Recent Advances for Imidacloprid Detection Based on Functional Nanomaterials
Abstract
:1. Introduction
2. The Metabolites of IMI
3. Detection Methods
3.1. Sample Pretreatment and Chromatographic Analysis
| Matrices | Sample Pretreatment | Analytical Technique | Recovery | LOD | Reference |
|---|---|---|---|---|---|
| fruit | SPE: UiO-66-NH2 | UPLC-MS/MS | 92.39% | 0.04 μg L−1 | [50] |
| groundwater | SPE: MSU-1 | UPLC-MS/MS | 80–86% | below 0.1 µg L−1 | [51] |
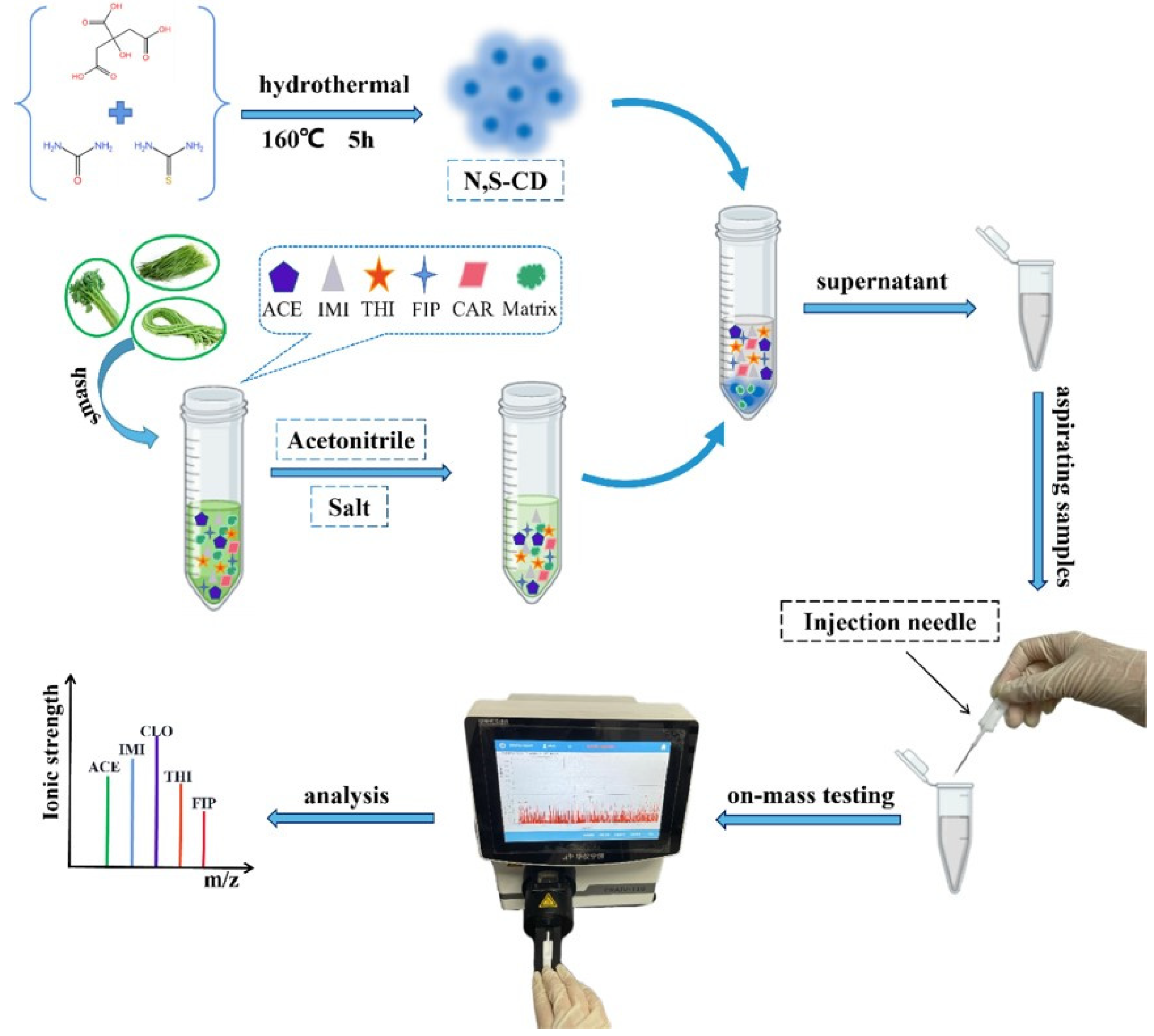
| vegetables | QuEChERS: N,S-CD | μ-MS | 82.2–109.7% | 0.5–1.0 ng g−1 | [23] |
| honey, tomato, lettuce and Chinese cabbage samples | SPE: a Fe3O4@Ph-HCP | HPLC | 80.1–111% | 0.30–0.67 ng g−1 (honey), 1–1.5 ng g−1 (tomato, lettuce and Chinese cabbage) | [54] |
| lemon juice, honey | SPE: b Rut-MOP | HPLC | 82–118% | 0.03–0.04 ng mL− 1 (lemon juice), 2.5–3.0 ng g−1 (honey) | [55] |
| vegetable | c MSPE: d (Fe3O4@COF-(NO2)2) | HPLC | 81.7–103.5% | 0.04 ng mL−1 | [56] |
| cucumber, tomato and tap water | SPE: e TPN/Fe3O4 NPs/GO | HPLC | 91.2–102.4% | 0.17 μg L−1 | [57] |
| honey | anion exchanger-f DPX | LC-MS/MS | 72–104% | 1.5 µg kg−1 | [34] |
| wheat samples | g D-µSPE: h CNPC | HPLC | 91–99% | 0.056 µg Kg− 1 | [58] |
| wheat, rice and fruit | QuEChERS | LC–MS/MS | 94.1–103.3% | - | [59] |
3.2. Electrochemical Sensors
3.2.1. Direct Electrochemical Detection
3.2.2. Electrochemical Sensors Based on MIPs
3.2.3. Electrochemical Sensor Based on Biometric Molecules
| Electrode Materials | Recognition Element | Technique | Linear Range | LOD | Reference |
|---|---|---|---|---|---|
| PoPD-RGO/GCE a | MIP | LSV | 0.75–70 μM | 0.4 μM | [78] |
| GN/MIPs/GCE b | MIP | LSV | 0.5–15 μM | 0.1 μM | [79] |
| GCE/TiO2NPs/IMD imprinted poly(levodopa) | MIP | SWV | 2.0–400 μM | 0.3 μM | [84] |
| UMV-Ce-MOF c | MIP | ECL | 2–120 nM | 0.34 nM | [81] |
| MIPs/ UCNPs@ZIF-8/GCE d | MIP | ECL | 0.1 ng mL−1 mg mL−1 | 0.01 ng mL−1 | [80] |
| Gold electrode | aptamer | EIS f | 0.1–50 n g mL−1 | 0.19 ng mL−1 | [82] |
| AuNP-SPCE e | antibody | chronoamperometry | 50–10,000 pM | 22 pM | [83] |
| SPCE | antibody | chronoamperometry | 50–10,000 pM | 24 pM | [85] |
3.2.4. Ratiometric Electrochemical Sensor
3.3. Optical Sensors
3.3.1. Fluorescent Method
3.3.2. Colorimetric and Surface Plasmon Resonance (SPR) Sensors
3.3.3. Surface-Enhanced Raman Spectroscopy
| Methods | Materials | Linear Range | LOD | Reference |
|---|---|---|---|---|
| colorimetry | PPC-Au NPs 1 | 0.05–1000 μM | 5.0 μM | [97] |
| colorimetry | I-IL-Au NPs 2 | Not given | 0.5 μM | [102] |
| SPR immunoassay | Nanoplasmonic chips | Not given | 11.103 ppb | [99] |
| SPR immunoassay | plasmonic biochip | Not given | 0.2 ng mL−1 | [103] |
| fluorescence | CoOOH-AuNCs | 0.1 ng mL−1 –50 ng mL−1 | 0.1 ng mL−1 | [91] |
| fluorescence | colloidal gold | 0.028 ng mL−1–0.5 ng mL−1 | 0.01 ng mL−1 | [90] |
| fluorescence | GO-UCNPs 3 | 0.08 ng mL−1–50 ng mL−1 | 0.08 ng mL−1 | [12] |
| SERS immunosensor | Fe3O4-AuNR@Ag | 10–400 nM | 9.58 nM | [101] |
| LSPR immunosensor | Y-shaped gold NPs | Not given | 1.0 ng mL−1 | [104] |
| ratiometric fluorescence | MIFP-SiCQDs@CdTe QDs 4 | 5 ng mL−1–0.5 μg mL−1 | 3.55 ng mL−1 | [105] |
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeschke, P.; Nauen, R. Neonicotinoids—From zero to hero in insecticide chemistry. Pest. Manag. Sci. 2008, 64, 1084–1098. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kong, X.; Xiao, Z.; Zhang, L.; Wang, F.; Zhang, H.; Li, Y.; Wang, Y. Structural determinants of imidacloprid-based nicotinic acetylcholine receptor inhibitors identified using 3D-QSAR, docking and molecular dynamics. J. Mol. Model. 2012, 18, 2279–2289. [Google Scholar] [CrossRef] [PubMed]
- Sriapha, C.; Trakulsrichai, S.; Intaraprasong, P.; Wongvisawakorn, S.; Tongpoo, A.; Schimmel, J.; Wananukul, W. Imidacloprid poisoning case series: Potential for liver injury. Clin. Toxicol. 2020, 58, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Millot, F.; Decors, A.; Mastain, O.; Quintaine, T.; Berny, P.; Vey, D.; Lasseur, R.; Bro, E. Field evidence of bird poisonings by imidacloprid-treated seeds: A review of incidents reported by the French SAGIR network from 1995 to 2014. Environ. Sci. Pollut. Res. 2017, 24, 5469–5485. [Google Scholar] [CrossRef]
- Wan, Y.; Han, Q.; Wang, Y.; He, Z. Five degradates of imidacloprid in source water, treated water, and tap water in Wuhan, central China. Sci. Total Environ. 2020, 741, 140227. [Google Scholar] [CrossRef]
- European Food Safety Authority. Neonicotinoids: Risk to Bees Confirmed. 2018. Available online: https://www.efsa.europa.eu/en/press/news/180228 (accessed on 1 June 2022).
- Yang, B.; Ma, W.; Wang, S.; Shi, L.; Li, X.; Ma, Z.; Zhang, Q.; Li, H. Determination of eight neonicotinoid insecticides in Chinese cabbage using a modified QuEChERS method combined with ultra performance liquid chromatography-tandem mass spectrometry. Food Chem. 2022, 387, 132935. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, Y.; Wang, Y.; Lu, Q.; Ruan, H.; Luo, J.; Yang, M. A comprehensive review on the pretreatment and detection methods of neonicotinoid insecticides in food and environmental samples. Food Chem. X 2022, 15, 100375. [Google Scholar] [CrossRef]
- Liu, L.; Guo, J.; Liu, X.; Wang, A.; Yu, X.; Ding, L. Photoelectrochemical Clothianidin Detection Based on a WO3/CdS Heterostructure Coated with a Molecularly Imprinted Thin Film. Anal. Sens. 2022, 2, e202200029. [Google Scholar] [CrossRef]
- Liu, L.; Guo, J.; Ding, L. Polyaniline Nanowire Arrays Deposited on Porous Carbon Derived from Raffia for Electrochemical Detection of Imidacloprid. Electroanalysis 2021, 33, 2048–2052. [Google Scholar] [CrossRef]
- Guo, J.-W.; Yang, Z.-W.; Liu, X.-L.; Zhang, L.-W.; Guo, W.-B.; Zhang, J.; Ding, L.-H. 2D Co metal-organic framework nanosheet as an oxidase-like nanozyme for sensitive biomolecule monitoring. Rare Met. 2023, 42, 797–805. [Google Scholar] [CrossRef]
- Guo, Y.; Zou, R.; Si, F.; Liang, W.; Zhang, T.; Chang, Y.; Qiao, X.; Zhao, J. A sensitive immunoassay based on fluorescence resonance energy transfer from up-converting nanoparticles and graphene oxide for one-step detection of imidacloprid. Food Chem. 2021, 335, 127609. [Google Scholar] [CrossRef]
- Gao, L.; He, C. Application of nanomaterials decorated with cyclodextrins as sensing elements for environment analysis. Environ. Sci. Pollut. Res. 2021, 28, 59499–59518. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Wang, Z.; Zhang, W.; Liu, X.; Li, M.; Li, G.; Zhang, B.; Singh, R. Optically Active Nanomaterials and Its Biosensing Applications—A Review. Biosensors 2023, 13, 85. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.-y.; Cheng, R.; Shi, L.; Ma, Z.; Zheng, X. Nanomaterials for water pollution monitoring and remediation. Environ. Chem. Lett. 2017, 15, 23–27. [Google Scholar] [CrossRef]
- Ahmadi, M.; Elmongy, H.; Madrakian, T.; Abdel-Rehim, M. Nanomaterials as sorbents for sample preparation in bioanalysis: A review. Anal. Chim. Acta 2017, 958, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Yan, J.; Zhao, Z.; Li, D. Synthesis of NiGa2O4 nanosheets for non-enzymatic glucose electrochemical sensor. Sens. Actuators B Chem. 2019, 296, 126705. [Google Scholar] [CrossRef]
- Ding, L.; Ma, C.; Li, L.; Zhang, L.; Yu, J. A photoelectrochemical sensor for hydrogen sulfide in cancer cells based on the covalently and in situ grafting of CdS nanoparticles onto TiO2 nanotubes. J. Electroanal. Chem. 2016, 783, 176–181. [Google Scholar] [CrossRef]
- An, H.; Li, M.; Ga, J.; Zhang, Z.; Ma, S.; Chen, Y. Incorporation of biomolecules in Metal-Organic Frameworks for advanced applications. Coord. Chem. Rev. 2019, 384, 90–106. [Google Scholar] [CrossRef]
- Bilal, M.; Asgher, M.; Cheng, H.; Yan, Y.; Iqbal, H.M.N. Multi-point enzyme immobilization, surface chemistry, and novel platforms: A paradigm shift in biocatalyst design. Crit. Rev. Biotechnol. 2019, 39, 202–219. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, L.; Yang, H.; Liu, H.; Ge, S.; Yu, J. Electrochemical biosensor for p53 gene based on HRP-mimicking DNAzyme-catalyzed deposition of polyaniline coupled with hybridization chain reaction. Sens. Actuators B Chem. 2018, 268, 210–216. [Google Scholar] [CrossRef]
- Mutharani, B.; Keerthi, M.; Chen, S.-M.; Ranganathan, P.; Chen, T.-W.; Lee, S.-Y.; Chang, W.-H. One-Pot Sustainable Synthesis of Ce2S3/Gum Arabic Carbon Flower Nanocomposites for the Detection of Insecticide Imidacloprid. ACS Appl. Mater. Interfaces 2020, 12, 4980–4988. [Google Scholar] [CrossRef]
- Mou, B.; Zuo, C.; Chen, L.; Xie, H.; Zhang, W.; Wang, Q.; Wen, L.; Gan, N. On-site Simultaneous Determination of Neonicotinoids, Carbamates, and Phenyl Pyrazole Insecticides in Vegetables by QuEChERS Extraction on Nitrogen and Sulfur co-doped Carbon Dots and Portable Mass Spectrometry. J. Chromatogr. A 2023, 1689, 463744. [Google Scholar] [CrossRef]
- Sheng, E.; Lu, Y.; Xiao, Y.; Li, Z.; Wang, H.; Dai, Z. Simultaneous and ultrasensitive detection of three pesticides using a surface-enhanced Raman scattering-based lateral flow assay test strip. Biosens. Bioelectron. 2021, 181, 113149. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.A.; Lehmler, H.-J.; Kolpin, D.W.; Hladik, M.L.; Vargo, J.D.; Schilling, K.E.; LeFevre, G.H.; Peeples, T.L.; Poch, M.C.; LaDuca, L.E.; et al. A critical review on the potential impacts of neonicotinoid insecticide use: Current knowledge of environmental fate, toxicity, and implications for human health. Environ. Sci. Process. Impacts 2020, 22, 1315–1346. [Google Scholar] [CrossRef] [PubMed]
- Loser, D.; Grillberger, K.; Hinojosa, M.G.; Blum, J.; Haufe, Y.; Danker, T.; Johansson, Y.; Möller, C.; Nicke, A.; Bennekou, S.H.; et al. Acute effects of the imidacloprid metabolite desnitro-imidacloprid on human nACh receptors relevant for neuronal signaling. Arch. Toxicol. 2021, 95, 3695–3716. [Google Scholar] [CrossRef] [PubMed]
- Brunet, J.-L.; Maresca, M.; Fantini, J.; Belzunces, L.P. Human intestinal absorption of imidacloprid with Caco-2 cells as enterocyte model. Toxicol. Appl. Pharmacol. 2004, 194, 1–9. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, X.; Rao, Q.; Chen, S.; Song, W. Imidacloprid dissipation, metabolism and accumulation in Agaricus bisporus fruits, casing soil and compost and dietary risk assessment. Chemosphere 2020, 254, 126837. [Google Scholar] [CrossRef]
- Suchail, S.; Guez, D.; Belzunces, L.P. Discrepancy between acute and chronic toxicity induced by imidacloprid and its metabolites in Apis mellifera. Environ. Toxicol. Chem. 2001, 20, 2482–2486. [Google Scholar] [CrossRef]
- Eng, M.L.; Hao, C.; Watts, C.; Sun, F.; Morrissey, C.A. Characterizing imidacloprid and metabolites in songbird blood with applications for diagnosing field exposures. Sci. Total Environ. 2021, 760, 143409. [Google Scholar] [CrossRef]
- Jiao, X.; Li, Z.; He, J.; Wang, C. Enhanced photodegradation of applied dithianon fungicides on plant leaves by dissolved substances in atmosphere under simulated sunlight. Chemosphere 2020, 254, 126807. [Google Scholar] [CrossRef]
- Codling, G.; Al Naggar, Y.; Giesy, J.P.; Robertson, A.J. Concentrations of neonicotinoid insecticides in honey, pollen and honey bees (Apis mellifera L.) in central Saskatchewan, Canada. Chemosphere 2016, 144, 2321–2328. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Dong, F.; Mei, X.; Ning, J.; She, D. Distribution, Dissipation, and Metabolism of Neonicotinoid Insecticides in the Cotton Ecosystem under Foliar Spray and Root Irrigation. J. Agric. Food Chem. 2019, 67, 12374–12381. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Zhang, C.; Chen, Z.; He, F.; Wei, J.; Tan, H.; Li, X. Simultaneous determination of neonicotinoid insecticides and insect growth regulators residues in honey using LC–MS/MS with anion exchanger-disposable pipette extraction. J. Chromatogr. A 2018, 1557, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Hernández, L.; Hernández-Domínguez, D.; Bernal, J.; Neusüß, C.; Martín, M.T.; Bernal, J.L. Capillary electrophoresis–mass spectrometry as a new approach to analyze neonicotinoid insecticides. J. Chromatogr. A 2014, 1359, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yu, P.; Zhou, C.; Tong, L.; Li, D.; Yu, Z.; Zhao, Y. Analysis of pesticide residues in commercially available chenpi using a modified QuEChERS method and GC-MS/MS determination. J. Pharm. Anal. 2020, 10, 60–69. [Google Scholar] [CrossRef]
- Li, S.; Xu, H.; Sui, Y.; Mei, X.; Shi, J.; Cai, S.; Xiong, T.; Carrillo, C.; Castagnini, J.M.; Zhu, Z.; et al. Comparing the LC-MS Phenolic Acids Profiles of Seven Different Varieties of Brown Rice (Oryza sativa L.). Foods 2022, 11, 1552. [Google Scholar] [CrossRef]
- Ingle, R.G.; Zeng, S.; Jiang, H.; Fang, W.-J. Current developments of bioanalytical sample preparation techniques in pharmaceuticals. J. Pharm. Anal. 2022, 12, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.-H.; Shi, Y.-P. A porous boron nitride nanorods-based QuEChERS analysis method for detection of five neonicotinoid pesticide residues in goji berries. J. Chromatogr. A 2022, 1670, 462968. [Google Scholar] [CrossRef]
- Song, Z.; Li, J.; Lu, W.; Li, B.; Yang, G.; Bi, Y.; Arabi, M.; Wang, X.; Ma, J.; Chen, L. Molecularly imprinted polymers based materials and their applications in chromatographic and electrophoretic separations. TrAC Trends Anal. Chem. 2022, 146, 116504. [Google Scholar] [CrossRef]
- Tao, X.-y.; Zhang, Y.; Zhou, Y.; Liu, Z.-f.; Feng, X.-s. Nicotine in Complex Samples: Recent Updates on the Pretreatment and Analysis Method. Crit. Rev. Anal. Chem. 2021. [Google Scholar] [CrossRef]
- Abdel-Ghany, M.F.; Hussein, L.A.; El Azab, N.F.; El-Khatib, A.H.; Linscheid, M.W. Simultaneous determination of eight neonicotinoid insecticide residues and two primary metabolites in cucumbers and soil by liquid chromatography-tandem mass spectrometry coupled with QuEChERS. J. Chromatogr. B 2016, 1031, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.-T.; Zheng, X.; Li, H.-F.; Lin, J.-M. Application of carbon-based nanomaterials in sample preparation: A review. Anal. Chim. Acta 2013, 784, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Che, D.; Cheng, J.; Ji, Z.; Zhang, S.; Li, G.; Sun, Z.; You, J. Recent advances and applications of polydopamine-derived adsorbents for sample pretreatment. TrAC Trends Anal. Chem. 2017, 97, 1–14. [Google Scholar] [CrossRef]
- Zhang, H.; Xiong, P.; Li, G.; Liao, C.; Jiang, G. Applications of multifunctional zirconium-based metal-organic frameworks in analytical chemistry: Overview and perspectives. TrAC Trends Anal. Chem. 2020, 131, 116015. [Google Scholar] [CrossRef]
- Selahle, S.K.; Mpupa, A.; Nomngongo, P.N. Combination of zeolitic imidazolate framework-67 and magnetic porous porphyrin organic polymer for preconcentration of neonicotinoid insecticides in river water. J. Chromatogr. A 2022, 1661, 462685. [Google Scholar] [CrossRef]
- Wang, X.-H.; Li, W.; Jiang, H.-X.; Chen, Y.; Gao, R.-Z.; Tang, A.-N.; Kong, D.-M. Heteropore covalent organic framework-based composite membrane prepared by in situ growth on non-woven fabric for sample pretreatment of food non-targeted analysis. Microchim. Acta 2021, 188, 235. [Google Scholar] [CrossRef]
- Wang, R.; Jiang, H.-X.; Jia, H.; Li, W.; Chen, Y.; Tang, A.-N.; Shao, B.; Kong, D.-M. Easily operated COF-based monolithic sponges as matrix clean-up materials for non-targeted analysis of chemical hazards in oil-rich foods. Talanta 2023, 255, 124250. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, G.; Wu, D.; Li, X.; Yu, Y.; Luo, P.; Chen, J.; Dai, C.; Wu, Y. Recent advances in emerging nanomaterials based food sample pretreatment methods for food safety screening. TrAC Trends Anal. Chem. 2019, 121, 115669. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Zhang, W.; Jiang, H.; Pu, Y.; Cao, J.; Jiang, W. Zirconium(IV)-based metal-organic framework for determination of imidacloprid and thiamethoxam pesticides from fruits by UPLC-MS/MS. Food Chem. 2020, 344, 128650. [Google Scholar] [CrossRef]
- Kharbouche, L.; Martínez Galera, M.; Díaz Galiano, F.J.; Gil García, M.D. Pre-concentration of 218 multiclass pesticide in groundwater samples using MSU-1 mesoporous sorbent. Microchem. J. 2023, 184, 108168. [Google Scholar] [CrossRef]
- Sanchez-Hernandez, L.; Hernandez-Dominguez, D.; Martin, M.T.; Nozal, M.J.; Higes, M.; Yague, J.L.B. Residues of neonicotinoids and their metabolites in honey and pollen from sunflower and maize seed dressing crops. J. Chromatogr. A 2016, 1428, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Long, L.; Ge, J.; Li, H.; Zhang, M.; Wan, Q.; Yu, X. Effect of Imidacloprid Uptake from Contaminated Soils on Vegetable Growth. J. Agric. Food Chem. 2019, 67, 7232–7242. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, Z.; Ma, S.; Hao, L.; Liu, W.; Wang, Q.; Wang, C.; Wang, Z.; Wu, Q. Synthesis of nitrogen-rich magnetic hypercrosslinked polymer as robust adsorbent for the detection of neonicotinoids in honey, tomatoes, lettuce and Chinese cabbage. J. Chromatogr. A 2022, 1677, 463326. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Meng, X.; Xu, M.; Li, M.; Li, S.; Wang, Q.; Liu, W.; Hao, L.; Wang, J.; Wang, C.; et al. Green synthesis of novel magnetic porous organic polymer for magnetic solid phase extraction of neonicotinoids in lemon juice and honey samples. Food Chem. 2022, 383, 132599. [Google Scholar] [CrossRef]
- Lu, J.; Wang, R.; Luan, J.; Li, Y.; He, X.; Chen, L.; Zhang, Y. A functionalized magnetic covalent organic framework for sensitive determination of trace neonicotinoid residues in vegetable samples. J. Chromatogr. A 2020, 1618, 460898. [Google Scholar] [CrossRef]
- Moradi Shahrebabak, S.; Saber-Tehrani, M.; Faraji, M.; Shabanian, M.; Aberoomand-Azar, P. Simultaneous magnetic solid phase extraction of acidic and basic pesticides using triazine-based polymeric network modified magnetic nanoparticles/graphene oxide nanocomposite in water and food samples. Microchem. J. 2019, 146, 630–639. [Google Scholar] [CrossRef]
- Alizadeh, R.; Mashalavi, B.; Yeganeh Faal, A.; Seidi, S. Development of ultrasound assisted dispersive micro solid phase extraction based on CuO nanoplate-polyaniline composite as a new sorbent for insecticides analysis in wheat samples. Microchem. J. 2021, 168, 106422. [Google Scholar] [CrossRef]
- Wu, C.; Dong, F.; Mei, X.; Ning, J.; She, D. Isotope-labeled internal standards and grouping scheme for determination of neonicotinoid insecticides and their metabolites in fruits, vegetables and cereals—A compensation of matrix effects. Food Chem. 2020, 311, 125871. [Google Scholar] [CrossRef]
- Li, H.; Qi, H.; Chang, J.; Gai, P.; Li, F. Recent progress in homogeneous electrochemical sensors and their designs and applications. TrAC Trends Anal. Chem. 2022, 156, 116712. [Google Scholar] [CrossRef]
- Oliveira, A.E.F.; Bettio, G.B.; Pereira, A.C. Optimization of an Electrochemical Sensor for Determination of Imidacloprid Based on β-cyclodextrin Electropolymerization on Glassy Carbon Electrode. Electroanalysis 2018, 30, 1929–1937. [Google Scholar] [CrossRef]
- Urbanova, V.; Bakandritsos, A.; Jakubec, P.; Szambo, T.; Zboril, R. A facile graphene oxide based sensor for electrochemical detection of neonicotinoids. Biosens. Bioelectron. 2017, 89, 532–537. [Google Scholar] [CrossRef]
- Johnson, Z.T.; Williams, K.; Chen, B.; Sheets, R.; Jared, N.; Li, J.; Smith, E.A.; Claussen, J.C. Electrochemical Sensing of Neonicotinoids Using Laser-Induced Graphene. ACS Sens. 2021, 6, 3063–3071. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, X.; Wang, Q.; Zhe, T.; Bai, Y.; Bu, T.; Zhang, M.; Wang, L. Electrochemical behavior of reduced graphene oxide/cyclodextrins sensors for ultrasensitive detection of imidacloprid in brown rice. Food Chem. 2020, 333, 127495. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Liu, X.; Wang, A.; Yu, X.; Ding, L. Antifouling electrochemical sensor-based on mesoporous silica film for imidacloprid detection in Traditional Chinese medicine. Microchem. J. 2022, 183, 107964. [Google Scholar] [CrossRef]
- Kumaravel, A.; Chandrasekaran, M. Electrochemical determination of imidacloprid using nanosilver Nafion®/nanoTiO2 Nafion® composite modified glassy carbon electrode. Sens. Actuators B Chem. 2011, 158, 319–326. [Google Scholar] [CrossRef]
- Lezi, N.; Economou, A. Voltammetric Determination of Neonicotinoid Pesticides at Disposable Screen-Printed Sensors Featuring a Sputtered Bismuth Electrode. Electroanalysis 2015, 27, 2313–2321. [Google Scholar] [CrossRef]
- Nasr-Esfahani, P.; Ensafi, A.A.; Rezaei, B. Fabrication of a highly sensitive and selective modified electrode for imidacloprid determination based on designed nanocomposite graphene quantum dots/ionic liquid/multiwall carbon nanotubes/polyaniline. Sens. Actuators B Chem. 2019, 296, 126682. [Google Scholar] [CrossRef]
- Lei, W.; Han, Z.; Si, W.; Hao, Q.; Zhang, Y.; Xia, M.; Wang, F. Sensitive and Selective Detection of Imidacloprid by Graphene-Oxide-Modified Glassy Carbon Electrode. Chemelectrochem 2014, 1, 1063–1067. [Google Scholar] [CrossRef]
- Si, W.; Lei, W.; Hao, Q.; Xia, X.; Zhang, H.; Li, J.; Li, Q.; Cong, R. Facile Synthesis of Nitrogen-doped Graphene Derived from Graphene Oxide and Vitamin B3 as High-performance Sensor for Imidacloprid Determination. Electrochim. Acta 2016, 212, 784–790. [Google Scholar] [CrossRef]
- Chen, M.; Meng, Y.; Zhang, W.; Zhou, J.; Xie, J.; Diao, G. beta-Cyclodextrin polymer functionalized reduced-graphene oxide: Application for electrochemical determination imidacloprid. Electrochim. Acta 2013, 108, 1–9. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, C.; Zou, X.; Zhang, H.; Xu, Y. A β-CD/MWCNT-modified-microelectrode array for rapid determination of imidacloprid in vegetables. Food Anal. Methods 2019, 12, 2326–2333. [Google Scholar] [CrossRef]
- BelBruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, P.; Costa-Rama, E.; Seguro, I.; Pacheco, J.G.; Nouws, H.P.A.; Cordeiro, M.N.D.S.; Delerue-Matos, C. Molecularly imprinted polymer-based electrochemical sensors for environmental analysis. Biosens. Bioelectron. 2021, 172, 112719. [Google Scholar] [CrossRef] [PubMed]
- Ramanavicius, S.; Samukaite-Bubniene, U.; Ratautaite, V.; Bechelany, M.; Ramanavicius, A. Electrochemical molecularly imprinted polymer based sensors for pharmaceutical and biomedical applications (review). J. Pharm. Biomed. Anal. 2022, 215, 114739. [Google Scholar] [CrossRef]
- Nawaz, N.; Abu Bakar, N.K.; Mahmud, H.N.M.E.; Jamaludin, N.S. Molecularly imprinted polymers-based DNA biosensors. Anal. Biochem. 2021, 630, 114328. [Google Scholar] [CrossRef]
- Garcia, Y.; Vera, M.; Giraldo, J.D.; Garrido-Miranda, K.; Jimenez, V.A.; Urbano, B.F.; Pereira, E.D. Microcystins Detection Methods: A Focus on Recent Advances Using Molecularly Imprinted Polymers. Anal. Chem. 2022, 94, 464–478. [Google Scholar] [CrossRef]
- Kong, L.; Jiang, X.; Zeng, Y.; Zhou, T.; Shi, G. Molecularly imprinted sensor based on electropolmerized poly(o-phenylenediamine) membranes at reduced graphene oxide modified electrode for imidacloprid determination. Sens. Actuators B Chem. 2013, 185, 424–431. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, H.T.; Xie, T.J.; Yang, X.; Dong, A.J.; Zhang, H.; Wang, J.; Wang, Z.Y. Molecularly imprinted polymer on graphene surface for selective and sensitive electrochemical sensing imidacloprid. Sens. Actuators B Chem. 2017, 252, 991–1002. [Google Scholar] [CrossRef]
- Tang, F.; Hua, Q.; Wang, X.; Luan, F.; Wang, L.; Li, Y.; Zhuang, X.; Tian, C. A novel electrochemiluminescence sensor based on a molecular imprinting technique and UCNPs@ZIF-8 nanocomposites for sensitive determination of imidacloprid. Analyst 2022, 147, 3917–3923. [Google Scholar] [CrossRef]
- Ma, X.; Pang, C.; Li, S.; Li, J.; Wang, M.; Xiong, Y.; Su, L.; Luo, J.; Xu, Z.; Lin, L. Biomimetic Synthesis of Ultrafine Mixed-Valence Metal-Organic Framework Nanowires and Their Application in Electrochemiluminescence Sensing. ACS Appl. Mater. Interfaces 2021, 13, 41987–41996. [Google Scholar] [CrossRef]
- Bor, G.; Man, E.; Ugurlu, O.; Ceylan, A.E.; Balaban, S.; Durmus, C.; Pinar Gumus, Z.; Evran, S.; Timur, S. In vitro Selection of Aptamer for Imidacloprid Recognition as Model Analyte and Construction of a Water Analysis Platform. Electroanalysis 2020, 32, 1922–1929. [Google Scholar] [CrossRef]
- Pérez-Fernández, B.; Mercader, J.V.; Abad-Fuentes, A.; Checa-Orrego, B.I.; Costa-García, A.; Escosura-Muñiz, A.d.l. Direct competitive immunosensor for Imidacloprid pesticide detection on gold nanoparticle-modified electrodes. Talanta 2020, 209, 120465. [Google Scholar] [CrossRef] [PubMed]
- Ghodsi, J.; Rafati, A.A. A novel molecularly imprinted sensor for imidacloprid pesticide based on poly(levodopa) electro-polymerized/TiO2 nanoparticles composite. Anal. Bioanal. Chem. 2018, 410, 7621–7633. [Google Scholar] [CrossRef] [PubMed]
- Perez-Fernandez, B.; Mercader, J.V.; Checa-Orrego, B.I.; de la Escosura-Muniz, A.; Costa-Garcia, A. A monoclonal antibody-based immunosensor for the electrochemical detection of imidacloprid pesticide. Analyst 2019, 144, 2936–2941. [Google Scholar] [CrossRef]
- Wei, J.; Liu, C.; Wu, T.; Zeng, W.; Hu, B.; Zhou, S.; Wu, L. A review of current status of ratiometric molecularly imprinted electrochemical sensors: From design to applications. Anal. Chim. Acta 2022, 1230, 340273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, D.; Li, Y. Ratiometric Electrochemical Sensors Associated with Self-Cleaning Electrodes for Simultaneous Detection of Adrenaline, Serotonin, and Tryptophan. ACS Appl. Mater. Interfaces 2019, 11, 13557–13563. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kan, X. A ratiometric strategy-based electrochemical sensing interface for the sensitive and reliable detection of imidacloprid. Analyst 2018, 143, 2150–2156. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, C.; Han, K.; Wei, X.; Xu, Y.; Zou, X.; Zhang, H.; Chen, Z. A signal on-off ratiometric electrochemical sensor coupled with a molecular imprinted polymer for selective and stable determination of imidacloprid. Biosens. Bioelectron. 2020, 154, 112091. [Google Scholar] [CrossRef]
- Tan, G.; Zhao, Y.; Wang, M.; Chen, X.; Wang, B.; Li, Q.X. Ultrasensitive quantitation of imidacloprid in vegetables by colloidal gold and time-resolved fluorescent nanobead traced lateral flow immunoassays. Food Chem. 2020, 311, 126055. [Google Scholar] [CrossRef]
- Li, H.; Jin, R.; Kong, D.; Zhao, X.; Liu, F.; Yan, X.; Lin, Y.; Lu, G. Switchable fluorescence immunoassay using gold nanoclusters anchored cobalt oxyhydroxide composite for sensitive detection of imidacloprid. Sens. Actuators B Chem. 2019, 283, 207–214. [Google Scholar] [CrossRef]
- Yu, L.; Song, Z.; Peng, J.; Yang, M.; Zhi, H.; He, H. Progress of gold nanomaterials for colorimetric sensing based on different strategies. TrAC Trends Anal. Chem. 2020, 127, 115880. [Google Scholar] [CrossRef]
- Sun, J.; Lu, Y.; He, L.; Pang, J.; Yang, F.; Liu, Y. Colorimetric sensor array based on gold nanoparticles: Design principles and recent advances. TrAC Trends Anal. Chem. 2020, 122, 115754. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Y.; Ma, K.; Wang, Z. Optical colorimetric sensor arrays for chemical and biological analysis. Sci. China Chem. 2018, 61, 643–655. [Google Scholar] [CrossRef]
- Liu, B.; Zhuang, J.; Wei, G. Recent advances in the design of colorimetric sensors for environmental monitoring. Environ. Sci. Nano 2020, 7, 2195–2213. [Google Scholar] [CrossRef]
- Yan, P.; Zheng, X.; Liu, S.; Dong, Y.; Fu, T.; Tian, Z.; Wu, Y. Colorimetric Sensor Array for Identification of Proteins and Classification of Metabolic Profiles under Various Osmolyte Conditions. ACS Sens. 2023, 8, 133–140. [Google Scholar] [CrossRef]
- Tan, S.; Zhao, H.; Tian, D.; Wang, F.; Liu, J.; Li, H. Piperidine–calix [4] arene modified gold nanoparticles: Imidacloprid colorimetric sensing. Sens. Actuators B Chem. 2014, 204, 522–527. [Google Scholar] [CrossRef]
- Zhao, T.; Liang, X.; Guo, X.; Yang, X.; Guo, J.; Zhou, X.; Huang, X.; Zhang, W.; Wang, Y.; Liu, Z.; et al. Smartphone-based colorimetric sensor array using gold nanoparticles for rapid distinguishment of multiple pesticides in real samples. Food Chem. 2023, 404, 134768. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-H.; Lo, S.-C.; Tung, Y.-J.; Kuo, C.-W.; Tai, Y.-H.; Hsieh, S.-Y.; Lee, K.-L.; Hsiao, S.-R.; Sheen, J.-F.; Hsu, J.-C.; et al. Multichannel nanoplasmonic platform for imidacloprid and fipronil residues rapid screen detection. Biosens. Bioelectron. 2020, 170, 112677. [Google Scholar] [CrossRef]
- Creedon, N.; Lovera, P.; Moreno, J.G.; Nolan, M.; O’Riordan, A. Highly Sensitive SERS Detection of Neonicotinoid Pesticides. Complete Raman Spectral Assignment of Clothianidin and Imidacloprid. J. Phys. Chem. A 2020, 124, 7238–7247. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, N.; Han, C.; Chen, Z.; Zhai, X.; Li, Z.; Zheng, K.; Zhu, J.; Wang, X.; Zou, X.; et al. Competitive immunosensor for sensitive and optical anti-interference detection of imidacloprid by surface-enhanced Raman scattering. Food Chem. 2021, 358, 129898. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, Z.; Cui, Z.; Li, H. Ionic liquid functionalized gold nanoparticles: Synthesis, rapid colorimetric detection of imidacloprid. Sens. Actuators B Chem. 2014, 191, 313–319. [Google Scholar] [CrossRef]
- Lee, K.-L.; You, M.-L.; Tsai, C.-H.; Lin, E.-H.; Hsieh, S.-Y.; Ho, M.-H.; Hsu, J.-C.; Wei, P.-K. Nanoplasmonic biochips for rapid label-free detection of imidacloprid pesticides with a smartphone. Biosens. Bioelectron. 2016, 75, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Vestri, A.; Rippa, M.; Marchesano, V.; Sagnelli, D.; Margheri, G.; Zhou, J.; Petti, L. LSPR immuno-sensing based on iso-Y nanopillars for highly sensitive and specific imidacloprid detection. J. Mater. Chem. B. 2021, 9, 9153–9161. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Zhang, W.; Wang, S.; Han, Y.; Feng, D.; Ma, Y.; Deng, B.; Chen, Z.; Mao, J.; Xu, F.; et al. A ratiometric fluorescent sensor based on molecularly imprinted multilevel mesoporous silica for highly sensitive detection of imidacloprid. Dyes Pigment. 2022, 208, 110775. [Google Scholar] [CrossRef]









| Electrode Materials | Technique | Linear Range | LOD | Reference |
|---|---|---|---|---|
| GO/GCE | SWV a | 10–200 μM | 8.3 μM | [62] |
| GO/GCE | CV b | 0.8–10 μM | 0.36 μM | [69] |
| NGE-N/GCE e | DPV c | 4–20.0 μM | 0.55 μM | [70] |
| LIG f | SWV | Not given | 384 nM | [63] |
| β-CDP/rGO/GCE g | DPV | 0.05–15.0 μM, 20–150.0 μM | 0.02 μM | [71] |
| E-rGO/α-CD/GCE | LSV d | 0.5–40.0 μM | 20 nM | [64] |
| β-CD/MWCNT-MEA h | DPV | 5.0–100.0 μM | 0.629 μM | [72] |
| RPC@PANI/GCE i | CV | 0.1–70 μg mL−1 | 0.03 μg mL−1 | [10] |
| MSF/ErGO/GCE j | CV | 1.0–50 μg mL−1 50–400 μg mL−1 | 0.3 μg mL−1 | [65] |
| Ce2S3/GACFs/GCE k | DPV | 0.391–274 μM | 32 nM | [22] |
| GQDs/IL/MWCNTs/GCE l | DPV | 0.03–12 μM | 9 nM | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Wang, Y.; Liu, X.; Ding, L. Recent Advances for Imidacloprid Detection Based on Functional Nanomaterials. Chemosensors 2023, 11, 300. https://doi.org/10.3390/chemosensors11050300
Chen S, Wang Y, Liu X, Ding L. Recent Advances for Imidacloprid Detection Based on Functional Nanomaterials. Chemosensors. 2023; 11(5):300. https://doi.org/10.3390/chemosensors11050300
Chicago/Turabian StyleChen, Shu, Yawen Wang, Xiuli Liu, and Longhua Ding. 2023. "Recent Advances for Imidacloprid Detection Based on Functional Nanomaterials" Chemosensors 11, no. 5: 300. https://doi.org/10.3390/chemosensors11050300
APA StyleChen, S., Wang, Y., Liu, X., & Ding, L. (2023). Recent Advances for Imidacloprid Detection Based on Functional Nanomaterials. Chemosensors, 11(5), 300. https://doi.org/10.3390/chemosensors11050300






