaminants—Current State and Future Perspectives. Chemosensors 2023, 11, 35. [Google Scholar] [CrossRef]
- Tan, S.; Wang, Q.; Tan, Q.; Zhao, S.; Huang, L.; Wang, B.; Song, X.; Lan, M. Ratiometric Fluorescence Probe Based on Deep-Red Emissive CdTe Quantum Dots and Eu3+ Hybrid for Oxytetracycline Detection. Chemosensors 2023, 11, 62. [Google Scholar] [CrossRef]
- Tay, Y.Y.; Lin, X.H.; Li, S.F.Y. Nanogel for Selective Recognition of Nanoparticles in Water Samples. Chemosensors 2023, 11, 72. [Google Scholar] [CrossRef]
- Saruhan, B.; Fomekong, R.L.; Nahirniak, S. High-Sensitivity and -Selectivity Gas Sensors with Nanoparticles, Nanostructures, and Thin Films. Chemosensors 2023, 11, 81. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Zhang, M.; Feng, Z.; Yu, D.-G.; Wang, K. Electrospun Nanofiber Membranes for Air Filtration: A Review. Nanomaterials 2022, 12, 1077. [Google Scholar] [CrossRef]
- Thavasi, V.; Singh, G.; Ramakrishna, S. Electrospun Nanofibers in Energy and Environmental Applications. Energy Environ. Sci. 2008, 1, 205–221. [Google Scholar] [CrossRef]
- Sherlin, V.A.; Baby, J.N.; Sriram, B.; Hsu, Y.-F.; Wang, S.-F.; George, M. Construction of ANbO3 (A= Na, K)/f-Carbon Nanofiber Composite: Rapid and Real-Time Electrochemical Detection of Hydroxychloroquine in Environmental Samples. Environ. Res. 2022, 215, 114232. [Google Scholar] [CrossRef]
- Grant, J.J.; Pillai, S.C.; Perova, T.S.; Hehir, S.; Hinder, S.J.; McAfee, M.; Breen, A. Electrospun Fibres of Chitosan/PVP for the Effective Chemotherapeutic Drug Delivery of 5-Fluorouracil. Chemosensors 2021, 9, 70. [Google Scholar] [CrossRef]
- Song, W.; Tang, Y.; Qian, C.; Kim, B.J.; Liao, Y.; Yu, D.-G. Electrospinning Spinneret: A Bridge between the Visible World and the Invisible Nanostructures. Innov. 2023, 4, 100381. [Google Scholar] [CrossRef]
- Rahman, N.S.A.; Greish, Y.E.; Mahmoud, S.T.; Qamhieh, N.N.; El-Maghraby, H.F.; Zeze, D. Fabrication and Characterization of Cellulose Acetate-Based Nanofibers and Nanofilms for H2S Gas Sensing Application. Carbohydr. Polym. 2021, 258, 117643. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Yuan, H.; Ren, T.; Liu, Z.; Qiao, J.; Ma, Q.; Guo, X.; Ma, G.; Wu, Y. Fluorescent Nanocellulose-Based Hydrogel Incorporating Titanate Nanofibers for Sorption and Detection of Cr(VI). Int. J. Biol. Macromol. 2022, 215, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Pandey, A.; Singh, S.; Singh, S.P. Iron Nanoparticles Decorated Hierarchical Carbon Fiber Forest for the Magnetic Solid-Phase Extraction of Multi-Pesticide Residues from Water Samples. Chemosphere 2021, 282, 131058. [Google Scholar] [CrossRef] [PubMed]
- Perez-Rafols, C.; Serrano, N.; Manuel Diaz-Cruz, J.; Arino, C.; Esteban, M. Glutathione Modified Screen-Printed Carbon Nanofiber Electrode for the Voltammetric Determination of Metal Ions in Natural Samples. Talanta 2016, 155, 8–13. [Google Scholar] [CrossRef]
- Neri, G. Thin 2D: The New Dimensionality in Gas Sensing. Chemosensors 2017, 5, 21. [Google Scholar] [CrossRef]
- Facure, M.H.M.; Mercante, L.A.; Correa, D.S. Polyacrylonitrile/Reduced Graphene Oxide Free-Standing Nanofibrous Membranes for Detecting Endocrine Disruptors. ACS Appl. Nano Mater. 2022, 5, 6376–6384. [Google Scholar] [CrossRef]
- Karimi Afshar, S.; Abdorashidi, M.; Dorkoosh, F.A.; Akbari Javar, H. Electrospun Fibers: Versatile Approaches for Controlled Release Applications. Int. J. Polym. Sci. 2022, 2022, 9116168. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, S.; Qin, Y.; Li, C. Functional Electrospun Nanocomposites for Efficient Oxygen Reduction Reaction. Chem. Res. Chin. Univ. 2021, 37, 379–393. [Google Scholar] [CrossRef]
- Xu, X.; Lv, H.; Zhang, M.; Wang, M.; Zhou, Y.; Liu, Y.; Yu, D.-G. Recent Progress in Electrospun Nanofibers and Their Applications in Heavy Metal Wastewater Treatment. Front. Chem. Sci. Eng. 2023, 17, 1–27. [Google Scholar] [CrossRef]
- Huang, C.; Xu, X.; Fu, J.; Yu, D.-G.; Liu, Y. Recent Progress in Electrospun Polyacrylonitrile Nanofiber-Based Wound Dressing. Polymers 2022, 14, 3266. [Google Scholar] [CrossRef]
- Han, W.; Wang, L.; Li, Q.; Ma, B.; He, C.; Guo, X.; Nie, J.; Ma, G. A Review: Current Status and Emerging Developments on Natural Polymer-Based Electrospun Fibers. Macromol. Rapid Commun. 2022, 43, 2200456. [Google Scholar] [CrossRef]
- Wang, X.; Feng, C. Chiral Fiber Supramolecular Hydrogels for Tissue Engineering. Wiley Interdiscip. Rev.-Nanomed. Nanobiotechnol. 2022, 14, e1847. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.-G.; Du, Y.; Chen, J.; Song, W.; Zhou, T. A Correlation Analysis between Undergraduate Students’ Safety Behaviors in the Laboratory and Their Learning Efficiencies. Behav. Sci. 2023, 13, 127. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Y.; Lv, G.; Bu, W. Redox Dyshomeostasis Strategy for Tumor Therapy Based on Nanomaterials Chemistry. Chem. Sci. 2022, 13, 2202–2217. [Google Scholar] [CrossRef]
- Sivan, M.; Madheswaran, D.; Hauzerova, S.; Novotny, V.; Hedvicakova, V.; Jencova, V.; Kostakova, E.K.; Schindler, M.; Lukas, D. AC Electrospinning: Impact of High Voltage and Solvent on the Electrospinnability and Productivity of Polycaprolactone Electrospun Nanofibrous Scaffolds. Mater. Today Chem. 2022, 26, 101025. [Google Scholar] [CrossRef]
- Sivan, M.; Madheswaran, D.; Valtera, J.; Kostakova, E.K.; Lukas, D. Alternating Current Electrospinning: The Impacts of Various High-Voltage Signal Shapes and Frequencies on the Spinnability and Productivity of Polycaprolactone Nanofibers. Mater. Des. 2022, 213, 110308. [Google Scholar] [CrossRef]
- Liu, H.; Bai, Y.; Huang, C.; Wang, Y.; Ji, Y.; Du, Y.; Xu, L.; Yu, D.-G.; Bligh, S.W.A. Recent Progress of Electrospun Herbal Medicine Nanofibers. Biomolecules 2023, 13, 184. [Google Scholar] [CrossRef]
- Shen, Y.; Yu, X.; Cui, J.; Yu, F.; Liu, M.; Chen, Y.; Wu, J.; Sun, B.; Mo, X. Development of Biodegradable Polymeric Stents for the Treatment of Cardiovascular Diseases. Biomolecules 2022, 12, 1245. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, J.; Deng, C.; Wang, R.; Zhang, H. Protocol for Atmospheric Water Harvesting Using in Situ Polymerization Honeycomb Hygroscopic Polymers. STAR Protoc. 2022, 3, 101780. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, Y.; Lv, H.; Shi, H.; Zhou, W.; Liu, Y.; Yu, D.-G. Processes of Electrospun Polyvinylidene Fluoride-Based Nanofibers, Their Piezoelectric Properties, and Several Fantastic Applications. Polymers 2022, 14, 4311. [Google Scholar] [CrossRef]
- Kang, S.; Hou, S.; Chen, X.; Yu, D.-G.; Wang, L.; Li, X.; Williams, G.R. Energy-Saving Electrospinning with a Concentric Teflon-Core Rod Spinneret to Create Medicated Nanofibers. Polymers 2020, 12, 2421. [Google Scholar] [CrossRef]
- Guan, W.; Zhou, W.; Lu, J.; Lu, C. Luminescent Films for Chemo- and Biosensing. Chem. Soc. Rev. 2015, 44, 6981–7009. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhao, P.; Song, W.; Wang, M.; Yu, D.-G. Electrospun Zein/Polyoxyethylene Core-Sheath Ultrathin Fibers and Their Antibacterial Food Packaging Applications. Biomolecules 2022, 12, 1110. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.-G.; Li, Q.; Song, W.; Xu, L.; Zhang, K.; Zhou, T. Advanced Technique-Based Combination of Innovation Education and Safety Education in Higher Education. J. Chem. Educ. 2023, 100, 507–516. [Google Scholar] [CrossRef]
- Ge, R.; Ji, Y.; Ding, Y.; Huang, C.; He, H.; Yu, D.-G. Electrospun Self-Emulsifying Core-Shell Nanofibers for Effective Delivery of Paclitaxel. Front. Bioeng. Biotechnol. 2023, 11, 1112338. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jiang, W.; Zhou, J.; Yu, D.-G.; Liu, H. The Applications of Ferulic-Acid-Loaded Fibrous Films for Fruit Preservation. Polymers 2022, 14, 4947. [Google Scholar] [CrossRef]
- Li, F.; Song, H.; Yu, W.; Ma, Q.; Dong, X.; Wang, J.; Liu, G. Electrospun TiO2//SnO2 Janus Nanofibers and Its Application in Ethanol Sensing. Mater. Lett. 2020, 262, 127070. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, X.; Liu, P.; Zhang, Y.; Song, W.; Yu, D.-G.; Lu, X. Electrospun Healthcare Nanofibers from Medicinal Liquor of Phellinus Igniarius. Adv. Compos. Hybrid Mater. 2022, 5, 3045–3056. [Google Scholar] [CrossRef]
- Zhao, P.; Chen, W.; Feng, Z.; Liu, Y.; Liu, P.; Xie, Y.; Yu, D.-G. Electrospun Nanofibers for Periodontal Treatment: A Recent Progress. Int. J. Nanomed. 2022, 17, 4137–4162. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Liu, Y.; Zhao, P.; Bai, Y.; Cui, W.; Shen, S.; Liu, Y.; Wang, Z.; Yu, D.G. Insight into the Superior Piezophotocatalytic Performance of BaTiO3//ZnO Janus Nanofibrous Heterostructures in the Treatment of Multi-Pollutants from water. Appl. Cata. B Environ. 2023, 330, 122623. [Google Scholar] [CrossRef]
- Wang, P.; Lv, H.; Cao, X.; Liu, Y.; Yu, D.-G. Recent Progress of the Preparation and Application of Electrospun Porous Nanofibers. Polymers 2023, 15, 921. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Li, R.; Liu, G.; Jia, W.; Sun, M.; Liu, Y.; Luo, Y.; Cheng, Z. Phase Separation-Based Electrospun Janus Nanofibers Loaded with Rana Chensinensis Skin Peptides/Silver Nanoparticles for Wound Healing. Mater. Des. 2021, 207, 109864. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, M.; Yan, C.; Liu, H.; Yu, D.-G. Advances in the Application of Electrospun Drug-Loaded Nanofibers in the Treatment of Oral Ulcers. Biomolecules 2022, 12, 1254. [Google Scholar] [CrossRef]
- Wang, M.; Ge, R.; Zhao, P.; Williams, G.R.; Yu, D.-G.; Annie Bligh, S.W. Exploring wettability difference-driven wetting by utilizing electrospun chimeric Janus microfiber comprising cellulose acetate and polyvinylpyrrolidone. Mater. Desi. 2023, 226, 111652. [Google Scholar] [CrossRef]
- Yu, D.-G.; Zhao, P. The Key Elements for Biomolecules to Biomaterials and to Bioapplications. Biomolecules 2022, 12, 1234. [Google Scholar] [CrossRef]
- Lv, H.; Guo, S.; Zhang, G.; He, W.; Wu, Y.; Yu, D.-G. Electrospun Structural Hybrids of Acyclovir-Polyacrylonitrile at Acyclovir for Modifying Drug Release. Polymers 2021, 13, 4286. [Google Scholar] [CrossRef] [PubMed]
- Knapczyk-Korczak, J.; Zhu, J.; Ura, D.P.; Szewczyk, P.K.; Gruszczynski, A.; Benker, L.; Agarwal, S.; Stachewicz, U. Enhanced Water Harvesting System and Mechanical Performance from Janus Fibers with Polystyrene and Cellulose Acetate. ACS Sustain. Chem. Eng. 2021, 9, 180–188. [Google Scholar] [CrossRef]
- Wang, M.-L.; Yu, D.-G.; Annie Bligh, S.W. Progress in preparing electrospun Janus fibers and their applications. Appl. Mater. Today 2023, 31, 101766. [Google Scholar] [CrossRef]
- Li, X.; Niu, X.; Chen, Y.; Yuan, K.; He, W.; Yang, S.; Tang, T.; Yu, D.-G. Electrospraying Micro-Nano Structures on Chitosan Composite Coatings for Enhanced Antibacterial Effect. Prog. Org. Coat. 2023, 174, 107310. [Google Scholar] [CrossRef]
- Yang, Y.; Chang, S.; Bai, Y.; Du, Y.; Yu, D.-G. Electrospun Triaxial Nanofibers with Middle Blank Cellulose Acetate Layers for Accurate Dual-Stage Drug Release. Carbohydr. Polym. 2020, 243, 116477. [Google Scholar] [CrossRef]
- Nagiah, N.; Murdock, C.J.; Bhattacharjee, M.; Nair, L.; Laurencin, C.T. Development of Tripolymeric Triaxial Electrospun Fibrous Matrices for Dual Drug Delivery Applications. Sci. Rep. 2020, 10, 609. [Google Scholar] [CrossRef]
- Wang, M.; Hou, J.; Yu, D.-G.; Li, S.; Zhu, J.; Chen, Z. Electrospun Tri-Layer Nanodepots for Sustained Release of Acyclovir. J. Alloy. Compd. 2020, 846, 156471. [Google Scholar] [CrossRef]
- Lubasova, D.; Niu, H.; Zhao, X.; Lin, T. Hydrogel Properties of Electrospun Polyvinylpyrrolidone and Polyvinylpyrrolidone/Poly(Acrylic Acid) Blend Nanofibers. RSC Adv. 2015, 5, 54481–54487. [Google Scholar] [CrossRef]
- Li, C.; Yang, J.; He, W.; Xiong, M.; Niu, X.; Li, X.; Yu, D.-G. A Review on Fabrication and Application of Tunable Hybrid Micro–Nano Array Surfaces. Adv. Mater. Interfaces. 2023, 10, 2202160. [Google Scholar] [CrossRef]
- Wang, C.; Kaneti, Y.V.; Bando, Y.; Lin, J.; Liu, C.; Li, J.; Yamauchi, Y. Metal–Organic Framework-Derived One-Dimensional Porous or Hollow Carbon-Based Nanofibers for Energy Storage and Conversion. Mater. Horiz. 2018, 5, 394–407. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, Z.; Zhao, K. Fabrication of Hollow and Porous Polystyrene Fibrous Membranes by Electrospinning Combined with Freeze-Drying for Oil Removal from Water. J. Appl. Polym. Sci. 2019, 136, 47262. [Google Scholar] [CrossRef]
- Yao, L.; Sun, C.; Lin, H.; Li, G.; Lian, Z.; Song, R.; Zhuang, S.; Zhang, D. Electrospun Bi-Decorated BixTiyOz/TiO2 Flexible Carbon Nanofibers and Their Applications on Degradating of Organic Pollutants under Solar Radiation. J. Mater. Sci. Technol. 2022, 150, 114–123. [Google Scholar] [CrossRef]
- Irfan, M.; Uenlue, F.; Le, K.; Fischer, T.; Ullah, H.; Mathur, S. Electrospun Networks of ZnO-SnO2 Composite Nanowires as Electron Transport Materials for Perovskite Solar Cells. J. Nanomater. 2022, 2022, 6043406. [Google Scholar] [CrossRef]
- Guo, F.; Guo, Z.; Gao, L.; Qi, D.; Yue, G.; Wang, N.; Zhao, Y.; Li, N.; Xiong, J. Electrospun Core-Shell Hollow Structure Cocatalysts for Enhanced Photocatalytic Activity. J. Nanomater. 2021, 2021, 9980810. [Google Scholar] [CrossRef]
- Liu, T.; Guo, Y.; Zhang, Z.; Miao, Z.; Zhang, X.; Su, Z. Fabrication of Hollow CuO/PANI Hybrid Nanofibers for Non-Enzymatic Electrochemical Detection of H2O2 and Glucose. Sens. Actuator B-Chem. 2019, 286, 370–376. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, Y.; Hou, X.; Zhou, H.; Qiao, H.; Wei, Q. Preparation of TiO2 Nanofibrous Membranes with Hierarchical Porosity for Efficient Photocatalytic Degradation. J. Phys. Chem. C 2018, 122, 8946–8953. [Google Scholar] [CrossRef]
- Yin, D.; Liu, J.; Bo, X.; Guo, L. Cobalt-Iron Selenides Embedded in Porous Carbon Nanofibers for Simultaneous Electrochemical Detection of Trace of Hydroquinone, Catechol and Resorcinol. Anal. Chim. Acta 2020, 1093, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Poudel, M.B.; Kim, H.J. Confinement of Zn-Mg-Al-Layered Double Hydroxide and Alpha-Fe2O3 Nanorods on Hollow Porous Carbon Nanofibers: A Free-Standing Electrode for Solid-State Symmetric Supercapacitors. Chem. Eng. J. 2022, 429, 132345. [Google Scholar] [CrossRef]
- Cao, X.; Chen, W.; Zhao, P.; Yang, Y.; Yu, D.-G. Electrospun Porous Nanofibers: Pore-Forming Mechanisms and Applications for Photocatalytic Degradation of Organic Pollutants in Wastewater. Polymers 2022, 14, 3990. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Meng, G.; Huang, Q.; Qian, Y. Electrospun 1,4-DHAQ-Doped Cellulose Nanofiber Films for Reusable Fluorescence Detection of Trace Cu2+ and Further for Cr3+. Environ. Sci. Technol. 2012, 46, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Atapour, M.; Amoabediny, G.; Ahmadzadeh-Raji, M. Integrated Optical and Electrochemical Detection of Cu2+ Ions in Water Using a Sandwich Amino Acidgold Nanoparticle-Based Nano-Biosensor Consisting of a Transparent-Conductive Platform. RSC Adv. 2019, 9, 8882–8893. [Google Scholar] [CrossRef]
- Bao, J.; Huang, T.; Wang, Z.; Yang, H.; Geng, X.; Xu, G.; Samalo, M.; Sakinati, M.; Huo, D.; Hou, C. 3D Graphene/Copper Oxide Nano-Flowers Based Acetylcholinesterase Biosensor for Sensitive Detection of Organophosphate Pesticides. Sens. Actuators B Chem. 2019, 279, 95–101. [Google Scholar] [CrossRef]
- Li, M.; Peng, X.; Han, Y.; Fan, L.; Liu, Z.; Guo, Y. Ti3C2 MXenes with Intrinsic Peroxidase-Like Activity for Label-Free and Colorimetric Sensing of Proteins. Microchem. J. 2021, 166, 106238. [Google Scholar] [CrossRef]
- Asghar, N.; Mustafa, G.; Yasinzai, M.; Al-Soud, Y.A.; Lieberzeit, P.A.; Latif, U. Real-Time and Online Monitoring of Glucose Contents by Using Molecular Imprinted Polymer-Based Ides Sensor. Appl. Biochem. Biotechnol. 2019, 189, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Ji, P.; Wang, B.; Wang, H.; Chen, S. Color-Tunable Luminescent Macrofibers Based on CdTe QDs-Loaded Bacterial Cellulose Nanofibers for Ph and Glucose Sensing. Sens. Actuators B-Chem. 2018, 254, 110–119. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, D.-G.; Liu, Y.; Liu, Y.-N. Progress of Electrospun Nanofibrous Carriers for Modifications to Drug Release Profiles. J. Funct. Biomater. 2022, 13, 289. [Google Scholar] [CrossRef]
- Song, W.; Zhang, M.; Huang, X.; Chen, B.; Ding, Y.; Zhang, Y.; Yu, D.G.; Kim, I. Smart L-Borneol-Loaded Hierarchical Hollow Polymer Nanospheres with Antipollution and Antibacterial Capabilities. Mater. Today Chem. 2022, 26, 101252. [Google Scholar] [CrossRef]
- Abdel-Karim, R.; Reda, Y.; Abdel-Fattah, A. Review-Nanostructured Materials-Based Nanosensors. J. Electrochem. Soc. 2020, 167, 037554. [Google Scholar] [CrossRef]
- Afzali, M.; Mostafavi, A.; Nekooie, R.; Jahromi, Z. A Novel Voltammetric Sensor Based on Palladium Nanoparticles/Carbon Nanofibers/Ionic Liquid Modified Carbon Paste Electrode for Sensitive Determination of Anti-Cancer Drug Pemetrexed. J. Mol. Liq. 2019, 282, 456–465. [Google Scholar] [CrossRef]
- Adhikari, J.; Rizwan, M.; Keasberry, N.A.; Ahmed, M.U. Current Progresses and Trends in Carbon Nanomaterials-Based Electrochemical and Electrochemiluminescence Biosensors. J. Chin. Chem. Soc. 2020, 67, 937–960. [Google Scholar] [CrossRef]
- Giummarella, N.; Zhang, L.; Henriksson, G.; Lawoko, M. Structural Features of Mildly Fractionated Lignin Carbohydrate Complexes (LCC) from Spruce. RSC Adv. 2016, 6, 42120–42131. [Google Scholar] [CrossRef]
- Guo, C.; Wang, J.; Chen, X.; Li, Y.; Wu, L.; Zhang, J.; Tao, C. Construction of a Biosensor Based on a Combination of Cytochrome C, Graphene, and Gold Nanoparticles. Sensors 2018, 19, 40. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Song, T.; Liu, X.; Lin, Q.; He, B.; Ren, J. Cellulose-Based Biosensor for Bio-Molecules Detection in Medical Diagnosis: A Mini-Review. Curr. Med. Chem. 2020, 27, 4593–4612. [Google Scholar] [CrossRef]
- Kamel, S.; Khattab, T.A. Recent Advances in Cellulose-Based Biosensors for Medical Diagnosis. Biosensors 2020, 10, 67. [Google Scholar] [CrossRef]
- Ulhakim, M.T.; Rezki, M.; Dewi, K.K.; Abrori, S.A.; Harimurti, S.; Septiani, N.L.W.; Kurnia, K.A.; Setyaningsih, W.; Darmawan, N.; Yuliarto, B. Review—Recent Trend on Two-Dimensional Metal-Organic Frameworks for Electrochemical Biosensor Application. J. Electrochem. Soc. 2020, 167, 136509. [Google Scholar] [CrossRef]
- Zhuo, Y.; Hu, H.; Wang, Y.; Marin, T.; Lu, M. Photonic Crystal Slab Biosensors Fabricated with Helium Ion Lithography (HIL). Sens. Actuators Phys. 2019, 297, 111493. [Google Scholar] [CrossRef]
- Abraham, P.; Renjini, S.; Vijayan, P.; Nisha, V.; Sreevalsan, K.; Anithakumary, V. Review—Review on the Progress in Electrochemical Detection of Morphine Based on Different Modified Electrodes. J. Electrochem. Soc. 2020, 167, 037559. [Google Scholar] [CrossRef]
- Wu, D.; Rios-Aguirre, D.; Chounlakone, M.; Camacho-Leon, S.; Voldman, J. Sequentially Multiplexed Amperometry for Electrochemical Biosensors. Biosens. Bioelectron. 2018, 117, 522–529. [Google Scholar] [CrossRef]
- Daubinger, P.; Kieninger, J.; Unmüssig, T.; Urban, G.A. Electrochemical Characteristics of Nanostructured Platinum Electrodes—A Cyclic Voltammetry Study. Phys. Chem. Chem. Phys. 2014, 16, 8392–8399. [Google Scholar] [CrossRef]
- Li, D.; Qi, R.; Zhang, L.-Z. Electrochemical Impedance Spectroscopy Analysis of V–I Characteristics and a Fast Prediction Model for PEM-Based Electrolytic Air Dehumidification. Int. J. Hydrogen Energy 2019, 44, 19533–19546. [Google Scholar] [CrossRef]
- Zhu, Y.; Hang, Y.; Huang, X.; Song, C. Sensitive Determination of Semicarbazide in Flour by Differential Pulse Voltammetry. J. Anal. Chem. 2019, 74, 913–919. [Google Scholar] [CrossRef]
- Kamel, R.M.; Shahat, A.; Hegazy, W.H.; Khodier, E.M.; Awual, M.R. Efficient Toxic Nitrite Monitoring and Removal from Aqueous Media with Ligand Based Conjugate Materials. J. Mol. Liq. 2019, 285, 20–26. [Google Scholar] [CrossRef]
- Lucia Campana, A.; Leonardo Florez, S.; Juliana Noguera, M.; Fuentes, O.P.; Ruiz Puentes, P.; Cruz, J.C.; Osma, J.F. Enzyme-Based Electrochemical Biosensors for Microfluidic Platforms to Detect Pharmaceutical Residues in Wastewater. Biosensors 2019, 9, 41. [Google Scholar] [CrossRef]
- Vanýsek, P. Two Common Electroanalytical Techniques—Cyclic Voltammetry and Impedance Capacitance Data from Cyclic Voltammetry. ECS Trans. 2019, 41, 15–24. [Google Scholar] [CrossRef]
- Wen, T.; Yuan, L.; Tian, C.; Jin, Y.; Liu, T.; Yu, J. Preparation and Characterization of (Ca2+, Al3+)-Infiltrated CaO-Al2O3 Auxiliary Electrode for Electrochemical Sulfur Sensor. J. Ind. Eng. Chem. 2022, 106, 393–399. [Google Scholar] [CrossRef]
- Semenova, E.; Navolotskaya, D.; Ermakov, S. Interrupted Amperometry: The New Possibilities in Electrochemical Measurements. Pure Appl. Chem. 2017, 89, 1459–1469. [Google Scholar] [CrossRef]
- McCutcheon, K.; Bandara, A.B.; Zuo, Z.; Heflin, J.R.; Inzana, T.J. The Application of a Nanomaterial Optical Fiber Biosensor Assay for Identification of Brucella Nomenspecies. Biosensors 2019, 9, 64. [Google Scholar] [CrossRef]
- McNichols, R.J.; Cote, G.L. Optical Glucose Sensing in Biological Fluids: An Overview. J. Biomed. Opt. 2000, 5, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, C.; Sun, Z.; Mao, H.; Zhang, L.; Yu, X.; Zhao, J.; Chen, X. Black Phosphorus Based Fiber Optic Biosensor for Ultrasensitive Cancer Diagnosis. Biosens. Bioelectron. 2019, 137, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Feng, T.; Zhao, N.; Zhang, J.; Wang, H.; Liang, N.; Zhao, L. A Carbon Nanoparticle-Peptide Fluorescent Sensor Custom-Made for Simple and Sensitive Detection of Trypsin. J. Pharm. Anal. 2020, 10, 482–489. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Sun, H.; Wang, L.; Zhang, J.; Huang, X.; Deng, L.; Xi, J.; Ma, T. A New Type of Optical Fiber Glucose Biosensor with Enzyme Immobilized by Electrospinning. IEEE Sens. J. 2021, 21, 16078–16085. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J. Optical Biosensors: An Exhaustive and Comprehensive Review. Analyst 2020, 145, 1605–1628. [Google Scholar] [CrossRef]
- Wu, X.; Yin, J.; Liu, J.; Gu, Y.; Wang, S.; Wang, J. Colorimetric Detection of Glucose Based on the Binding Specificity of a Synthetic Cyclic Peptide. Analyst 2020, 145, 7234–7241. [Google Scholar] [CrossRef]
- Zheng, S.; Fang, Y.; Chen, Y.; Kong, Q.; Wang, F.; Chen, X. Benzothiazole Derivatives Based Colorimetric and Fluorescent Probes for Detection of Amine/Ammonia and Monitoring the Decomposition of Urea by Urease. Spectrochim. Acta Part-Mol. Biomol. Spectrosc. 2022, 267, 120616. [Google Scholar] [CrossRef]
- Li, Y.; Wen, Y.; Wang, L.; He, J.; Al-Deyab, S.S.; El-Newehy, M.; Yu, J.; Ding, B. Simultaneous Visual Detection and Removal of Lead(II) Ions with Pyromellitic Dianhydride-Grafted Cellulose Nanofibrous Membranes. J. Mater. Chem. A 2015, 3, 1818–18189. [Google Scholar] [CrossRef]
- Najarzadekan, H.; Sereshti, H. Development of a Colorimetric Sensor for Nickel Ion Based on Transparent Electrospun Composite Nanofibers of Polycaprolactam-Dimethylglyoxime/Polyvinyl Alcohol. J. Mater. Sci. 2016, 51, 8645–8654. [Google Scholar] [CrossRef]
- Abou-Omar, M.N.; Attia, M.S.; Afify, H.G.; Amin, M.A.; Boukherroub, R.; Mohamed, E.H. Novel Optical Biosensor Based on a Nano-Gold Coated by Schiff Base Doped in Sol/Gel Matrix for Sensitive Screening of Oncomarker CA-125. ACS Omega 2021, 6, 20812–20821. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-M.; Uh, M.; Jeong, D.H.; Lee, H.-Y.; Park, J.-H.; Lee, S.-K. Localized Surface Plasmon Resonance Biosensor Using Nanopatterned Gold Particles on the Surface of an Optical Fiber. Sens. Actuators B Chem. 2019, 280, 183–191. [Google Scholar] [CrossRef]
- Marchetti, M.; Ronda, L.; Percudani, R.; Bettati, S. Immobilization of Allantoinase for the Development of an Optical Biosensor of Oxidative Stress States. Sensors 2019, 20, 196. [Google Scholar] [CrossRef]
- Tang, Y.; Kang, A.; Yang, X.; Hu, L.; Tang, Y.; Li, S.; Xie, Y.; Miao, Q.; Pan, Y.; Zhu, D. A Robust OFF-ON Fluorescent Biosensor for Detection and Clearance of Bacterial Endotoxin by Specific Peptide Based Aggregation Induced Emission. Sens. Actuators B Chem. 2020, 304, 127300. [Google Scholar] [CrossRef]
- Long, C.; Jiang, Z.; Shangguan, J.; Qing, T.; Zhang, P.; Feng, B. Applications of Carbon Dots in Environmental Pollution Control: A Review. Chem. Eng. J. 2021, 406, 126848. [Google Scholar] [CrossRef]
- Yuan, H.; Peng, J.; Ren, T.; Luo, Q.; Luo, Y.; Zhang, N.; Huang, Y.; Guo, X.; Wu, Y. Novel Fluorescent Lignin-Based Hydrogel with Cellulose Nanofibers and Carbon Dots for Highly Efficient Adsorption and Detection of Cr(VI). Sci. Total Environ. 2021, 760, 143395. [Google Scholar] [CrossRef]
- Cai, J.; Ding, L.; Gong, P.; Huang, J. A Colorimetric Detection of MicroRNA-148a in Gastric Cancer by Gold Nanoparticle–RNA Conjugates. Nanotechnology 2020, 31, 095501. [Google Scholar] [CrossRef]
- Cao, X.; Xia, Z.; Yan, W.; He, S.; Xu, X.; Wei, Z.; Ye, Y.; Zheng, H. Colorimetric Biosensing of Nopaline Synthase Terminator Using Fe3O4@Au and Hemin-Functionalized Reduced Graphene Oxide. Anal. Biochem. 2020, 602, 113798. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, N.; Abarghoei, S.; Dadmehr, M.; Hosseini, M.; Sabahi, H.; Ganjali, M.R. Paper Based Colorimetric Detection of Mirna-21 Using Ag/Pt Nanoclusters. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2020, 227, 117529. [Google Scholar] [CrossRef] [PubMed]
- Mulder, D.W.; Phiri, M.M.; Vorster, B.C. Gold Nanostar Colorimetric Detection of Fructosyl Valine as a Potential Future Point of Care Biosensor Candidate for Glycated Haemoglobin Detection. Biosensors 2019, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Cao, Y.; Xie, Z.; Zhao, Y.; Zhang, C.; Chen, Z. Multi-Parameter Photoelectric Data Fitting for Microfluidic Sweat Colorimetric Analysis. Sens. Actuators B Chem. 2022, 372, 132644. [Google Scholar] [CrossRef]
- Wei, J.-H.; Yi, J.-W.; Han, M.-L.; Li, B.; Liu, S.; Wu, Y.-P.; Ma, L.-F.; Li, D.-S. A Water-Stable Terbium(III)-Organic Framework as a Chemosensor for Inorganic Ions, Nitro-Containing Compounds and Antibiotics in Aqueous Solutions. Chem.-Asian J. 2019, 14, 3694–3701. [Google Scholar] [CrossRef]
- Yu, Z.; Huang, W.; Xu, S.; Ke, S. Benzothiazole-Based Colorimetric Chemosensors Bearing Naphthol Aldehyde Unit: Synthesis, Characterization, Selective Detection of Hypochlorite and Its Application as Test Strips. Microchem. J. 2021, 164, 106009. [Google Scholar] [CrossRef]
- Fan, H.; Zhang, M.; Bhandari, B.; Yang, C. Food Waste as a Carbon Source in Carbon Quantum Dots Technology and Their Applications in Food Safety Detection. Trends Food Sci. Technol. 2020, 95, 86–96. [Google Scholar] [CrossRef]
- Ye, M.-L.; Zhu, Y.; Lu, Y.; Gan, L.; Zhang, Y.; Zhao, Y.-G. Magnetic Nanomaterials with Unique Nanozymes-like Characteristics for Colorimetric Sensors: A Review. Talanta 2021, 230, 122299. [Google Scholar] [CrossRef]
- Sahu, S.; Sharma, S.; Kurrey, R.; Ghosh, K.K. Recent Advances on Gold and Silver Nanoparticle-Based Colorimetric Strategies for the Detection of Different Substances and SARS-CoV-2: A Comprehensive Review. Environ. Sci.-Nano 2022, 9, 3684–3710. [Google Scholar] [CrossRef]
- Hu, L.; Yan, X.-W.; Li, Q.; Zhang, X.-J.; Shan, D. Br-PADAP Embedded in Cellulose Acetate Electrospun Nanofibers: Colorimetric Sensor Strips for Visual Uranyl Recognition. J. Hazard. Mater. 2017, 329, 205–210. [Google Scholar] [CrossRef]
- Deng, G.; Deng, X.; Deng, J.; Lu, X.; Kang, X.; Song, Y. Performance Evaluation of an Electrospun Nanofiber Mat as Samplers for the Trap of Trace Heavy Metals in Atmospheric Particles and Its Application. Anal. Sci. 2020, 36, 1453–1459. [Google Scholar] [CrossRef]
- Lee, C.-G.; Na, K.-H.; Kim, W.-T.; Park, D.-C.; Yang, W.-H.; Choi, W.-Y. TiO2/ZnO Nanofibers Prepared by Electrospinning and Their Photocatalytic Degradation of Methylene Blue Compared with TiO2 Nanofibers. Appl. Sci. 2019, 9, 3404. [Google Scholar] [CrossRef]
- Hua, W.; Wang, M.; Li, P.; Shen, K.; Wang, X.; Hsiao, B.S. Sulfonylcalix[4]Arene Functionalized Nanofiber Membranes for Effective Removal and Selective Fluorescence Recognition of Terbium(III) Ions. New J. Chem. 2018, 42, 6191–6202. [Google Scholar] [CrossRef]
- Chen, R.; Yang, Y.; Wang, N.; Hao, L.; Li, L.; Guo, X.; Zhang, J.; Hu, Y.; Shen, W. Application of Packed Porous Nanofibers-Solid-Phase Extraction for the Detection of Sulfonamide Residues from Environmental Water Samples by Ultra High Performance Liquid Chromatography with Mass Spectrometry. J. Sep. Sci. 2015, 38, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Li, X.; Yang, B.; Rong, F.; Xu, Q. Disks Solid Phase Extraction Based Polypyrrole Functionalized Core-Shell Nanofibers Mat. Talanta 2015, 144, 129–135. [Google Scholar] [CrossRef]
- Abedalwafa, M.A.; Tang, Z.; Qiao, Y.; Mei, Q.; Yang, G.; Li, Y.; Wang, L. An Aptasensor Strip-Based Colorimetric Determination Method for Kanamycin Using Cellulose Acetate Nanofibers Decorated DNA-Gold Nanoparticle Bioconjugates. Microchim. Acta 2020, 187, 360. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, M.; Veeramuthu, L.; Liang, F.-C.; Chen, W.-C.; Cho, C.-J.; Chen, C.-W.; Chen, J.-Y.; Yan, Y.; Chang, S.-H.; Kuo, C.-C. Evolution of Electrospun Nanofibers Fluorescent and Colorimetric Sensors for Environmental Toxicants, PH, Temperature, and Cancer Cells? A Review with Insights on Applications. Chem. Eng. J. 2020, 397, 125431. [Google Scholar] [CrossRef]
- Terra, I.A.A.; Sanfelice, R.C.; Valente, G.T.; Correa, D.S. Optical Sensor Based on Fluorescent PMMA/PFO Electrospun Nanofibers for Monitoring Volatile Organic Compounds. J. Appl. Polym. Sci. 2018, 135, 46128. [Google Scholar] [CrossRef]
- Han, C.; Li, X.; Liu, Y.; Li, X.; Shao, C.; Ri, J.; Ma, J.; Liu, Y. Construction of In2O3/ZnO Yolk-Shell Nanofibers for Room-Temperature NO2 Detection under UV Illumination. J. Hazard. Mater. 2021, 403, 124093. [Google Scholar] [CrossRef]
- Ngoensawat, U.; Pisuchpen, T.; Sritana-anant, Y.; Rodthongkum, N.; Hoven, V.P. Conductive Electrospun Composite Fibers Based on Solid-State Polymerized Poly(3,4-Ethylenedioxythiophene) for Simultaneous Electrochemical Detection of Metal Ions. Talanta 2022, 241, 123253. [Google Scholar] [CrossRef]
- Deng, Z.X.; Tao, J.W.; Zhao, L.J.; Zhang, W.; Wang, Y.B.; Mu, H.J.; Wu, H.J.; Xu, X.X.; Zheng, W. Effect of Protein Adsorption on Bioelectrochemistry of Electrospun Core-Shell MWCNTs/Gelatin-Hb Nanobelts on Electrode Surface. Proc. Biochem. 2020, 96, 73–79. [Google Scholar] [CrossRef]
- Jin, X.; Wu, X.; Zhang, F.; Zhao, H.; Zhong, W.; Cao, Y.; Ma, X.; Leng, X.; Zhou, H.; She, M. Cu2+/ATP Reversible Ratiometric Fluorescent Probe through Strip, Hydrogel, and Nanofiber, and Its Application in Living Cells and Edaphic Ecological Safety Assessment. Dyes. Pigment. 2020, 182, 108677. [Google Scholar] [CrossRef]
- Srinivasan, S.; Nesakumar, N.; Rayappan, J.B.B.; Kulandaiswamy, A.J. Electrochemical Detection of Imidacloprid Using Cu-rGO Composite Nanofibers Modified Glassy Carbon Electrode. Bull. Environ. Contam. Toxicol. 2020, 104, 449–454. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, P.; Yang, Y.; Yu, D.G. Electrospun beads-on-the-string nanoproducts: Preparation and drug delivery application. Curr. Drug Deliv. 2022, 19, 1567201819666220525095844. [Google Scholar]
- Zhao, P.; Li, H.; Bu, W. A Forward Vision for Chemodynamic Therapy: Issues and Opportunities. Angrew Chem. Int. Ed. 2023, 62, e202210415. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Sun, C.; Lin, H.; Li, G.; Lian, Z.; Song, R.; Zhuang, S.; Zhang, D. Enhancement of AFB1 Removal Effificiency via Adsorption/Photocatalysis Synergy Using Surface-Modifified Electrospun PCL-g-C3N4/CQDs Membranes. Biomolecules 2023, 13, 550. [Google Scholar] [CrossRef]
- Huang, J.; Feng, C. Aniline Dimers Serving as Stable and Efficient Transfer Units for Intermolecular Charge-Carrier Transmission. Iscience 2023, 26, 105762. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Q.; Gao, J.; He, J.; Zhang, H.; Ding, J. Stereo Coverage and Overall Stiffness of Biomaterial Arrays Underly Parts of Topography Effects on Cell Adhesion. ACS Appl. Mater. Interf. 2023, 15, 6142–6155. [Google Scholar] [CrossRef]
- Huang, H.; Song, Y.; Zhang, Y.; Li, Y.; Li, J.; Lu, X.; Wang, C. Electrospun Nanofibers: Current Progress and Applications in Food Systems. J. Agr. Food Chem. 2022, 70, 1391–1409. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Zhang, Z.B.; Ren, Z.T.; Chen, Y.C.; Huang, J.Y.; Lei, Z.X.; Qian, X.M.; Lai, Y.K.; Zhang, S.N. A quadruple biomimetic hydrophilic/hydrophobic Janus composite material integrating Cu(OH)2 micro-needles and embedded bead-on-string nanofiber membrane for efficient fog harvesting. Chem. Eng. J. 2022, 455, 140863. [Google Scholar] [CrossRef]
- Xu, J.; Zhong, M.; Song, N.; Wang, C.; Lu, X. General Synthesis of Pt and Ni Co-Doped Porous Carbon Nanofibers to Boost HER Performance in Both Acidic and Alkaline Solutions. Chin. Chem. Lett. 2023, 34, 107359. [Google Scholar] [CrossRef]
- Zhu, M.M.; Yu, J.Y.; Li, Z.L.; Ding, B. Self-Healing Fibrous Membranes. Angrew. Chem. Int. Ed. 2022, 61, e202208949. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Peng, Y.; Wu, Y.; Cao, S.; Deng, H.; Cao, Z. Chitosan/silk fibroin composite bilayer PCL nanofibrous mats for bone regeneration with enhanced antibacterial properties and improved osteogenic potential. Int. J. Biolog. Macromol. 2023, 230, 123265. [Google Scholar] [CrossRef] [PubMed]







| Detection Method | Materials | Structure | Target Analyte | Linear Range | LOD | Application Area | Ref. |
|---|---|---|---|---|---|---|---|
| Electrochemical | NH2-MIL-101(Fe)/CNF@AuNPs | Single | Tetracycline | 0.1–105 nM | 0.01 nM | Water | [41] |
| Electrochemical | CuO/PANI hybrid NFs | Hollow | H2O2 | 0.005–9.255 mM | 0.11 mM | – | [111] |
| Electrochemical | CoFe2Se4/PCF | Porous | HQ | 0.5–200 μM | 0.13 μM | Water | [113] |
| CC | 0.5–190 μM | 0.15 μM | |||||
| RS | 5–350 μM | 1.36 μM | |||||
| Fluorescent | 3D porous fluorescent lignin | Porous | Hexavalent chromium | 15–200 mg/L | 11.2 mg/L | Water | [159] |
| Colorimetric | Br-PADAP/CA NFs | Single | Uranyl | 0–500 ppb | 50 ppb | Water | [170] |
| Fluorescent | Sulfonylcalix-NFM | Porous | Terbium(III) ions | 0.1–100 ppm | 0.5 ppm | Water | [173] |
| Colorimetric | cDNA@Au/GA-CA NFM | Single | Kanamycin | 2.5–80 nM | 2.5 nM | Water | [176] |
| Fluorescent | PMMA/PFO NFs | Single | Chloroform | 10–300 ppm | 47.9 ppm | Air | [178] |
| 350–500 ppm | 15.4 ppm | ||||||
| Electrochemical | PEDOT/PVA/AgNPs NFs | Single | Zn(II) | 10–80 ppb | 6 ppb | – | [180] |
| Cd(II) | 3 ppb | ||||||
| Pb(II) | 8 ppb | ||||||
| Electrochemical | MWCNTs/gelatin-Hb nanobelts | Core–shell | H2O2 | 5.0 × 10−6–55 × 10−6 mol/L | 0.0293 μmol/L | – | [181] |
| Fluorescent | RB/PMMA NFs | Single | Copper ion | 0.0–10 μM | 0.11 μM | Soil | [182] |
| ATP | 0.08 μM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Yu, D.-G.; Yi, T. Electrospun Nanofibers as Chemosensors for Detecting Environmental Pollutants: A Review. Chemosensors 2023, 11, 208. https://doi.org/10.3390/chemosensors11040208
Du Y, Yu D-G, Yi T. Electrospun Nanofibers as Chemosensors for Detecting Environmental Pollutants: A Review. Chemosensors. 2023; 11(4):208. https://doi.org/10.3390/chemosensors11040208
Chicago/Turabian StyleDu, Yutong, Deng-Guang Yu, and Tao Yi. 2023. "Electrospun Nanofibers as Chemosensors for Detecting Environmental Pollutants: A Review" Chemosensors 11, no. 4: 208. https://doi.org/10.3390/chemosensors11040208
APA StyleDu, Y., Yu, D.-G., & Yi, T. (2023). Electrospun Nanofibers as Chemosensors for Detecting Environmental Pollutants: A Review. Chemosensors, 11(4), 208. https://doi.org/10.3390/chemosensors11040208







