Recyclable Multifunctional Magnetic Fe3O4@SiO2@Au Core/Shell Nanoparticles for SERS Detection of Hg (II)
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Instrumentations
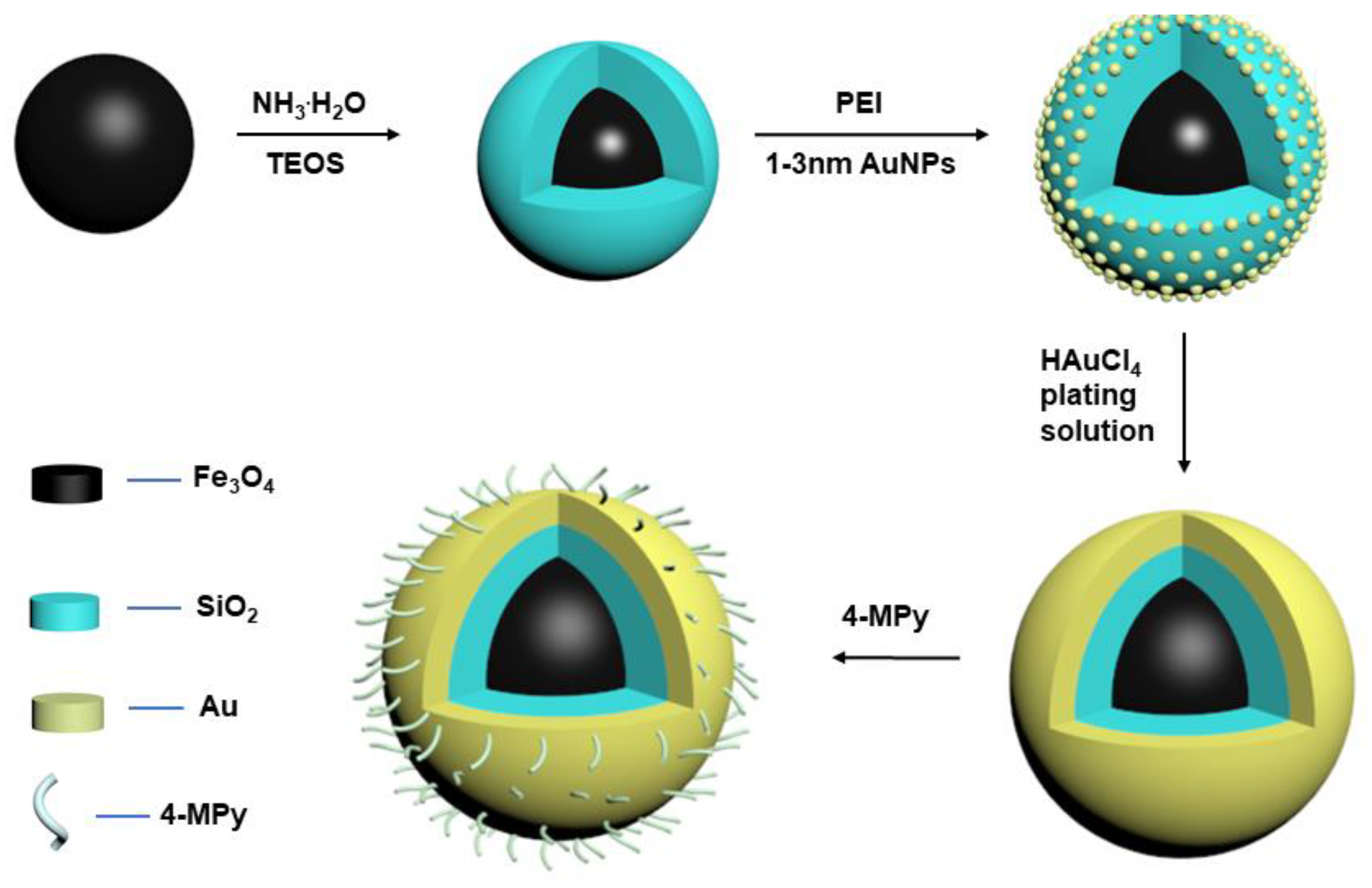
2.3. Synthesis of Fe3O4@SiO2@Au NPs
2.3.1. Synthesis of Monodisperse Fe3O4 MNPs
2.3.2. Synthesis of Fe3O4@SiO2 MNPs
2.3.3. Functionalization of Fe3O4@SiO2 MNPs Surfaces
2.3.4. Preparation of Gold Nanoparticles
2.3.5. Preparation of Gold-Attached Fe3O4@SiO2 MNPs
2.3.6. Preparation of Three-Layer Core/Shell Fe3O4@SiO2@Au Magnetic Nanocomposites
2.4. Enhancement Factor (EF) Calculation
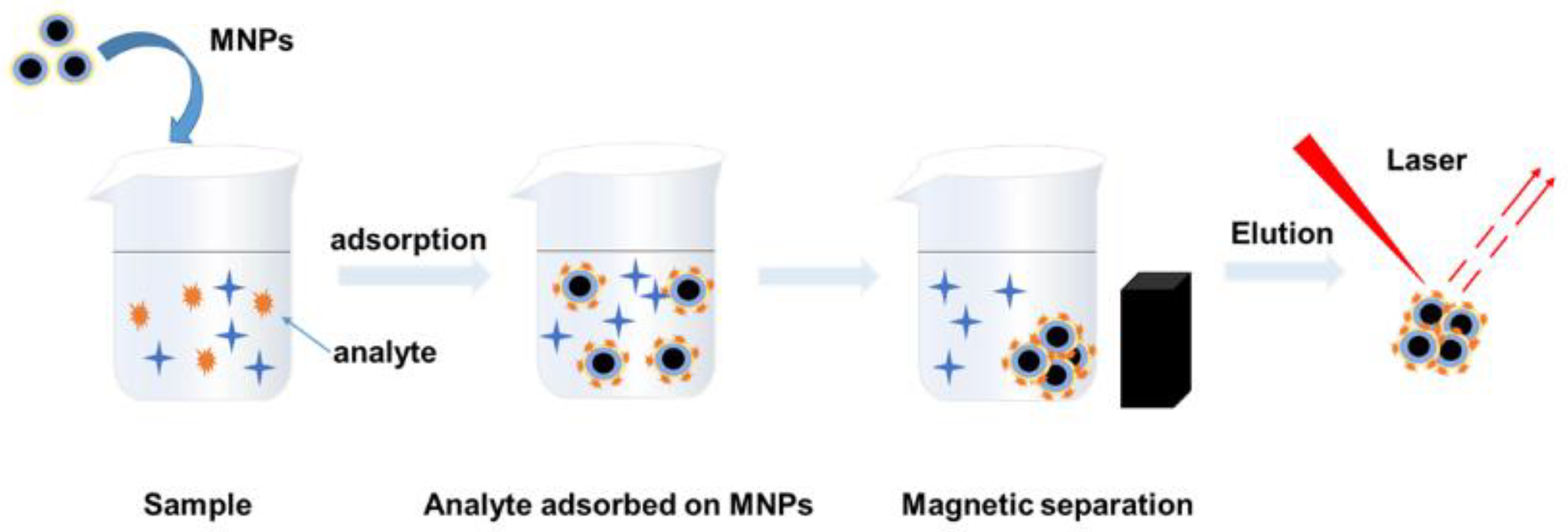
2.5. SERS Measurements of Hg (II) Ions
2.6. Detection of Mercury Ions in Cells
3. Results and Discussion
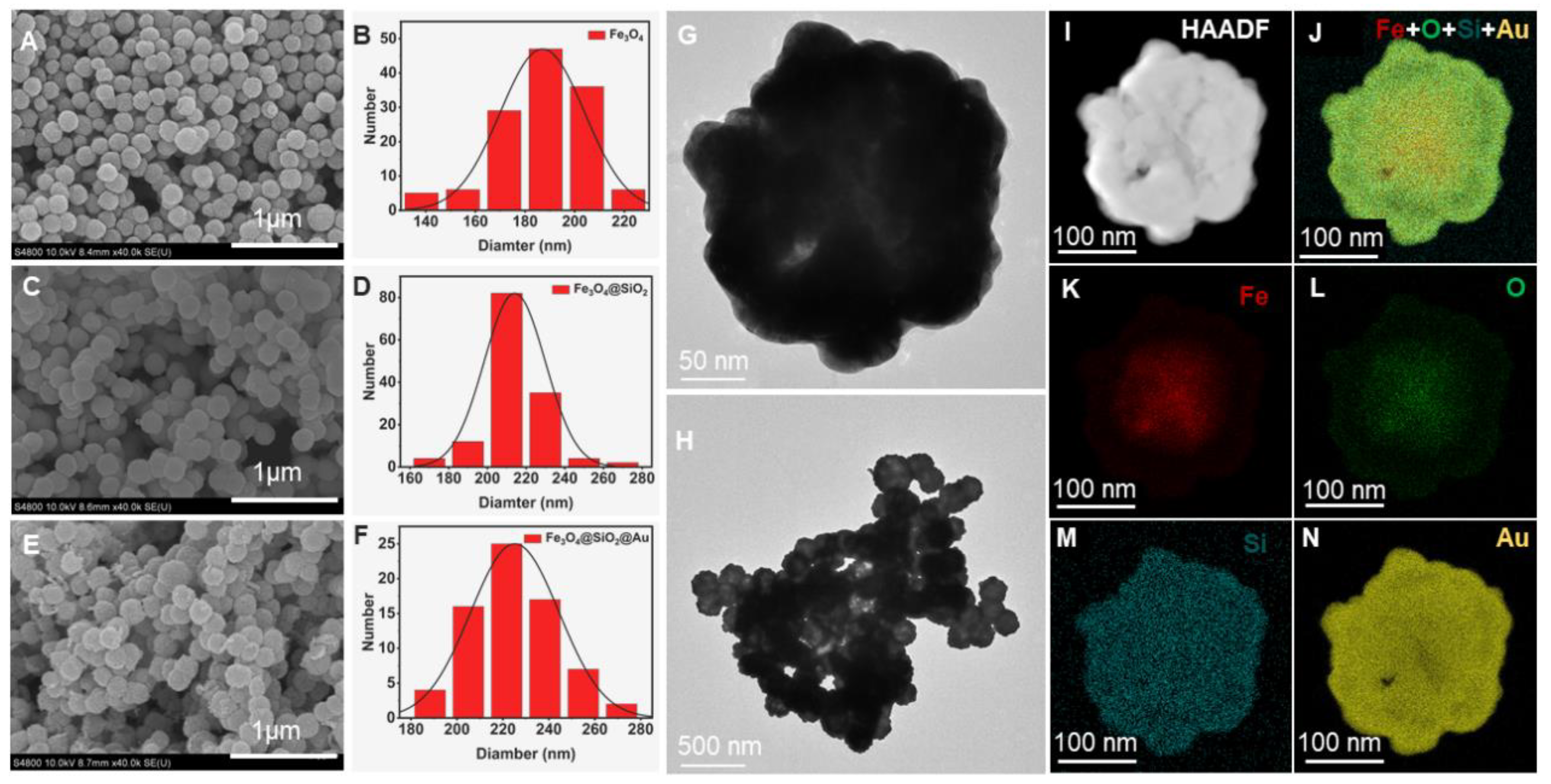
3.1. Fabrication and Characterization of Fe3O4@SiO2@Au
3.2. SERS Measurement of Hg (II) Ions
3.3. The Explanation of 416 cm−1 Raman Band by Hybrid Density Functional (h-DFT)
3.4. The Selectivity Analysis of as Prepared Sensors
3.5. The Application of Hg2+ Detection in Real Water Samples
3.6. Test of Detection of Hg2+ for Cell Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, Q.; Teng, X.; Li, Q.; Ma, Z.; Ying, Y.; Wu, Y.; Wen, Y.; Guo, X.; Yang, H. A Raman chip for rapid and specific detection of trace mercury ions in seawater. Sens. Actuators B Chem. 2021, 346, 130468. [Google Scholar] [CrossRef]
- Fu, S.; Guo, X.; Wang, H.; Yang, T.; Wen, Y.; Yang, H. Detection of trace mercury ions in water by a novel Raman probe. Sens. Actuators B Chem. 2014, 199, 108–114. [Google Scholar] [CrossRef]
- Sarfo, D.K.; Sivanesan, A.; Izake, E.L.; Ayoko, G.A. Rapid detection of mercury contamination in water by surface enhanced Raman spectroscopy. RSC Adv. 2017, 7, 21567–21575. [Google Scholar] [CrossRef]
- Wu, H.; Wu, Q.; Zhang, J.; Gu, Q.; Wei, L.; Guo, W.; He, M. Chromium ion removal from raw water by magnetic iron composites and Shewanella oneidensis MR-1. Sci. Rep. 2019, 9, 3687–3703. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, Z.M. Mercury toxicity, molecular response and tolerance in higher plants. Biometals 2012, 25, 847–857. [Google Scholar] [CrossRef]
- Li, P.; Du, B.; Chan, H.L.; Feng, X.; Li, B. Mercury bioaccumulation and its toxic effects in rats fed with methylmercury polluted rice. Sci. Total Environ. 2018, 633, 93–99. [Google Scholar] [CrossRef]
- Lubick, N. IMMUNITY: Mercury Alters Immune System Response in Artisanal Gold Miners. Environ. Health Perspect. 2010, 118, A243–A245. [Google Scholar] [CrossRef] [PubMed]
- Pollard, K.M.; Hultman, P. Effects of mercury on the immune system. Met. Ions Biol. Syst. 1997, 34, 421–440. [Google Scholar] [PubMed]
- Fowler, B.A. Mechanisms of kidney cell injury from metals. Environ. Health Perspect. 1993, 100, 57–63. [Google Scholar] [CrossRef]
- Zalups, R.K. Molecular interactions with mercury in the kidney. Pharmacol. Rev. 2000, 52, 113–143. [Google Scholar]
- Clarkson, T.W.; Magos, L.; Myers, G.J. The Toxicology of Mercury—Current Exposures and Clinical Manifestations. N. Engl. J. Med. 2003, 349, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Kabir, E.; Jahan, S.A. A review on the distribution of Hg in the environment and its human health impacts. J. Hazard. Mater. 2016, 306, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Peplow, D.; Augustine, S. Neurological abnormalities in a mercury exposed population among indigenous Wayana in Southeast Suriname. Environ. Sci. Process. Impacts 2014, 16, 2415–2422. [Google Scholar] [CrossRef] [PubMed]
- Gump, B.B.; Dykas, M.J.; MacKenzie, J.A.; Dumas, A.K.; Hruska, B.; Ewart, C.K.; Parsons, P.J.; Palmer, C.D.; Bendinskas, K. Background lead and mercury exposures: Psychological and behavioral problems in children. Environ. Res. 2017, 158, 576–582. [Google Scholar] [CrossRef]
- Li, Y.; Chen, C.; Li, B.; Sun, J.; Wang, J.; Gao, Y.; Zhao, Y.; Chai, Z. Elimination efficiency of different reagents for the memory effect of mercury using ICP-MS. J. Anal. At. Spectrom. 2006, 21, 94–96. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, Z.; Zheng, H.; Jin, L.; Hu, S. Significant signal enhancement of dielectric barrier discharge plasma induced vapor generation by using non-ionic surfactants for determination of mercury and cadmium by atomic fluorescence spectrometry. J. Anal. At. Spectrom. 2016, 31, 383–389. [Google Scholar] [CrossRef]
- Panichev, N.A.; Panicheva, S.E. Determination of total mercury in fish and sea products by direct thermal decomposition atomic absorption spectrometry. Food Chem. 2015, 166, 432–441. [Google Scholar] [CrossRef]
- Savoie, J.; St-Louis, R.; Clément, M. Facilitating local analysis in northern regions: Microwave plasma-atomic emission spectrometry for mercury determination in wild Atlantic salmon. Int. J. Environ. Anal. Chem. 2018, 98, 582–591. [Google Scholar] [CrossRef]
- Zhang, X.; Young, M.A.; Lyandres, O.; Van Duyne, R.P. Rapid Detection of an Anthrax Biomarker by Surface-Enhanced Raman Spectroscopy. J. Am. Chem. Soc. 2005, 127, 4484–4489. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Sun, M.; Xu, L.; Wang, L.; Kuang, H.; Xu, C. A SERS active gold nanostar dimer for mercury ion detection. Chem. Commun. 2013, 49, 4989–4991. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, R.M.; Brooker, A.; Goodacre, R. Surface-Enhanced Raman Spectroscopy for Bacterial Discrimination Utilizing a Scanning Electron Microscope with a Raman Spectroscopy Interface. Anal. Chem. 2004, 76, 5198–5202. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Koh, C.S.L.; Lee, H.K.; Chew, W.S.; Ling, X.Y. Microchemical Plant in a Liquid Droplet: Plasmonic Liquid Marble for Sequential Reactions and Attomole Detection of Toxin at Microliter Scale. ACS Appl. Mater. Interfaces 2017, 9, 39635–39640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; de Aberasturi, D.J.; Henriksen-Lacey, M.; Langer, J.; Liz-Marzán, L.M. Live-Cell Surface-Enhanced Raman Spectroscopy Imaging of Intracellular pH: From Two Dimensions to Three Dimensions. ACS Sens. 2020, 5, 3194–3206. [Google Scholar] [CrossRef] [PubMed]
- Hurst, S.J.; Fry, H.C.; Gosztola, D.J.; Rajh, T. Utilizing Chemical Raman Enhancement: A Route for Metal Oxide Support-Based Biodetection. J. Phys. Chem. C 2011, 115, 620–630. [Google Scholar] [CrossRef]
- Lee, S.J.; Moskovits, M. Visualizing Chromatographic Separation of Metal Ions on a Surface-Enhanced Raman Active Medium. Nano Lett. 2011, 11, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wen, Y.; Xu, L.; Xu, Q.; Song, S.; Zuo, X.; Yan, J.; Zhang, W.; Liu, G. Development of mercury (II) ion biosensors based on mercury-specific oligonucleotide probes. Biosens. Bioelectron. 2016, 75, 433–445. [Google Scholar] [CrossRef]
- Wang, G.; Lim, C.; Chen, L.; Chon, H.; Choo, J.; Hong, J.; Demello, A.J. Surface-enhanced Raman scattering in nanoliter droplets: Towards high-sensitivity detection of mercury (II) ions. Anal. Bioanal. Chem. 2009, 394, 1827–1832. [Google Scholar] [CrossRef]
- Kang, Y.; Wu, T.; Liu, B.; Wang, X.; Du, Y. Selective determination of mercury(II) by self-referenced surface-enhanced Raman scattering using dialkyne-modified silver nanoparticles. Microchim. Acta 2014, 181, 1333–1339. [Google Scholar] [CrossRef]
- Duan, J.; Yang, M.; Lai, Y.; Yuan, J.; Zhan, J. A colorimetric and surface-enhanced Raman scattering dual-signal sensor for Hg2+ based on Bismuthiol II-capped gold nanoparticles. Anal. Chim. Acta 2012, 723, 88–93. [Google Scholar] [CrossRef]
- Chung, E.; Gao, R.; Ko, J.; Choi, N.; Lim, D.W.; Lee, E.K.; Chang, S.-I.; Choo, J. Trace analysis of mercury(ii) ions using aptamer-modified Au/Ag core–shell nanoparticles and SERS spectroscopy in a microdroplet channel. Lab A Chip 2013, 13, 260–266. [Google Scholar] [CrossRef]
- Xu, L.; Yin, H.; Ma, W.; Kuang, H.; Wang, L.; Xu, C. Ultrasensitive SERS detection of mercury based on the assembled gold nanochains. Biosens. Bioelectron. 2015, 67, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, K.; Zou, B.; Gao, T.; Zhang, X.; Du, Z.; Zhou, S. Magnetic-based silver composite microspheres with nanosheet-assembled shell for effective SERS substrate. J. Mater. Chem. C 2013, 1, 2441–2447. [Google Scholar] [CrossRef]
- Wang, C.; Rong, Z.; Wang, J.; Jiang, N.; Pang, Y.; Xiao, R.; Wang, S. Seed-mediated synthesis of high-performance silver-coated magnetic nanoparticles and their use as effective SERS substrates. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 393–401. [Google Scholar] [CrossRef]
- Chi, Y.; Yuan, Q.; Li, Y.; Tu, J.; Zhao, L.; Li, N.; Li, X. Synthesis of Fe3O4@SiO2–Ag magnetic nanocomposite based on small-sized and highly dispersed silver nanoparticles for catalytic reduction of 4-nitrophenol. J. Colloid Interface Sci. 2012, 383, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Wang, M.; Luo, X.; Hu, Q.; Hou, R.; Chen, W.; Chen, D.; Wang, J.; Liu, J. SiO2 Stabilized Magnetic Nanoparticles as a Highly Effective Catalyst for the Degradation of Basic Fuchsin in Industrial Dye Wastewaters. Molecules 2018, 23, 2573. [Google Scholar] [CrossRef]
- Li, J.F.; Zhang, Y.J.; Ding, S.Y.; Panneerselvam, R.; Tian, Z.Q. Core-Shell Nanoparticle-Enhanced Raman Spectroscopy. Chem. Rev. 2017, 117, 5002–5069. [Google Scholar] [CrossRef]
- Yuan, H.; Ji, W.; Chu, S.; Liu, Q.; Qian, S.; Guang, J.; Wang, J.; Han, X.; Masson, J.-F.; Peng, W. Mercaptopyridine-Functionalized Gold Nanoparticles for Fiber-Optic Surface Plasmon Resonance Hg2+ Sensing. ACS Sens. 2019, 4, 704–710. [Google Scholar] [CrossRef]
- Gao, J.; Ran, X.; Shi, C.; Cheng, H.; Cheng, T.; Su, Y. One-step solvothermal synthesis of highly water-soluble, negatively charged superparamagnetic Fe3O4 colloidal nanocrystal clusters. Nanoscale 2013, 5, 7026–7033. [Google Scholar] [CrossRef]
- Liu, X.; Yang, X.; Li, K.; Liu, H.; Xiao, R.; Wang, W.; Wang, C.; Wang, S. Fe3O4@ Au SERS tags-based lateral flow assay for simultaneous detection of serum amyloid A and C-reactive protein in unprocessed blood sample. Sens. Actuators B Chem. 2020, 320, 128350. [Google Scholar] [CrossRef]
- Wang, C.; Xu, J.; Wang, J.; Rong, Z.; Li, P.; Xiao, R.; Wang, S. Polyethylenimine-interlayered silver-shell magnetic-core microspheres as multifunctional SERS substrates. J. Mater. Chem. C 2015, 3, 8684–8693. [Google Scholar] [CrossRef]
- Duff, D.G.; Baiker, A.; Edwards, P.P. A new hydrosol of gold clusters. 1. Formation and particle size variation. Langmuir 1993, 9, 2301–2309. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Y.; Wang, C.; Jia, X.; Li, J.; Xiao, R.; Wang, S. Facile synthesis of high-performance SiO2@Au core–shell nanoparticles with high SERS activity. RSC Adv. 2018, 8, 30825–30831. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yao, G.; Sun, K.; Huang, Q. beta-Cyclodextrin coated SiO(2)@Au@Ag core-shell nanoparticles for SERS detection of PCBs. Phys. Chem. Chem. Phys. 2015, 17, 21149–21157. [Google Scholar] [CrossRef]
- Lu, Y.; Zhong, J.; Yao, G.; Huang, Q. A label-free SERS approach to quantitative and selective detection of mercury (II) based on DNA aptamer-modified SiO2@Au core/shell nanoparticles. Sens. Actuators B Chem. 2018, 258, 365–372. [Google Scholar] [CrossRef]
- Hunyadi, S.E.; Murphy, C.J. Bimetallic silver–gold nanowires: Fabrication and use in surface-enhanced Raman scattering. J. Mater. Chem. 2006, 16, 3929–3935. [Google Scholar] [CrossRef]
- Wang, C.; Shang, M.; Wei, H.; Zhang, M.; Zou, W.; Meng, X.; Chen, W.; Shao, H.; Lai, Y. Specific and sensitive on-site detection of Cr(VI) by surface-enhanced Raman spectroscopy. Sens. Actuators B Chem. 2021, 346, 130594. [Google Scholar] [CrossRef]
- Wang, J.; Wu, J.; Zhang, Y.; Zhou, X.; Hu, Z.; Liao, X.; Sheng, B.; Yuan, K.; Wu, X.; Cai, H.; et al. Colorimetric and SERS dual-mode sensing of mercury (II) based on controllable etching of Au@Ag core/shell nanoparticles. Sens. Actuators B Chem. 2021, 330, 129364. [Google Scholar] [CrossRef]
- Liu, C.; Xu, T.; Cheng, G.; Zhang, X. Target-triggered regioselective assembly of nanoprobes for Raman imaging of dual cancer biomarkers in living cells. Sens. Actuators B Chem. 2021, 330, 129319. [Google Scholar] [CrossRef]
- Iturrioz-Rodríguez, N.; Martín-Rodríguez, R.; Renero-Lecuna, C.; Aguado, F.; González-Legarreta, L.; González, J.; Fanarraga, M.L.; Perdigón, A.C. Free-labeled nanoclay intracellular uptake tracking by confocal Raman imaging. Appl. Surf. Sci. 2021, 537, 147870. [Google Scholar] [CrossRef]
- Gjergjizi, B.; Çoğun, F.; Yıldırım, E.; Eryılmaz, M.; Selbes, Y.; Sağlam, N.; Tamer, U. SERS-based ultrafast and sensitive detection of luteinizing hormone in human serum using a passive microchip. Sens. Actuators B Chem. 2018, 269, 314–321. [Google Scholar] [CrossRef]
- Dong, Y.; Wen, B.; Chen, Y.; Cao, P.; Zhang, C. Autoclave-free facile approach to the synthesis of highly tunable nanocrystal clusters for magnetic responsive photonic crystals. RSC Adv. 2016, 6, 64434–64440. [Google Scholar] [CrossRef]
- Li, K.; Liang, A.; Jiang, C.; Li, F.; Liu, Q.; Jiang, Z. A stable and reproducible nanosilver-aggregation-4-mercaptopyridine surface-enhanced Raman scattering probe for rapid determination of trace Hg2+. Talanta 2012, 99, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Do, W.H.; Lee, C.J.; Kim, D.Y.; Jung, M.J. Adsorption of 2-mercaptopyridine and 4-mercaptopyridine on a silver surfaces investigated by SERS spectroscopy. J. Ind. Eng. Chem. 2012, 18, 2141–2146. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, B.; Xu, W.; Li, B.; Fan, Y. Surface-enhanced Raman spectroscopy study on the structure changes of 4-mercaptopyridine adsorbed on silver substrates and silver colloids. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2002, 58, 2827–2834. [Google Scholar] [CrossRef]
- Guo, H.; Ding, L.; Zhang, T.; Mo, Y. 4-Mercaptopyridine adsorbed on pure palladium island films: A combined SERS and DFT investigation. J. Mol. Struct. 2013, 1035, 231–235. [Google Scholar] [CrossRef]
- Guo, X.; Chen, F.; Wang, F.; Wu, Y.; Ying, Y.; Wen, Y.; Yang, H.; Ke, Q. Recyclable Raman chip for detection of trace Mercury ions. Chem. Eng. J. 2020, 390, 124528–124536. [Google Scholar] [CrossRef]
- Huang, Z.; Meng, G.; Huang, Q.; Chen, B.; Zhu, C.; Zhang, Z. Large-area Ag nanorod array substrates for SERS: AAO template-assisted fabrication, functionalization, and application in detection PCBs. J. Raman Spectrosc. 2013, 44, 240–246. [Google Scholar] [CrossRef]
- Ordal, M.A.; Long, L.L.; Bell, R.J.; Bell, S.E.; Bell, R.R.; Alexander Jr., R. W.; Ward, C.A. Optical properties of the metals Al, Co, Cu, Au, Fe, Pb, Ni, Pd, Pt, Ag, Ti, and W in the infrared and far infrared. Appl. Opt. 1983, 22, 1099–1119. [Google Scholar] [CrossRef]
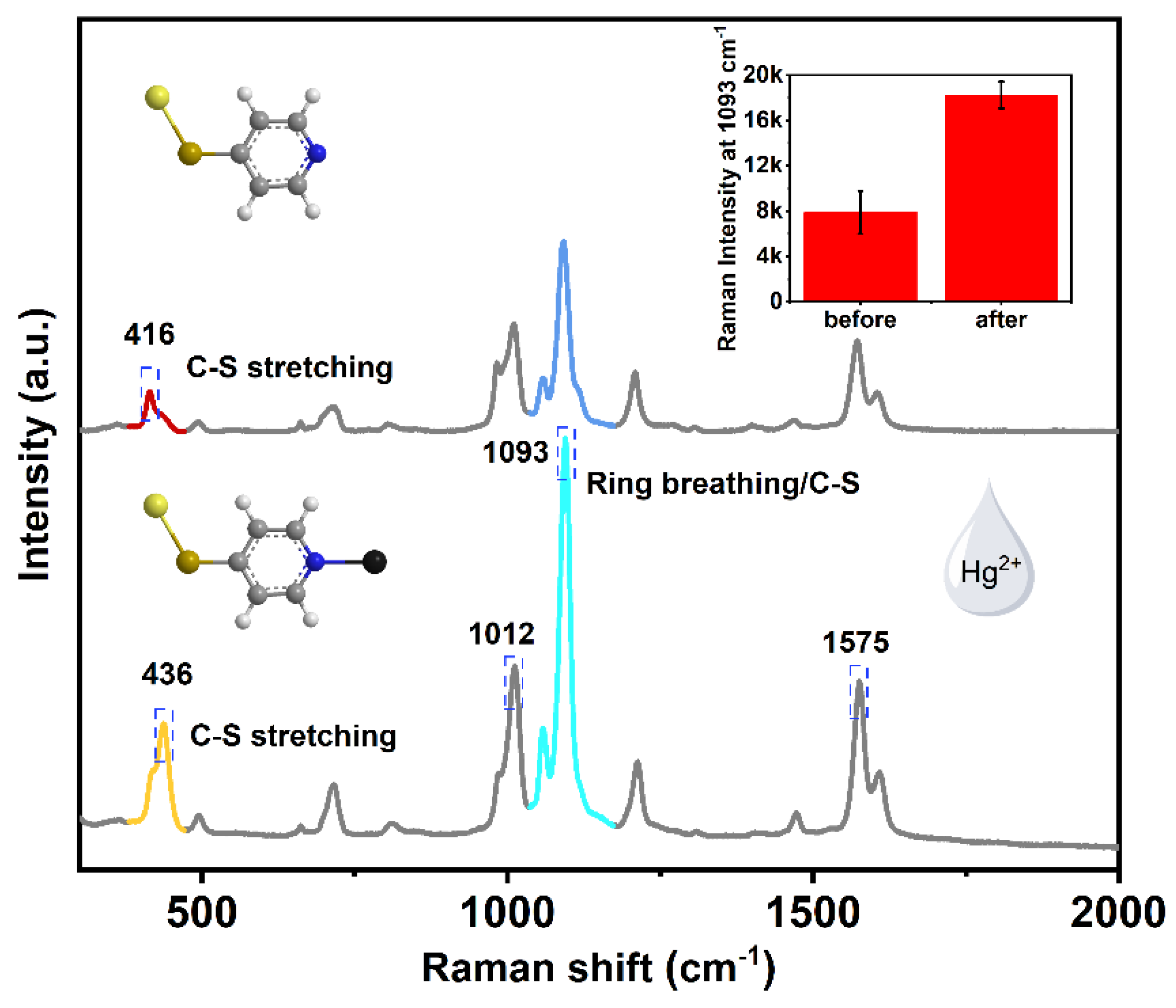
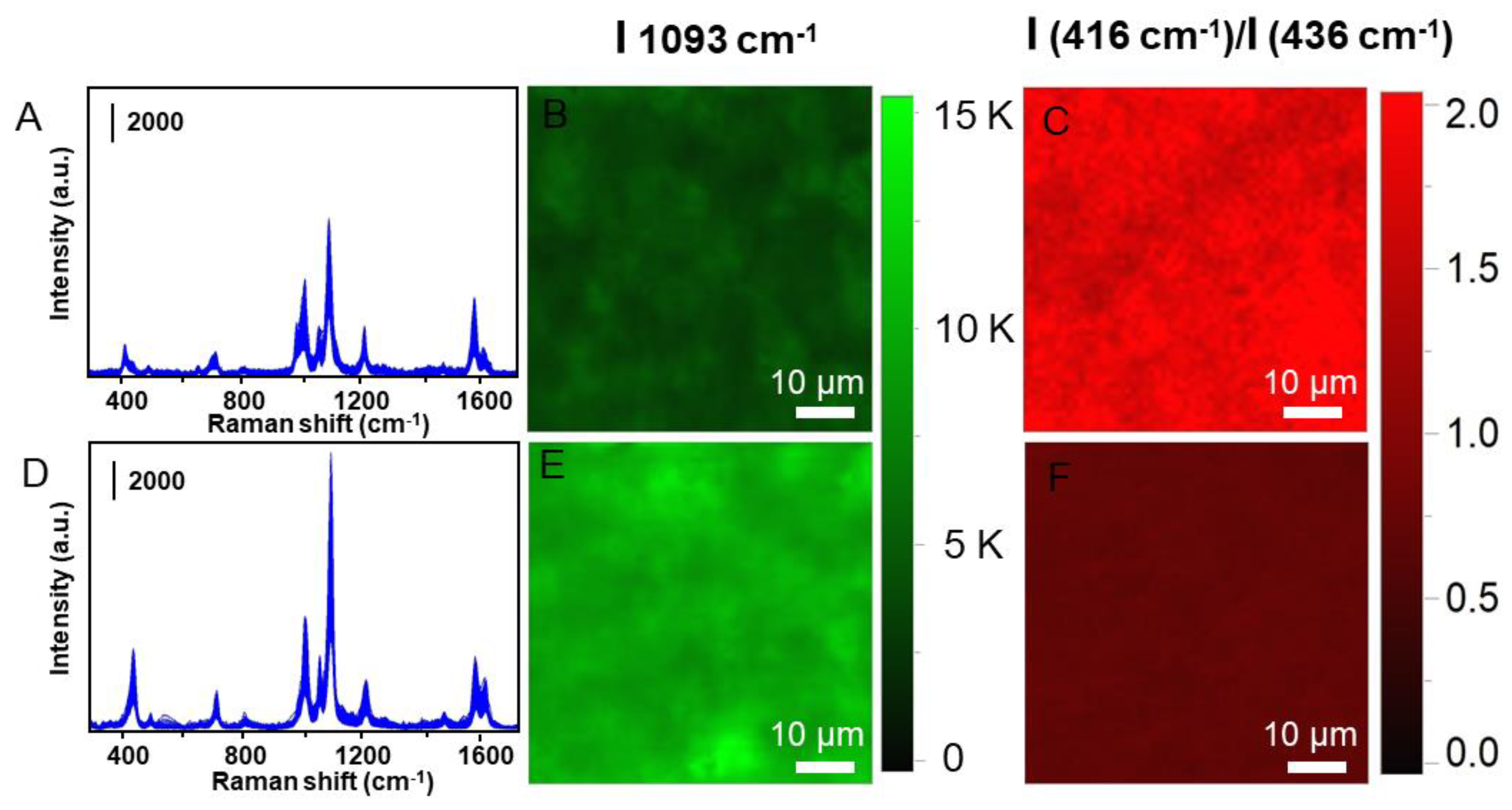


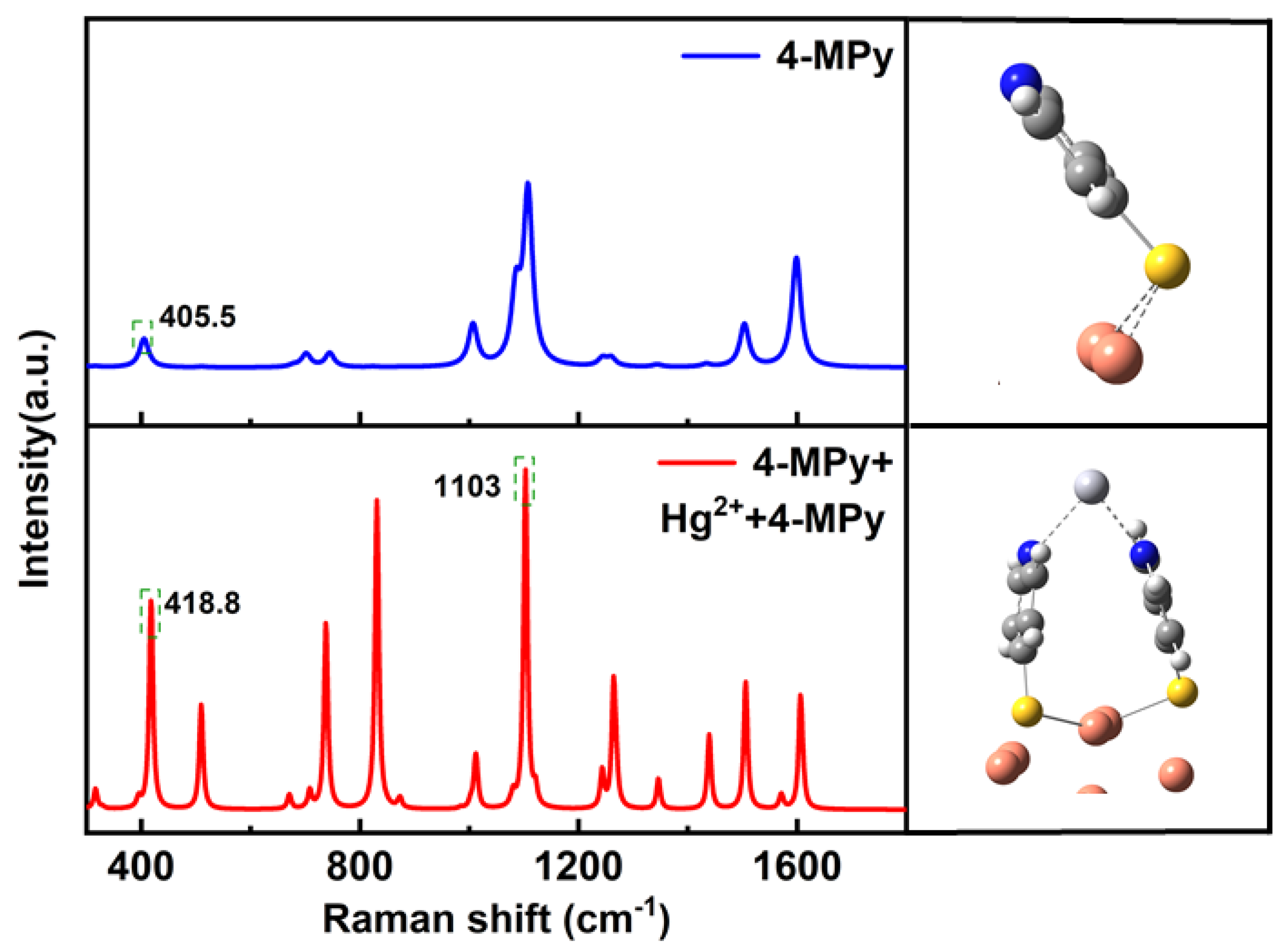
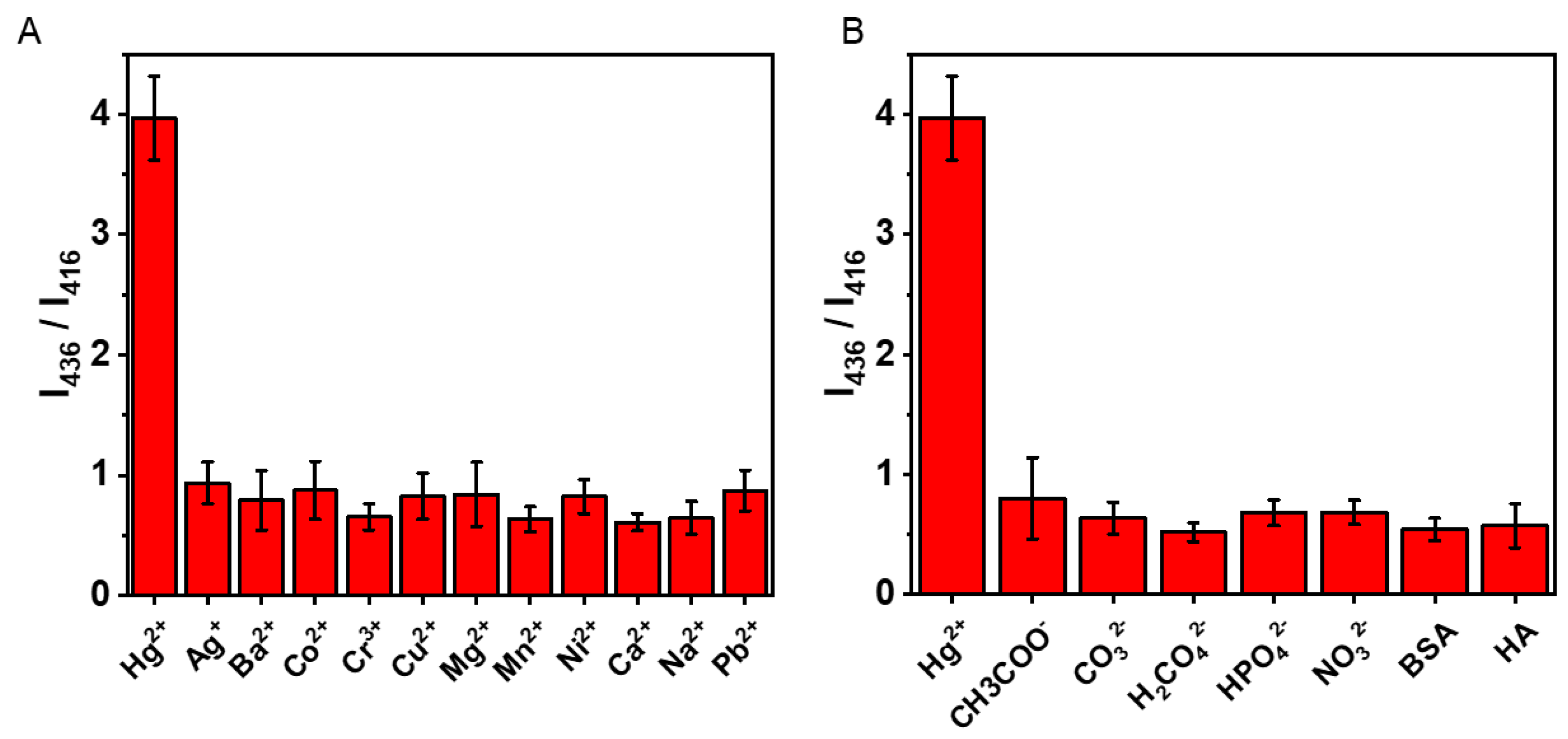
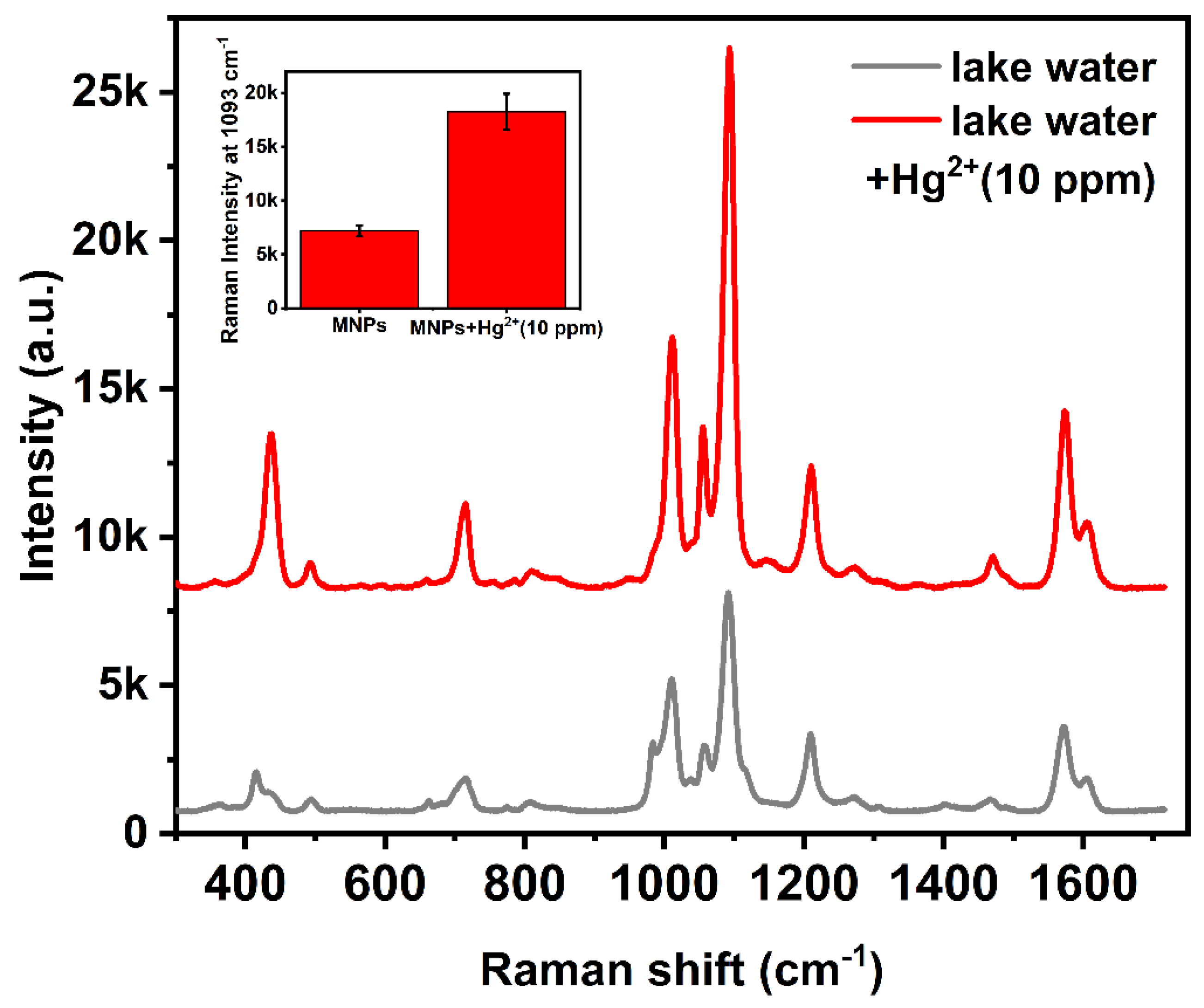

| Wavenumber/cm−1 | Assignment | Reference |
|---|---|---|
| 416/436 | C-S stretching | [52] |
| 715 | β(CC)/(C-S) | [53] |
| 1012 | Ring breathing | [54] |
| 1075 | β(CH) | [54] |
| 1093 | Ring breathing/C-S | [52] |
| 1212 | β(CH) | [55] |
| 1471 | ν(C=C)/ν(C=N) | [53] |
| 1575 | ν(C=C) with deprotonated nitrogen | [52] |
| 1610 | ν(C=C) with protonated nitrogen | [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Wang, H.; Xu, S.; Li, H.; Lu, Y.; Zhu, C. Recyclable Multifunctional Magnetic Fe3O4@SiO2@Au Core/Shell Nanoparticles for SERS Detection of Hg (II). Chemosensors 2023, 11, 347. https://doi.org/10.3390/chemosensors11060347
Liu C, Wang H, Xu S, Li H, Lu Y, Zhu C. Recyclable Multifunctional Magnetic Fe3O4@SiO2@Au Core/Shell Nanoparticles for SERS Detection of Hg (II). Chemosensors. 2023; 11(6):347. https://doi.org/10.3390/chemosensors11060347
Chicago/Turabian StyleLiu, Chao, Hui Wang, Shengmin Xu, Hongbao Li, Yilin Lu, and Chuhong Zhu. 2023. "Recyclable Multifunctional Magnetic Fe3O4@SiO2@Au Core/Shell Nanoparticles for SERS Detection of Hg (II)" Chemosensors 11, no. 6: 347. https://doi.org/10.3390/chemosensors11060347
APA StyleLiu, C., Wang, H., Xu, S., Li, H., Lu, Y., & Zhu, C. (2023). Recyclable Multifunctional Magnetic Fe3O4@SiO2@Au Core/Shell Nanoparticles for SERS Detection of Hg (II). Chemosensors, 11(6), 347. https://doi.org/10.3390/chemosensors11060347







