Abstract
Electrochemical sensors present a wide range of interesting applications in the areas of environmental, industrial, and chemical analysis. This review presents an overview of two types of sensors: electrocatalytic ones, which involve oxidation and reduction reactions through electron transfer, and photoelectrocatalytic ones, which involve a current response due to the incidence of light and redox reactions. Another point discussed was how these sensors’ detection capacity and behavior can be affected by several factors related to the material used to make the electrode. In this way, inorganic, organic, and hybrid materials were compared in electrocatalytic and photoelectrocatalytic sensors. The use of inorganic materials is interesting due to the fact of their abundance, low cost, and good electroactivity. Among organics, conductive polymers and carbonaceous materials are often cited due to the fact of their conductivity and their different possibilities for synthesis, being possible to mold their shape. Finally, hybrid materials unite these two classes, presenting different properties not found in a single substance.
1. Introduction
Chemical sensors are devices that convert chemical information (concentration, partial pressure, specific sample components, etc.) into a measurable signal. They are composed of a recognition part and a transducer part. The recognition is responsible for interacting with the molecules or ions of interest, and the transducer converts the chemical interactions into a measurable signal. If the electrical signal is the main signal, this sensor is classified as an electrochemical sensor [1].
In this context, a good sensor has some specific characteristics that must be evaluated, such as accuracy (related to the proximity of the sensor output value to the actual measured value), precision (standard deviation), trueness, repeatability (degrees of agreement among measurements), reproducibility, sensitivity, limit of detection, limit of quantification, selectivity, deviations due to the fact of operational stability, and response time [2].
Based on this, sensors that detect materials through electrocatalytic and photoelectrocatalytic activity stand out [3,4,5,6]. This technology has advantages and disadvantages compared to other sensors [7]. As advantages, we can mention the wide limit of detection, high stability, low cost, small size, and quick answer. As disadvantages, we can mention that pH and temperature parameters can influence sensor performance and sensitivity. In the case of photoelectrocatalyst sensors, a material with suitable band structures is required, and it is necessary to apply an external potential.
In this scope, electrocatalysis is a catalytic process involving oxidation and reduction reactions through electron transfer [8]. Electrocatalysis consists of reducing the overpotential by the presence of an organic or inorganic catalyst, decreasing oxidation and reduction electrochemical potentials to lower values. Both the catalytic material and the substance are of equal importance in electrocatalysis. The nature of the electrode describes the electrochemical activity and selectivity, in addition to determining the thermodynamic parameters of the reaction. The substance describes the progress of the reaction and the products formed. Several studies are being carried out varying these two fundamentals of electrocatalysis. These electrochemical reactions with an adsorbed species, either as a reagent or product, can change the reaction kinetics and are used for detection in sensors. In the recognition process based on electrocatalytic sensors, the electrochemical reaction usually leads to a change in current, potential, or conductivity, called amperometric, potentiometric, and conductometric sensor systems, respectively [9].
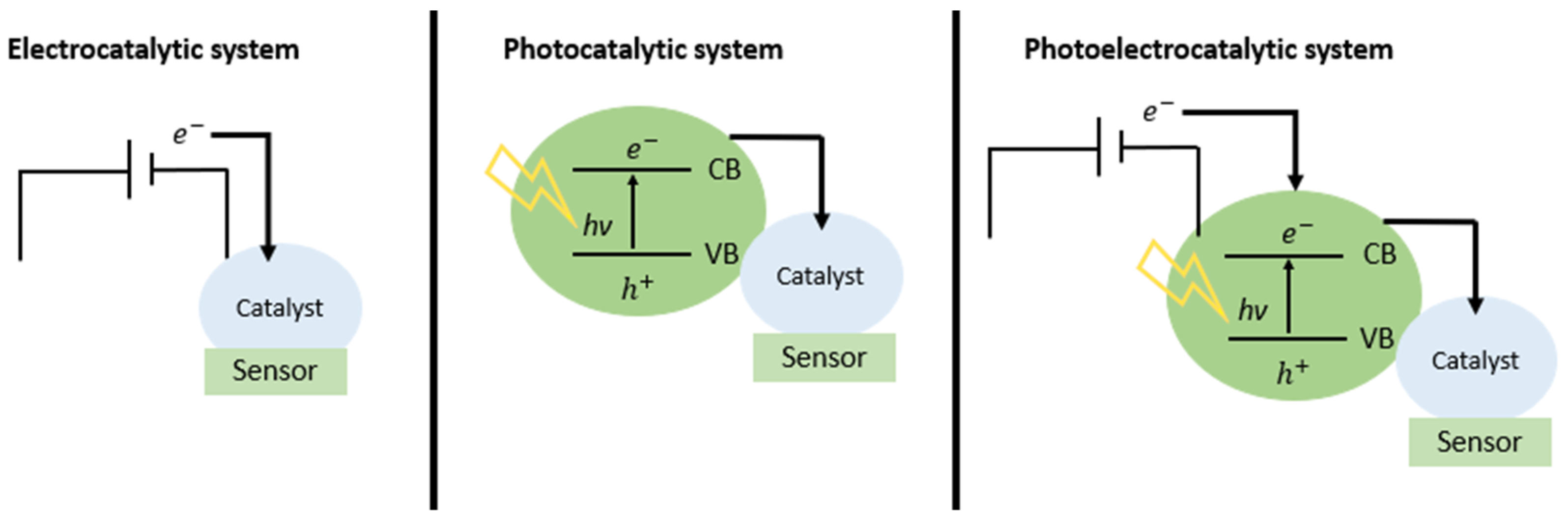
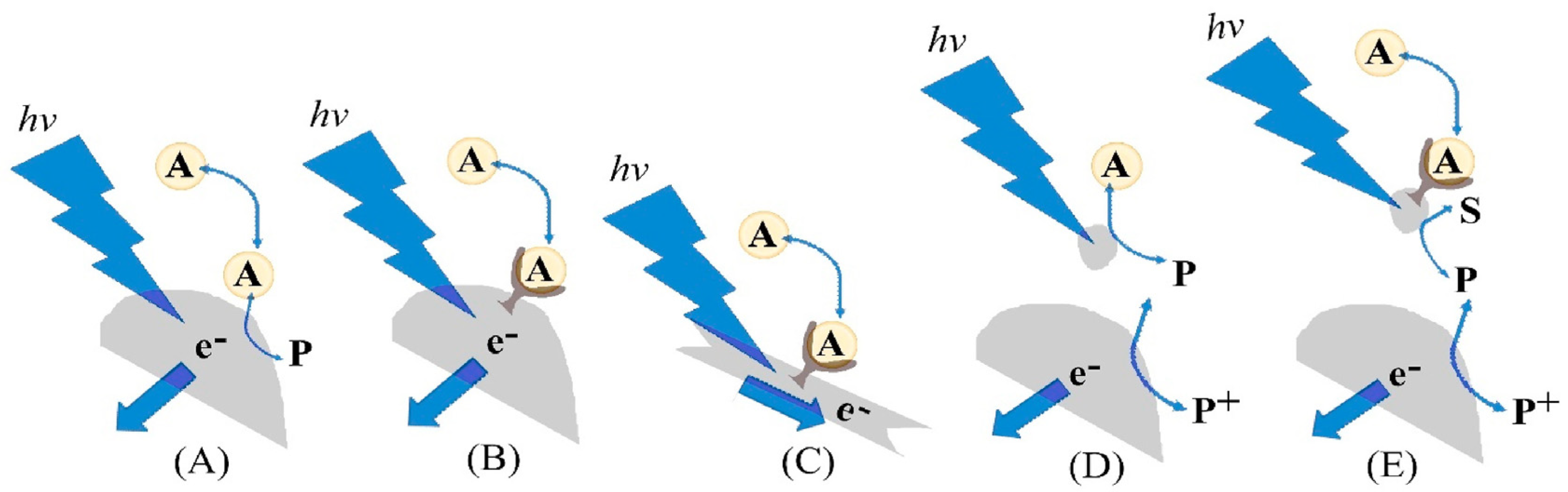
In other turns, the interest in photoelectrochemical sensors has increased in recent years, as it is a promising way of determining an analyte based on the current increase due to the fact of light’s incidence. The comparison between electrocatalytic, photocatalytic and photoelectrocatalytic systems is shown in Figure 1. When compared to conventional electrochemical methods, a photoelectrochemical (PEC) analysis system has higher sensitivity and low background signal due to the complete separation of the excitation source (light) and the detection signal (current) [10,11]. In the PEC process, when a photon is absorbed with energy greater than or equal to the semiconductor gap, electrons are excited from the valence band (VB) to the conduction band (CB), and this causes the generation of oxidizing sites and reducers on the surface of this semiconductor, known as electron/hole pair. These two charge carriers, electron and hole, are responsible for generating current in semiconductors. Thus, when the semiconductor is in contact with the electrolyte, the redox reaction occurs, allowing the transfer of charge between the semiconductor and the redox species at the semiconductor–electrolyte interface.
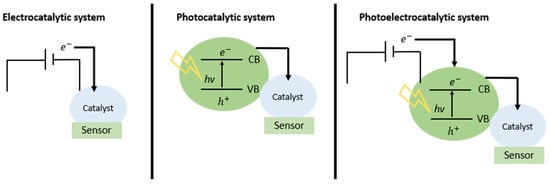
Figure 1.
Comparison of electrocatalytic, photocatalytic, and photoelectrocatalytic technologies for sensing.
In general, semiconductors (organic/inorganic) are used as photoactive material, forming electron–hole pairs at the interface when illuminated by light, thus causing the redox reaction of the ground or excited states of molecules [10,11]. The interactions between the analyte and the semiconductor involved in PEC-assisted detection can be addressed. Coupling the photoexcitation process with electrochemical detection leads to improved unique advantages of optical and electrochemical sensors. Still, the PEC consists of a detection system with a low cost and the feasibility of simplifying the instrument, among others [12].
Thus, sensitivity and selectivity toward the target compound are essential factors. One of the strategies to achieve the selective and sensitive binding of the target compounds in the developed electrochemical sensor would be the modification of the electrode surface through the immobilization of different substances and recognition elements [13]. In this way, studying the material to be used is extremely important.
Catalytic processes are also strongly influenced by the size and structure of the catalyst particle. Several studies indicate the high efficiency of nanostructured materials [14]. Among the advantages is the increase in the surface area available for the catalysis reaction when compared to materials with larger deposition sizes, which increases the amount of electroactive material. Another advantage is that nanomaterials have organized structures, such as nanotubes, which prevent the narrowing of the internal channels, favoring the mass diffusion of the substance inside the material and increasing the conductivity.
The materials used in the manufacture of sensors have active and passive functions. Passive materials are those used for electrical connections and to provide the mechanical structure of the sensor. Active materials, on the other hand, are important for the detection process and may have specific characteristics for their purpose, such as being photosensitive or being able to perform specific connections with certain molecules [15]. Table 1 presents the materials covered in this review, with their main characteristics and their main applications in the field of sensing.

Table 1.
Materials covered in this review, with their main characteristics and their main applications in the field of sensing.
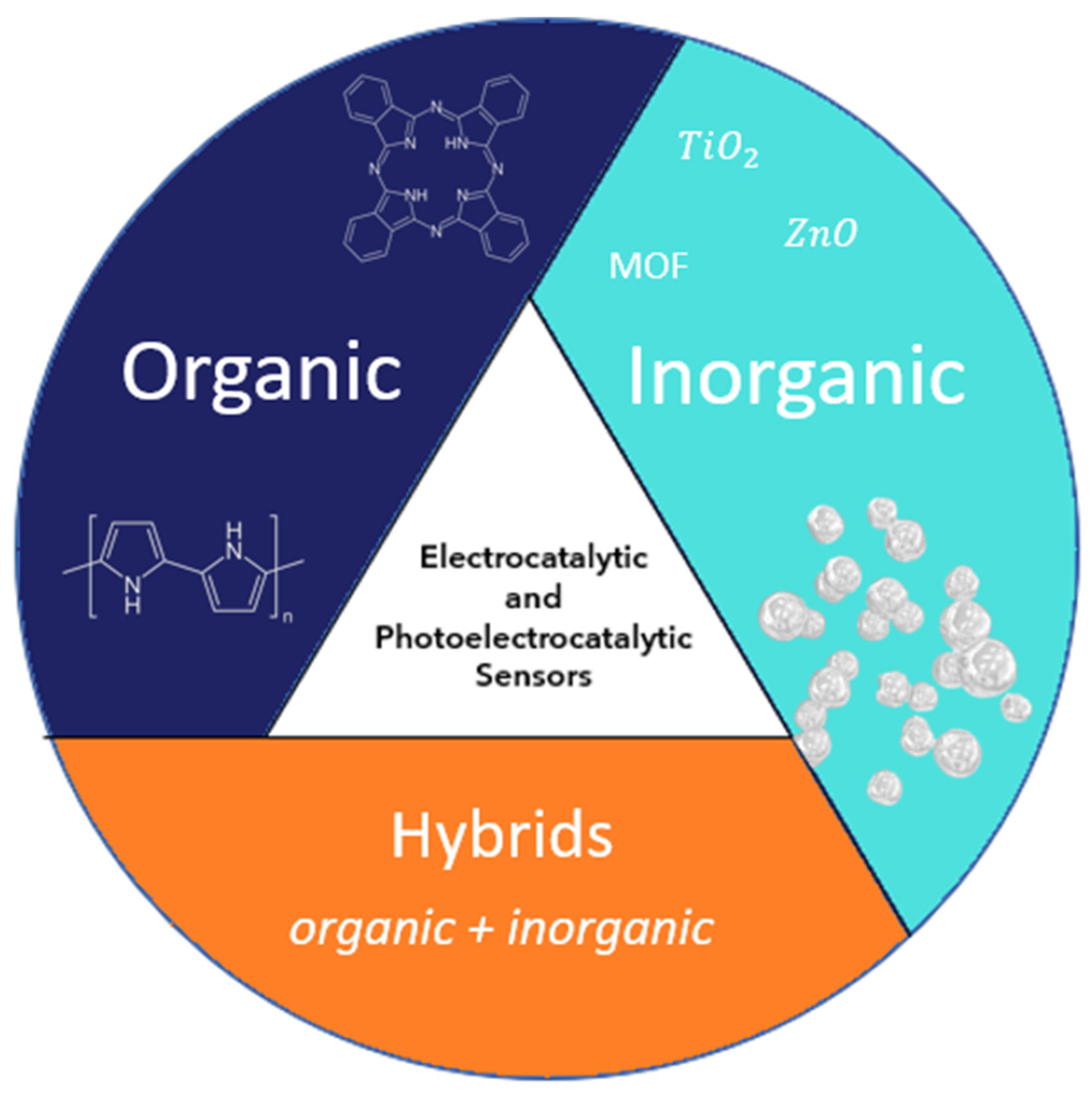
In this review, we discuss the main fundamentals of sensing by electrocatalysis and photoelectrocatalysis, using sensors based on organic, inorganic, and hybrid materials. This review will provide a guide for researchers summarizing the options available for applying sensor detection using different substrates. Figure 2 provides a summary diagram of the topics covered in the article.
Figure 2.
Summary diagram of the topics covered in this article.
2. Inorganic Materials for Sensors
2.1. Detection by Electrocatalysis
Platinum, palladium, silver, and gold are widely used as electrodes and electrocatalysts in different electrochemical reactions. Even though they have good properties, some issues, such as poor stability and specificity, limited resources, and high cost, restrict the commercial application of these materials. On the other hand, transition metals are abundant and have low cost and good electroactivity, making them exciting materials for electrocatalytic sensing platforms. Another group of materials that have drawn increasing interest and have been applied as electrochemical sensors are metal–organic frameworks. More details on the properties of these materials and their applications as electrocatalytic sensors are explored below.
2.1.1. Noble Metals
Noble metals are known as good electrocatalysts due to the fact of their sorption properties with a strong influence from the material surface. These metals are used as nanoparticles to take advantage of their maximized surface area and changes in the physical properties, enhancing the electrocatalytic performance [16]. Gold nanoparticles are vastly used in composite materials to improve electroanalytical measurements, strengthening the ability and sensitivity of detection [17,18].
A nanocomposite of gold nanochain and multiwalled carbon nanotubes was fabricated as a voltammetric sensor for bisphenol A (BPA) detection. The synergic effect of the two materials in the resulting composite was indicated by the decrease in the peak potential and significant enhancement in the peak current value of the electrocatalytic oxidation of BPA. The BPA sensor showed a linear range of 0.5–2000 µM, with a detection limit of 12 nM and showed practicability for real samples [19].
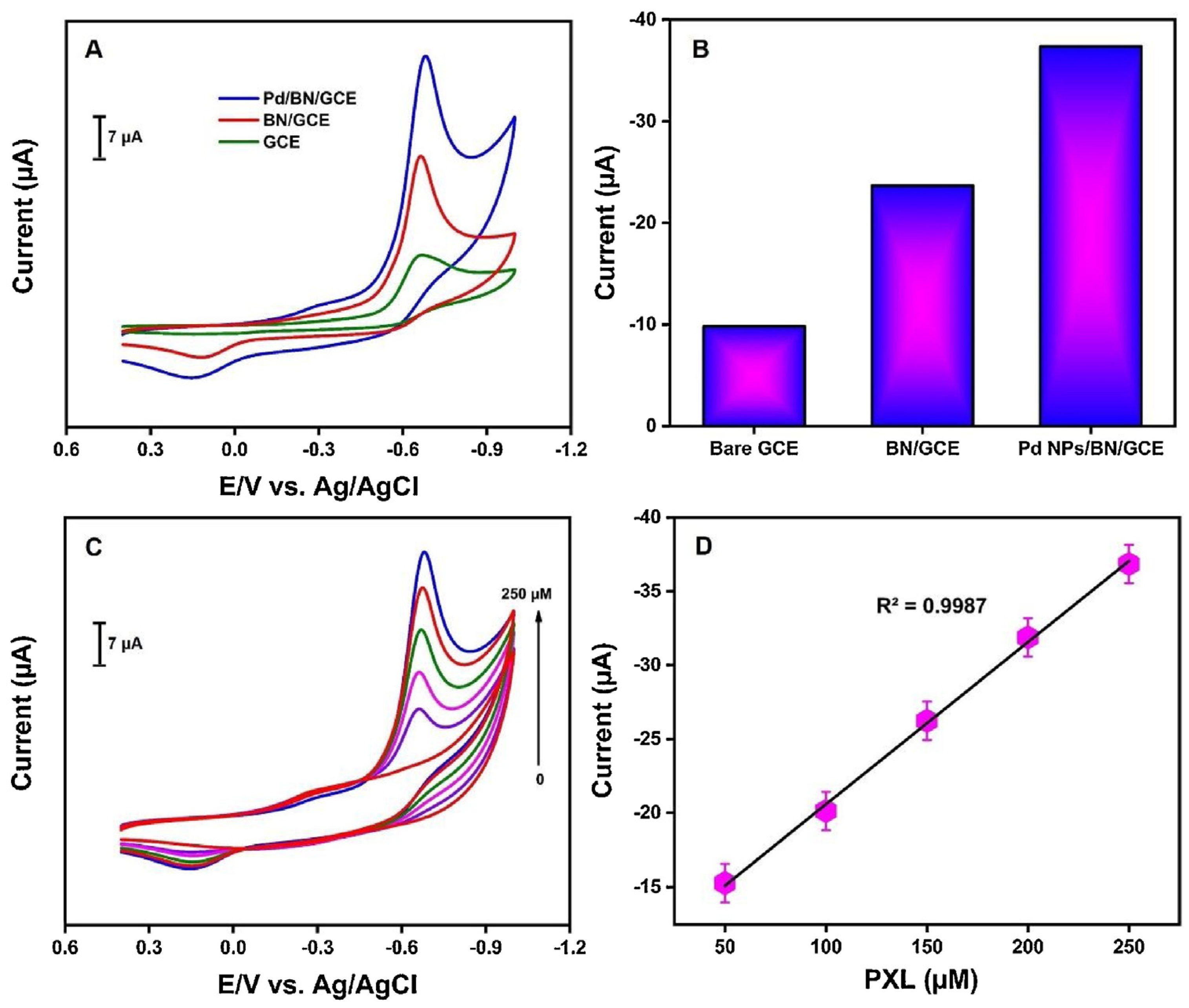
Palladium nanoparticles (PdNPs) stand out as electrocatalytic materials due to the fact of their good stability, fast electron transfer, size distribution control, and large surface–volume ratio. PdNPs have been successfully used in the detection of organophosphorus pesticides. In the application for electrochemical detection of paraoxon ethyl (PXL), PdNPs were adorned in the heterojunction with boron nitride and immobilized in a glassy carbon electrode (GCE). The Pd/BN/GCE sensor exhibited excellent electrochemical performance for the detection of PXL, with superior current response compared to the bare GCE or the BN/GCE sensor (Figure 3A,B), which demonstrated an improvement in the catalytic activity by introducing PdNPs. The sensor exhibited a linear current response with PXL concentration (Figure 3C,D), with a low detection limit of 0.003 μM and sensitivity of 2.23 μA μm−1 cm−2, attributed to the synergistic effect of a large surface area, high electrical conductivity, and numerous sites [20].
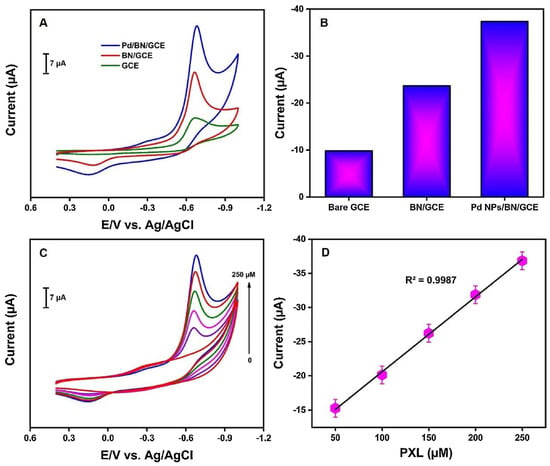
Figure 3.
(A) CV responses of the different electrodes (GCE, BN/GCE, and Pd/BC/GCE) in 0.05 M PBS solution (pH 7) with the presence of 250 μM of PXL; (B) bar graph of the different electrodes; (C) CV responses of the PdNPs/BN/GCE to different concentrations of PXL (0–250 μM) in 0.05 M PBS (pH 7); (D) plot of current versus different concentrations of PXL. Reprinted with permission from Ref. [20].
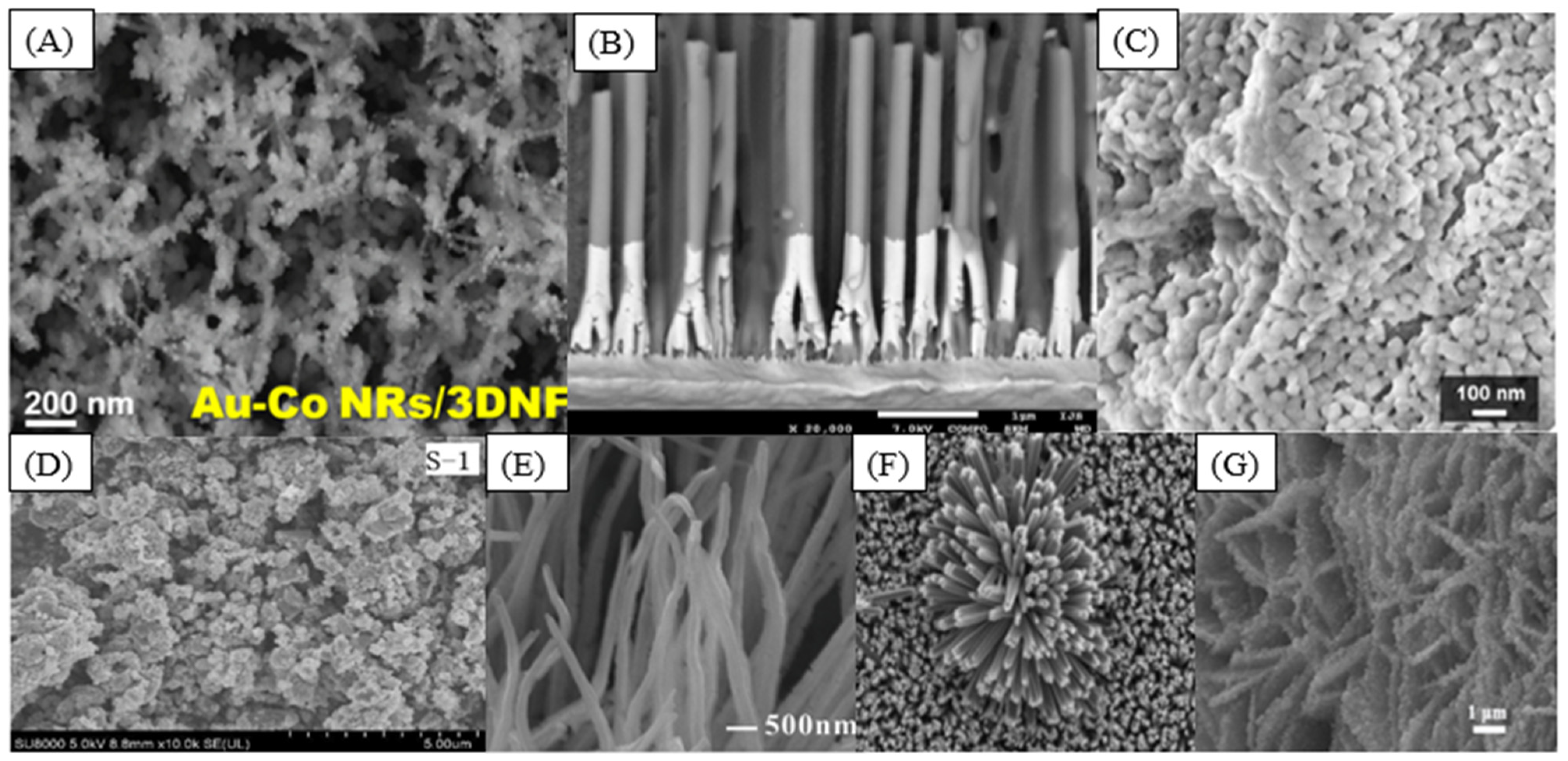
Bimetallic nanoparticles have drawn significant interest over the last decade. These materials are synthesized by the combination of different metallic nanoparticles, and the catalytic properties of the resulting nanomaterial can be improved compared to the monometallic catalysts due to the change in the chemical properties owing to the interaction of two metals [21,22,23]. The synergic effect of applying two noble metals has been explored, and the use of these materials was a breakthrough. A nanohybrid based on gold and cobalt nanorods (Figure 4A) presented a remarkable selectivity toward H2O2 detection [23]. Bimetallic palladium–gold electrocatalytic sensors for hydrogen peroxide and glucose detection were synthesized [22,24]. The hydrogen peroxide electroreduction is enhanced with the concentration of gold because of the synergetic effect and bifunctional mechanism of the reaction [22]. PdNPs have more electrocatalytic activity toward glucose oxidation than AuNP; however, the electrocatalytic activity was further improved by the synergistic effects of the Au-Pd bimetallic nanoparticles [25].
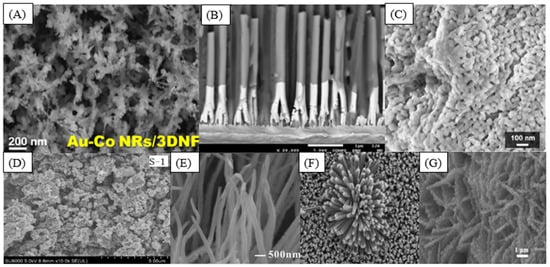
Figure 4.
SEM image of (A) Au-Co NRs on a 3D nickel foam (reprinted with permission from Ref. [23]); (B) cross-section of completely homogeneous Ni NWs (reprinted with permission from Ref. [26]); (C) leaf-templated Co3O4 (reprinted with permission from Ref. [27]); (D) Fe2(MoO4)3 sample (reprinted with permission from Ref. [28]); (E) CuO nanoarray (reprinted with permission from Ref. [29]); (F) TiO2 nanorods (reprinted with permission from Ref. [30]); (G) NiO@Ni-MOF on Ti mesh (reprinted with permission from Ref. [31]).
Silver nanoparticles (AgNPs) also present good electrocatalytic performance. The dispersion of AgNPs in an iron oxide matrix was studied as an electrocatalytic nitrate sensor. The results reported show an electrocatalytic activity of the nanocomposite electrode toward nitrate reduction attributed to the presence of the silver nanoparticles. The electrode presented a low detection potential in neutral media, with a wide linear range [32].
Another noble metal used in electrocatalysis is rhodium. Rhodium can be used in its metallic form, with alloys, rhodium phosphide, and selenide, or even as hybrid nanomaterials. However, a problem related to its use is that its Gibbs free energy of H* at the Rh site is negative, obtaining very strong adsorption [33]. Thus, the incorporation of this metal with other materials is necessary. Dong et al. [34] synthesized rhodium oxide nanocorals for pH and glucose sensing. The material had a detection limit of 3.1 μM and sensitivity of 11.46 μA mM−1 cm−2, and the authors also tested the accuracy in human serum samples. In other turn, Shin et al. [35] synthesized nanofibers composed of binary palladium–rhodium oxide for CO detection. The authors explored different atomic ratios to reach a better synergistic effect between the two metals and a highly conductive phase, in which the ratio of Pd 0.55 and Rh 0.45 showed the highest sensitivity.
Other works using noble metals for electrocatalytic detection are cited in Table 2.

Table 2.
Electrocatalytic sensor based on noble metals.
2.1.2. Transition Metal-Based Materials
Nickel
Nickel is one of the most important transition metals due to the fact of its higher natural abundance, feasibility for numerous applications, and low cost. Many nickel-based materials exhibit excellent electrocatalytic activity toward the oxidation of a wide range of small compounds, such as glucose, insulin, ethanol, carbohydrates, anions, ammonia, and urea [43,44]. Nickel hydroxide (NiOH2) and nickel (III) oxyhydroxide (NiOOH) are important materials, but nickel oxide (NiO), pure nickel, and nickel cations (Ni2+) can also detect substances by electrocatalysis [43,45]. Table 3 cites several works that used nickel-based material for electrocatalytic sensing.

Table 3.
Electrocatalytic sensor based on nickel.
Most electrocatalysis studies are based on the mediation of Ni(OH)2/NiOOH redox couple in an alkaline solution. It has been suggested that Ni2+ undergoes oxidation to its active Ni3+ form, which catalyzes the target molecule’s electrochemical oxidation [53,54]. One disadvantage of this material is the necessity of using alkaline media to produce the active electrocatalyst.
As a p-type semiconductor, Ni(OH)2 presents poor electric conductivity which limits its electrocatalytic performance. The α-Ni(OH)2 phase exhibited a higher conductivity when compared to β-Ni(OH)2 phase. On the other hand, α-Ni(OH)2 is not stable and spontaneously transforms to β-Ni(OH)2 by aging and charge–discharge cycles [43,44]. The substitution of nickel sites in the crystalline structure for other metals, such as cobalt and copper, can improve the conductivity and electrochemical activity by producing vacancies in the structure and increasing the stacking fault disorder [44].
Nickel-based electrodes exhibit good electroanalytic performance for small organic molecules, and it is well known for its application as a nonenzymatic glucose sensor in alkaline media [55,56,57]. An electrocatalytic sensor for the detection of formaldehyde was prepared using nickel nanowires to form the redox pair of Ni(OH)2/NiOOH on the surface via the cycling voltammetry modification process in KOH. As suggested, the nanowires (SEM image in Figure 4B) showed unique electrical and catalytic properties compared to nickel nanoparticles [26]. The amperometric response indicated that the modified electrode exhibits enhanced electron transfer, high formaldehyde oxidation currents, and high signal-to-noise ratios [26].
The incorporation of cobalt into the Ni(OH)2 structure was evaluated as an electrochemical sensor. The result was an enhancement in the electrocatalytic properties by shifting the formation of NiOOH in the cathodic direction [58]. To improve the conductivity and also electrocatalytic behavior, hybrid materials with conductive carbon-based materials can promote a synergistic effect and good performance in electroanalysis [59]. For example, Yang et al. [60] built a sensor using an ordered mesoporous carbon/nickel oxide (OMC-NiO) nanocomposite for GCE modification. OMC has good electrocatalytic and electrochemical properties, which have been improved by the incorporation of NiO nanocrystals. The OMC-NiO/GCE sensor was successfully applied in the detection of epinephrine by DPV, with detection limits of 0.085 μM and a large linear range (8.0–50.0 μM).
Layered double hydroxides are two-dimensional nanomaterials with distinctive physicochemical properties. Nickel–cobalt-based (NiCo-LDHs) electrocatalysts present layered structures with a high specific surface area, chemical stability, and electrical conductivity. The structural defects promote abundant electroactive sites, decreasing the overpotential for oxidation reactions [61,62]. NiCo-LDH synthesized by a hydrothermal process presented good prospects for application in the electrochemical detection of pesticides with a wide linear detection range from the nanomolar to micromolar [61,63].
Cobalt
Cobalt is another abundant transition metal that has emerged as an attractive material for electrochemical reactions due to the fact of its catalytic performance [64]. Cobalt-based materials have been recognized as potential electrocatalysts because of their good electroactivity and superior electrochemical stability compared to noble metals. Efforts have been devoted to preparing materials with various morphologies and composites to improve the electrocatalytic performance [64,65]. Similar to nickel hydroxide, amorphous cobalt hydroxide (Co(OH)2) is more electroactive than crystalline compounds, as the active site of amorphous compounds is more accessible [65]. Works using cobalt-based material for electrocatalytic detection are cited in Table 4.
The addition of a second metal catalyst to form a complex nanostructure or the addition of vacancies enhances the electroactivity by increasing the number of electroactive sites and electronic conductivity [62,66]. Cobalt vacancy-rich Co(OH)2 ultrathin nanosheets were synthesized. As a glucose and L-cysteine sensor, the oxidation of the target molecule occurred through the interaction among the oxidized CoO(OH). Compared to being defect-free, the electrode with vacancies showed a faster response and higher catalytic response current. The main idea is a facile and scalable synthesis of active material. Moreover, the results indicate excellent analytical performance in human blood serum samples [64].
Cobalt oxide (Co3O4) has Co2+ and Co3+ cations distributed in the structure, and a large number of unoccupied interstitial sites provide excellent catalytic activity [27,67,68]. A 3D hierarchical porous Co3O4 nanomaterial, shown in Figure 4C, was synthesized as a nonenzymatic sensor for glucose or H2O2 detection [27].

Table 4.
Electrocatalytic sensor based on cobalt.
Table 4.
Electrocatalytic sensor based on cobalt.
| Material | Species to Be Detected Electrocatalytically | Type of Detection Technique | Limit of Detection | Reference |
|---|---|---|---|---|
| Cobalt hydroxide nanosheets with abundant cobalt vacancies | Glucose | Amperometric | 295nM | [62] |
| Glassy carbon electrode with a nanocomposite from cobalt nanoparticle and tungsten carbide | Peroxide sensor | Amperometric | 6.3 nM | [69] |
| Nanostructured spinel cobalt manganese oxides | Hydrogen peroxide sensor | Amperometric | 15 μM | [70] |
| Cobalt phosphide nanowire array grown in situ on titanium mesh | Glucose | Amperometric | 0.1 μM | [71] |
| CeO2 nanospheres codoped with Cu and Co | MicroRNA | Differential pulse voltammetry and electrochemical impedimetric spectroscopy | 33 aM | [72] |
| Graphene oxide/silica-cobalt mesostructured nanocomposite | Salmonella spp. | Electrochemical impedimetric spectroscopy | 101 cfu mL−1 | [73] |
| CNT-nickel–cobalt oxide/Nafion | Insulin | Amperometric | 0.22 μg/mL | [74] |
Iron
Among the materials used for electrocatalysis, iron-based structures have several advantages over other non-noble metals. When combined with metallic alloys, other studies showed an increase in the electrocatalytic activity due to the presence of Fe [75,76]. Its abundance in nature and low price make using iron more attractive in catalytic reactions, especially in large-scale systems due to the easy processing of industrial waste [77]. In addition to the economic advantage, ferric catalysts’ electrochemical properties reveal superior results compared to other metallic materials [78]. Another advantage is the facility to modify the structure of the deposited material, favoring its selectivity [79]. Works using iron-based materials for electrocatalytic detection are shown in Table 5.

Table 5.
Electrocatalytic sensor based on iron.
The efficiency of catalysis depends on both the support material and the synthesis mode. In the case of iron-based catalysts, their deposition occurs through the formation of aggregates with crystalline or amorphous structures similar to those that appear in nature, such as hematite (α-Fe2O3), maghemite (γ-Fe2O3), and magnetite (Fe3O4), among others [87]. These structures make the material more stable because of the distribution of the atoms and increase in the catalytic activity due to the existence of empty sites in the structure. These empty spaces can be filled with other elements, as shown in Figure 4D, with the iron molybdenum catalyst [28]. The most applied methods for synthesizing iron-based materials are hydrothermal, coprecipitation, electrodeposition, sol-gel, and microemulsion [88]. The industrial uses of ferric catalysts are in supercapacitors, water treatment, lithium batteries, and drug delivery due to the fact of their magnetic and optical excellent properties [87,89,90].
Studies show that the use of iron-based materials in sensors is a viable economic option [91]. They have been used in several fields such as gas sensors and biosensors for detecting organic molecules, including glucose, dopamine, amino acids, and pesticides. Its performance as a sensor replaces enzyme-based sensors, reducing costs and improving the operation stability in biological samples [92,93].
An interesting research field in electrocatalytic sensing, also using iron, is the use of magnetic particles, which are an important class of micro/nanomaterials that exhibit magnetic properties when subjected to external magnetic fields. Among these particles, we can mention the use of Fe3O4. The work of Thamilselvan et al. [94] is an example of the development of a portable electrocatalytic sensor based on gold nanoparticles decorated with Fe3O4 magnetic nanocomposites for dopamine detection. The behavior of this device showed an amperometric detection, with a linear response of 0–0.8 μM and a detection limit of 2.7 nM.
In the work of Zokhtareh et al. [95], a sensor based on Fe3O4 nanoparticles for the detection of metronidazole (MNZ) was constructed. The Fe3O4 NPs were immobilized along with graphene nanosheets on the surface of GCE (GR/Fe3O4NPs/GCE). The sensor showed good catalytic activity for MNZ, with two working ranges of 0.05–5 μM and 5–120 μM, a limit of detection of 0.23 nM, a limit of quantification of 0.76 nM, and a sensitivity of 7.34 μA μM. In addition, the magnetic property of Fe3O4 was explored by Zhang et al. [96] to fix the material on magnetic substrates, such as in magnetic glassy carbon electrodes, which enables magnetic adsorption recycling and sensor recovery.
Copper
Electrocatalysis with copper-based materials happens due to the presence of an unpaired electron in the structure of the molecule [29]. Some advantages of using copper instead of other materials in catalytic reactions are a greater operational stability, thermal resistance to high temperatures, ease of system preparation, analysis speed, and lower cost [79,97]. One example is the detection of hydrogen peroxide (H2O2) with a copper-based sensor, instead of sensors that require the immobilization of enzymes. Through the electrooxidation of H2O2, it can be detected simply and cost-effectively in samples contaminated by industrial waste [98].
The copper catalyst synthesis methods are similar to those of other metallic oxides, mainly hydrothermal methods, chemical precipitation, emulsification, thermal oxidation, and electrodeposition [92,99]. The detection capacity due to the electrochemical reaction is increased with the formation of micro- and nanostructures during the deposition when compared with the bulk material [97]. Its electrochemical activity is inferior to nickel-based materials, but it can be favored with nanostructures, as shown in Figure 4E, which increase the contact between the sample and the sensor surface [29]. Nanostructures depend on both the deposition method and the precursor solution [100]. Copper is used as an electrocatalyst in glucose, alcohol, and carbon monoxide oxidation reactions at low temperatures [99].
Some of the applications of copper sensors are as a biosensor for glucose detection [29,100], hydrogen peroxide electrocatalyst [98,99], nitrite sensor [79], and caffeine sensor [97]; other applications can be seen in Table 6. In the case of glucose sensors, copper oxide acts as an electrocatalyst that oxidizes glucose into gluconolactone, which is subsequently hydrolyzed into gluconic acid [29]. The results obtained in studies on H2O2 sensors showed detection limits of 0.21 µM, indicating better results than other enzymatic and nickel-based sensors [99]. Li et al. compare the performance of an electrochemical sensor with the HPLC results, showing that it was possible to quantify nitrite with greater precision in natural samples of apple juice and pickles [79].

Table 6.
Electrocatalytic sensor based on copper.
Titanium
Titanium has several applications in electrocatalysis, mainly in the form of its oxide (TiO2), and it is used in thermal catalytic reactions, photocatalysis, and sensors [108]. It has the advantages of being nontoxic, biocompatible, photo-corrosion resistant, and cost effective, in addition to offering mild temperature and pressure operating conditions and a low manufacturing cost [67].
TiO2 presents three types of structure in nature, which are anatase, brookite, and rutile. Rutile is the most stable due to the fact of its compressed tetragonal conformation, and brookite is the most unstable [109]. The synthesis of the catalyst can be carried out by hydrothermal methodology, first forming anatase at low temperatures and later forming rutile with increasing temperatures up to 700 °C [68]. It is important to note that rutile has superior electrochemical properties than the material in the anatase form. Other methodologies can be used to synthesize TiO2, but because it is biocompatible, the technique of immobilizing biomolecules has great prominence in the use of titanium oxide. By immobilizing enzymes in the oxide structure, it is possible to maintain the activity of such molecules and build biosensors based on TiO2 [110]. The electrocatalytic reaction occurs in two stages, firstly, the adsorption of the molecule on the electrode surface by physisorption or chemisorption, followed by a direct reaction with the oxide or mediated by an immobilized enzyme in the material structure. The electrical response of the reaction reaches the sensor and allows for the definition of the amount of analyte in the sample. Titanium-based materials have different uses in sensors, and the main ones are gaseous sensors for identifying toxic or flammable gases; contaminant sensors in natural water courses or the food industry; and biosensors for the analysis of biological material in several areas [111].
One current challenge for the practical application of sensors is the material’s long recovery time and high operating temperatures. This obstacle can be overcome partly by combining TiO2 in alloys with other metals and modifying the sensor’s nanostructure [69]. The nanostructure allows for several conformations facilitating the contact between the surface and the sample, such as TiO2 nanorods, as shown in Figure 4F [30]. Future studies are important for the development of new synthesis technologies to overcome these application challenges, since the advantages of operation and cost–benefit drive the industry to apply such sensors.
TiO2-based electrochemical sensors have also been successfully applied for the detection of hazardous analytics such as pesticides. In the work of Malode et al. [112], TiO2 nanoparticles were synthesized with cetyltrimethylammonium bromide (CTAB) for the modification of carbon paste electrode (TiO2/CTAB-CPE). TiO2 NPs have good electrical characteristics that favor the rapid electron transfer and increase the analytical sensitivity. The sensor was used to detect aminotriazole (ATZ) in soil and water samples and showed improvement in catalytic properties compared to the bare electrode. The sensor presented low limits of detection and quantification for the ATZ of 2.53 nM and 8.44 nM, respectively.
Other works using titanium for electrocatalytic detection are shown in Table 7.

Table 7.
Electrocatalytic sensor based on titanium.
Metal–Organic Framework (MOF)
Metal–organic frameworks (MOFs) are a class of crystalline materials characterized by their high porosity and internal surface area. These materials consist of metallic ions or clusters bonded through covalent bonds with organic linkers resulting in chemical and physical properties more commonly associated with nonmolecular solids, such as porosity, magnetism, and electrical conductivity [120].
Catalysis is hardly studied as an application of MOF materials due to the fact of their pore structure with metal nodes and organic linkers that can be functionalized as a catalyst. Considering the field of electrochemistry, the principal properties are a large surface area, presence of electrochemically active centers, and high permeability of ions and target substances [31,121,122]. MOFs have been studied for application as energy storage devices [123,124] and electrochemical sensors [31,121,125,126,127,128]. Works using MOFs for electrocatalytic detection are shown in Table 8.
The limitations of MOFs as electrochemical sensors are their relatively poor chemical stability and conductivity. The direct utilization of MOFs in electrochemical sensors is not common [31,122,129]. Combining MOFs with highly conductive materials and noble metal nanoparticles to form composites can improve the electrochemical performance [31,121]. A novel electrochemical sensing platform based on NiO@MOF was developed by Gao et al. for luteolin determination through differential pulse voltammetry. The structure presented in Figure 4G showed a large surface area, active sites, fast electron transport, and good electrolyte penetration of the result material, leading to excellent electrocatalytic activity and resulting in an LOD of 3 pM and good stability and reproducibility [31]. A core–shell composite N-doped-Co-MOF@polydopamine decorated with silver nanoparticles (AgNPs) was synthesized and tested as a nonenzymatic glucose sensor. The presence of AgNP enhances the electron transfer, indicating a higher catalytic activity of the material toward the electrochemical oxidation of glucose. The sensor’s performance showed a linear range, from 1 µM to 2 mM, with a detection limit of up to 0.5 µM, and was verified by detecting glucose in human serum samples. These works show the great potential of using MOF structures for the detection of substances at low concentrations [130].

Table 8.
Electrocatalytic sensor based on MOFs.
Table 8.
Electrocatalytic sensor based on MOFs.
| Material | Specie to Be Detected Electrocatalytically | Type of Detection Technique | Limit of Detection | Reference |
|---|---|---|---|---|
| Co-MOF/titanium nanosheet | Hydrogen peroxide | Amperometric | 0.25 μM | [131] |
| Fe-MOF/Pt nanoparticles | Tinidazole | Differential pulse voltammetry | 43 nM | [132] |
| Ag Nanoparticles in cluster-based Co-MOF | Glucose | Amperometric | 1.32 μM | [133] |
| Fe-MOF/rGO nanocomposite | Hydrogen peroxide | Amperometric | 0.5 μM | [134] |
| MOF-derived MnO@C nanocomposite | Cancer biomarker | Differential pulse voltammetry and chronoamperometry | 0.31 pM (differential pulse voltammetry) and 0.25 pM (chronoamperometric) | [135] |
| Ni-MOF | Glucose | Amperometric | 0.66 μM | [136] |
| MOF-818 metal–organic framework-reduced graphene oxide/multiwalled carbon nanotubes composite | Caffeic acid, chlorogenic acid, and gallic acid | Differential pulse voltammetry | 5.2 nM (caffeic acid), 5.7 nM (chlorogenic), and 0.18 μM (gallic acid) | [137] |
2.2. Detection by Photoelectrocatalysis
In addition to the use of electrochemical sensors as previously described, their combination with solar energy harvesting gives origin to the photoelectrochemical (PEC) approach. In general, the photoelectrochemical approach can be described as a photo-induced process that involves charge separation and the charge transport/process. This process can also be exploited in analytical detection. Highly sensitive, strong, lower background signal, and selective electrodes can be obtained depending on the device’s architecture.
As per Blaskievicz et al. [138], the photoelectroanalytical mechanism can be described based on the consumption of analyte A during the reaction with holes after the photoexcitation process (Figure 5A). The analytical target might be oxidized directly on the electrode surface, and the external circuit collects the electrons as a current response (Figure 5A). In addition, a receptor (biomolecules such as proteins and enzymes) can be immobilized on the electrode surface, improving its selectivity (Figure 5B). The photoconductivity/resistivity can also be monitored in the system with receptor molecules (Figure 5C). Furthermore, an indirect detection process is available. For instance, a semiconductor particle is not directly immobilized at the electrode surface. After the excitation process, it reacts with the analyte to provide a reaction intermediate, which is then detected at the electrode surface (Figure 5D). Blaskievicz further describes a hybrid process that involves the production of intermediate species using receptor molecules, which is in the sequence detected on the electrode surface (Figure 5E).
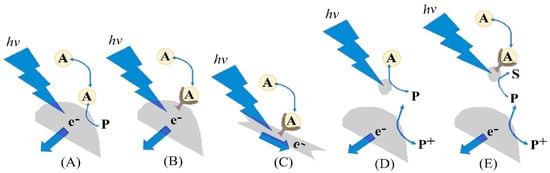
Figure 5.
Illustration of the photoelectroanalytical mechanisms based on (A) analyte A acting as a quencher to provide photoanode signals after photoexcitation; (B) a receptor on the photoabsorber increasing the selectivity; (C) photoexcitation monitored by conductivity or impedance; (D) an external photoabsorber producing an intermediate P that is detected; (E) an external photoabsorber with a receptor and photocatalytic conversion of a substrate. Reprinted with permission from Ref. [138].
In fact, infinite variations of mechanisms to improve the response can be proposed. However, some essential criteria should be adopted to maximize the performance of photoelectrochemical sensors, such as the electrode material’s stability, resistance to electrochemical and photoelectrochemical corrosion, nontoxicity, energy absorption capacity, low-cost synthesis process, and good absorption of visible light. Furthermore, other factors also need to be considered during the use of biomolecules. For instance, biomolecules may have a high cost and low thermal and chemical stability in addition to a complex immobilization procedure.
Many photoactive materials have been investigated for the preparation of PEC sensors. In this context, TiO2 stands out for having an excellent oxidation capacity, high physical–chemical stability, and low toxicity. However, TiO2, ZnO, and other studied semiconductors have a high band gap value, and the excitation of these semiconductors only occurs in the incidence of ultraviolet light. These semiconductors are called usual because their photoelectrocatalytic activity is better known and explored in the literature. Other semiconductors have been studied because they are excited with light in the visible region, and among these compounds, iron and bismuth vanadates stand out, among others.
2.2.1. Usual Semiconductors
One of the most used semiconductors for application in photoelectrocatalytic sensors is TiO2. TiO2 has an excellent photocatalytic performance, adjustable morphology, good stability, and biocompatibility [139]. The stability of the TiO2-based material in photocatalytic reactions allows for its satisfactory application in photosensors [70,140].
Cui et al. [139] used TiO2 nanotubes regulated by oxygen vacancy for application to the defect of tetracycline hydrochloride. The photocurrent response of the photoelectrochemical sensor decreased with an increasing tetracycline concentration due to the fact of adsorption, which consumed part of the oxygen vacancies on the surface of the synthesized material. This system had a detection limit of 0.33 nM and showed selectivity because of the mutual electrostatic interaction between electron-donating groups containing negatively charged tetracycline and the positively charged nanotubes. This study is very interesting and provides information on the fabrication of adsorptive photoelectrochemical sensors regulated by oxygen vacancy. The use of TiO2 is also interesting because this semiconductor can be activated by visible light. The work by Liu et al. [138] exemplifies this use; in this study, gold-modified TiO2 nanotubes were used for glucose sensing. This sensor can be activated by visible red light (625 nm) and has a detection limit of 1.3 μM.
Another widely used metal oxide semiconductor is zinc oxide (ZnO), which has high electron mobility, a high melting point, and good chemical stability. In this context, Han et al. [141] used a p-n heterojunction made with ZnO nanorods and MoS2 flakes for the sensing of propyl gallate. This substance is used as a synthetic antioxidant to prevent the oxidative deterioration of the oil, but it is considered toxic to human health. The material showed optoelectronic behavior, and under open circuit potential and visible light it showed a good response in the determination of propyl gallate, with a detection limit of 1.2 × 10−8 mol L−1. This good response was probably due to the heterojunction, which presented an adequate band level, making the photogenerated electrons and holes separate more easily, leading to an improvement in the sensitivity. In addition, the five-membered Zn chelating ring structure with oxygen atoms aids in selective detection.
2.2.2. Vanadates
Recently, Da Silva Araujo et al. [142] show a new and simple photoelectrochemical (PEC) sensor for the determination of acetaminophen (AC). This sensor was made using a glassy carbon electrode (GCE) modified with bismuth vanadate nanoparticles and dihexadecyl phosphate film. The sensing was based on the photocurrent response by the interaction between GCE and BiVO4 and the energy of visible light, thus using the chronoamperometric method to determine AC in commercial drugs and tap water. The proposed method presented satisfactory accuracy and precision, in addition to presenting a sensor with easy construction and short response time.
In addition, regarding the vanadate electrodes for a photoelectrochemical sensor, Pelissari et al. [143] investigated iron vanadate (Fe2V4O13) synthesized using the adsorption and successive ionic layer reaction (SILAR) process. This method has a low cost, is easy to apply, and can be used for the photoelectrochemical oxidation of glucose by a nonenzymatic photoanode material. The X-ray and Raman diffraction techniques showed the obtainment of the monoclinic phase of Fe2V4O13, formed at 500 °C, without any secondary phase. In turn, the electrochemical characterization under light conditions showed the photoelectrochemical activity of the FTO (fluorine doped with tin oxide) modified with Fe2V4O13, which a good reproducibility in transient light conditions, low resistance to charge transfer, and flat band potential consistent with the LSV (linear sweep voltammetry). Using the chronoamperometry technique, the performance of the electrode as a nonenzymatic interaction of glucose was evaluated. The results showed good performance in the glucose photoelectrooxidation reaction, excellent reproducibility, good sensitivity, good detection limit, and good electrode selectivity against interfering species.
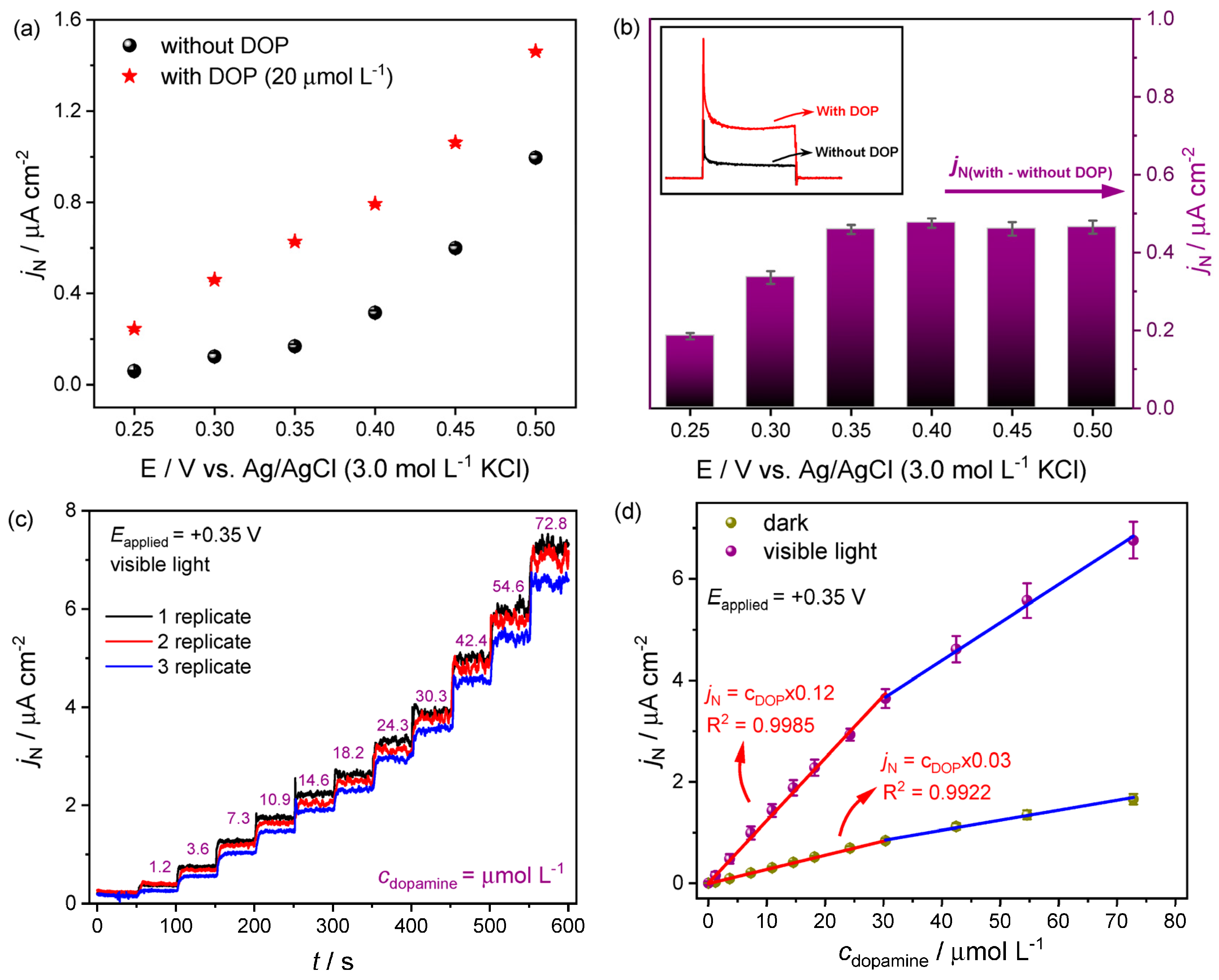
Moreover, iron vanadate was investigated by Camargo et al. [144] for dopamine detection. The SILAR process was used to obtain iron vanadate, and through structural, morphological, and electrochemical characterizations, a high photoelectroactivity of the photoanode in visible light irradiation was contacted. This process presented an easy construction of semiconductor material, with remarkable electroactivity and low cost. First, the photoelectrochemical behavior of the iron vanadate was evaluated in the absence and presence of dopamine molecules (Figure 6a). The experimental results indicated a good affinity between the semiconductor surface and the organic molecule [144]. In addition, electrochemical and photoelectrochemical sensors were obtained, and due to the excellent photoelectrochemical behavior of the iron vanadate semiconductor, the best response was obtained under visible light incidence (Figure 6). In addition, the selectivity of this sensor in the detection of dopamine against several interfering species was evaluated, and a good accuracy was obtained. The precision of the electrode for the determination of dopamine in artificial cerebrospinal fluid was also evaluated, and electronic structural calculations based on DFT were performed, the dominant dopamine species were determined, and their interaction with the iron vanadate photoanode was proposed. All these results indicate promising applications for the proposed method and the manufactured material.
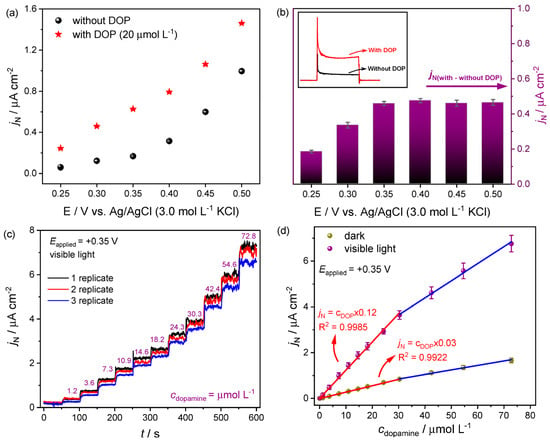
Figure 6.
(a) Photocurrent density responses for chronoamperometry measurements under different applied potentials; (b) difference between the photocurrent with and without dopamine solution; (c) photocurrent density response obtained for different dopamine concentrations; (d) analytical curve obtained by linear adjustment. Reprinted with permission from Ref. [144].
3. Organic Materials for Sensors
3.1. Detection by Electrocatalysis
The development of efficient electrocatalysts with reduced amounts of noble metals or without noble metals is highly desirable. In this sense, organic materials, such as phthalocyanines, carbonaceous materials, and conducting polymers, have been explored as electrocatalyst materials in different electrochemical reactions. These are materials, each one with its properties, which are easy to modify and have great potential for exploration in the field of electrocatalysis.
3.1.1. Phthalocyanines
An organic material that arouses interest for use in electrocatalysis is phthalocyanines, which are polycyclic compounds formed by four benzene rings linked to a central porphyrin ring. To become a semiconductor, the two hydrogen atoms occupying the central ring can be replaced by a metal ion. The great stability in the redox processes of phthalocyanines attracts interest in using them in electrocatalysis processes [145,146,147,148,149,150,151]. Materials based on transition metal phthalocyanine have been extensively studied for the improvement of reactions involving oxygen (ORR and OER). Tajbakhsh, E., et al. used cobalt phthalocyanines in reducing carbon dioxide [146]. Eyele-Mezui, S. et al. used phthalocyanine tetrasulfonate intercalated in metal-layered simple hydroxides (metal: Co, Cu, and Zn) in the evaluation of the electrocatalytic activity, with a focus on fuel cells, where the materials showed potential [145].
Phthalocyanines combined with carbonaceous materials can improve electrocatalytic properties such as stability and selectivity. Zhang, X. et al. developed hybrid structures of cobalt phthalocyanine/carbon nanotubes for CO2 reduction, achieving an efficiency of 96% at an overpotential of 0.52 V [151]. Kumar, Y. et al. developed Fe-containing bimetallic–FeMn, FeNi, and FeCo–phthalocyanine complexes on multiwalled carbon nanotubes, using pyrolysis for the synthesis of mixed catalysts. The presence of the MNx catalytic center provided an increase in the oxygen reduction reaction, as well as a reduction in oxygen evolution [150].
3.1.2. Carbonaceous Materials
Carbon-based materials, such as graphene and carbon nanotubes, have a conductive surface with a high surface area. These characteristics make these materials perfect for use in electrochemical sensors and for modification by the accumulation of catalytic nanoparticles, in addition to presenting great potential as free electrocatalysts [152,153,154,155]. Li, X., et al. have successfully demonstrated the use of graphene as electrocatalysts in hydrogen evolution reactions [155].
Carbon nanotubes (CNTs) are nanocarbon materials with tubular structures composed of rolled-up graphene sheets. Studies using electrocatalysts based on carbon nanotubes (CNTs) demonstrate high efficiency in many typical electrocatalytic reactions. The easy structural operability and accessibility allow for the use of sustainable electrocatalysts based on these materials [156]. Sheng, J., et al. obtained carbon nanotubes through the pyrolysis of imidazolate zeolite structures containing Fe, and the high performance of these noble metal-free catalysts in ZABs presents great potential in real applications [157].
Carbon dots are another class of material that can be used in electrocatalysis. These structures can be defined as near-0D carbon-based materials with a size below 20 nm. The main characteristics of these materials are linked to fluorescence, which is their intrinsic property, in addition to having high quantum yield, low toxicity, and low cost [158]. Dash et al. used carbon dots to modify the glassy carbon electrodes to detect chlorpyrifos. This molecule is used in agriculture as an insecticide and is considered toxic to the environment. The authors obtained a sensor with a detection limit of 1.5 nm, with good reproducibility and good repeatability, in addition to the fact that the detection of chlorpyrifos was compared with the detections by HPLC [159].
These works support the great versatility of carbonaceous materials as electrocatalytic sensors and indicate the possibility of further exploring their use.
3.1.3. Conducting Polymers
One of the most used materials for electrocatalytic sensors is conducting polymers. Conducting polymers (CPs) are a generation of organic materials with metallic or semiconductor features, such as electrical, optical, and electronic properties, associated with the characteristics of conventional organic polymers, including ease of synthesis, corrosion resistance, flexibility, and low cost. Chemically, conductive polymers have alternating single and double carbon–carbon bonds along the polymeric chains and are considered conjugated organic polymeric entities [160]. By presenting this highly conjugated polymeric chain formation, these materials are extremely attractive for several applications, presenting reversible chemical, electrochemical and physical properties controlled by a doping and de-doping process [161].
These materials are insulators or semiconductors in the neutral or undoped form. The polymer becomes conductive only upon the removal of a p-bond electron from the conjugated polymer backbone to form a radial cation defect [162]. To achieve high conductivity, CPs can be doped using different methods. Due to the fact of their unique chemical structure, the doping mechanism is completely different from those occurring in semiconductor materials, where a hole is formed in the valence band. The dopants in the polymer are subjected to redox processes in which the charges are transferred with the subsequent formation of charge carriers [163,164].
The doping process occurs through chain oxidation, called p-doping, or reduction, called n-doping. In p-doping, a hole is created that causes an electron deficiency in the backbone due to the fact of electrons migrating from the HOMO of the polymeric backbone to the doping species. In n-doping, electrons are transferred to the LUMO of the polymer backbone, and electron density is generated [165]. In this way, these oxidation/reduction processes create charge carriers in the form of polarons (radical ions), bipolarons (dications or dianions), or solitons in the polymer.
The polarons and bipolarons can move along the polymer backbone causing localized geometric changes and are responsible for the metallic character of these materials [166]. The most common is p-doping, where the conjugated system stabilizes the positive charge carriers by delocalization [167,168].
Representatively, pristine undoped polypyrrole act as a semiconductor material with a bandgap of 3.16 eV. When it is p-type doping, the polymer backbone is oxidized, a π-electron is removed from the neutral PPy, and a local deformation from the benzenoid structure to a quinoid one occurs to generate a polaron. The polaron creates a localized electronic level within the band structure, and upon further oxidation, a second electron is removed resulting in the formation of doubly charged bipolarons [166,168]. In high oxidized states, overlapping bipolarons levels lead to the formation of two bipolaronic bands in the bandgap, which reduces the energy bandgap from 3.16 eV to 1.4 eV. To electrostatically balance the positive charges, a dopant anion is incorporated after the formation of a positive charge in the polymeric chain [165].
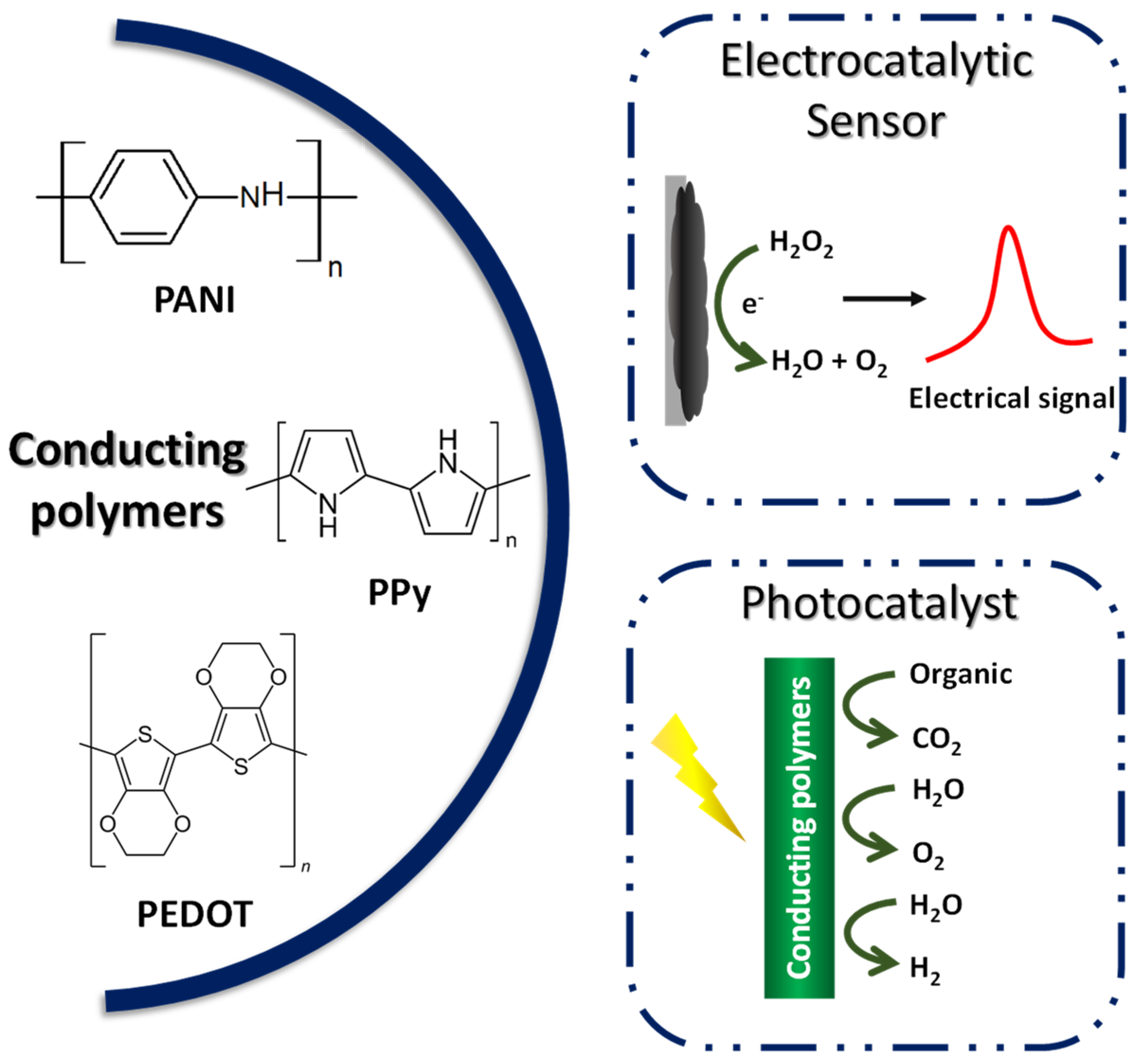
Compared to other organic polymers, conductive polymers have an advantage due to the fact of their adjustable chemical structure, which can be modified to change the conductivity of the polymer [160], allied with the low density, corrosion resistance, and control shape and morphology. Because of this, CPs have been used in several fields such as batteries [169], supercapacitors, sensors and biosensors [170], electrochromic devices [171], and electrocatalysts [172]. The most reported conducting polymers for the mentioned applications include polyaniline (PANI), polypyrrole (Ppy), and poly(3,4-ethylenedioxythiophene) (PEDOT) [173], as exemplified in Figure 7.
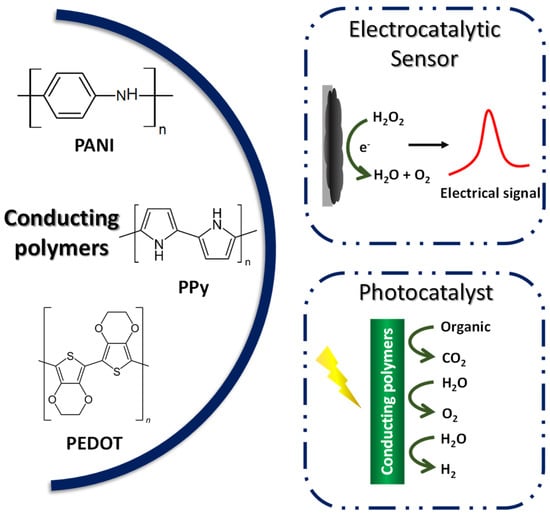
Figure 7.
Application of conductive polymers in electrocatalysis and photocatalysis.
The unique one-dimensional delocalized conjugated structures and excellent conductivity, electrochemical, and optical properties make CPs widely explored as electrocatalysis and photocatalysis materials. In addition, CP catalysts are cheap and effective and can be easily obtained on a large scale [174]. The inherent CP catalysts and compounds are synthesized chemically or electrochemically as powders and films. Chemical synthesis is preferable for large-scale production, while electrochemical synthesis stands out for controlling the thickness and morphology by the electrochemical parameters [165].
CPs have intrinsic electrocatalytic properties toward certain redox reactions. In addition, the interchain cavities that these materials present can be used for the incorporation of metal oxides and nanoparticles of catalyst metals, to generate heterogeneous electrocatalysts with enhanced catalytic activity. In addition, CPs have been used in the immobilization of redox mediators, thus increasing the electron transfer rate in catalytic cycles. Within this approach, the immobilization of biomolecules such as proteins, enzymes, or oligonucleotides stands out. Therefore, CPs demonstrate potential for use in electrocatalytic reactions, such as oxygen evolution reactions, O2 reduction, hydrogen evolution reactions, solar cells, and biosensors [167].
Unlike most other synthetic polymers, conductive polymers, as mentioned above, have robust conjugate structures, which are excellent candidates for electrode materials for electrocatalysis. A thin layer of a conductive polymer, deposited on the surface of the substrate electrode, can increase the kinetics of the electrode’s redox processes in some types of solution [175,176]. Three main processes are important to be considered when using conductive polymers as an electrode during the electrocatalytic conversion of solution species: (1) heterogeneous transfer of electrons between the electrode and a conductive polymeric layer and the electron transfer within the polymeric film; (2) diffusion of species from the solution so that the electrocatalytic conversion takes place; (3) chemical reaction that takes place between the solution species and the conductive polymer [176].
These mentioned processes can be observed in the work by Soares et al., who used PEDOT:PSS|AuNPs as an electrocatalytic sensor in catechol detection. The modified electrode showed a high electrochemical response and strong interactions with the analyte, which allowed for the development of different forms of transduction based on electrochemical reactions and changes in Rct values. In the work, the strong catechol adsorption on the substrate was attributed to hydrogen bonding in the oxyethylene ring of PEDOT, confirmed by Raman spectroscopy [177].
The dopant used in polymer synthesis can directly influence the conductivity of the material. For example, when PEDOT polymer is doped with an anionic system, the high density of the positive charge carriers within the PEDOT phase, necessary for electronic conductivity, is enabled by the charge compensation of the anions. The polymer maintains its electroneutrality by the insertion of anions or by the expulsion of cations. When using large dopants that turn out to be immobile, such as polystyrenesulfonate (PSS), the ionic charge compensation is mainly provided by mobile cations of the electrolyte, which makes cation transport dominant in PEDOT:PSS during oxidation–reduction exchange. When the anions used are small and exchangeable (for example, tosylate (Tos)), it results in an electrode that favors the transport of anions to balance the charge compensation [175,178,179,180].
Another advantage of CPs over conventional metal electrocatalysts is that they can be used to improve sensor selectivity. This advantage was explored in the work of Tang et al. [181], where the efficient junction of the characteristics of Ppy, such as hydrophobicity and π bonds, combined with good electrical conductivity of graphene oxide, was used to prepare a selective sensor for NH3. For the sensor construction, a thin film of Ppy was electrodeposited in reduced graphene oxide (rGO). The sensor demonstrated a fast, reversible, and linear response in the range of 1 to 4 ppm, with excellent selectivity of 6.1% ppm for ammonia detection. The performance obtained by the Ppy/rGO sensor is due to the synergistic effect between the ultrathin layer of Ppy and rGO. The rGO sheets, in addition to acting as a support for Ppy, allow an efficient path for electron transfer, accelerating the response and sensor recovery, while the hydrophobic nature of Ppy protects the sensor from humidity. Still, the excellent selectivity of the sensor was attributed to the adsorption of NH3 molecules in the ultrathin layer of Ppy and electron transfer between the NH3 and Ppy layers.
Despite all the catalytic properties of interest, CPs still have some disadvantages. For example, the low ion transfer rate during the redox reaction eventually leads to area capacitance saturation as the polymer film thickness increases [182]. To overcome this limitation, the introduction of other materials, such as metallic oxides is interesting. Shyamala, S. et al. used this strategy of doping polyaniline nanocomposites with zirconia in the oxidation of formic acid (FAO) for an ethanol oxidation reaction (EOR) [182]. Uwaya, G. E. et al. used polyaniline/nickel oxide in the detection of epinephrine (EP), where the obtained nanocomposite exhibited a better electrocatalytic response with a greater current response and a lower resistance to charge transfer [183].
Another approach to improve the catalytic activity and conductivity are the introduction of metal nanoparticles (e.g., Au and Pt) or carbon nanomaterials in the polymer matrix [160], resulting in hybrid catalysts. This type of catalyst will be further discussed in the next section.
3.2. Detection by Photoelectrocatalysis
Among the organic semiconductors, carbon nitrides stand out, having achieved a lot of interest in recent years, as well as their application as sensors [12,184,185]. g-C3N4 consists of a polymeric material that contains nitrogen (N) and carbon (C) atoms with sp2 hybridization and forms a highly delocalized conjugate system connected via s-triazine or s-heptazine motifs [186].
Li et al. [186] investigated a photoelectrochemical detection platform for the highly sensitive and selective detection of gallic acid, based on the g-C3N4@CNT heterojunction. This research is interesting, as gallic acid is found in a wide range of natural plants and is relevant to the health of humans. The results display that abundant amino groups of g-C3N4 provide excellent selectivity for the sensor. In addition, the sensor was investigated for the analysis of GA in black tea samples, providing a novel and quick method for the detection of GA in food samples.
Li et al. [187] show the detection of metronidazole (MNZ) using a simple, easy, and sensitive photoelectrochemical bioassay (PEC) protocol. In this research, samples of common oral drugs were used under visible light irradiation, where new g-C3N4 nanoarchitectures similar to hierarchical corals (cg-C3N4) were explored for the first time as a PEC detection platform.
4. Hybrid Materials for Sensors
4.1. Detection by Electrocatalysis
A hybrid material is a material that includes two parts mixed at the molecular scale. [14]. This mixture of at least two materials, with different properties, results in unique characteristics compared to the isolated material.
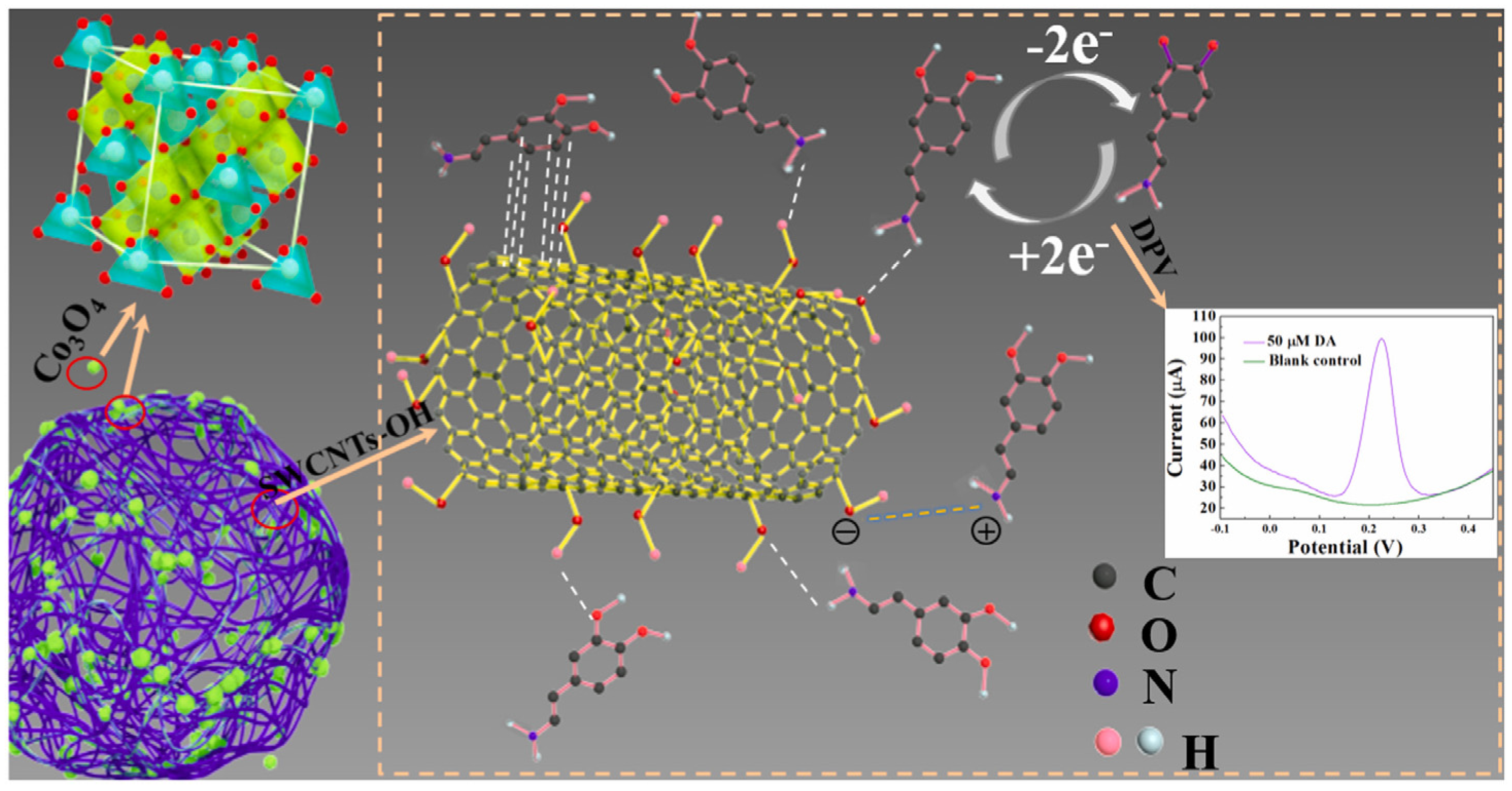
The addition of single-walled carbon nanotubes (SWCNTs) to cobalt oxide enhanced electrochemical sensing platforms. It is important to point out that the carbonaceous material not only increased the conductivity but also improved the electrocatalytic activity of Co3O4/SWCNTs-OH as a result of the synergistic effect. The catalysis mechanism of dopamine (DA) involves the reversible conversion into o-dopaminoquinone (DOQ), as summarized in Figure 8. Briefly, the DA adsorption onto the sensor is facilitated by the negatively charged surface by hydrogen bonds between the -OH groups of the SWCNTs-OH film and the -NH2/-OH groups on DA. In addition, the π-π stacking interactions between the aromatic skeleton of the SWCNTs-OH and the benzene ring of DA favor a sensitive response. Due to the fact of intermolecular interactions, many DA cations can be rapidly and efficiently adsorbed on the composite-based Co3O4/SWCNTs-OH film, which results in a rapid and sensitive response. The electrochemical performances for dopamine detection showed good sensitivity, selectivity, and reproducibility [67].
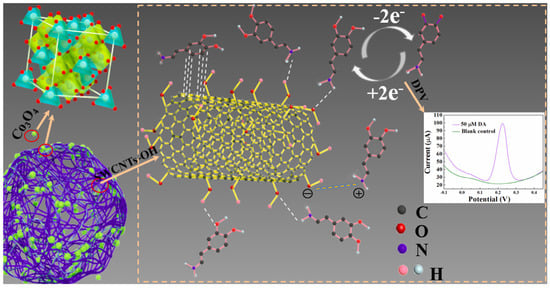
Figure 8.
Schematic catalytic mechanism of DA at Co3O4/SWCNTs-OH/GCE in PB (pH = 6.0). Reprinted with permission from Ref. [67].
Another example is the work of Wang and Hu [188], who obtained an inorganic–organic hybrid material based on PMo12 and used it for volume modification of a carbon paste electrode. This material showed good electrocatalytic activity for the reduction of nitrite, bromate, and hydrogen peroxide. In addition, it presented advantages, such as the use of cheap materials, easy preparation, surface encrustation, and stability, due to the insolubility of the hybrid material, which is an important characteristic for practical application.
In the field of electrocatalytic sensors, the detection capacity is affected by several factors that are directly related to the physical and/or chemical properties of the electrode material. Among these factors, we can mention the electrocatalytic activity, surface area, conductivity, chemical and electrochemical stability, and surface properties, among others [189].
It is often quite challenging to find all these requirements in a single material, so using hybrid materials helps with the objective and final application. In this way, it is possible to have numerous possible combinations of organic and inorganic materials, improving one or more of the necessary properties for electrocatalytic sensors [189].
Many works reported in the literature use hybrid materials for application in electrocatalytic sensors. A significant part of these works uses carbonic structures as the main substrate, such as conductive polymers, carbon nanotubes, and graphene, among others, and is decorated with metallic nanostructures, as can be seen in Table 9. These nanostructures provide greater surface area and help in the mass transport, catalysis, and control over the local microenvironment when compared to the same volume of material consisting of larger particles [190]. This also means that the electrocatalytic activity can be intensified.
In turn, carbonaceous materials have a high conductivity, high surface area, low resistance to charge transfer, and the possibility of functionalization. In addition, they can aid in the attachment of other materials to support the electrode surface, aiding in the stability and durability. In this way, by joining these structures it is possible to improve the electrical conductivity, electrochemical capacitance, mechanical resistance, and consequently, the electrocatalytic sensing.
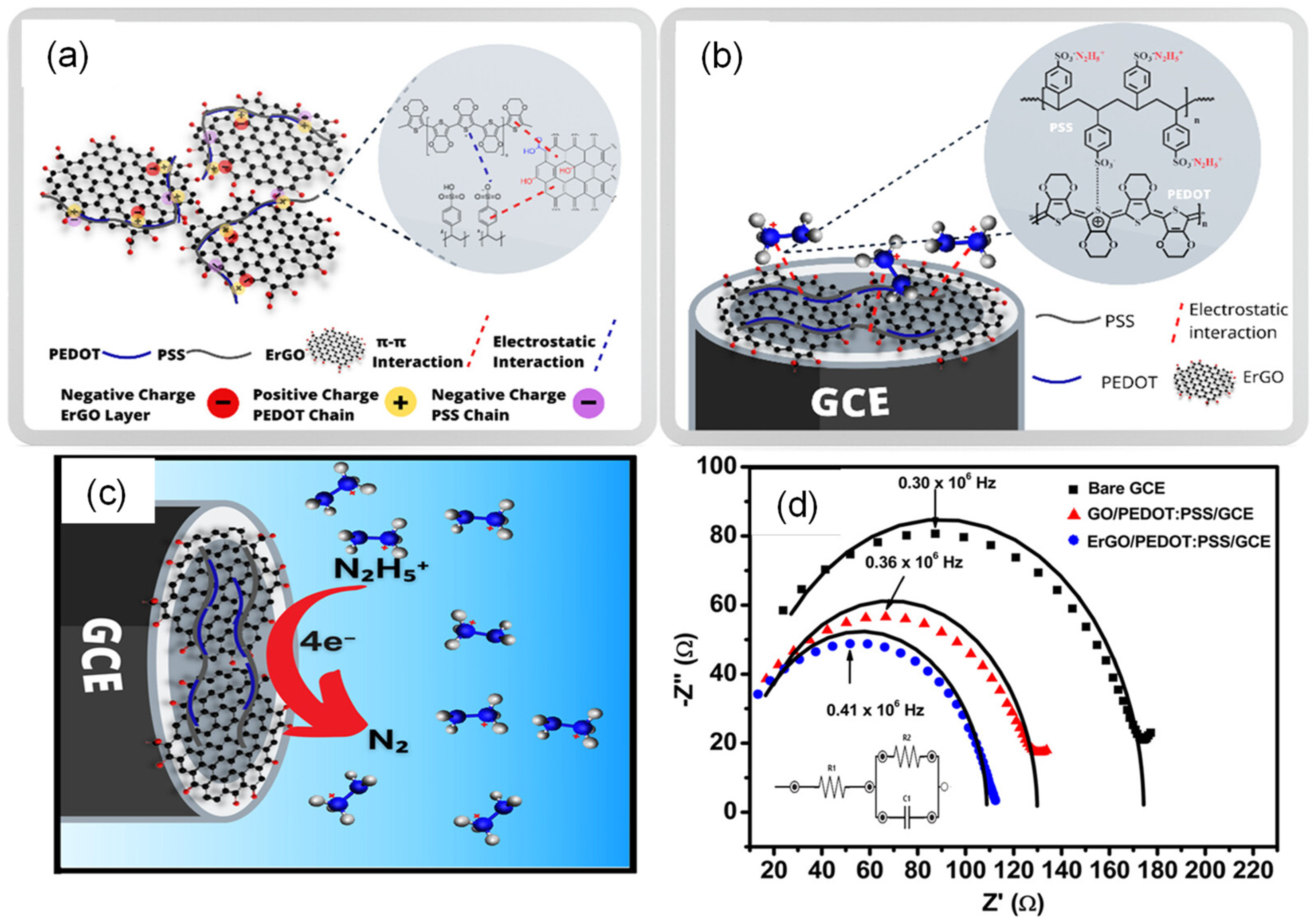
The synergy between carbonaceous and CPs materials is exploited to improve conductivity, as shown in Rahman et al.’s work [179]. A sensor was built with the composite of electrochemically reduced graphene oxide (ErGO) and PEDOT:PSS deposited on glassy carbon electrodes. The chemical bond among the materials comes from the π-π interactions between the graphene layers and PEDOT:PSS, in addition to the electrostatic interaction of the negative charges of GO layers with positively charged PEDOT chains, as shown in Figure 9a. The composite material was used for the detection of hydrazine, where the sulfonic functional groups (−SO3−) of the PSS structure interact with the hydrazinium cations, forming the complex (−SO3− N2H5+), which causes the electrochemical reaction on the electrode surface (Figure 9c,d). As demonstrated by the EIS in Figure 9d, the composite decreases the charge transfer resistance. The authors conclude that ErGO increases the electron transfer rate and improves the conductivity, while PEDOT:PSS acts as a matrix that donates holes to the electro-oxidation process. Thus, the synergy among the materials significantly improves the electrocatalytic property of the sensor. The sensor was applied in the detection of hydrazine by DPV and amperometry. The limits of quantification detection were 0.01 and 0.03 μM, respectively, showing good selectivity in different tested interferents.
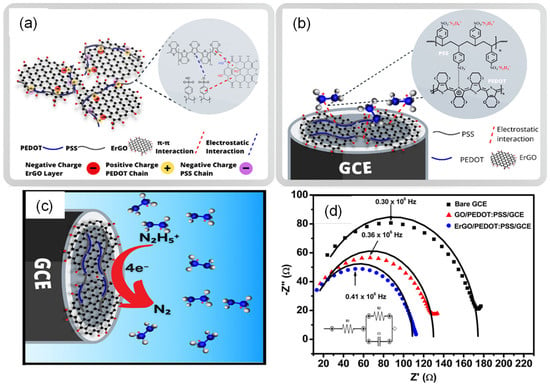
Figure 9.
(a) Schematic of the interaction between ErGO with PEDOT:PSS; (b) interaction between the hydrazinium cation and the ErGO/PEDOT:PSS composite; (c) mechanism of hydrazine oxidation at the surface of ErGO/PEDOT:PSS/GCE; (d) Nyquist plot obtained for the different electrodes (bare GCE, GO/PEDOT/GCE, and ErGO/PEDOT:PSS/GCE). Adapted and reprinted with permission from Ref. [179].
Selective sensors based on hybrid materials containing conducting polymers have been used in the detection of neurotransmitters, which are the most important biomarkers for regulating many functions in cells and tissues, being a powerful tool to improve the prevention, detection, and treatment of different diseases and degenerative disorders [191,192,193,194]. Demirkan et al.’s work [194] is an example of the use of hybrid materials for the detection of ascorbic acid (AA), dopamine (DA), and uric acid (AU). In this work, palladium nanoparticles supported on polypyrrole/reduced graphene oxide (rGO/Pd@PPy NPs) were used. Through the characterizations, it was observed that the Pb nanoparticles were well distributed in the polymeric film and had good detection limits. Furthermore, due to the synergistic effects of its components, this sensor showed an electrocatalytic performance, effective electron transfer capability, and better sensitivity.
Xu, G. et al. [192] developed PEDOT-modified laser-etched graphene electrodes (PEDOT-LSGs) for dopamine electrochemical detection. The material showed a good limit of detection with a linear range of 1–150 µM. The sensitivity of this sensor was related to the use of the material used in which the fast electron transport properties of the porous graphene combined with the electrocatalytic activity of the PEDOT deposit.
An interesting hybrid sensor was fabricated combining the characteristics of metallic catalysts and nanocatalysts materials (Fe3O4 and PdNPs), with the properties of Ppy. The screen-printed electrode was modified by the nanocomposite and applied to the simultaneous electrochemical detection of methotrexate and folic acid. The electrocatalytic material induced a remarkable improvement relative to the oxidation of methotrexate, with the change of the oxidation potential to a less positive amount, and allowed an increase in the peak current. It was possible to co-detect methotrexate and folic acid with the separation of the peaks at approximately 200 mV, without any interference. The work demonstrates the positive synergistic effect between the materials used to improve the electrocatalytic properties [195].

Table 9.
Electrocatalytic sensor based on carbonic structures.
Table 9.
Electrocatalytic sensor based on carbonic structures.
| Main Substrate | Metal Nanostructures | Specie to Be Detected Electrocatalytically | Reference |
|---|---|---|---|
| Carbon ionic liquid electrode | Silver nanoparticles | Hydrogen peroxide | [196] |
| PEDOT | Silver nanograins | Hydrogen peroxide | [197] |
| Carbon nanotubes | Palladium nanoparticles | Glucose | [198] |
| Reduced graphene oxide | ZnO nanoparticles | L-cysteine | [199] |
| Graphene | Platinum nanoparticle | Hydrogen peroxide and trinitrotoluene | [200] |
| Polyaniline | Palladium nanoparticles | Hydrazine | [201] |
| Poly(m-phenylenediamine) | Silver nanoparticles | Hydrogen peroxide | [202] |
Other work using conductive polymers in the detection of the neurotransmitter dopamine is that of Daria Minta et al. [203]. The authors modified a glassy carbon electrode (GCE) with a ternary polyaniline/Fe2O3-SnO2/reduced-graphene oxide (PFSG) nanocomposite to improve dopamine (DA) and uric acid (UA) detection. The characterizations showed an improvement in the detection parameters due to the introduction of polyaniline (PANI), increasing the electrocatalytic properties of the electrode concerning the detected analytes. This sensor allowed for the detection of low concentrations of DA (0.076 µM) and UA (1.6 µM) and showed excellent performance in their simultaneous detection with the limits of detection and excellent long-term stability of DA and UA, maintaining 100% and 90% of their initial signs, respectively, after one month of use.
Malode et al. developed a sensor for the detection of aminotriazole, a herbicide that prevents the biosynthesis of carotenoids. For this, they used multiwalled carbon nanotubes together with calcium-doped zinc oxide nanoparticles. The sensor presented a detection limit of 3.58 × 10−9 M/L and a quantification limit of 1.26 × 10−8 M/L [204]. In turn, Singh et al. [205] developed sensors based on biogenic nanocomposites of CuFeO2 and polyaniline for the electrocatalytic detection of hydrazine. The retention of hydrazine in plants can increase along the food chain, which can inhibit germination, withering the leaves, considered a danger to animal and human health. The material produced showed a high sensitivity of 47.36 μA mM−1 cm−2, with a detection limit of 0.0313 mM, and demonstrated antibacterial activity.
Another approach to using conductive polymers as high-efficiency and affordable electrocatalytic sensors is in water separation applications. Teng, J. et al. [206] developed a shell–core structured catalyst of polyaniline-coated hybrid Ni3Mo2P-MoO3 nanoarrays designed for oxygen evolution.
4.2. Detection by Photoelectrocatalysis
Regarding the hybrid platforms integrating the organic and inorganic semiconductors, the g-C3N4 and different inorganic oxides are more significant for detection. In this way, many studies can be pointed out as relatively enhanced for the development of sensors.
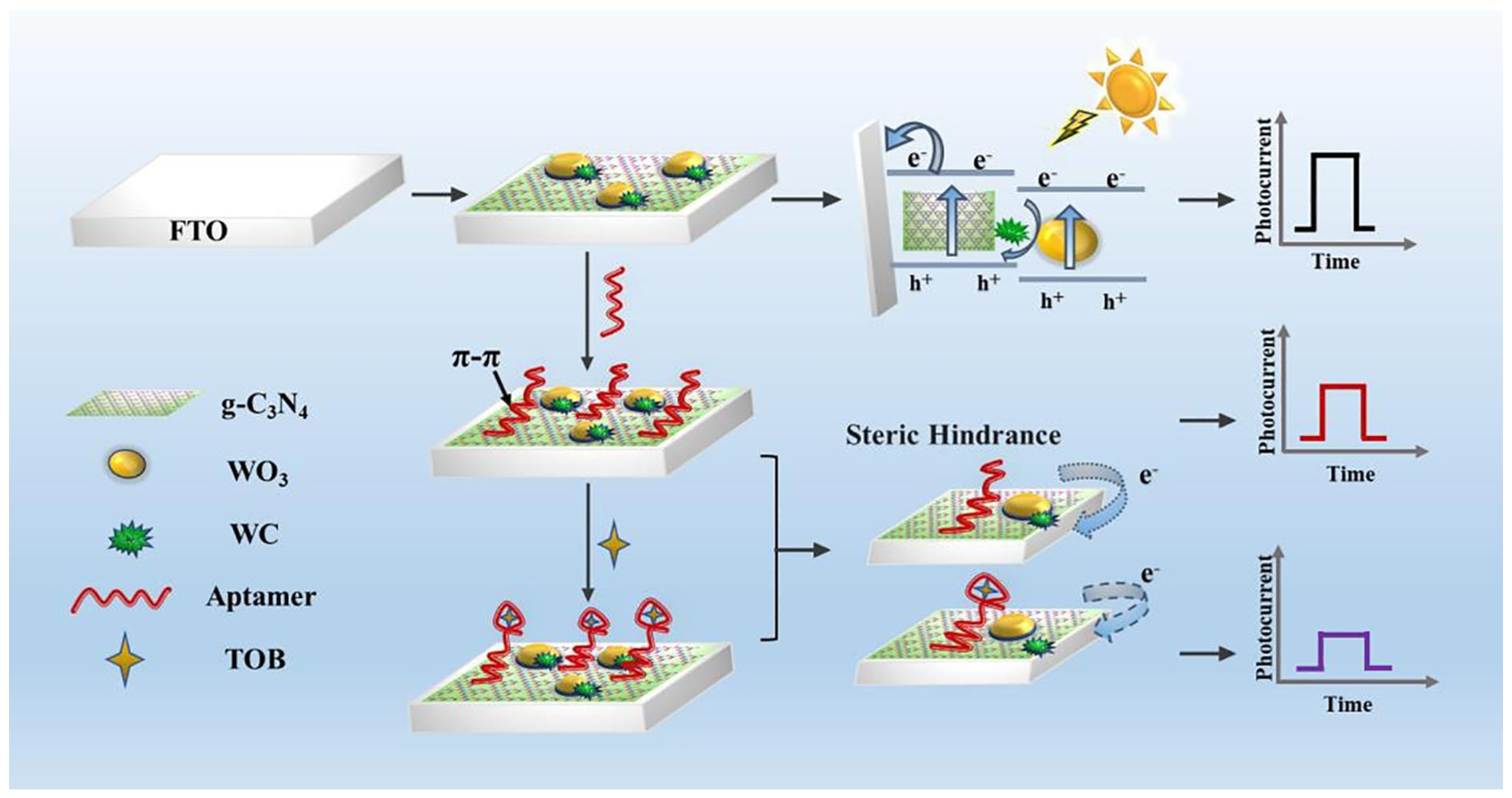
Recently, Qiao et al. [207] showed a sensor for the detection of tobramycin (TOB). This new photoelectrochemical (PEC) aptasensor was based on a “turn-off” PEC mode (Figure 10). The working electrode was modified by g-C3N4/WC/WO3 composites and TOB aptamer probes, which in turn can anchor onto g-C3N4/WC/WO3 through π-π stacking interactions to avoid interference from other modifications. A decrease in the photocurrent was met due to the fact of steric hindrance and prevented electron transfer when TOB was captured by aptamer probes anchored on the modified fluorine-doped tin oxide (FTO) electrode. The proposed sensor shows high specificity, satisfactory detectability, and excellent reproducibility, and it may provide a new thought for detecting other pollutants.
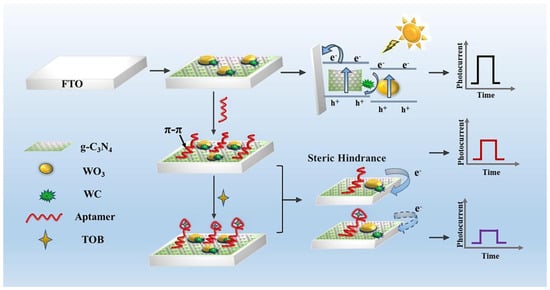
Figure 10.
Schematic illustration of the PEC aptasensor for TOB detection. Reprinted with permission from Ref. [207].
Moreover, Zhang et al. [186] showed sensitive monitoring of a carcinoembryonic antigen (CEA) based on a photoelectrochemical signaling (PEC) biosensor. This sensor was made using graphite-type carbon nitride sensitized by copper and indium disulfide as photosensitive material and cobalt oxyhydroxide as light-blocking material. The results showed high sensitivity, favorable selectivity, and good accuracy.
5. Conclusions
This review discussed the recent progress of electrocatalytic and photoelectrocatalytic sensors based on inorganic, organic, and hybrid materials. While photoelectrocatalytic sensors require a light source for detection, unlike electrocatalytic sensors, they have the advantage of enhanced selectivity. Both classes of sensors can detect a wide range of species, such as ions, small organic molecules, pesticides, and drugs. Regarding active materials, noble metals are known as good electrocatalysts with great perspectives for use as electrocatalytic sensors. Nanostructured materials present even more advantages with an increase in the electroactive area, a greater presence of active sites, and an organized structure that allows for better diffusion of the analyte, resulting in more efficient detection. Therefore, many studies are carried out for the construction of electrocatalytic sensors based on these materials and their composites. Due to the high cost of noble metals, alternative materials have been studied to produce electrocatalytic sensors with good performance at a reduced cost. Inorganic materials, such as transition metals and their oxides and hydroxide, show great prospects as electrocatalytic and photoelectrocatalytic sensors. In turn, among organic materials, carbonaceous materials, phthalocyanines, and conductive polymers stand out due to the fact of their electrocatalytic properties. Nevertheless, there are many challenges to be overcome to insure the reliability of these sensor materials (organic and inorganic) for commercial application. Hybrid materials emerge as a good choice, promoting unique characteristics compared to isolated materials and boosting the electroactivity, conductivity, or robustness of the sensors. Most of the current studies predict the synergic effect of combining two different materials to produce more efficient sensors. The use of hybrid materials with facile synthesis, focusing on good sensitivity and selectivity should be considered as a prospect in this field.
Author Contributions
I.J., writing, review, editing, and original draft preparation; T.L.V., writing; V.K., writing; C.M.P., writing; J.M., writing; L.P.C., writing; L.H.D.A., writing and review; M.V., writing and review. All authors have read and agreed to the published version of the manuscript.
Funding
CNPq (303038/2019–5 and 408635/2018–5) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES)—Finance Code 001. In addition, INCT in Bioanalytics (FAPESP grant no. 2014/50867–3 and CNPq grant no. 465389/2014–7).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are thankful for the financial support from CNPq, CAPES, and Fundação Araucaria.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ivaska, A.; Bobacka, J. Electroanalytical Techniques. In Process Analysis; Elsevier: Amsterdam, The Netherlands; Abo Akademi University: Turku-Abo, Finland, 2005. [Google Scholar] [CrossRef]
- Dincer, C.; Bruch, R.; Costa-Rama, E.; Fernández-Abedul, M.T.; Merkoçi, A.; Manz, A.; Urban, G.A.; Güder, F. Disposable Sensors in Diagnostics, Food, and Environmental Monitoring. Adv. Mater. 2019, 31, 1806739. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, W.; Zhu, W.; Wu, H.; Wang, F.; Xu, X. A photoelectrochemical sensor for highly sensitive detection of glucose based on Au–NiO1– hybrid nanowires. Sens. Actuators B Chem. 2020, 304, 127330. [Google Scholar] [CrossRef]
- Zhou, M.; Guo, S. Electrocatalytic Interface Based on Novel Carbon Nanomaterials for Advanced Electrochemical Sensors. ChemCatChem 2015, 7, 2744–2764. [Google Scholar] [CrossRef]
- Wang, Y.; Zu, M.; Zhou, X.; Lin, H.; Peng, F.; Zhang, S. Designing efficient TiO2-based photoelectrocatalysis systems for chemical engineering and sensing. Chem. Eng. J. 2020, 381, 122605. [Google Scholar] [CrossRef]
- Furst, A.L.; Hill, M.G.; Barton, J.K. Electrocatalysis in DNA sensors. Polyhedron 2014, 84, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, J.; Barse, B.; Gatto, G.; Broncova, G.; Kumar, A. Electrochemical Sensors and Their Applications: A Review. Chemosensors 2022, 10, 363. [Google Scholar] [CrossRef]
- Li, R.; Li, C. Photocatalytic Water Splitting on Semiconductor-Based Photocatalysts. In Advances in Catalysis; Academic Press Inc.: Cambridge, MA, USA, 2017; Volume 60, pp. 1–57. [Google Scholar]
- Keçili, R.; Denizli, A. Molecular Imprinting-Based Smart Nanosensors for Pharmaceutical Applications. In Molecular Imprinting for Nanosensors and Other Sensing Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 19–43. [Google Scholar]
- Rajeshwar, K. Encyclopedia of Electrochemistry; The University of Texas at Arlington: Arlington, TX, USA, 2007; Volume 6. [Google Scholar]
- Peter, L.M. Semiconductor Electrochemistry. In Photoelectrochemical Solar Fuel Production; Springer International Publishing: Cham, Switzerland, 2016; pp. 3–40. [Google Scholar]
- Low, S.S.; Chen, Z.; Li, Y.; Lu, Y.; Liu, Q. Design principle in biosensing: Critical analysis based on graphitic carbon nitride (G-C3N4) photoelectrochemical biosensor. TrAC Trends Anal. Chem. 2021, 145, 116454. [Google Scholar] [CrossRef]
- Shi, J.; Marshall, D. Surface Modification Approaches for Electrochemical Biosensors. In Biosensors—Emerging Materials and Applications; InTech: Charlotte, NC, USA; Purdue University: West Lafayette, IN, USA, 2011. [Google Scholar]
- Kickelbick, G. Hybrid Materials: Synthesis, Characterization, and Applications; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007. [Google Scholar]
- Bansal, D. PC-Based Data Acquisition. In Real-Time Data Acquisition in Human Physiology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 21–55. [Google Scholar]
- Kim, H.; Yoo, T.Y.; Bootharaju, M.S.; Kim, J.H.; Chung, D.Y.; Hyeon, T. Noble Metal-Based Multimetallic Nanoparticles for Electrocatalytic Applications. Adv. Sci. 2022, 9, 2104054. [Google Scholar] [CrossRef] [PubMed]
- Trojanowicz, M. Applications of Gold Nanoparticles in Electroanalysis. In Comprehensive Analytical Chemistry; Elsevier B.V.: Amsterdam, The Netherlands, 2014; Volume 66, pp. 429–476. [Google Scholar]
- Amin, H.M.A.; El-Kady, M.F.; Atta, N.F.; Galal, A. Gold Nanoparticles Decorated Graphene as a High Performance Sensor for Determination of Trace Hydrazine Levels in Water. Electroanalysis 2018, 30, 1749–1758. [Google Scholar] [CrossRef]
- Chang, F.; Ren, K.; Li, S.; Su, Q.; Peng, J.; Tan, J. A voltammetric sensor for bisphenol A using gold nanochains and carbon nanotubes. Ecotoxicol. Environ. Saf. 2023, 252, 114588. [Google Scholar] [CrossRef]
- Renganathan, V.; Balaji, R.; Chen, S.-M.; Kokulnathan, T. Coherent design of palladium nanostructures adorned on the boron nitride heterojunctions for the unparalleled electrochemical determination of fatal organophosphorus pesticides. Sens. Actuators B Chem. 2020, 307, 127586. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Sharma, S.; Naushad, M.; Dwivedi, R.P.; ALOthman, Z.A.; Mola, G.T. Novel development of na-noparticles to bimetallic nanoparticles and their composites: A review. J. King Saud Univ. Sci. 2019, 31, 257–269. [Google Scholar] [CrossRef]
- Alal, O.; Caglar, A.; Kivrak, H.; Sahin, O. Dendrimer Templated Synthesis of Carbon Nanotube Supported PdAu Catalyst and its Application as Hydrogen Peroxide Sensor. Electroanalysis 2019, 31, 1646–1655. [Google Scholar] [CrossRef]
- Bach, L.; Thi, M.; Son, N.; Bui, Q.; Nhac-Vu, H.-T.; Ai-Le, P. Mesoporous gold nanoparticles supported cobalt nanorods as a free-standing electrochemical sensor for sensitive hydrogen peroxide detection. J. Electroanal. Chem. 2019, 848, 113359. [Google Scholar] [CrossRef]
- Waqas, M.; Lan, J.; Zhang, X.; Fan, Y.; Zhang, P.; Liu, C.; Jiang, Z.; Wang, X.; Zeng, J.; Chen, W. Fabrication of Non-enzymatic Electrochemical Glucose Sensor Based on Pd−Mn Alloy Nanoparticles Supported on Reduced Graphene Oxide. Electroanalysis 2020, 32, 1226–1236. [Google Scholar] [CrossRef]
- Li, X.; Du, X. Molybdenum disulfide nanosheets supported Au-Pd bimetallic nanoparticles for non-enzymatic electrochemical sensing of hydrogen peroxide and glucose. Sens. Actuators B Chem. 2017, 239, 536–543. [Google Scholar] [CrossRef]
- Trafela, Š.; Zavašnik, J.; Šturm, S.; Rožman, K. Formation of a Ni(OH)2/NiOOH active redox couple on nickel nanowires for formaldehyde detection in alkaline media. Electrochim. Acta 2019, 309, 346–353. [Google Scholar] [CrossRef]
- Han, L.; Yang, D.-P.; Liu, A. Leaf-templated synthesis of 3D hierarchical porous cobalt oxide nanostructure as direct electrochemical biosensing interface with enhanced electrocatalysis. Biosens. Bioelectron. 2015, 63, 145–152. [Google Scholar] [CrossRef]
- Kong, L.-T.; Zhang, M.; Liu, X.; Ma, F.-Y.; Wei, B.; Wumaier, K.; Zhao, J.-G.; Lu, Z.-P.; Sun, J.-G.; Chen, J.; et al. Green and rapid synthesis of iron molybdate catalyst by mechanochemistry and their catalytic performance for the oxidation of methanol to formaldehyde. Chem. Eng. J. 2019, 364, 390–400. [Google Scholar] [CrossRef]
- Yang, J.; Chen, H.; Zhu, C.; Huang, Z.; Ou, R.; Gao, S.; Yang, Z. A miniature CuO nanoarray sensor for noninvasive detection of trace salivary glucose. Anal. Biochem. 2022, 656, 114857. [Google Scholar] [CrossRef]
- Dey, S.; Mehta, N.S. Synthesis and applications of titanium oxide catalysts for lower temperature CO oxidation. Curr. Res. Green Sustain. Chem. 2020, 3, 100022. [Google Scholar] [CrossRef]
- Gao, F.; Tu, X.; Ma, X.; Xie, Y.; Zou, J.; Huang, X.; Qu, F.; Yu, Y.; Lu, L. NiO@Ni-MOF nanoarrays modified Ti mesh as ultrasensitive electrochemical sensing platform for luteolin detection. Talanta 2020, 215, 120891. [Google Scholar] [CrossRef] [PubMed]
- Bonyani, M.; Mirzaei, A.; Leonardi, S.G.; Bonavita, A.; Neri, G. Electrochemical Properties of Ag@iron Oxide Nanocomposite for Application as Nitrate Sensor. Electroanalysis 2015, 27, 2654–2662. [Google Scholar] [CrossRef]
- Jiang, A.; Chen, J.; Liu, S.; Wang, Z.; Li, Q.; Xia, D.; Dong, M. Intermetallic Rhodium Alloy Nanoparticles for Electrocatalysis. ACS Appl. Nano Mater. 2021, 4, 13716–13723. [Google Scholar] [CrossRef]
- Dong, Q.; Huang, Y.; Song, D.; Wu, H.; Cao, F.; Lei, Y. Dual functional rhodium oxide nanocorals enabled sensor for both non-enzymatic glucose and solid-state pH sensing. Biosens. Bioelectron. 2018, 112, 136–142. [Google Scholar] [CrossRef]
- Shin, S.; Kwon, T.; Lee, Y. Palladium-rhodium binary oxide composite nanofibers with various composition ratios for highly efficient electrochemical sensing of carbon monoxide in neutral aqueous media. Appl. Surf. Sci. 2022, 598, 153847. [Google Scholar] [CrossRef]
- Daly, R.; Narayan, T.; Shao, H.; O’riordan, A.; Lovera, P. Platinum-Based Interdigitated Micro-Electrode Arrays for Reagent-Free Detection of Copper. Sensors 2021, 21, 3544. [Google Scholar] [CrossRef] [PubMed]
- Bin, Q.; Wang, M.; Wang, L. Ag nanoparticles decorated into metal-organic framework (Ag NPs/ZIF-8) for electrochemical sensing of chloride ion. Nanotechnology 2020, 31, 125601. [Google Scholar] [CrossRef]
- Miao, Z.; Zhang, D.; Chen, Q. Non-enzymatic Hydrogen Peroxide Sensors Based on Multi-wall Carbon Nanotube/Pt Nanoparticle Nanohybrids. Materials 2014, 7, 2945–2955. [Google Scholar] [CrossRef] [PubMed]
- Lan, Q.; Ren, C.; Lambert, A.; Zhang, G.; Li, J.; Cheng, Q.; Hu, X.; Yang, Z. Platinum Nanoparticle-decorated Graphene Oxide@Polystyrene Nanospheres for Label-free Electrochemical Immunosensing of Tumor Markers. ACS Sustain. Chem. Eng. 2020, 8, 4392–4399. [Google Scholar] [CrossRef]
- Sun, H.; Chao, J.; Zuo, X.; Su, S.; Liu, X.; Yuwen, L.; Fan, C.; Wang, L. Gold nanoparticle-decorated MoS2 nanosheets for simultaneous detection of ascorbic acid, dopamine and uric acid. RSC Adv. 2014, 4, 27625–27629. [Google Scholar] [CrossRef]
- Jahandari, S.; Taher, M.A.; Fazelirad, H.; Sheikhshoai, I. Anodic stripping voltammetry of silver(I) using a carbon paste electrode modified with multi-walled carbon nanotubes. Microchim. Acta 2013, 180, 347–354. [Google Scholar] [CrossRef]
- Sidambaram, P.; Colleran, J. Nanomole Silver Detection in Chloride-Free Phosphate Buffer Using Platinum and Gold Micro- and Nanoelectrodes. J. Electrochem. Soc. 2019, 166, B532–B541. [Google Scholar] [CrossRef]
- Miao, Y.; Ouyang, L.; Zhou, S.; Xu, L.; Yang, Z.; Xiao, M.; Ouyang, R. Electrocatalysis and electroanalysis of nickel, its oxides, hydroxides and oxyhydroxides toward small molecules. Biosens. Bioelectron. 2014, 53, 428–439. [Google Scholar] [CrossRef]
- Hall, D.S.; Lockwood, D.J.; Bock, C.; MacDougall, B.R. Nickel Hydroxides and Related Materials: A Review of Their Structures, Synthesis and Properties. Proc. R. Soc. A Math. Phys. Eng. Sci. 2015, 471, 20140792. [Google Scholar] [CrossRef]
- Raoof, J.-B.; Omrani, A.; Ojani, R.; Monfared, F. Poly(N-methylaniline)/nickel modified carbon paste electrode as an efficient and cheep electrode for electrocatalytic oxidation of formaldehyde in alkaline medium. J. Electroanal. Chem. 2009, 633, 153–158. [Google Scholar] [CrossRef]
- Neiva, E.G.; Bergamini, M.F.; Oliveira, M.M.; Marcolino, L.H.; Zarbin, A.J. PVP-capped nickel nanoparticles: Synthesis, characterization and utilization as a glycerol electrosensor. Sens. Actuators B Chem. 2014, 196, 574–581. [Google Scholar] [CrossRef]
- Uwaya, G.E.; Wen, Y.; Bisetty, K. A combined experimental-computational approach for electrocatalytic detection of epinephrine using nanocomposite sensor based on polyaniline/nickel oxide. J. Electroanal. Chem. 2022, 911, 116204. [Google Scholar] [CrossRef]
- Neiva, E.G.; Oliveira, M.M.; Marcolino, L.H.; Zarbin, A.J. Nickel nanoparticles with hcp structure: Preparation, deposition as thin films and application as electrochemical sensor. J. Colloid Interface Sci. 2016, 468, 34–41. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, P.R.; Schibelbain, A.F.; Neiva, E.G.; Zarbin, A.J.; Marcolino, L.H.; Bergamini, M.F. Nickel hexacyanoferrate supported at nickel nanoparticles for voltammetric determination of rifampicin. Sens. Actuators B Chem. 2018, 260, 816–823. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Karimi, F.; Orooji, Y.; Mansouri, G.; Razmjou, A.; Aygun, A.; Sen, F. A new nickel-based co-crystal complex electrocatalyst amplified by NiO dope Pt nanostructure hybrid; a highly sensitive approach for determination of cysteamine in the presence of serotonin. Sci. Rep. 2020, 10, 11699. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, E.; Salimi, A.; Shams, E. Electrocatalytic activity of nickel oxide nanoparticles as mediatorless system for NADH and ethanol sensing at physiological pH solution. Biosens. Bioelectron. 2013, 45, 260–266. [Google Scholar] [CrossRef]
- Adeniyi, O.; Nwahara, N.; Mwanza, D.; Nyokong, T.; Mashazi, P. High-performance non-enzymatic glucose sensing on nanocomposite electrocatalysts of nickel phthalocyanine nanorods and nitrogen doped-reduced graphene oxide nanosheets. Appl. Surf. Sci. 2023, 609, 155234. [Google Scholar] [CrossRef]
- Vedharathinam, V.; Botte, G.G. Understanding the electro-catalytic oxidation mechanism of urea on nickel electrodes in alkaline medium. Electrochim. Acta 2012, 81, 292–300. [Google Scholar] [CrossRef]
- Jafarian, M.; Mahjani, M.; Heli, H.; Gobal, F.; Heydarpoor, M. Electrocatalytic oxidation of methane at nickel hydroxide modified nickel electrode in alkaline solution. Electrochem. Commun. 2003, 5, 184–188. [Google Scholar] [CrossRef]
- Zhao, Z.; Huang, Y.; Huang, Z.; Mei, H.; Xie, Y.; Long, D.; Zhu, F.; Gong, W. Nonenzymetic glucose sensitive device based on morchella shaped nickel-copper layered double hydroxide. Appl. Surf. Sci. 2022, 597, 153658. [Google Scholar] [CrossRef]
- Pal, N.; Banerjee, S.; Bhaumik, A. A facile route for the syntheses of Ni(OH)2 and NiO nanostructures as potential candidates for non-enzymatic glucose sensor. J. Colloid Interface Sci. 2018, 516, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Sithini, T.N.; Thiyagasundaram, T.; Zen, J.-M. A nickel hydroxide platform prepared on a hydroxyl-enriched screen-printed carbon electrode for oxidative electrocatalysis. Anal. Methods 2022, 14, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Wolfart, F.; Lorenzen, A.L.; Nagata, N.; Vidotti, M. Nickel/cobalt alloys modified electrodes: Synthesis, characterization and optimization of the electrocatalytical response. Sens. Actuators B Chem. 2013, 186, 528–535. [Google Scholar] [CrossRef]
- Fu, R.; Lu, Y.; Ding, Y.; Li, L.; Ren, Z.; Si, X.; Wu, Q. A novel non-enzymatic glucose electrochemical sensor based on CNF@Ni-Co layered double hydroxide modified glassy carbon electrode. Microchem. J. 2019, 150, 104106. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, P.; Xie, Z.; Ni, M.; Wang, C.; Yang, P.; Xie, Y.; Fei, J. Selective determination of epinephrine using electrochemical sensor based on ordered mesoporous carbon/nickel oxide nanocomposite. Talanta 2021, 233, 122545. [Google Scholar] [CrossRef]
- Kokulnathan, T.; Wang, T.-J.; Ahmed, F.; Arshi, N. Fabrication of flower-like nickel cobalt-layered double hydroxide for electrochemical detection of carbendazim. Surf. Interfaces 2023, 36, 102570. [Google Scholar] [CrossRef]
- Balasubramanian, P.; He, S.-B.; Deng, H.-H.; Peng, H.-P.; Chen, W. Defects engineered 2D ultrathin cobalt hydroxide nanosheets as highly efficient electrocatalyst for non-enzymatic electrochemical sensing of glucose and l-cysteine. Sens. Actuators B Chem. 2020, 320, 128374. [Google Scholar] [CrossRef]
- Kokulnathan, T.; Wang, T.-J.; Kumar, E.A.; Ahmed, F. Construction of nickel cobalt-layered double hydroxide/functionalized–halloysite nanotubes composite for electrochemical detection of organophosphate insecticide. Chem. Eng. J. 2022, 433, 133639. [Google Scholar] [CrossRef]
- Zhong, H.; Campos-Roldán, C.A.; Zhao, Y.; Zhang, S.; Feng, Y.; Alonso-Vante, N. Recent Advances of Cobalt-Based Electrocatalysts for Oxygen Electrode Reactions and Hydrogen Evolution Reaction. Catalysts 2018, 8, 559. [Google Scholar] [CrossRef]
- Zhang, W.; Cui, L.; Liu, J. Recent advances in cobalt-based electrocatalysts for hydrogen and oxygen evolution reactions. J. Alloys Compd. 2020, 821, 153542. [Google Scholar] [CrossRef]
- Tran, T.; Thi, M.; Son, N.; Bui, Q.; Ai-Le, P.; Nhac-Vu, H.-T. Novel nanoneedle structures of zinc-doped cobalt hydroxide as a self-supported sensor for sensitive glucose detection. Mater. Res. Bull. 2019, 120, 110580. [Google Scholar] [CrossRef]
- Zou, J.; Guan, J.-F.; Zhao, G.-Q.; Jiang, X.-Y.; Liu, Y.-P.; Yu, J.-G.; Li, W.-J. Construction of a highly sensitive signal electrochemical sensor based on self-assembled cobalt oxide-hydroxylated single-walled carbon nanotubes composite for detection of dopamine in bovine serum samples. J. Environ. Chem. Eng. 2021, 9, 105831. [Google Scholar] [CrossRef]
- Sreekanth, T.; Sindhu, R.; Kumar, E.P.; Abhilash, M.; Wei, X.; Kim, J.; Yoo, K. Controllable synthesis of urea-assisted Co3O4 nanostructures as an effective catalyst for urea electrooxidation. Colloids Surfaces A Physicochem. Eng. Asp. 2023, 657, 130576. [Google Scholar] [CrossRef]
- Annalakshmi, M.; Balasubramanian, P.; Chen, S.-M.; Chen, T.-W. Enzyme-free electrocatalytic sensing of hydrogen peroxide using a glassy carbon electrode modified with cobalt nanoparticle-decorated tungsten carbide. Microchim. Acta 2019, 186, 265. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-C.; Lan, W.-J.; Chen, C.-H. Redox preparation of mixed-valence cobalt manganese oxide nanostructured materials: Highly efficient noble metal-free electrocatalysts for sensing hydrogen peroxide. Nanoscale 2014, 6, 334–341. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, X.; Kong, R.; Du, G.; Asiri, A.M.; Lu, Q.; Sun, X. Cobalt phosphide nanowire array as an effective electrocatalyst for non-enzymatic glucose sensing. J. Mater. Chem. B 2017, 5, 1901–1904. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Li, Q.; Wang, L.; You, W.; Zhang, J.; Che, R. Copper- and Cobalt-Codoped CeO2 Nanospheres with Abundant Oxygen Vacancies as Highly Efficient Electrocatalysts for Dual-Mode Electrochemical Sensing of MicroRNA. Anal. Chem. 2019, 91, 2659–2666. [Google Scholar] [CrossRef] [PubMed]
- Appaturi, J.N.; Pulingam, T.; Muniandy, S.; Dinshaw, I.J.; Fen, L.B.; Johan, M.R. Supported cobalt nanoparticles on graphene oxide/mesoporous silica for oxidation of phenol and electrochemical detection of H2O2 and Salmonella spp. Mater. Chem. Phys. 2019, 232, 493–505. [Google Scholar] [CrossRef]
- Arvinte, A.; Westermann, A.C.; Sesay, A.M.; Virtanen, V. Electrocatalytic oxidation and determination of insulin at CNT-nickel–cobalt oxide modified electrode. Sens. Actuators B Chem. 2010, 150, 756–763. [Google Scholar] [CrossRef]
- Sander, M.; Hofstetter, T.B.; Gorski, C.A. Electrochemical Analyses of Redox-Active Iron Minerals: A Review of Nonmediated and Mediated Approaches. Environ. Sci. Technol. 2015, 49, 5862–5878. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, K.; Bai, S.; Guan, C.; Wei, H.; Chu, H. High productivity of tartronate from electrocatalytic oxidation of high concentration glycerol through facilitating the intermediate conversion. Appl. Catal. B Environ. 2022, 317, 121784. [Google Scholar] [CrossRef]
- Hou, X.; Shen, W.; Huang, X.; Ai, Z.; Zhang, L. Ascorbic acid enhanced activation of oxygen by ferrous iron: A case of aerobic degradation of rhodamine B. J. Hazard. Mater. 2016, 308, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Wahab, R.; Khan, F.; Al-Khedhairy, A.A. Hematite iron oxide nanoparticles: Apoptosis of myoblast cancer cells and their arithmetical assessment. RSC Adv. 2018, 8, 24750–24759. [Google Scholar] [CrossRef]
- Li, Y.; Wang, T.; Wang, T.; Li, L.; Gong, J.; Zhang, L.; Chen, W. Copper oxide nanoleaves covered with loose nickel oxide nanoparticles for sensitive and selective non-enzymatic nitrite sensors. Mater. Res. Bull. 2022, 149, 111712. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, W.; Sun, Y.; Jiang, Y.; Han, N.; Zou, J.; Si, W.; Wang, F.; Núñez-Delgado, A.; Liu, S. Highly active iron-nitrogen-boron-carbon bifunctional electrocatalytic platform for hydrogen peroxide sensing and oxygen reduction. Environ. Res. 2021, 201, 111563. [Google Scholar] [CrossRef] [PubMed]
- Heli, H.; Majdi, S.; Sattarahmady, N. Ultrasensitive sensing of N-acetyl-l-cysteine using an electrocatalytic transducer of nanoparticles of iron(III) oxide core–cobalt hexacyanoferrate shell. Sens. Actuators B Chem. 2010, 145, 185–193. [Google Scholar] [CrossRef]
- Adekunle, A.S.; Agboola, B.O.; Pillay, J.; Ozoemena, K.I. Electrocatalytic detection of dopamine at single-walled carbon nanotubes–iron (III) oxide nanoparticles platform. Sens. Actuators B Chem. 2010, 148, 93–102. [Google Scholar] [CrossRef]
- Mani, V.; Vilian, A.T.E.; Chen, S.-M. Graphene Oxide Dispersed Carbon Nanotube and Iron Phthalocyanine Composite Modified Electrode for the Electrocatalytic Determination of Hydrazine. Int. J. Electrochem. Sci. 2012, 7, 12774–12785. [Google Scholar]
- Rezaei, B.; Damiri, S. Voltammetric behavior of multi-walled carbon nanotubes modified electrode-hexacyanoferrate(II) electrocatalyst system as a sensor for determination of captopril. Sens. Actuators B Chem. 2008, 134, 324–331. [Google Scholar] [CrossRef]
- Nehru, R.; Hsu, Y.-F.; Wang, S.-F. Electrochemical determination of caffeic acid in antioxidant beverages samples via a facile synthesis of carbon/iron-based active electrocatalyst. Anal. Chim. Acta 2020, 1122, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhou, C.; An, J.; Yang, L.; Yang, Y.; Liu, X. Ultra-fast synthesis of iron decorated multiwalled carbon nanotube composite materials: A sensitive electrochemical sensor for determining dopamine. J. Alloys Compd. 2022, 897, 163257. [Google Scholar] [CrossRef]
- Movlaee, K.; Ganjali, M.R.; Norouzi, P.; Neri, G. Iron-Based Nanomaterials/Graphene Composites for Advanced Electrochemical Sensors. Nanomaterials 2017, 7, 406. [Google Scholar] [CrossRef] [PubMed]
- Wahab, R.; Khan, F.; Ahmad, N.; Alam, M. Copper and iron based bimetallic nanocomposite: An enhanced and operative phenol sensor. Phys. E Low-Dimens. Syst. Nanostruct. 2022, 144, 115419. [Google Scholar] [CrossRef]
- Chen, S.-H.; Zhu, J.-J.; Li, P.-H.; Sun, Y.-F.; Yang, M.; Huang, X.-J. In-situ growth of zero-valent iron in FeOx/Mn3O4 to improve the surficial redox for high-efficient electrocatalysis of Pb(II). Chem. Eng. J. 2022, 430, 132959. [Google Scholar] [CrossRef]
- Hao, X.; Yan, T.; Wang, Z.; Liu, S.; Liang, Z.; Shen, Y.; Pritzker, M. Unipolar pulse electrodeposition of nickel hexacyanoferrate thin films with controllable structure on platinum substrates. Thin Solid Film. 2012, 520, 2438–2448. [Google Scholar] [CrossRef]
- Šutinys, E.; Dzedzickis, A.; Samukaitė-Bubnienė, U.; Bučinskas, V. Novel synthetic iron (III) oxide-based force sensor. Sens. Actuators A Phys. 2021, 331, 113043. [Google Scholar] [CrossRef]
- Guo, C.; Zheng, Y.; Ran, J.; Xie, F.; Jaroniec, M.; Qiao, S. Engineering High-Energy Interfacial Structures for High-Performance Oxygen-Involving Electrocatalysis. Angew. Chem. 2017, 129, 8659–8663. [Google Scholar] [CrossRef]
- Xie, X.; Li, Y.; Liu, Z.-Q.; Haruta, M.; Shen, W. Low-temperature oxidation of CO catalysed by Co3O4 nanorods. Nature 2009, 458, 746–749. [Google Scholar] [CrossRef]
- Thamilselvan, A.; Manivel, P.; Rajagopal, V.; Nesakumar, N.; Suryanarayanan, V. Improved electrocatalytic activity of Au@Fe3O4 magnetic nanoparticles for sensitive dopamine detection. Colloids Surfaces B Biointerfaces 2019, 180, 1–8. [Google Scholar] [CrossRef]
- Zokhtareh, R.; Rahimnejad, M.; Najafpour-Darzi, G.; Karimi-Maleh, H. A novel sensing platform for electrochemical detection of metronidazole antibiotic based on green-synthesized magnetic Fe3O4 nanoparticles. Environ. Res. 2023, 216, 114643. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, M.; Pan, S.; Yu, L.; Zhang, S.; Liu, R. A magnetically induced self-assembled and label-free electrochemical aptasensor based on magnetic Fe3O4/Fe2O3@Au nanoparticles for VEGF165 protein detection. Appl. Surf. Sci. 2022, 580, 152362. [Google Scholar] [CrossRef]
- Balaji, R.; Zheng, X.-H.; Chen, S.-M.; Renganathan, V. The copper oxide nanoflakes modified electrodes for selective and real time electrochemical sensing of caffeine. Inorg. Chem. Commun. 2020, 118, 108014. [Google Scholar] [CrossRef]
- Song, H.; Ni, Y.; Kokot, S. A novel electrochemical sensor based on the copper-doped copper oxide nano-particles for the analysis of hydrogen peroxide. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 465, 153–158. [Google Scholar] [CrossRef]
- Daemi, S.; Ghasemi, S.; Ashkarran, A.A. Electrospun CuO-ZnO nanohybrid: Tuning the nanostructure for improved amperometric detection of hydrogen peroxide as a non-enzymatic sensor. J. Colloid Interface Sci. 2019, 550, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chai, G.; Zhao, X.; Dai, Y.; Qi, Y. Effect of different copper sources on the morphology of cuprous oxide and its application as a non-enzymatic glucose sensor. Sens. Actuators B Chem. 2020, 321, 128485. [Google Scholar] [CrossRef]
- Yan, Q.; Zhi, N.; Yang, L.; Xu, G.; Feng, Q.; Zhang, Q.; Sun, S. A highly sensitive uric acid electrochemical biosensor based on a nano-cube cuprous oxide/ferrocene/uricase modified glassy carbon electrode. Sci. Rep. 2020, 10, 10607. [Google Scholar] [CrossRef]
- Amiripour, F.; Azizi, S.N.; Ghasemi, S. Gold-copper bimetallic nanoparticles supported on nano P zeolite modified carbon paste electrode as an efficient electrocatalyst and sensitive sensor for determination of hydrazine. Biosens. Bioelectron. 2018, 107, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, J.; Feng, H.; Zhang, Y.; Jiang, J.; Feng, Y.; Chen, M.; Qian, D. A facile one-step in situ synthesis of copper nanostructures/graphene oxide as an efficient electrocatalyst for 2-naphthol sensing application. Electrochim. Acta 2015, 153, 352–360. [Google Scholar] [CrossRef]
- Chiani, E.; Azizi, S.N.; Ghasemi, S. Superior electrocatalyst based on mesoporous silica nanoparticles/carbon nanotubes modified by platinum-copper bimetallic nanoparticles for amperometric detection of hydrazine. Int. J. Hydrogen Energy 2022, 47, 20087–20102. [Google Scholar] [CrossRef]
- Vinothkumar, V.; Prasad, G.V.; Chen, S.-M.; Sangili, A.; Jang, S.-J.; Lim, H.C.; Kim, T.H. One-step synthesis of calcium-doped copper oxide nanoparticles as an efficient bifunctional electrocatalyst for sensor and supercapacitor applications. J. Energy Storage 2023, 59, 106415. [Google Scholar] [CrossRef]
- Wei, C.; Liu, Y.; Li, X.; Zhao, J.; Ren, Z.; Pang, H. Nitrogen-Doped Carbon-Copper Nanohybrids as Electrocatalysts in H2O2 and Glucose Sensing. Chemelectrochem 2014, 1, 799–807. [Google Scholar] [CrossRef]
- Almutairi, E.M.; Ghanem, M.A.; Al-Warthan, A.; Kuniyil, M.; Adil, S.F. Hydrazine High-Performance Oxidation and Sensing Using a Copper Oxide Nanosheet Electrocatalyst Prepared via a Foam-Surfactant Dual Template. Nanomaterials 2022, 13, 129. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Ghicov, A.; Schmuki, P. Self-ordering electrochemistry: A review on growth and functionality of TiO2 nanotubes and other self-aligned MOx structures. Chem. Commun. 2009, 20, 2791–2808. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Bai, J.; Zhou, B. Titanium Dioxide Nanomaterials for Sensor Applications. Chem. Rev. 2014, 114, 10131–10176. [Google Scholar] [CrossRef] [PubMed]
- Malode, S.J.; Prabhu K., K.; Shetti, N.P.; Reddy, K.R. Highly sensitive electrochemical assay for selective detection of Aminotriazole based on TiO2/poly(CTAB) modified sensor. Environ. Technol. Innov. 2021, 21, 101222. [Google Scholar] [CrossRef]
- Kokulnathan, T.; Kumar, E.A.; Wang, T.-J. Design and In Situ Synthesis of Titanium Carbide/Boron Nitride Nanocomposite: Investigation of Electrocatalytic Activity for the Sulfadiazine Sensor. ACS Sustain. Chem. Eng. 2020, 8, 12471–12481. [Google Scholar] [CrossRef]
- Khan, A.L.; Jain, R. Polypyrrole/titanium dioxide nanocomposite sensor for the electrocatalytic quantification of sulfamoxole. Ionics 2018, 24, 2473–2488. [Google Scholar] [CrossRef]
- Reys, J.R.M.; Lima, P.R.; Cioletti, A.G.; Ribeiro, A.S.; de Abreu, F.C.; Goulart, M.O.; Kubota, L.T. An amperometric sensor based on hemin adsorbed on silica gel modified with titanium oxide for electrocatalytic reduction and quantification of artemisinin. Talanta 2008, 77, 909–914. [Google Scholar] [CrossRef]
- Chen, T.-W.; Chinnapaiyan, S.; Chen, S.-M.; Mahmoud, A.H.; Elshikh, M.S.; Ebaid, H.; Yassin, M.T. Facile sonochemical synthesis of rutile-type titanium dioxide microspheres decorated graphene oxide composite for efficient electrochemical sensor. Ultrason. Sonochemistry 2020, 62, 104872. [Google Scholar] [CrossRef] [PubMed]
- Kumar, E.A.; Kokulnathan, T.; Wang, T.-J.; Anthuvan, A.J.; Chang, Y.-H. Two-dimensional titanium carbide (MXene) nanosheets as an efficient electrocatalyst for 4-nitroquinoline N-oxide detection. J. Mol. Liq. 2020, 312, 113354. [Google Scholar] [CrossRef]
- Annalakshmi, M.; Balasubramanian, P.; Chen, S.-M.; Chen, T.-W. Amperometric sensing of nitrite at nanomolar concentrations by using carboxylated multiwalled carbon nanotubes modified with titanium nitride nanoparticles. Microchim. Acta 2019, 186, 8. [Google Scholar] [CrossRef]
- Guo, Q.; Wu, T.; Liu, L.; Hou, H.; Chen, S.; Wang, L. Flexible and conductive titanium carbide–carbon nanofibers for the simultaneous determination of ascorbic acid, dopamine and uric acid. J. Mater. Chem. B 2018, 6, 4610–4617. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J. Keper Metal-Organic Framework Materials. In Porous Materials; Wiley: Hoboken, NJ, USA, 2010; ISBN 9780470997499. [Google Scholar]
- Liu, X.; Yang, H.; Diao, Y.; He, Q.; Lu, C.; Singh, A.; Kumar, A.; Liu, J.; Lan, Q. Recent advances in the electrochemical applications of Ni-based metal organic frameworks (Ni-MOFs) and their derivatives. Chemosphere 2022, 307, 135729. [Google Scholar] [CrossRef]
- Jiao, L.; Jiang, H.-L. Metal-organic frameworks for catalysis: Fundamentals and future prospects. Chin. J. Catal. 2023, 45, 1–5. [Google Scholar] [CrossRef]
- Downes, C.; Marinescu, S.C. Electrocatalytic Metal–Organic Frameworks for Energy Applications. Chemsuschem 2017, 10, 4374–4392. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wang, Y.; Dong, J.; He, C.-T.; Yin, H.; An, P.; Zhao, K.; Zhang, X.; Gao, C.; Zhang, L.; et al. Ultrathin metal–organic framework nanosheets for electrocatalytic oxygen evolution. Nat. Energy 2016, 1, 16184. [Google Scholar] [CrossRef]
- Qin, N.; Pan, A.; Yuan, J.; Ke, F.; Wu, X.; Zhu, J.; Liu, J.; Zhu, J. One-Step Construction of a Hollow Au@Bimetal–Organic Framework Core–Shell Catalytic Nanoreactor for Selective Alcohol Oxidation Reaction. ACS Appl. Mater. Interfaces 2021, 13, 12463–12471. [Google Scholar] [CrossRef] [PubMed]
- Sheikh-Mohseni, M.H.; Nezamzadeh-Ejhieh, A. Modification of carbon paste electrode with Ni-clinoptilolite nanoparticles for electrocatalytic oxidation of methanol. Electrochim. Acta 2014, 147, 572–581. [Google Scholar] [CrossRef]
- Zhai, X.; Cao, Y.; Sun, W.; Cao, S.; Wang, Y.; He, L.; Yao, N.; Zhao, D. Core-shell composite N-doped-Co-MOF@polydopamine decorated with Ag nanoparticles for nonenzymatic glucose sensors. J. Electroanal. Chem. 2022, 918, 116491. [Google Scholar] [CrossRef]
- Mohan, B.; Kumari, R.; Singh, G.; Singh, K.; Pombeiro, A.J.; Yang, X.; Ren, P. Covalent organic frameworks (COFs) and metal-organic frameworks (MOFs) as electrochemical sensors for the efficient detection of pharmaceutical residues. Environ. Int. 2023, 175, 107928. [Google Scholar] [CrossRef]
- Johnson, E.M.; Ilic, S.; Morris, A.J. Design Strategies for Enhanced Conductivity in Metal–Organic Frameworks. ACS Central Sci. 2021, 7, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.-Z.; Wang, Y.-S.; Liu, B.; Jiao, H.; Xu, L. A Non–Enzymatic Electrochemical Sensor of Cu@Co–MOF Composite for Glucose Detection with High Sensitivity and Selectivity. Chemosensors 2022, 10, 416. [Google Scholar] [CrossRef]
- Saeb, E.; Asadpour-Zeynali, K. Enhanced electrocatalytic reduction activity of Fe-MOF/Pt nanoparticles as a sensitive sensor for ultra-trace determination of Tinidazole. Microchem. J. 2022, 172, 106976. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, W.-J.; Lu, Y.-K.; Liu, G.; Hou, L.; Wang, Y.-Y. Nonenzymatic Glucose Sensing and Magnetic Property Based On the Composite Formed by Encapsulating Ag Nanoparticles in Cluster-Based Co-MOF. Inorg. Chem. 2019, 58, 16743–16751. [Google Scholar] [CrossRef]
- Yang, S. Facile Synthesis of Fe-MOF/rGO nanocomposite as an Efficient Electrocatalyst for Nonenzymatic H2O2 Sensing. Int. J. Electrochem. Sci. 2019, 14, 7703–7716. [Google Scholar] [CrossRef]
- Liu, F.; Geng, L.; Ye, F.; Zhao, S. MOF-derivated MnO@C nanocomposite with bidirectional electrocatalytic ability as signal amplification for dual-signal electrochemical sensing of cancer biomarker. Talanta 2022, 239, 123150. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Liu, Q.; Lu, S.; Chen, G.; Gao, S.; Lu, W.; Sun, X. High-performance non-enzymatic glucose detection: Using a conductive Ni-MOF as an electrocatalyst. J. Mater. Chem. B 2020, 8, 5411–5415. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Bo, X.; Guo, L. MOF-818 metal-organic framework-reduced graphene oxide/multiwalled carbon nanotubes composite for electrochemical sensitive detection of phenolic acids. Talanta 2020, 218, 121123. [Google Scholar] [CrossRef]
- Blaskievicz, S.F.; Mascaro, L.H.; Zhao, Y.; Marken, F. Semiconductor photoelectroanalysis and photobioelectroanalysis: A perspective. TrAC Trends Anal. Chem. 2021, 135, 116154. [Google Scholar] [CrossRef]
- Liu, W.; Duan, W.; Jia, L.; Wang, S.; Guo, Y.; Zhang, G.; Zhu, B.; Huang, W.; Zhang, S. Surface Plasmon-Enhanced Photoelectrochemical Sensor Based on Au Modified TiO2 Nanotubes. Nanomaterials 2022, 12, 2058. [Google Scholar] [CrossRef]
- Cui, H.; Yao, C.; Cang, Y.; Liu, W.; Zhang, Z.; Miao, Y.; Xin, Y. Oxygen vacancy-regulated TiO2 nanotube photoelectrochemical sensor for highly sensitive and selective detection of tetracycline hydrochloride. Sens. Actuators B Chem. 2022, 359, 131564. [Google Scholar] [CrossRef]
- Tanaka, K.; Capule, M.F.; Hisanaga, T. Effect of crystallinity of TiO2 on its photocatalytic action. Chem. Phys. Lett. 1991, 187, 73–76. [Google Scholar] [CrossRef]
- Han, F.; Song, Z.; Nawaz, M.H.; Dai, M.; Han, D.; Han, L.; Fan, Y.; Xu, J.; Han, D.; Niu, L. MoS2/ZnO-Heterostructures-Based Label-Free, Visible-Light-Excited Photoelectrochemical Sensor for Sensitive and Selective Determination of Synthetic Antioxidant Propyl Gallate. Anal. Chem. 2019, 91, 10657–10662. [Google Scholar] [CrossRef]
- Araújo, M.D.S.; Barretto, T.R.; Galvão, J.C.R.; Tarley, C.R.T.; Dall’Antônia, L.H.; de Matos, R.; Medeiros, R.A. Visible Light Photoelectrochemical Sensor for Acetaminophen Determination using a Glassy Carbon Electrode Modified with BiVO4 Nanoparticles. Electroanalysis 2021, 33, 663–671. [Google Scholar] [CrossRef]
- Pelissari, M.R.D.S.; Neto, N.F.A.; Camargo, L.P.; Dall’antonia, L.H. Characterization and Photo-Induced Electrocatalytic Evaluation for BiVO4 Films Obtained by the SILAR Process. Electrocatalysis 2021, 12, 211–224. [Google Scholar] [CrossRef]
- Camargo, L.P.; Pelissari, M.R.D.S.; da Silva, P.R.C.; Batagin-Neto, A.; Medeiros, R.A.; Dias, M.A.; Dall’antonia, L.H. Visible Light Photoelectrochemical Sensor for Dopamine: Determination Using Iron Vanadate Modified Electrode. Molecules 2022, 27, 6410. [Google Scholar] [CrossRef] [PubMed]
- Eyele-Mezui, S.; Vialat, P.; Higy, C.; Bourzami, R.; Leuvrey, C.; Parizel, N.; Turek, P.; Rabu, P.; Rogez, G.; Mousty, C. Electrocatalytic Properties of Metal Phthalocyanine Tetrasulfonate Intercalated in Metal Layered Simple Hydroxides (Metal: Co, Cu, and Zn). J. Phys. Chem. C 2015, 119, 13335–13342. [Google Scholar] [CrossRef]
- Tajbakhsh, E.; McKearney, D.; Leznoff, D.B.; Warren, J.J. Heterogenous Preparations of Solution-Processable Cobalt Phthalocyanines for Carbon Dioxide Reduction Electrocatalysis. Inorganics 2023, 11, 43. [Google Scholar] [CrossRef]
- Buschow, K.H.J. Wright Phthalocyanines. In Encyclopedia of Materials: Science and Technology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 6987–6991. [Google Scholar]
- Valverde-González, A.; Guan, L.Z.; Ferrer, M.L.; Iglesias, M.; Maya, E.M. Iron Phthalocyanine-Knitted Polymers as Electrocatalysts for the Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2020, 12, 32681–32688. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, Z.; Bu, J.; Ma, W.; Zhang, L.; Zhong, H.; Cheng, L.; Li, S.; Wang, T.; Zhang, J. Metal phthalocyanines as efficient electrocatalysts for acetylene semihydrogenation. Chem. Eng. J. 2022, 431, 134129. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Z.; Zhang, X.; Li, L.; Li, Y.; Xu, H.; Li, X.; Yu, X.; Zhang, Z.; Liang, Y.; et al. Highly selective and active CO2 reduction electrocatalysts based on cobalt phthalocyanine/carbon nanotube hybrid structures. Nat. Commun. 2017, 8, 14675. [Google Scholar] [CrossRef]
- Kumar, Y.; Kibena-Põldsepp, E.; Kozlova, J.; Rähn, M.; Treshchalov, A.; Kikas, A.; Kisand, V.; Aruväli, J.; Tamm, A.; Douglin, J.C.; et al. Bifunctional Oxygen Electrocatalysis on Mixed Metal Phthalocyanine-Modified Carbon Nanotubes Prepared via Pyrolysis. ACS Appl. Mater. Interfaces 2021, 13, 41507–41516. [Google Scholar] [CrossRef]
- Zhan, X.; Tong, X.; Gu, M.; Tian, J.; Gao, Z.; Ma, L.; Xie, Y.; Chen, Z.; Ranganathan, H.; Zhang, G.; et al. Phosphorus-Doped Graphene Electrocatalysts for Oxygen Reduction Reaction. Nanomaterials 2022, 12, 1141. [Google Scholar] [CrossRef] [PubMed]
- Pogacean, F.; Socaci, C.; Pruneanu, S.; Biris, A.R.; Coros, M.; Magerusan, L.; Katona, G.; Turcu, R.; Borodi, G. Graphene based nanomaterials as chemical sensors for hydrogen peroxide—A comparison study of their intrinsic peroxidase catalytic behavior. Sens. Actuators B Chem. 2015, 213, 474–483. [Google Scholar] [CrossRef]
- Wang, J.; Kong, H.; Zhang, J.; Hao, Y.; Shao, Z.; Ciucci, F. Carbon-based electrocatalysts for sustainable energy applications. Prog. Mater. Sci. 2021, 116, 100717. [Google Scholar] [CrossRef]
- Li, X.; Duan, X.; Han, C.; Fan, X.; Li, Y.; Zhang, F.; Zhang, G.; Peng, W.; Wang, S. Chemical activation of nitrogen and sulfur co-doped graphene as defect-rich carbocatalyst for electrochemical water splitting. Carbon 2019, 148, 540–549. [Google Scholar] [CrossRef]
- Harik, V. Nanotechnology of Carbon Nanotubes. In Mechanics of Carbon Nanotubes; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–24. [Google Scholar]
- Sheng, J.; Zhu, S.; Jia, G.; Liu, X.; Li, Y. Carbon nanotube supported bifunctional electrocatalysts containing iron-nitrogen-carbon active sites for zinc-air batteries. Nano Res. 2021, 14, 4541–4547. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef]
- Dash, S.R.; Bag, S.S.; Golder, A.K. Carbon Dots Derived from Waste Psidium Guajava Leaves for Electrocatalytic Sensing of Chlorpyrifos. Electroanalysis 2022, 34, 1141–1149. [Google Scholar] [CrossRef]
- Stadler, P. Isotropic metallic transport in conducting polymers. Synth. Met. 2019, 254, 106–113. [Google Scholar] [CrossRef]
- Idumah, C.I. Novel trends in conductive polymeric nanocomposites, and bionanocomposites. Synth. Met. 2021, 273, 116674. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Nejad, F.G.; Shoaie, I.S.; Khalilzadeh, M.A.; Asl, M.S.; Van Le, Q.; Zhang, K.; Jang, H.W.; Shokouhimehr, M. Recent developments in conducting polymers: Applications for electrochemistry. RSC Adv. 2020, 10, 37834–37856. [Google Scholar] [CrossRef]
- Guo, X.; Facchetti, A. The journey of conducting polymers from discovery to application. Nat. Mater. 2020, 19, 922–928. [Google Scholar] [CrossRef]
- Xia, L.; Wei, Z.; Wan, M. Conducting polymer nanostructures and their application in biosensors. J. Colloid Interface Sci. 2010, 341, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Namsheer, K.; Rout, C.S. Conducting polymers: A comprehensive review on recent advances in synthesis, properties and applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar] [CrossRef]
- Bredas, J.L.; Street, G.B. Polarons, bipolarons, and solitons in conducting polymers. Acc. Chem. Res. 1985, 18, 309–315. [Google Scholar] [CrossRef]
- Ibanez, J.G.; Rincón, M.E.; Gutierrez-Granados, S.; Chahma, M.; Jaramillo-Quintero, O.A.; Frontana-Uribe, B.A. Conducting Polymers in the Fields of Energy, Environmental Remediation, and Chemical–Chiral Sensors. Chem. Rev. 2018, 118, 4731–4816. [Google Scholar] [CrossRef]
- Le, T.-H.; Kim, Y.; Yoon, H. Electrical and Electrochemical Properties of Conducting Polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, K.; Ye, K.; Yan, J.; Wang, G.; Cao, D. Hierarchical conducting polymer coated conjugated polyimide anode towards durable lithium-ion batteries. J. Power Sources 2022, 552, 232226. [Google Scholar] [CrossRef]
- Hryniewicz, B.M.; Volpe, J.; Bach-Toledo, L.; Kurpel, K.C.; Deller, A.E.; Soares, A.L.; Nardin, J.M.; Marchesi, L.F.; Simas, F.F.; Oliveira, C.C.; et al. Development of polypyrrole (nano)structures decorated with gold nanoparticles toward immunosensing for COVID-19 serological diagnosis. Mater. Today Chem. 2022, 24, 100817. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, X.; Zhang, G.; Wang, S.; Zhu, S.; Wu, X.; Wang, Y.; Wang, Q.; Hu, C. Conducting polymer/silver nanowires stacking composite films for high-performance electrochromic devices. Sol. Energy Mater. Sol. Cells 2019, 200, 109919. [Google Scholar] [CrossRef]
- Khalid, M.; Honorato, A.M.; Varela, H.; Dai, L. Multifunctional electrocatalysts derived from conducting polymer and metal organic framework complexes. Nano Energy 2018, 45, 127–135. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Shi, G. Conducting Polymer-Based Catalysts. J. Am. Chem. Soc. 2016, 138, 2868–2876. [Google Scholar] [CrossRef] [PubMed]
- Vagin, M.; Che, C.; Gueskine, V.; Berggren, M.; Crispin, X. Ion-Selective Electrocatalysis on Conducting Polymer Electrodes: Improving the Performance of Redox Flow Batteries. Adv. Funct. Mater. 2020, 30, 2007009. [Google Scholar] [CrossRef]
- Malinauskas, A. Electrocatalysis at Conducting Polymers. Synth. Met. 1999, 107, 75–83. [Google Scholar] [CrossRef]
- Soares, A.L.; Zamora, M.L.; Marchesi, L.F.; Vidotti, M. Adsorption of catechol onto PEDOT films doped with gold nanoparticles: Electrochemical and spectroscopic studies. Electrochim. Acta 2019, 322, 134773. [Google Scholar] [CrossRef]
- Ren, Y.; Lin, Z.; Mao, X.; Tian, W.; Voorhis, T.; Hatton, T.A. Superhydrophobic, Surfactant-doped, Conducting Polymers for Electrochemically Reversible Adsorption of Organic Contaminants. Adv. Funct. Mater. 2018, 28, 1801466. [Google Scholar] [CrossRef]
- Rahman, H.A.; Rafi, M.; Putra, B.R.; Wahyuni, W.T. Electrochemical Sensors Based on a Composite of Electrochemically Reduced Graphene Oxide and PEDOT:PSS for Hydrazine Detection. ACS Omega 2022, 8, 3258–3269. [Google Scholar] [CrossRef]
- Getachew, T.; Addis, F.; Mehretie, S.; Yip, H.-L.; Xia, R.; Admassie, S. Electrocatalytic reduction of oxygen at platinum nanoparticles dispersed on electrochemically reduced graphene oxide/PEDOT:PSS composites. RSC Adv. 2020, 10, 30519–30528. [Google Scholar] [CrossRef]
- Tang, X.; Raskin, J.-P.; Kryvutsa, N.; Hermans, S.; Slobodian, O.; Nazarov, A.N.; Debliquy, M. An ammonia sensor composed of polypyrrole synthesized on reduced graphene oxide by electropolymerization. Sens. Actuators B Chem. 2020, 305, 127423. [Google Scholar] [CrossRef]
- Shyamala, S.; Kalaiarasi, S.; Karpagavinayagam, P.; Vedhi, C.; Muthuchudarkodi, R. Electrochemical studies and electrocatalytic applications of Zirconia-Polyaniline nanocomposite. J. Electroanal. Chem. 2022, 923, 116834. [Google Scholar] [CrossRef]
- Uwaya, G.E.; Bisetty, K. Electrocatalytic Detection of Epinephrine at Polyaniline-Biosynthesized Nickel Oxide Modified Electrode-Supported By Computational Study. Electrochem. Soc. Meet. Abstr. 2021, 240, 54. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Rajabzadeh-Khosroshahi, M.; Tabar, F.S.; Ajalli, N.; Samadi, A.; Yazdani, M.; Yazdian, F.; Rahdar, A.; Díez-Pascual, A.M. Two-Dimensional Graphitic Carbon Nitride (g-C3N4) Nanosheets and Their Derivatives for Diagnosis and Detection Applications. J. Funct. Biomater. 2022, 13, 204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Lv, S.; Zhou, Q.; Tang, D. CoOOH nanosheets-coated g-C3N4/CuInS2 nanohybrids for photoelectrochemical biosensor of carcinoembryonic antigen coupling hybridization chain reaction with etching reaction. Sens. Actuators B Chem. 2020, 307, 127631. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Chen, X.; Huang, M.; Fu, Q.; Li, R.; Wang, Y.; Li, C.; Zhao, P.; Xie, Y.; et al. A non-enzymatic photoelectrochemical sensor based on g-C3N4@CNT heterojunction for sensitive detection of antioxidant gallic acid in food. Food Chem. 2022, 389, 133086. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yuan, Y.; Pan, X.; Zhang, L.; Gong, J. Boosted photoelectrochemical immunosensing of metronidazole in tablet using coral-like g-C3N4 nanoarchitectures. Biosens. Bioelectron. 2019, 123, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-L.; Wang, E.-B.; Lan, Y.; Hu, C.-W. Renewable PMo 12-Based Inorganic-Organic Hybrid Material Bulk-Modified Carbon Paste Electrode: Preparation. Electrochem. Electrocatal. 2002, 14, 15–16. [Google Scholar] [CrossRef]
- Janaky, C.; Visy, C. Conducting polymer-based hybrid assemblies for electrochemical sensing: A materials science perspective. Anal. Bioanal. Chem. 2013, 405, 3489–3511. [Google Scholar] [CrossRef]
- Welch, C.M.; Compton, R.G. The use of nanoparticles in electroanalysis: A review. Anal. Bioanal. Chem. 2006, 384, 601–619. [Google Scholar] [CrossRef]
- Terán-Alcocer, Á.; Bravo-Plascencia, F.; Cevallos-Morillo, C.; Palma-Cando, A. Electrochemical Sensors Based on Conducting Polymers for the Aqueous Detection of Biologically Relevant Molecules. Nanomaterials 2021, 11, 252. [Google Scholar] [CrossRef]
- Xu, G.; Jarjes, Z.A.; Desprez, V.; Kilmartin, P.A.; Travas-Sejdic, J. Sensitive, selective, disposable electrochemical dopamine sensor based on PEDOT-modified laser scribed graphene. Biosens. Bioelectron. 2018, 107, 184–191. [Google Scholar] [CrossRef]
- Wang, H.-H.; Chen, X.-J.; Li, W.-T.; Zhou, W.-H.; Guo, X.-C.; Kang, W.-Y.; Kou, D.-X.; Zhou, Z.-J.; Meng, Y.-N.; Tian, Q.-W.; et al. ZnO nanotubes supported molecularly imprinted polymers arrays as sensing materials for electrochemical detection of dopamine. Talanta 2018, 176, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Demirkan, B.; Bozkurt, S.; Cellat, K.; Arıkan, K.; Yılmaz, M.; Şavk, A.; Çalımlı, M.H.; Nas, M.S.; Atalar, M.N.; Alma, M.H.; et al. Palladium supported on polypyrrole/reduced graphene oxide nanoparticles for simultaneous biosensing application of ascorbic acid, dopamine, and uric acid. Sci. Rep. 2020, 10, 2946. [Google Scholar] [CrossRef] [PubMed]
- Tajik, S.; Beitollahi, H.; Shahsavari, S.; Nejad, F.G. Simultaneous and selective electrochemical sensing of methotrexate and folic acid in biological fluids and pharmaceutical samples using Fe3O4/ppy/Pd nanocomposite modified screen printed graphite electrode. Chemosphere 2022, 291, 132736. [Google Scholar] [CrossRef] [PubMed]
- Safavi, A.; Maleki, N.; Farjami, E. Electrodeposited Silver Nanoparticles on Carbon Ionic Liquid Electrode for Electrocatalytic Sensing of Hydrogen Peroxide. Electroanalysis 2009, 21, 1533–1538. [Google Scholar] [CrossRef]
- Balamurugan, A.; Chen, S.-M. Silver Nanograins Incorporated PEDOT Modified Electrode for Electrocatalytic Sensing of Hydrogen Peroxide. Electroanalysis 2009, 21, 1419–1423. [Google Scholar] [CrossRef]
- Baghayeri, M.; Veisi, H.; Veisi, H.; Maleki, B.; Karimi-Maleh, H.; Beitollahi, H. Multi-walled carbon nanotubes decorated with palladium nanoparticles as a novel platform for electrocatalytic sensing applications. RSC Adv. 2014, 4, 49595–49604. [Google Scholar] [CrossRef]
- Yang, S.; Li, G.; Qu, C.; Wang, G.; Wang, D. Simple synthesis of ZnO nanoparticles on N-doped reduced graphene oxide for the electrocatalytic sensing of l-cysteine. RSC Adv. 2017, 7, 35004–35011. [Google Scholar] [CrossRef]
- Guo, S.; Wen, D.; Zhai, Y.; Dong, S.; Wang, E. Platinum Nanoparticle Ensemble-on-Graphene Hybrid Nanosheet: One-Pot, Rapid Synthesis, and Used as New Electrode Material for Electrochemical Sensing. ACS Nano 2010, 4, 3959–3968. [Google Scholar] [CrossRef]
- Ivanov, S.; Lange, U.; Tsakova, V.; Mirsky, V.M. Electrocatalytically active nanocomposite from palladium nanoparticles and polyaniline: Oxidation of hydrazine. Sens. Actuators B Chem. 2010, 150, 271–278. [Google Scholar] [CrossRef]
- Tian, J.; Li, H.; Lu, W.; Luo, Y.; Wang, L.; Sun, X. Preparation of Ag nanoparticle-decorated poly(m-phenylenediamine) microparticles and their application for hydrogen peroxide detection. Analyst 2011, 136, 1806–1809. [Google Scholar] [CrossRef]
- Minta, D.; Moyseowicz, A.; Gryglewicz, S.; Gryglewicz, G. A Promising Electrochemical Platform for Dopamine and Uric Acid Detection Based on a Polyaniline/Iron Oxide-Tin Oxide/Reduced Graphene Oxide Ternary Composite. Molecules 2020, 25, 5869. [Google Scholar] [CrossRef] [PubMed]
- Malode, S.J.; Prabhu, K.K.; Shetti, N.P. Electrocatalytic behavior of a heterostructured nanocomposite sensor for aminotriazole. New J. Chem. 2020, 44, 19376–19384. [Google Scholar] [CrossRef]
- Singh, P.; Singh, K.R.; Verma, R.; Prasad, P.; Verma, R.; Das, S.N.; Singh, J.; Singh, R.P. Preparation, antibacterial activity, and electrocatalytic detection of hydrazine based on biogenic CuFeO2/PANI nanocomposites synthesized using Aloe barbadensis miller. New J. Chem. 2022, 46, 8805–8816. [Google Scholar] [CrossRef]
- Teng, J.; Liu, D.; Zhang, X.; Guo, J. PANI coated NiMoOP nanoarrays as efficient electrocatalyst for oxygen evolution. J. Electroanal. Chem. 2022, 908, 116129. [Google Scholar] [CrossRef]
- Qiao, L.; Zhu, Y.; Zeng, T.; Zhang, Y.; Zhang, M.; Song, K.; Yin, N.; Tao, Y.; Zhao, Y.; Zhang, Y.; et al. “Turn-off” photoelectrochemical aptasensor based on g-C3N4/WC/WO3 composites for tobramycin detection. Food Chem. 2023, 403, 134287. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).