Electrochemical Mercury Biosensor Based on Electrocatalytic Properties of Prussian Blue and Inhibition of Catalase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Equipment
2.3. Fabrication of the Glass|FTO|PB|Cat Electrode
2.4. Electrochemical Measurements
3. Results and Discussion
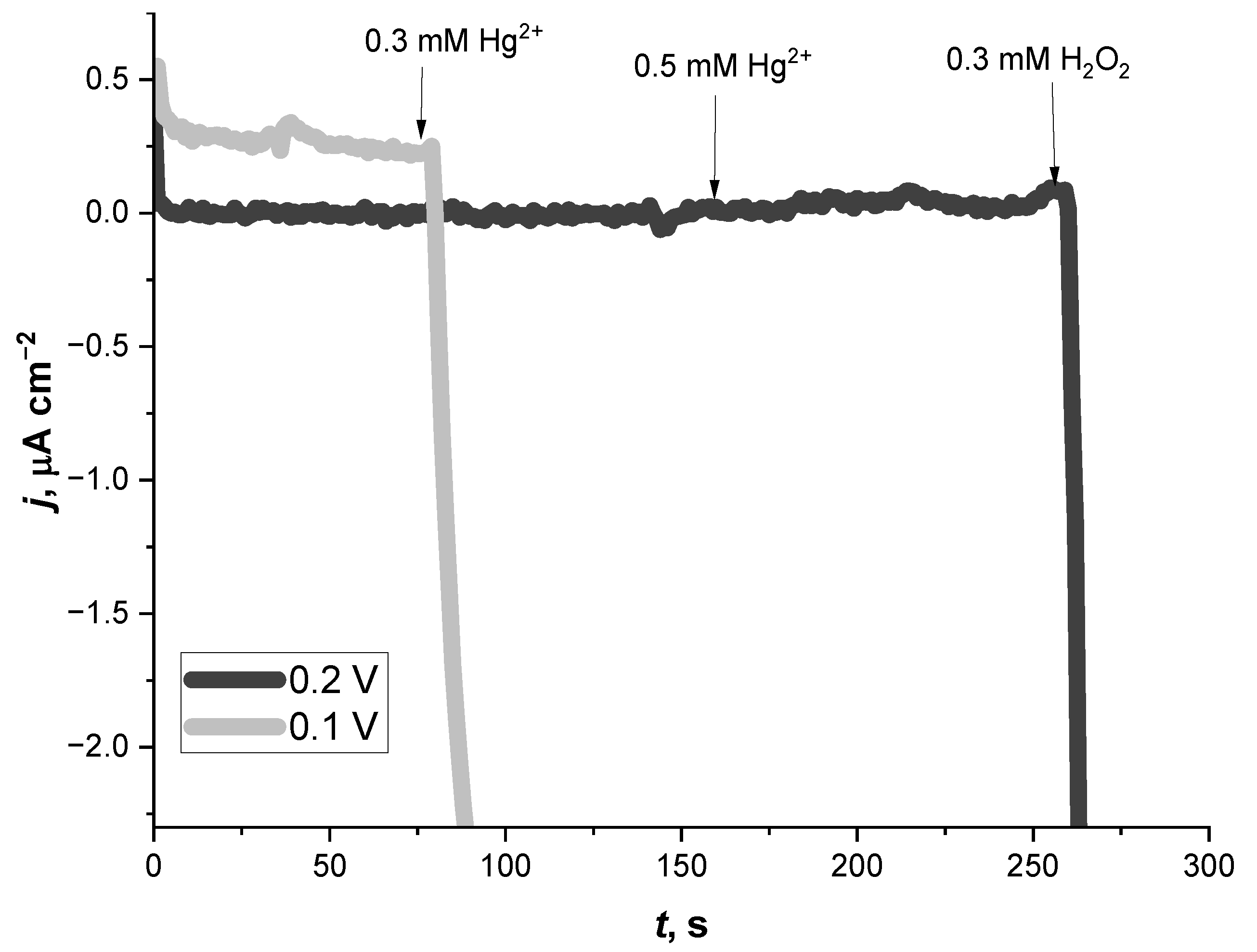
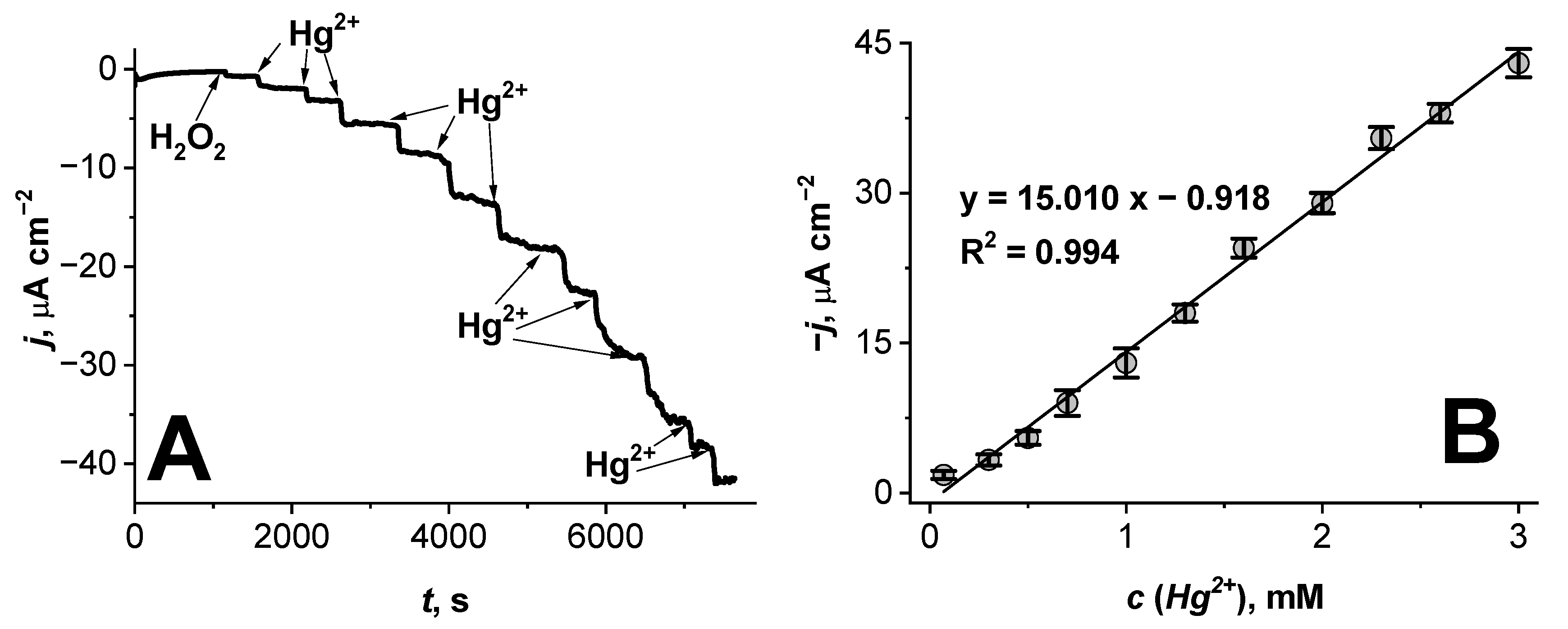
3.1. Amperometric Study of the Hg2+ Ion Biosensor Based on the Glass|FTO|PB|Cat Electrode
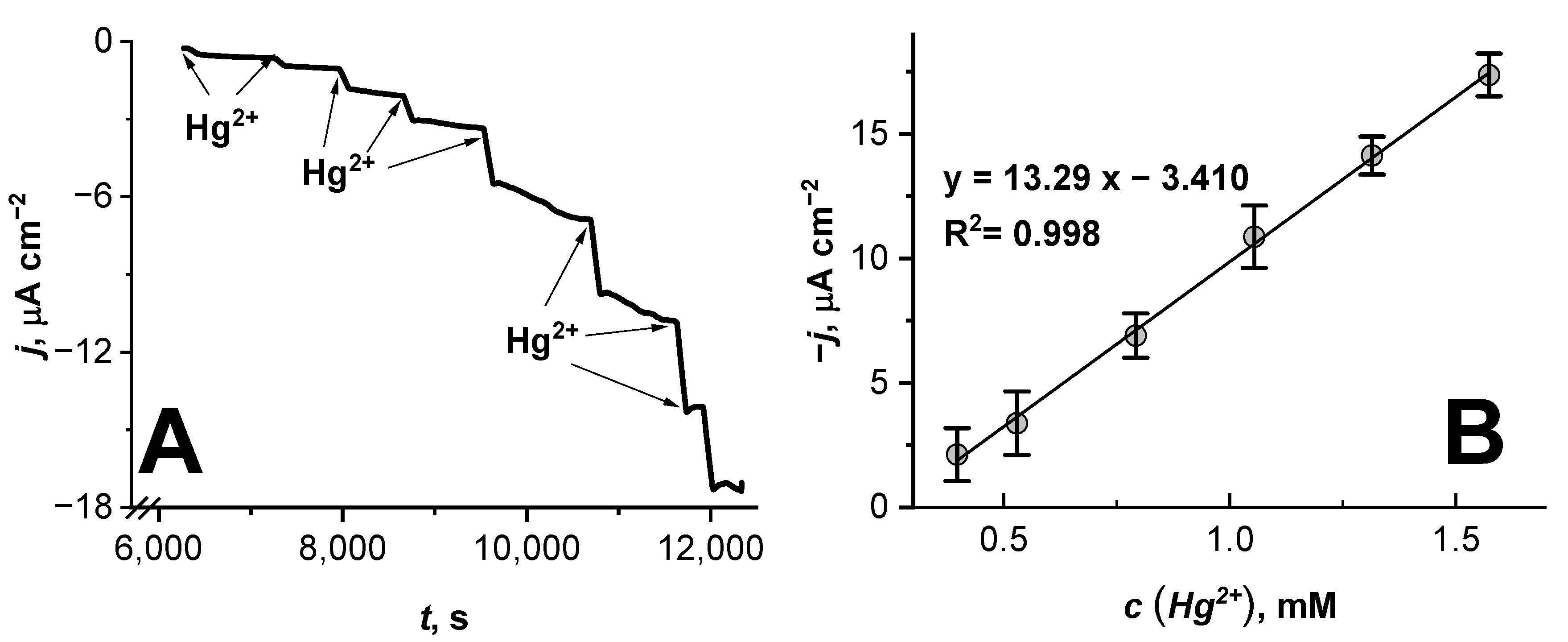
3.2. Stability of the Hg2+ Ion Biosensor Based on the Glass|FTO|PB|Cat Electrode
3.3. Investigation of the Hg2+ Biosensor in Tap Water
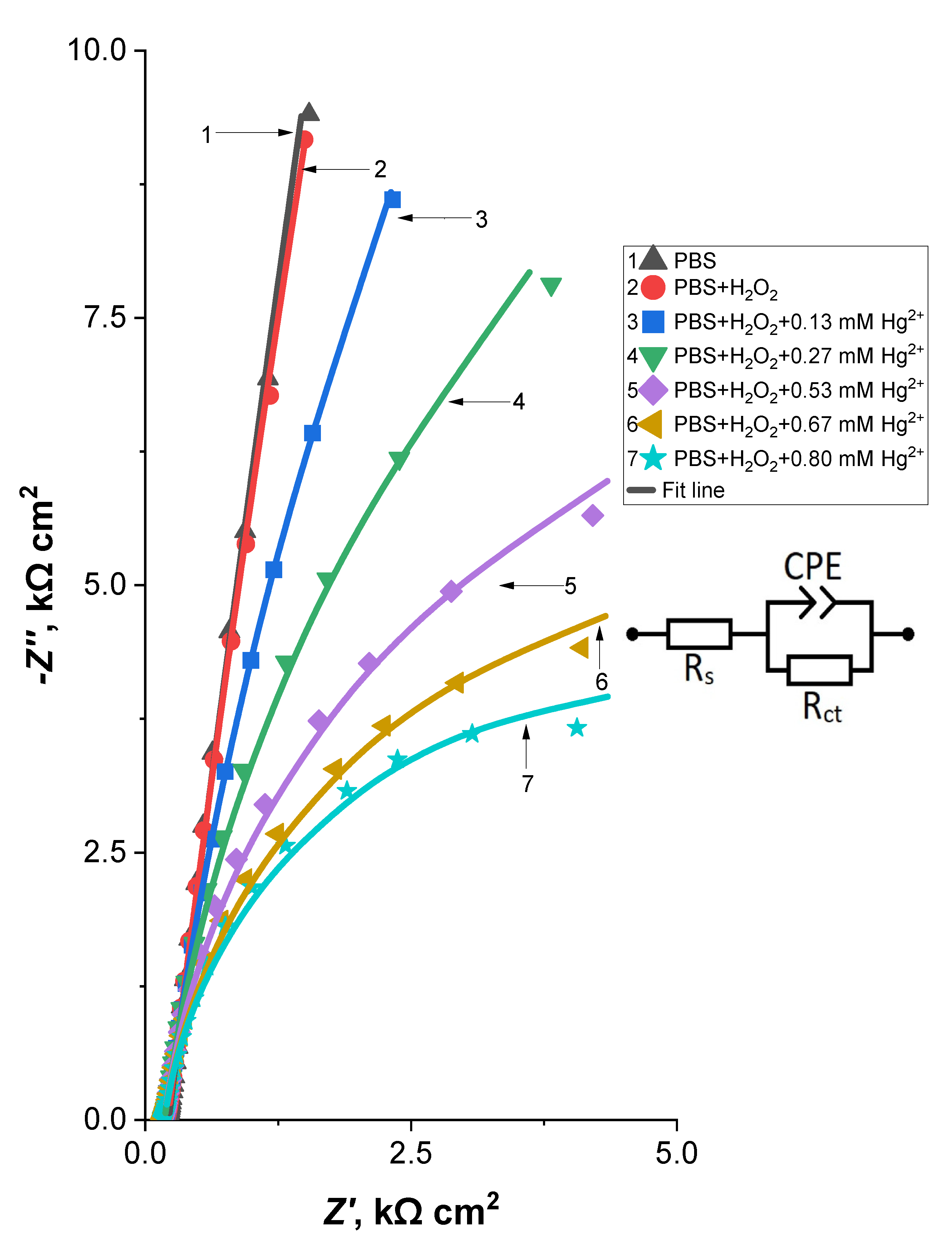
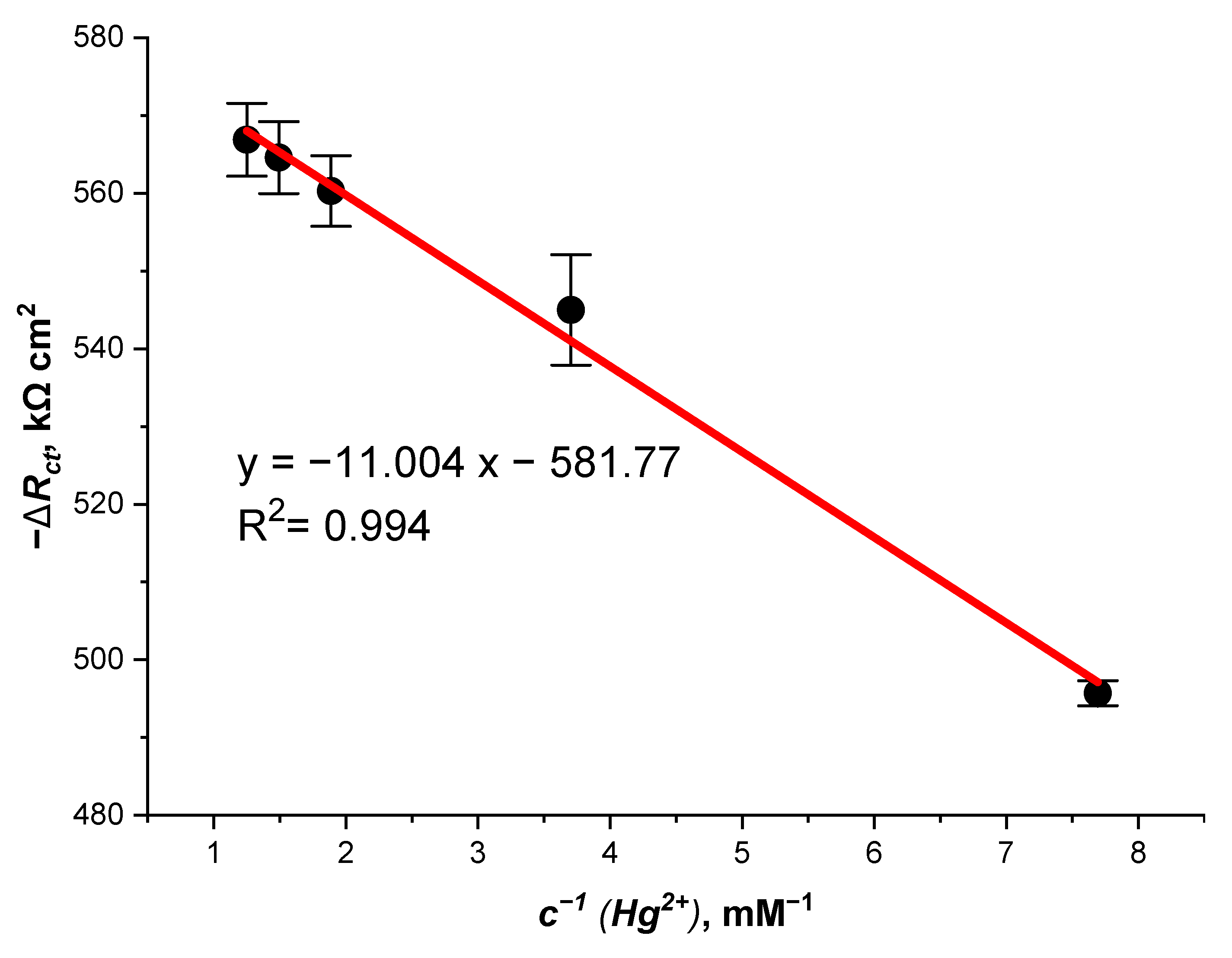
3.4. Investigation of the Hg2+ Biosensor Using Fast Fourier Transform Electrochemical Impedance Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bernhoft, R.A. Mercury Toxicity and Treatment: A Review of the Literature. J. Environ. Public Health 2012, 2012, 460508. [Google Scholar] [CrossRef]
- Berlin, M.; Jerksell, L.G.; von Ubisch, H. Uptake and Retention of Mercury in the Mouse Brain. Arch. Environ. Health Int. J. 1966, 12, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Berlin, M.; Fazackerley, J.; Nordberg, G.; Kand, M. The Uptake of Mercury in the Brains of Mammals Exposed to Mercury Vapor and to Mercuric Salts. Arch. Environ. Health Int. J. 1969, 18, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Kosta, L.; Byrne, A.R.; Zelenko, V. Correlation between selenium and mercury in man following exposure to inorganic mercury. Nature 1975, 254, 238–239. [Google Scholar] [CrossRef] [PubMed]
- Pendergrass, J.C.; Haley, B.E.; Vimy, M.J.; Winfield, S.A.; Lorscheider, F.L. Mercury vapor inhalation inhibits binding of GTP to tubulin in rat brain: Similarity to a molecular lesion in Alzheimer diseased brain. Neurotoxicology 1997, 18, 315–324. [Google Scholar]
- Clifton, J.C. Mercury Exposure and Public Health. Pediatr. Clin. N. Am. 2007, 54, 237.e1–237.e45. [Google Scholar] [CrossRef]
- Tinkov, A.A.; Ajsuvakova, O.; Skalnaya, M.; Popova, E.; Sinitskii, A.; Nemereshina, O.; Gatiatulina, E.; Nikonorov, A.; Skalny, A.V. Mercury and metabolic syndrome: A review of experimental and clinical observations. Biometals 2015, 28, 231–254. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.; Zhu, Y.; Zhang, Y. Molecular interaction of inorganic mercury(ii) with catalase: A spectroscopic study in combination with molecular docking. RSC Adv. 2015, 5, 79874–79881. [Google Scholar] [CrossRef]
- Maghsoudi, A.S.; Hassani, S.; Mirnia, K.; Abdollahi, M. Recent Advances in Nanotechnology-Based Biosensors Development for Detection of Arsenic, Lead, Mercury, and Cadmium. Int. J. Nanomed. 2021, 16, 803–832. [Google Scholar] [CrossRef]
- Gworek, B.; Dmuchowski, W.; Baczewska-Dąbrowska, A.H. Mercury in the terrestrial environment: A review. Environ. Sci. Eur. 2020, 32, 128. [Google Scholar] [CrossRef]
- Gray, J.E.; Theodorakos, P.M.; Fey, D.L.; Krabbenhoft, D.P. Mercury concentrations and distribution in soil, water, mine waste leachates, and air in and around mercury mines in the Big Bend region, Texas, USA. Environ. Geochem. Health 2015, 37, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Bernaus, A.; Gaona, X.; van Ree, D.; Valiente, M. Determination of mercury in polluted soils surrounding a chlor-alkali plant: Direct speciation by X-ray absorption spectroscopy techniques and preliminary geochemical characterisation of the area. Anal. Chim. Acta 2006, 565, 73–80. [Google Scholar] [CrossRef]
- Hylander, L.D.; Meili, M. 500 years of mercury production: Global annual inventory by region until 2000 and associated emissions. Sci. Total Environ. 2003, 304, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Higueras, P.; Oyarzun, R.; Biester, H.; Lillo, J.; Lorenzo, S. A first insight into mercury distribution and speciation in soils from the Almadén mining district, Spain. J. Geochem. Explor. 2003, 80, 95–104. [Google Scholar] [CrossRef]
- Ullrich, S.M.; Ilyushchenko, M.A.; Kamberov, I.M.; Tanton, T.W. Mercury contamination in the vicinity of a derelict chlor-alkali plant. Part I: Sediment and water contamination of Lake Balkyldak and the River Irtysh. Sci. Total Environ. 2007, 381, 1–16. [Google Scholar] [CrossRef]
- Geng, W.; Nakajima, T.; Takanashi, H.; Ohki, A. Determination of mercury in ash and soil samples by oxygen flask combustion method–Cold vapor atomic fluorescence spectrometry (CVAFS). J. Hazard. Mater. 2008, 154, 325–330. [Google Scholar] [CrossRef]
- Pourreza, N.; Ghanemi, K. Determination of mercury in water and fish samples by cold vapor atomic absorption spectrometry after solid phase extraction on agar modified with 2-mercaptobenzimidazole. J. Hazard. Mater. 2009, 161, 982–987. [Google Scholar] [CrossRef]
- Voegborlo, R.; Akagi, H. Determination of mercury in fish by cold vapour atomic absorption spectrometry using an automatic mercury analyzer. Food Chem. 2007, 100, 853–858. [Google Scholar] [CrossRef]
- Fernández, Z.H.; Rojas, L.A.V.; Álvarez, A.M.; Álvarez, J.R.E.; dos Santos, J.A.; González, I.P.; González, M.R.; Macias, N.A.; Sánchez, D.L.; Torres, D.H. Application of Cold Vapor-Atomic Absorption (CVAAS) Spectrophotometry and Inductively Coupled Plasma-Atomic Emission Spectrometry methods for cadmium, mercury and lead analyses of fish samples. Validation of the method of CVAAS. Food Control 2015, 48, 37–42. [Google Scholar] [CrossRef]
- Zheng, C.; Li, Y.; He, Y.; Ma, Q.; Hou, X. Photo-induced chemical vapor generation with formic acid for ultrasensitive atomic fluorescence spectrometric determination of mercury: Potential application to mercury speciation in water. J. Anal. At. Spectrom. 2005, 20, 746–750. [Google Scholar] [CrossRef]
- Allibone, J.; Fatemian, E.; Walker, P.J. Determination of mercury in potable water by ICP-MS using gold as a stabilising agent. J. Anal. At. Spectrom. 1999, 14, 235–239. [Google Scholar] [CrossRef]
- Baxter, D.C.; Frech, W. Determination of mercury by atomic absorption spectrometry using a platinum-lined graphite furnace for in situ preconcentration. Anal. Chim. Acta 1989, 225, 175–183. [Google Scholar] [CrossRef]
- Okamoto, Y.; Kumamaru, T.; Hara, S.; Matsuo, H.; Kiboku, M. Determination of Mercury by Graphite Furnace Atomic Absorption Spectrometry after Ion-Pair Extraction with Zephiramine in Small Scale. Bull. Chem. Soc. Jpn. 1987, 60, 3053–3055. [Google Scholar] [CrossRef]
- Zeiner, M.; Rezić, I.; Steffan, I. Analytical Methods for the Determination of Heavy Metals in the Textile Industry, Kemija u Industriji. Časopis Kemičara Kem. Inženjera Hrvat. 2007, 56, 587–595. [Google Scholar]
- Nuttall, K.L.; Gordon, W.H.; Ash, K.O. Inductively coupled plasma mass spectrometry for trace element analysis in the clinical laboratory. Ann. Clin. Lab. Sci. 1995, 25, 264–271. [Google Scholar]
- Malhotra, B.D.; Ali, A. Nanomaterials in Biosensors: Fundamentals and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–74. [Google Scholar] [CrossRef]
- Domínguez-Renedo, O.; Alonso-Lomillo, M.; Ferreira-Gonçalves, L.; Arcos-Martínez, M. Development of urease based amperometric biosensors for the inhibitive determination of Hg (II). Talanta 2009, 79, 1306–1310. [Google Scholar] [CrossRef]
- Ayenimo, J.G.; Adeloju, S.B. Rapid amperometric detection of trace metals by inhibition of an ultrathin polypyrrole-based glucose biosensor. Talanta 2016, 148, 502–510. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Ma, R.; Du, X.; Dong, W.; Chen, Y.; Chen, Q. An ultra-sensitive Au nanoparticles functionalized DNA biosensor for electrochemical sensing of mercury ions. Mater. Sci. Eng. C 2017, 75, 175–181. [Google Scholar] [CrossRef]
- Elsebai, B.; Ghica, M.E.; Abbas, M.N.; Brett, C.M. Catalase based hydrogen peroxide biosensor for mercury determination by inhibition measurements. J. Hazard. Mater. 2017, 340, 344–350. [Google Scholar] [CrossRef]
- Guascito, M.R.; Malitesta, C.; Mazzotta, E.; Turco, A. Inhibitive determination of metal ions by an amperometric glucose oxidase biosensor: Study of the effect of hydrogen peroxide decomposition. Sens. Actuators B Chem. 2008, 131, 394–402. [Google Scholar] [CrossRef]
- Kuralay, F.; Özyörük, H.; Yıldız, A. Inhibitive determination of Hg2+ ion by an amperometric urea biosensor using poly(vinylferrocenium) film. Enzym. Microb. Technol. 2007, 40, 1156–1159. [Google Scholar] [CrossRef]
- Mohammadi, H.; Amine, A.; Cosnier, S.; Mousty, C. Mercury–enzyme inhibition assays with an amperometric sucrose biosensor based on a trienzymatic-clay matrix. Anal. Chim. Acta 2005, 543, 143–149. [Google Scholar] [CrossRef]
- Tan, I.; Erhan, E.; Karagöz, P.; Özkan, M. Determination of mercury and nickel by amperometric biosensor prepared with thermostable lactate dehydrogenase. Trans. Nonferrous Met. Soc. China 2011, 21, 2332–2338. [Google Scholar] [CrossRef]
- Liu, J.-X.; Xu, X.-M.; Tang, L.; Zeng, G.-M. Determination of trace mercury in compost extract by inhibition based glucose oxidase biosensor. Trans. Nonferrous Met. Soc. China 2009, 19, 235–240. [Google Scholar] [CrossRef]
- Popkirov, G.S.; Schindler, R.N. A new impedance spectrometer for the investigation of electrochemical systems. Rev. Sci. Instrum. 1992, 63, 5366–5372. [Google Scholar] [CrossRef]
- Gabriunaite, I.; Valiūnienė, A.; Valincius, G. Formation and properties of phospholipid bilayers on fluorine doped tin oxide electrodes. Electrochim. Acta 2018, 283, 1351–1358. [Google Scholar] [CrossRef]
- Valiūnienė, A.; Kavaliauskaitė, G.; Virbickas, P.; Ramanavičius, A. Prussian blue based impedimetric urea biosensor. J. Electroanal. Chem. 2021, 895, 115473. [Google Scholar] [CrossRef]
- Valiūnienė, A.; Virbickas, P.; Medvikytė, G.; Ramanavičius, A. Urea Biosensor Based on Electrochromic Properties of Prussian Blue. Electroanalysis 2020, 32, 503–509. [Google Scholar] [CrossRef]
- Noël, J.-M.; Médard, J.; Combellas, C.; Kanoufi, F. Prussian Blue Degradation during Hydrogen Peroxide Reduction: A Scanning Electrochemical Microscopy Study on the Role of the Hydroxide Ion and Hydroxyl Radical. Chemelectrochem 2016, 3, 1178–1184. [Google Scholar] [CrossRef]
- Alfonso-Prieto, M.; Biarnés, X.; Vidossich, P.; Rovira, C. The Molecular Mechanism of the Catalase Reaction. J. Am. Chem. Soc. 2009, 131, 11751–11761. [Google Scholar] [CrossRef]
- Hermes, M.; Scholz, F. The electrochemical determination of ammonium based on the selective inhibition of the low-spin iron(II)/(III) system of Prussian blue. J. Solid State Electrochem. 1997, 1, 215–220. [Google Scholar] [CrossRef]
- Virbickas, P.; Valiūnienė, A.; Kavaliauskaitė, G.; Ramanavicius, A. Prussian White-Based Optical Glucose Biosensor. J. Electro-Chem. Soc. 2019, 166, B927–B932. [Google Scholar] [CrossRef]
- Chmayssem, A.; Hauchard, D. New Detection Method for Alkylphenol Traces in Water Based on an Integrated Electrochemical Cell Sensor. J. Water Sci. 2015, 28, 35–40. [Google Scholar] [CrossRef]
- Novgorodtseva, O.N.; Zelinsky, A. Effects of solution pH on sulfite oxidation at a gold electrode. J. Solid State Electrochem. 2019, 23, 2301–2306. [Google Scholar] [CrossRef]
- Ayenimo, J.G.; Adeloju, S.B. Inhibitive potentiometric detection of trace metals with ultrathin polypyrrole glucose oxidase biosensor. Talanta 2015, 137, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Guan, H.; Chi, D. An amperometric glucose oxidase biosensor based on liposome microreactor-chitosan nanocompo-site-modified electrode for determination of trace mercury. J. Solid State Electrochem. 2017, 21, 1175–1183. [Google Scholar] [CrossRef]
- Virbickas, P.; Ziziunaite, G.; Ramanavicius, A.; Valiūnienė, A. Prussian Blue Modified Amperometric Hg2+ Ion Biosensor Based on Glucose Oxidase Inhibition. Electroanalysis 2022, 35, e202200230. [Google Scholar] [CrossRef]
- Ashrafi, A.M.; Sýs, M.; Sedláčková, E.; Farag, A.S.; Adam, V.; Přibyl, J.; Richtera, L. Application of the Enzymatic Electrochemical Biosensors for Monitoring Non-Competitive Inhibition of Enzyme Activity by Heavy Metals. Sensors 2019, 19, 2939. [Google Scholar] [CrossRef]
- Website of the Department of Statistics and Data Science of Yale University. Chi-Square Goodness of Fit Test. Available online: http://www.stat.yale.edu/Courses/1997-98/101/chigf.htm (accessed on 27 April 2023).
- Virbickas, P.; Valiūnienė, A.; Baryševa, D.; Popkirov, G.; Ramanavičius, A. Determination of cyanide concentration by chronoamperometry, cyclic voltammetry and fast Fourier transform electrochemical impedance spectroscopy. J. Electroanal. Chem. 2021, 895, 115449. [Google Scholar] [CrossRef]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical Impedance Spectroscopy (EIS): Principles, Construction, and Biosensing Applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef]
- Li, S.; Zhou, Q.; Cong, H. Electrochemical Techniques, Impedance, and Spectroscopy. In Handbook of Advanced Non-Destructive Evaluation; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–27. [Google Scholar] [CrossRef]
- Singh, S.M.; Sivalingam, P.M. In vitro study on the interactive effects of heavy metals on catalase activity of Sarotherodon mossambicus (Peters). J. Fish Biol. 1982, 20, 683–688. [Google Scholar] [CrossRef]
- Yeboah, J.O.; Shi, G.; Shi, W. Effect of Heavy Metal Contamination on Soil Enzymes Activities. J. Geosci. Environ. Prot. 2021, 9, 135–154. [Google Scholar] [CrossRef]
- Karadag, H.; Ozhan, F. Effect of cyprodinil and fludioxonil pesticides on bovine liver catalase activity. Biotechnol. Biotechnol. Equip. 2015, 29, 40–44. [Google Scholar] [CrossRef] [PubMed]

| Biosensor | Linear Range, M | Sensitivity | Stability | Storing Conditions | Reference |
|---|---|---|---|---|---|
| Pt/PPy-GOx | 2.5 × 10−8–5 × 10−6 | 7.46 mV mM−1 | >90% initial sensitivity after 8 consecutive measurements | In PBS | [46] |
| (CS/GLM)8-GCE | 5 × 10−7–5 × 10−6 | - | 85% initial sensitivity after 72 h | At room temperature under dry conditions | [47] |
| Ultrathin Ppy|GOx | 4.8 × 10−7–3.3 × 10−6 | 4.0 µA cm−2 µM−1 | 40% initial sensitivity after 3 weeks | - | [28] |
| GCE|Cat | 5 × 10−11–5 × 10−10 | 0.44 inhibition% nM−1 | 40% initial sensitivity after 3 weeks (3 measurements) | - | [30] |
| Glass|FTO|PB|GOx | 2.7 × 10−5–2.5 × 10−4 | 0.164 inhibition% µM−1 | 72% initial sensitivity after one measurement | - | [48] |
| GCE/MWCNTs-RuO2/GOx/Nafion®® | 5 × 10−6–8 × 10−5 | 5 µA−1 mM−1 | - | - | [49] |
| Pt|PPD|Gox | 5 × 10−6–1.8 × 10−4 | 0.067 µA−1 µM−1 | 60% initial sensitivity after 24 h | In PBS | [31] |
| Glass|FTO|PB|Cat | 7 × 10−5–3 × 10−3 | 15 µA cm−2 mM−1 | 90% initial sensitivity after 2 weeks | In a refrigerator (t = 4 ± 1 °C) under dry conditions | This study |
| Days after Production of the Biosensor | Sensitivity, µA cm−2 mM−1 | R2 |
|---|---|---|
| Freshly prepared | 15.01 | 0.992 |
| 1 | 13.78 | 0.998 |
| 14 | 13.33 | 0.993 |
| 21 | 10.16 | 0.991 |
| 28 | 10.09 | 0.989 |
| 35 | 9.06 | 0.991 |
| 56 | 8.02 | 0.998 |
| 70 | 4.59 | 0.990 |
| Solution Composition | Rs, Ω cm2 | CPE, μF cm−2 | n (CPE) | Rct, kΩ cm2 |
|---|---|---|---|---|
| PBS (pH 7) | 219.2 | 9.45 | 0.92 | 960 |
| PBS + 0.3 µM H2O2 | 209.6 | 9.64 | 0.92 | 576 |
| PBS + 0.3 µM H2O2 + 0.13 mM HgCl2 | 195.8 | 9.92 | 0.92 | 80.3 |
| PBS + 0.3 µM H2O2 + 0.27 mM HgCl2 | 181.5 | 9.70 | 0.92 | 31 |
| PBS + 0.3 µM H2O2 + 0.53 mM HgCl2 | 178.2 | 10.03 | 0.92 | 15.7 |
| PBS + 0.3 µM H2O2 + 0.67 mM HgCl2 | 177.9 | 11.2 | 0.91 | 11.4 |
| PBS + 0.3 µM H2O2 + 0.80 mM HgCl2 | 176.3 | 11.1 | 0.91 | 9.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Virbickas, P.; Dėnas, N.; Valiūnienė, A. Electrochemical Mercury Biosensor Based on Electrocatalytic Properties of Prussian Blue and Inhibition of Catalase. Chemosensors 2023, 11, 311. https://doi.org/10.3390/chemosensors11050311
Virbickas P, Dėnas N, Valiūnienė A. Electrochemical Mercury Biosensor Based on Electrocatalytic Properties of Prussian Blue and Inhibition of Catalase. Chemosensors. 2023; 11(5):311. https://doi.org/10.3390/chemosensors11050311
Chicago/Turabian StyleVirbickas, Povilas, Narvydas Dėnas, and Aušra Valiūnienė. 2023. "Electrochemical Mercury Biosensor Based on Electrocatalytic Properties of Prussian Blue and Inhibition of Catalase" Chemosensors 11, no. 5: 311. https://doi.org/10.3390/chemosensors11050311
APA StyleVirbickas, P., Dėnas, N., & Valiūnienė, A. (2023). Electrochemical Mercury Biosensor Based on Electrocatalytic Properties of Prussian Blue and Inhibition of Catalase. Chemosensors, 11(5), 311. https://doi.org/10.3390/chemosensors11050311







