Enzymeless Electrochemical Glucose Sensors Based on Metal–Organic Framework Materials: Current Developments and Progresses
Abstract
:1. Introduction
2. Enzymeless Electrochemical Glucose Sensors Based on a Variety of Metal–Organic Framework Materials
2.1. Monometallic MOFs
2.2. Bimetallic MOFs
2.3. Composites of MOFs with Carbon Nanomaterials
2.4. Composites of MOFs with Metal Nanoparticles
2.5. Composites of MOFs with Metal Oxides
2.6. MOFs on Metal Foams
2.7. Pyrolyzed MOFs
2.8. Other MOFs
3. Discussion
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopa, N.S.; Rahman, M.M.; Ahmed, F.; Sutradhar, S.C.; Ryu, T.; Kim, W. A Ni-based redox-active metal-organic framework for sensitive and non-enzymatic detection of glucose. J. Electroanal. Chem. 2018, 822, 43–49. [Google Scholar] [CrossRef]
- Chen, C.; Zhong, Y.; Cheng, S.; Huanga, Y.; Li, T.; Shi, T.; Liao, G.; Tang, Z. In Situ Fabrication of Porous Nanostructures Derived from Bimetal-Organic Frameworks for Highly Sensitive Non-Enzymatic Glucose Sensors. J. Electrochem. Soc. 2020, 167, 027531. [Google Scholar] [CrossRef]
- Wang, F.; Chen, X.; Chen, L.; Yang, J.; Wang, Q. High-performance non-enzymatic glucose sensor by hierarchical flower-like nickel(II)-based MOF/carbon nanotubes composite. Mater. Sci. Eng. C 2019, 96, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Liu, Y.; Yang, P.; Chen, Y.; Tao, J.; Hu, J.; Zhao, P. Carbon nanohorns enhanced electrochemical properties of Cu-based metal organic framework for ultrasensitive serum glucose sensing. J. Electroanal. Chem. 2020, 862, 114018. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal–Organic Framework Materials as Chemical Sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal–organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Xu, Q.; Jiang, H.-L. Metal–organic frameworks meet metal nanoparticles: Synergistic effect for enhanced catalysis. Chem. Soc. Rev. 2017, 46, 4774–4808. [Google Scholar] [CrossRef]
- Jiao, L.; Wang, Y.; Jiang, H.-L.; Xu, Q. Metal–Organic Frameworks as Platforms for Catalytic Applications. Adv. Mater. 2018, 30, 1703663. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Li, Z.; Garcia, H. Catalysis and photocatalysis by metal organic frameworks. Chem. Soc. Rev. 2018, 47, 8134–8172. [Google Scholar] [CrossRef]
- Rasheed, H.U.; Lv, X.; Zhang, S.; Wei, W.; ullah, N.; Xie, J. Ternary MIL-100(Fe)@Fe3O4/CA magnetic nanophotocatalysts (MNPCs): Magnetically separable and Fenton-like degradation of tetracycline hydrochloride. Adv. Powder Technol. 2018, 29, 3305–3314. [Google Scholar] [CrossRef]
- Yin, H.-Q.; Yang, J.-C.; Yin, X.-B. Ratiometric Fluorescence Sensing and Real-Time Detection of Water in Organic Solvents with One-Pot Synthesis of Ru@MIL-101(Al)–NH2. Anal. Chem. 2017, 89, 13434–13440. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Jia, Q.; He, S.; Shan, S.; Su, H.; Zhi, Y.; He, L. Novel functionalized metal-organic framework MIL-101 adsorbent for capturing oxytetracycline. J. Alloy. Compd. 2017, 727, 114–122. [Google Scholar] [CrossRef]
- Mai, Z.; Liu, D. Synthesis and Applications of Isoreticular Metal–Organic Frameworks IRMOFs-n (n = 1, 3, 6, 8). Cryst. Growth Des. 2019, 19, 7439–7462. [Google Scholar] [CrossRef]
- Shaukat, R.A.; Saqib, Q.M.; Kim, J.; Song, H.; Khan, M.U.; Chougale, M.Y.; Bae, J.; Choi, M.J. Ultra-robust tribo- and piezo-electric nanogenerator based on metal organic frameworks (MOF-5) with high environmental stability. Nano Energy 2022, 96, 107128. [Google Scholar] [CrossRef]
- Ding, Y.; Wei, F.; Dong, C.; Li, J.; Zhang, C.; Han, X. UiO-66 based electrochemical sensor for simultaneous detection of Cd(II) and Pb(II). Inorg. Chem. Commun. 2021, 131, 108785. [Google Scholar] [CrossRef]
- Ruan, B.; Yang, J.; Zhang, Y.-J.; Ma, N.; Shi, D.; Jiang, T.; Tsai, F.-C. UiO-66 derivate as a fluorescent probe for Fe3+ detection. Talanta 2020, 218, 121207. [Google Scholar] [CrossRef]
- Sankar, S.S.; Karthick, K.; Sangeetha, K.; Karmakar, A.; Kundu, S. Transition-Metal-Based Zeolite Imidazolate Framework Nanofibers via an Electrospinning Approach: A Review. ACS Omega 2020, 5, 57–67. [Google Scholar] [CrossRef]
- Wagia, R.; Strashnov, I.; Anderson, M.W.; Attfield, M.P. Insight and Control of the Crystal Growth of Zeolitic Imidazolate Framework ZIF-67 by Atomic Force Microscopy and Mass Spectrometry. Cryst. Growth Des. 2018, 18, 695–700. [Google Scholar] [CrossRef]
- Zeraati, M.; Alizadeh, V.; Kazemzadeh, P.; Safinejad, M.; Kazemian, H.; Sargazi, G. A new nickel metal organic framework (Ni-MOF) porous nanostructure as a potential novel electrochemical sensor for detecting glucose. J. Porous Mater. 2022, 29, 257–267. [Google Scholar] [CrossRef]
- Chen, J.; Yin, H.; Zhao, S.; Gong, J.; Ji, Z.; Nie, Q.; Wang, L. Construction of highly efficient non-enzymatic glucose sensors based on micro-spherical Ni-metal-organic frameworks. Funct. Mater. Lett. 2020, 13, 2050022. [Google Scholar] [CrossRef]
- Wu, L.; Lu, Z.-W.; Ma, Y.; Zhang, J.-J.; Mo, G.-Q.; Du, H.-J.; Ye, J.-S. Cu(II) Metal-Organic Framework Encapsulated in Carbon Paste Electrode for High-Performance Non-Enzymatic Glucose Sensing. Chin. J. Anal. Chem. 2020, 48, e20038–e20046. [Google Scholar] [CrossRef]
- Chen, Q.; Chu, D.; Yan, L.; Lai, H.; Chu, X.-Q.; Ge, D.; Chen, X. Enhanced non-enzymatic glucose sensing based on porous ZIF-67 hollow nanoprisms. New J. Chem. 2021, 45, 10031–10039. [Google Scholar] [CrossRef]
- Kim, S.e.; Muthurasu, A. Metal-organic framework–assisted bimetallic Ni@Cu microsphere for enzyme-free electrochemical sensing of glucose. J. Electroanal. Chem. 2020, 873, 114356. [Google Scholar] [CrossRef]
- Kong, X.; Xia, B.; Xiao, Y.; Chen, H.; Li, H.; Chen, W.; Wu, P.; Shen, Y.; Wu, J.; Li, S.; et al. Regulation of Cobalt–Nickel LDHs’ Structure and Components for Optimizing the Performance of an Electrochemical Sensor. ACS Appl. Nano Mater. 2019, 2, 6387–6396. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Q.; Zhangsun, H.; Zhao, S.; Zhao, Y.; Wang, L. Carbon cloth-supported nanorod-like conductive Ni/Co bimetal MOF: A stable and high-performance enzyme-free electrochemical sensor for determination of glucose in serum and beverage. Food Chem. 2021, 349, 129202. [Google Scholar] [CrossRef]
- Xue, Z.; Jia, L.; Zhu, R.-R.; Du, L.; Zhao, Q.-H. High-performance non-enzymatic glucose electrochemical sensor constructed by transition nickel modified Ni@Cu-MOF. J. Electroanal. Chem. 2020, 858, 113783. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Y.; Ye, B. An efficient electrochemical glucose sensor based on porous nickel-based metal organic framework/carbon nanotubes composite (Ni-MOF/CNTs). J. Alloy. Compd. 2018, 767, 651–656. [Google Scholar] [CrossRef]
- Wu, L.; Lu, Z.; Ye, J. Enzyme-free glucose sensor based on layer-by-layer electrodeposition of multilayer films of multi-walled carbon nanotubes and Cu-based metal framework modified glassy carbon electrode. Biosens. Bioelectron. 2019, 135, 45–49. [Google Scholar] [CrossRef]
- Elizbit; Liaqat, U.; Hussain, Z.; Baig, M.M.; Khan, M.A.; Arif, D. Preparation of porous ZIF-67 network interconnected by MWCNTs and decorated with Ag nanoparticles for improved non-enzymatic electrochemical glucose sensing. J. Korean Ceram. Soc. 2021, 58, 598–605. [Google Scholar] [CrossRef]
- Chen, X.; Liu, D.; Cao, G.; Tang, Y.; Wu, C. In Situ Synthesis of a Sandwich-like Graphene@ZIF-67 Heterostructure for Highly Sensitive Nonenzymatic Glucose Sensing in Human Serums. ACS Appl. Mater. Interfaces 2019, 11, 9374–9384. [Google Scholar] [CrossRef]
- Wen, Y.; Meng, W.; Li, C.; Dai, L.; He, Z.; Wang, L.; Li, M.; Zhu, J. Enhanced glucose sensing based on a novel composite CoII-MOF/Acb modified electrode. Dalton Trans. 2018, 47, 3872–3879. [Google Scholar] [CrossRef] [PubMed]
- Asadian, E.; Shahrokhian, S.; Iraji Zad, A. Highly sensitive nonenzymetic glucose sensing platform based on MOF-derived NiCo LDH nanosheets/graphene nanoribbons composite. J. Electroanal. Chem. 2018, 808, 114–123. [Google Scholar] [CrossRef]
- Meng, W.; Wen, Y.; Dai, L.; He, Z.; Wang, L. A novel electrochemical sensor for glucose detection based on Ag@ZIF-67 nanocomposite. Sens. Actuators B Chem. 2018, 260, 852–860. [Google Scholar] [CrossRef]
- Chen, J.; Yin, H.; Zhou, J.; Wang, L.; Gong, J.; Ji, Z.; Nie, Q. Efficient Nonenzymatic Sensors Based on Ni-MOF Microspheres Decorated with Au Nanoparticles for Glucose Detection. J. Electron. Mater. 2020, 49, 4754–4763. [Google Scholar] [CrossRef]
- Zhang, L.; Ye, C.; Li, X.; Ding, Y.; Liang, H.; Zhao, G.; Wang, Y. A CuNi/C Nanosheet Array Based on a Metal–Organic Framework Derivate as a Supersensitive Non-Enzymatic Glucose Sensor. Nano-Micro Lett. 2017, 10, 28. [Google Scholar] [CrossRef]
- Wang, L.; Miao, X.; Qu, Y.; Duan, C.; Wang, B.; Yu, Q.; Gao, J.; Song, D.; Li, Y.; Yin, Z. Rattle-type Au@NiCo LDH hollow core-shell nanostructures for nonenzymatic glucose sensing. J. Electroanal. Chem. 2020, 858, 113810. [Google Scholar] [CrossRef]
- Yang, N.; Guo, K.; Zhang, Y.; Xu, C. Engineering the valence state of ZIF-67 by Cu2O for efficient nonenzymatic glucose detection. J. Mater. Chem. B 2020, 8, 2856–2861. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, L.; Yang, K. In situ synthesis of CuO nanoparticles decorated hierarchical Ce-metal-organic framework nanocomposite for an ultrasensitive non-enzymatic glucose sensor. Ionics 2019, 25, 4447–4457. [Google Scholar] [CrossRef]
- Shu, Y.; Yan, Y.; Chen, J.; Xu, Q.; Pang, H.; Hu, X. Ni and NiO Nanoparticles Decorated Metal–Organic Framework Nanosheets: Facile Synthesis and High-Performance Nonenzymatic Glucose Detection in Human Serum. ACS Appl. Mater. Interfaces 2017, 9, 22342–22349. [Google Scholar] [CrossRef]
- Arif, D.; Hussain, Z.; Sohail, M.; Liaqat, M.A.; Khan, M.A.; Noor, T. A Non-enzymatic Electrochemical Sensor for Glucose Detection Based on Ag@TiO2@ Metal-Organic Framework (ZIF-67) Nanocomposite. Front. Chem. 2020, 8, 573510. [Google Scholar] [CrossRef]
- Dong, Q.; He, Z.; Tang, X.; Zhang, Y.; Yang, L.; Huang, K.; Jiang, X.; Xiong, X. One-step synthesis of Mn3O4@ZIF-67 on carbon cloth: As an effective non-enzymatic glucose sensor. Microchem. J. 2022, 175, 107203. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, Q.; Yu, F.; Ran, G.-Y.; Wang, H.-Y.; Shepherd, N.D.; D’Alessandro, D.M.; Kurmoo, M.; Zuo, J.-L. A Metal–Organic Framework Based on a Nickel Bis(dithiolene) Connector: Synthesis, Crystal Structure, and Application as an Electrochemical Glucose Sensor. J. Am. Chem. Soc. 2020, 142, 20313–20317. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Qin, J.; Wang, X.F.; Ran, G.Y.; Wang, Q.; Liu, G.X.; Ma, J.P.; Ge, J.Y.; Wang, H.Y. Cu-Based Conductive MOF Grown in situ on Cu Foam as a Highly Selective and Stable Non-Enzymatic Glucose Sensor. Front. Chem. 2021, 9, 786970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, X.; Liang, H.; Lin, H.; Zhao, G. A non-enzymatic glucose sensor with enhanced anti-interference ability based on a MIL-53(NiFe) metal–organic framework. J. Mater. Chem. B 2019, 7, 7006–7013. [Google Scholar] [CrossRef]
- Li, Y.; Xie, M.; Zhang, X.; Liu, Q.; Lin, D.; Xu, C.; Xie, F.; Sun, X. Co-MOF nanosheet array: A high-performance electrochemical sensor for non-enzymatic glucose detection. Sens. Actuators B Chem. 2019, 278, 126–132. [Google Scholar] [CrossRef]
- Chen, C.; Xiong, D.; Gu, M.; Lu, C.; Yi, F.-Y.; Ma, X. MOF-Derived Bimetallic CoFe-PBA Composites as Highly Selective and Sensitive Electrochemical Sensors for Hydrogen Peroxide and Nonenzymatic Glucose in Human Serum. ACS Appl. Mater. Interfaces 2020, 12, 35365–35374. [Google Scholar] [CrossRef]
- Han, C.; Nie, L.; Han, X.; Zhuang, X.; Zhang, J.; Rui, Y.; Meng, W. A good-performance glucose sensor fabricated via rationally designing a new cobalt–metal–organic framework precursor. New J. Chem. 2020, 44, 14896–14905. [Google Scholar] [CrossRef]
- Xiao, X.; Peng, S.; Wang, C.; Cheng, D.; Li, N.; Dong, Y.; Li, Q.; Wei, D.; Liu, P.; Xie, Z.; et al. Metal/metal oxide@carbon composites derived from bimetallic Cu/Ni-based MOF and their electrocatalytic performance for glucose sensing. J. Electroanal. Chem. 2019, 841, 94–100. [Google Scholar] [CrossRef]
- Wang, L.; Hou, C.; Yu, H.; Zhang, Q.; Li, Y.; Wang, H. Metal–Organic Framework-Derived Nickel/Cobalt-Based Nanohybrids for Sensing Non-Enzymatic Glucose. ChemElectroChem 2020, 7, 4446–4452. [Google Scholar] [CrossRef]
- Zhou, H.; Zheng, M.; Tang, H.; Xu, B.; Tang, Y.; Pang, H. Amorphous Intermediate Derivative from ZIF-67 and Its Outstanding Electrocatalytic Activity. Small 2020, 16, 1904252. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Zhu, H.; Li, S.; Jiang, C.; Blue, R.J.; Su, Y. Functionalization of the support material based on N-doped carbon-reduced graphene oxide and its influence on the non-enzymatic detection of glucose. J. Alloy. Compd. 2019, 780, 98–106. [Google Scholar] [CrossRef]
- Hou, C.; Zhang, X.; Wang, L.; Zhang, F.; Huang, X.; Wang, Z. A buckypaper decorated with CoP/Co for nonenzymatic amperometric sensing of glucose. Microchim. Acta 2020, 187, 101. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; You, J. Synthesis of a three-dimensional porous Co3O4 network interconnected by MWCNTs and decorated with Au nanoparticles for enhanced nonenzymatic glucose sensing. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 126064. [Google Scholar] [CrossRef]
- Shi, L.; Li, Y.; Cai, X.; Zhao, H.; Lan, M. ZIF-67 derived cobalt-based nanomaterials for electrocatalysis and nonenzymatic detection of glucose: Difference between the calcination atmosphere of nitrogen and air. J. Electroanal. Chem. 2017, 799, 512–518. [Google Scholar] [CrossRef]
- Yin, H.; Zhan, T.; Chen, J.; Wang, L.; Gong, J.; Zhao, S.; Ji, Z.; Nie, Q. Polyhedral NiO/C porous composites derived by controlled pyrolysis of Ni-MOF for highly efficient non-enzymatic glucose detection. J. Mater. Sci. Mater. Electron. 2020, 31, 4323–4335. [Google Scholar] [CrossRef]
- Dolgopolova, E.A.; Brandt, A.J.; Ejegbavwo, O.A.; Duke, A.S.; Maddumapatabandi, T.D.; Galhenage, R.P.; Larson, B.W.; Reid, O.G.; Ammal, S.C.; Heyden, A.; et al. Electronic Properties of Bimetallic Metal-Organic Frameworks (MOFs): Tailoring the Density of Electronic States through MOF Modularity. J. Am. Chem. Soc. 2017, 139, 5201–5209. [Google Scholar] [CrossRef]
- Baumann, A.E.; Burns, D.A.; Liu, B.; Thoi, V.S. Metal-organic framework functionalization and design strategies for advanced electrochemical energy storage devices. Commun. Chem. 2019, 2, 86. [Google Scholar] [CrossRef]
- Jun, H.; Oh, S.; Lee, G.; Oh, M. Enhanced catalytic activity of MOF-74 via providing additional open metal sites for cyanosilylation of aldehydes. Sci. Rep. 2022, 12, 14735. [Google Scholar] [CrossRef]
- Zhai, Z.; Yan, W.; Dong, L.; Deng, S.; Wilkinson, D.P.; Wang, X.; Zhang, L.; Zhang, J. Catalytically active sites of MOF-derived electrocatalysts: Synthesis, characterization, theoretical calculations, and functional mechanisms. J. Mater. Chem. A 2021, 9, 20320–20344. [Google Scholar] [CrossRef]
- Zheng, W.; Lee, L.Y.S. Metal-Organic Frameworks for Electrocatalysis: Catalyst or Precatalyst? Acs Energy Lett. 2021, 6, 2838–2843. [Google Scholar] [CrossRef]
- Xuan, X.; Chen, S.; Zhao, S.; Yoon, J.Y.; Boczkaj, G.; Sun, X. Carbon Nanomaterials From Metal-Organic Frameworks: A New Material Horizon for CO2 Reduction. Front. Chem. 2020, 8, 573797. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Seow, J.Y.R.; Skinner, W.S.; Wang, Z.U.; Jiang, H.-L. Metal-organic frameworks: Structures and functional applications. Mater. Today 2019, 27, 43–68. [Google Scholar] [CrossRef]
- Singh, C.; Liberman, I.; Shimoni, R.; Ifraemov, R.; Hod, I. Pristine versus Pyrolyzed Metal-Organic Framework-based Oxygen Evolution Electrocatalysts: Evaluation of Intrinsic Activity Using Electrochemical Impedance Spectroscopy. J. Phys. Chem. Lett. 2019, 10, 3630. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Deng, Y.; Li, Y.; Li, T.; Xu, J.; Chen, S.-W.; Wang, J. Core-shell MOF@MOF composites for sensitive nonenzymatic glucose sensing in human serum. Anal. Chim. Acta 2020, 1110, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Zhao, Q.; Jia, L.; Chen, Q.; Jiang, J.; Yu, Q. Significantly enhanced activity of ZIF-67-supported nickel phosphate for electrocatalytic glucose oxidation. Electrochim. Acta 2019, 304, 456–464. [Google Scholar] [CrossRef]
- Sun, S.; Tang, Y.; Wu, C.; Wan, C. Phytic acid functionalized ZIF-67 decorated graphene nanosheets with remarkably boosted electrochemical sensing performance. Anal. Chim. Acta 2020, 1107, 55–62. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Yang, B.; Xiao, K.; Duan, H.; Zhao, H. The chemical stability of metal-organic frameworks in water treatments: Fundamentals, effect of water matrix and judging methods. Chem. Eng. J. 2022, 450, 138215. [Google Scholar] [CrossRef]
- Howarth, A.J.; Liu, Y.; Li, P.; Li, Z.; Wang, T.C.; Hupp, J.; Farha, O.K. Chemical, thermal and mechanical stabilities of metal-organic frameworks. Nat. Rev. Mater. 2016, 1, 1–15. [Google Scholar] [CrossRef]
- Chuang, C.-H.; Kung, C.-W. Metal-Organic Frameworks toward Electrochemical Sensors: Challenges and Opportunities. Electroanalysis 2020, 32, 1885–1895. [Google Scholar] [CrossRef]
- Heidary, N.; Chartrand, D.; Guiet, A.; Kornienko, N. Rational incorporation of defects within metal-organic frameworks generates highly active electrocatalytic sites. Chem. Sci. 2021, 12, 7324–7333. [Google Scholar] [CrossRef]
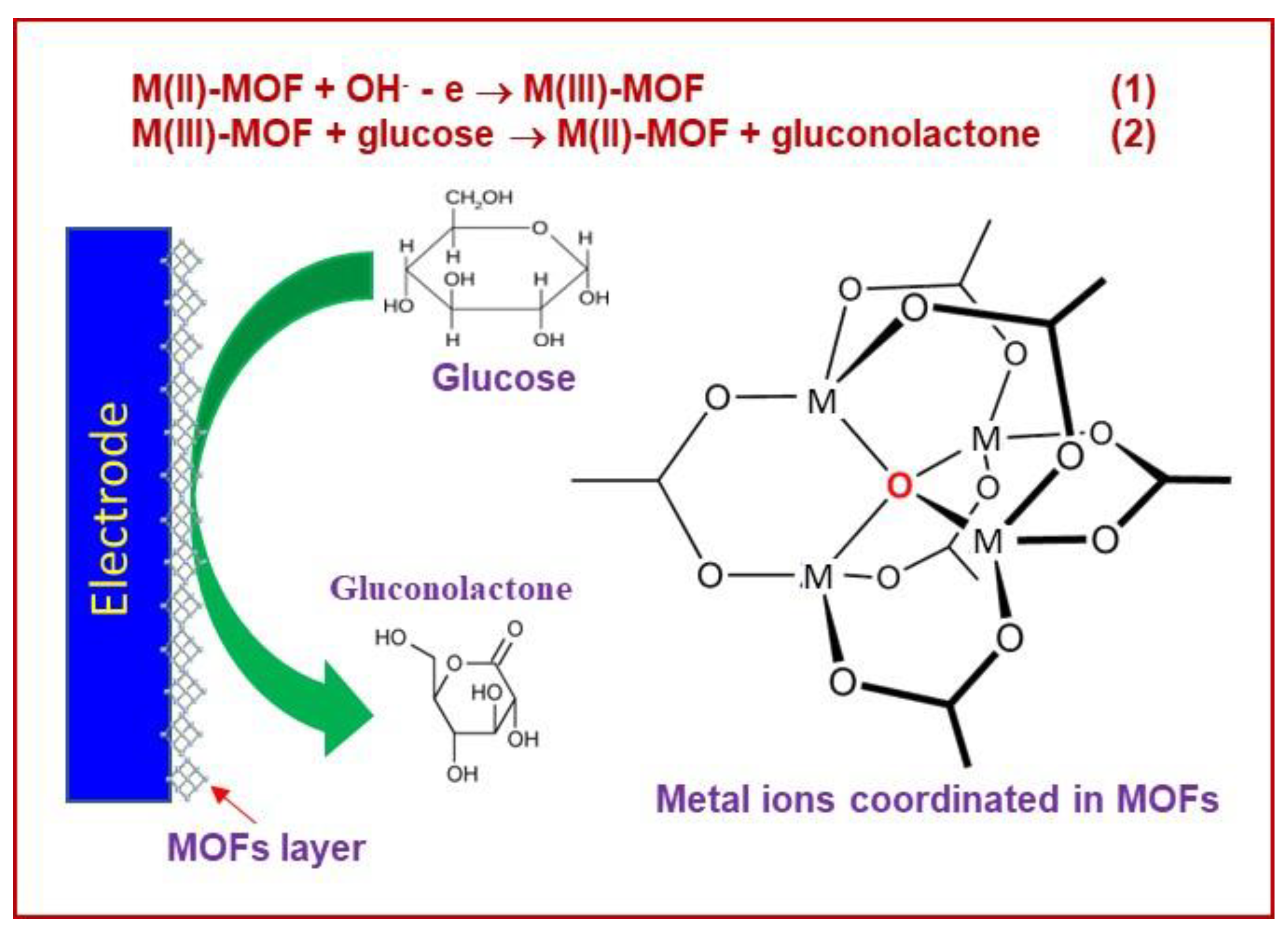
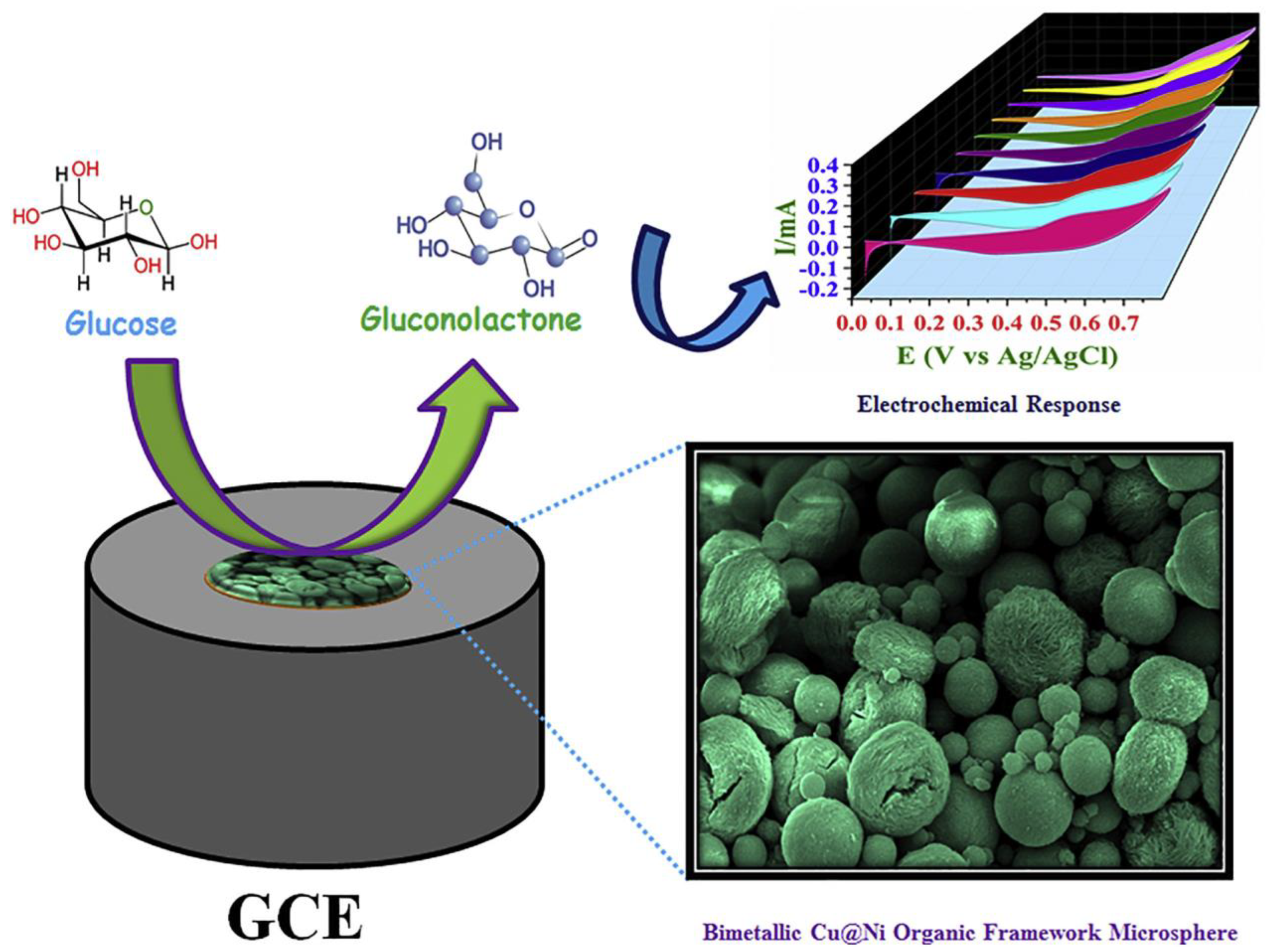

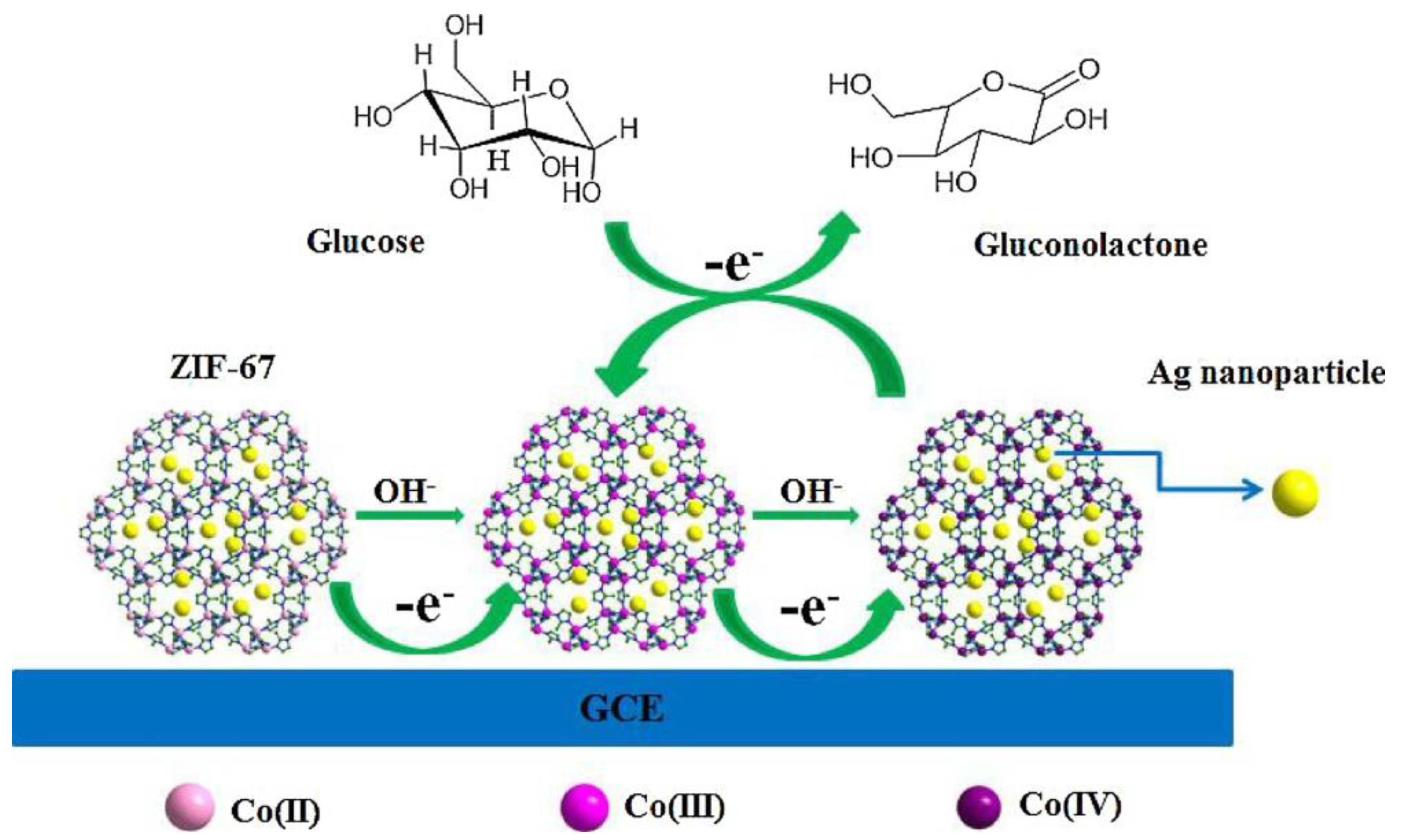

| MOF/Composite Classification | MOFs * | Detection Limit ** | Sensitivity | Linear Range | Reference |
|---|---|---|---|---|---|
| Monometallic MOFs | Ni-MOF | 0.76 µM | 2859.95 µA·mM−1·cm−2 | 1–1600 µM | [19] |
| Ni-BTC | 2.5 µM | 932.68 µA·mM−1·cm−2 273.04 µA·mM−1·cm−2 | 5–3000 μM 3500–6000 μM | [20] | |
| Cu-MOF | 0.11 μM | - | 5–3910 μM 3910–10,950 μM | [21] | |
| CPO-27-Ni | 1.46 μM | 40.95 μA·mM−1 | 0.04–0.5 mM 1–6 mM | [1] | |
| ZIF-67 HNPs | 0.96 μM | 445.7 μA·mM−1·cm−2 95.9 μA·mM−1·cm−2 | 0.005–3.3 mM 3.3–42.1 mM | [22] | |
| Bimetallic MOFs | Cu@Ni MOF | 0.4 μM | 496 μA·mM−1 | 0–5 mM | [23] |
| Co0.33Ni0.67-HLDH | 3.1 μM | 242.9 μA·mM−1 ·cm−2 | 0.01–2 mM | [24] | |
| Ni/Co(HHTP)MOF | 100 nM | 3250 μA·mM−1 ·cm−2 | 0.3 μM–2.312 mM | [25] | |
| Ni@Cu-MOF | 1.67 μM | 1703.33 μA·mM−1 ·cm−2 | 5–2500 μM | [26] | |
| Cu/Co-MOF | 2 μM | 18.68 mA·mM−1·cm−2 | 0.02–0.8 mM | [2] | |
| MOFs with carbon nanomaterials | Ni-MOF/CNTs | 0.82 μM | 13.85 mA·mM−1·cm−2 | 1 μM–1.6 mM | [27] |
| Ni(TPA)-SWCNT | 4.6 μM | - | 20 μM–4.4 mM | [3] | |
| Cu-MOF/MWCNTs | 0.4 μM | 3878 μA·mM−1·cm−2 | 0.5 μM–11.84 mM | [28] | |
| Ag@ZIF-67/MWCNTs | 0.49 μM | 13.014 μA·μM−1·cm−2 | 33–400 μM | [29] | |
| Cu-MOF/CNHs | 78 nM | - | 250 nM–1.2 mM | [4] | |
| GS@ZIF-67 | 0.36 μM | 1521.1 μA·mM−1·cm−2 | 1–805.5 μM | [30] | |
| Co-MOF/Acb | 1.7 μM | 0.255 μA·μM−1·cm−2 | 5–1000 μM | [31] | |
| Ni/Co LDH/GNRs | 0.6 μM | 344 μA·mM−1·cm−2 | 5 μM–0.8 mM | [32] | |
| MOFs with metal nanoparticles | Ag/ZIF-67 | 0.66 μM | 0.379 μA·μM−1·cm−2 | 2–1000 μM | [33] |
| Au@Ni-BTC | 1.5 μM | 1447.1 μA·mM−1·cm−2 | 5–7400 μM | [34] | |
| Cu/Ni-MOFs | 66.67 nM | 17.12 mA·mM−1·cm−2 | 0.20–2.72 mM | [35] | |
| Au@NiCo LDH | 0.028 μM | 864.7 μA·mM−1·cm−2 | 0.005–12 mM | [36] | |
| MOFs with metal oxides | Cu2O@ZIF-67 | 6.5 μM | 307.02 μA·mM−1·cm−2 181.34 μA·mM−1·cm−2 | 0.01–10 mM 10–16.3 mM | [37] |
| CuO/Ce-MOFs | 2 nM | 2058.5 μA·mM−1·cm−2 | 5 nM–8.6 mM | [38] | |
| Ni-MOF/Ni/NiO | 0.8 μM | 367.45 mA·M−1·cm−2 | 4–5664 μM | [39] | |
| Ag@TiO2@ZIF-67 | 0.99 μM | 0.788 μA·μM−1·cm−2 | 48 μM–1 mM | [40] | |
| Mn3O4@ZIF-67 | 0.24 μM | 3421.0 μA·mM−1·cm−2 | 0.8 μM–6.0 mM | [41] | |
| MOFs on metal foams | [Mn2(Ni(C2S2(C6H4COO)2)2)(H2O)2]·2DMF | 0.1 μM | 27.9 A·M−1·cm−2 | 2.0 μM–2.0 mM | [42] |
| Cu-MOFs/CF | 0.076 μM | 30,030 mA·μM−1·cm−2 | 0.001–0.95 mM | [43] | |
| (NiFe) MOFs/NF | 0.67 μM | 41.95 mA·mM−1·cm−2 | 2–1600 μM | [44] | |
| Co-MOFs/NF | 1.3 nM | 10,886 µA·mM−1·cm−2 | 0.001–3 mM | [45] | |
| CoFe-PBA/Co-ZIF/NF | 0.02 μM | 5270 μA·mM−1·cm−2 | 1.4 μM–1.5 mM | [46] | |
| MOFs pyrolyzed | Co-MOFs | - | 254.21 μA·mM−1·cm−2 102.80 μA·mM−1·cm−2 | 0.001–2 mM 2–8 mM | [47] |
| Cu/Ni-MOFs | 0.06 μM | - | 0.1 μM–2.2 mM | [48] | |
| NiCo-MOFs | 0.2 μM | 265.53 μA·mM−1·cm−2 | 0.5 μM–4.38 mM | [49] | |
| ZIF-67 | 3.9 μM | 1074.22 µA·mM−1·cm−2 | 0.5–1000 µM | [50] | |
| ZIF-67-GO | 0.34 μM | 3172 μA·mM−1·cm−2 | 0.5–10.0 μM | [51] | |
| ZIF-67-BP | 0.2 μM | 6427 μA·mM−1·cm−2 | 0.5 μM–1.8 mM | [52] | |
| ZIF-67/MWCNTs/Au | 0.1 μM | 1138.4 μA·mM−1·cm−2 | 0.2 μM–1.1 mM | [53] | |
| ZIF-67 | 5.69 μM | 0.227 mA·mM−1·cm−2 | 0.1–1.1 mM | [54] | |
| Ni-MOFs | 0.92 μM | 2918.2 μA·mM−1·cm−2 | 5 μM–4.1 mM | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Zhou, J.; Yan, R.; Wei, L.; Lei, C. Enzymeless Electrochemical Glucose Sensors Based on Metal–Organic Framework Materials: Current Developments and Progresses. Chemosensors 2023, 11, 290. https://doi.org/10.3390/chemosensors11050290
Liu C, Zhou J, Yan R, Wei L, Lei C. Enzymeless Electrochemical Glucose Sensors Based on Metal–Organic Framework Materials: Current Developments and Progresses. Chemosensors. 2023; 11(5):290. https://doi.org/10.3390/chemosensors11050290
Chicago/Turabian StyleLiu, Chang, Jian Zhou, Rongqiu Yan, Lina Wei, and Chenghong Lei. 2023. "Enzymeless Electrochemical Glucose Sensors Based on Metal–Organic Framework Materials: Current Developments and Progresses" Chemosensors 11, no. 5: 290. https://doi.org/10.3390/chemosensors11050290
APA StyleLiu, C., Zhou, J., Yan, R., Wei, L., & Lei, C. (2023). Enzymeless Electrochemical Glucose Sensors Based on Metal–Organic Framework Materials: Current Developments and Progresses. Chemosensors, 11(5), 290. https://doi.org/10.3390/chemosensors11050290






