Fast Monitoring of Quality and Adulteration of Blended Sunflower/Olive Oils Applying Near-Infrared Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Colorimetry
2.2. Conductivity and Total Solutes
2.3. Analysis of Fatty Acid Composition
2.4. NIR Spectroscopy
2.4.1. Laboratory NIR Device
2.4.2. Portable NIR Device
2.5. Spectral Data Processing
2.5.1. Repeatability and Reproducibility
2.5.2. Chemometric Modeling
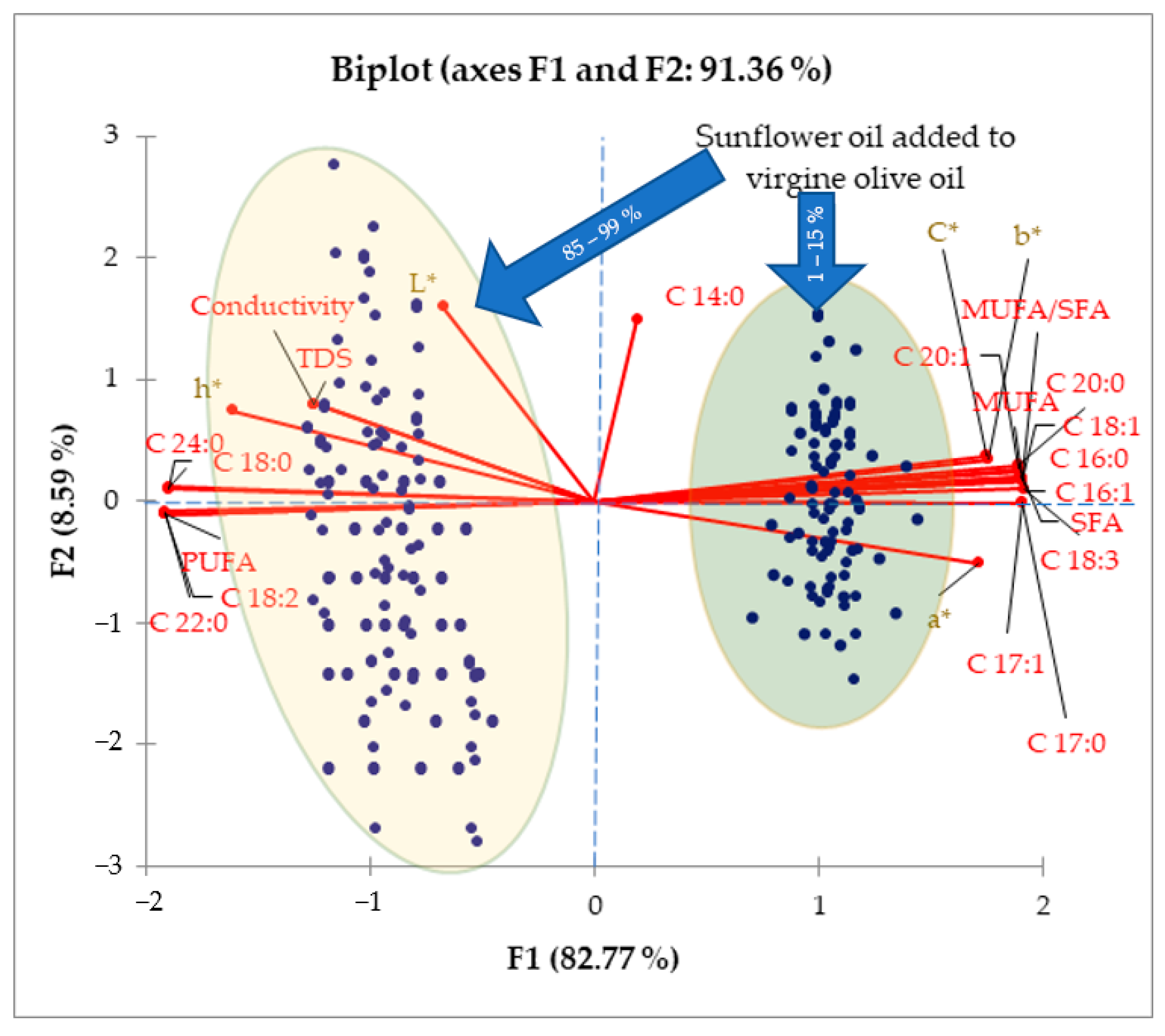
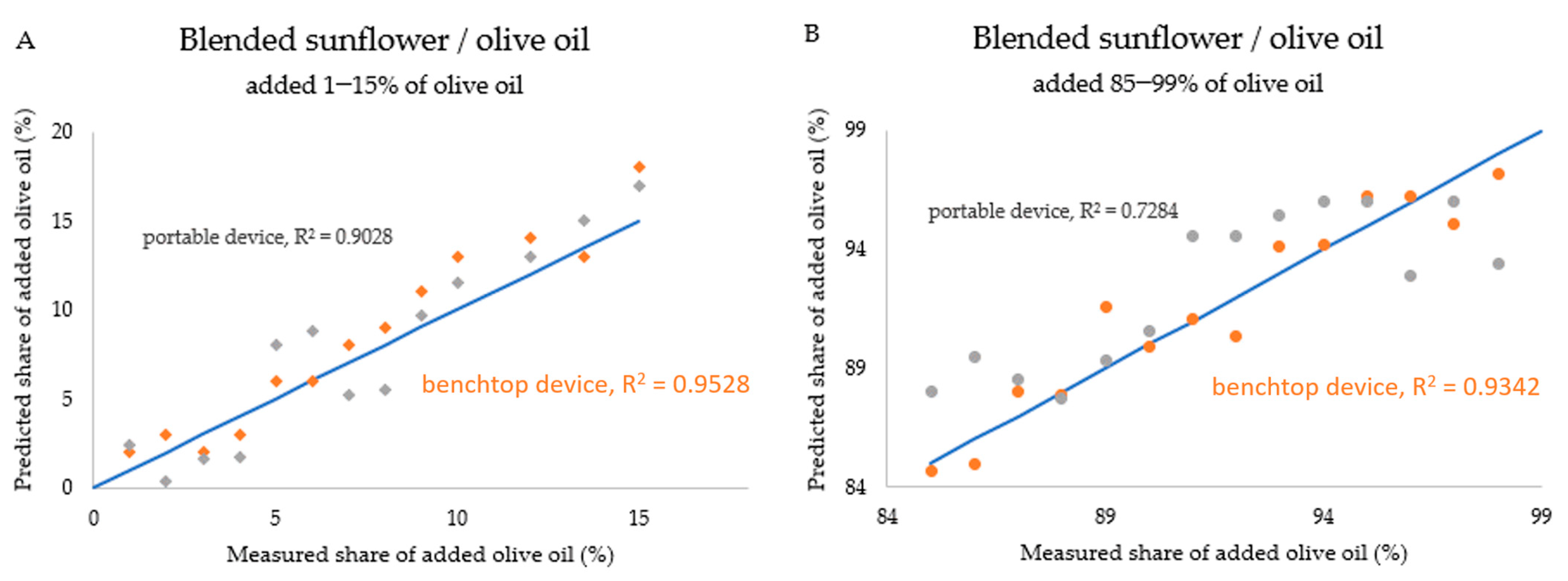
3. Results and Discussion
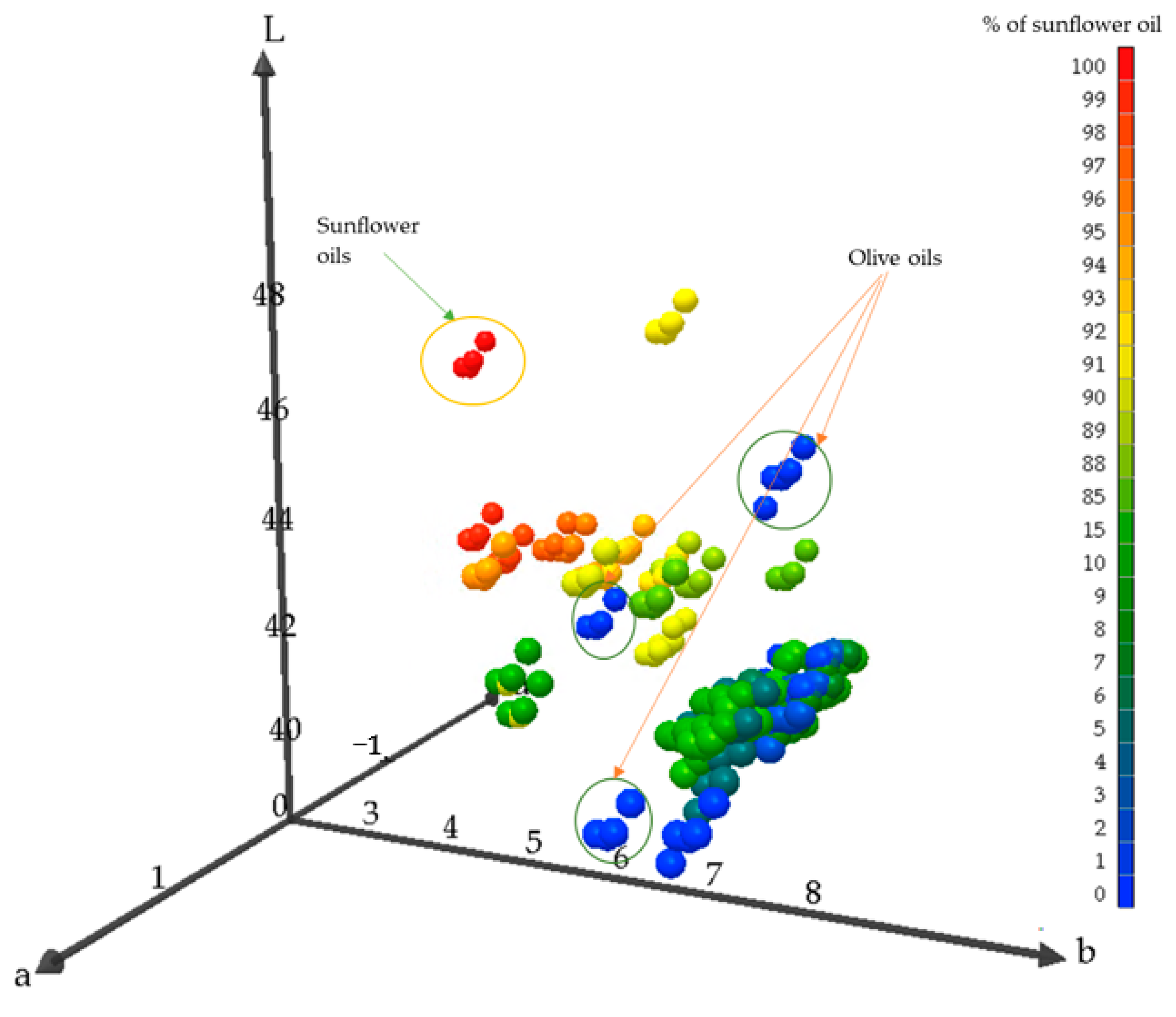
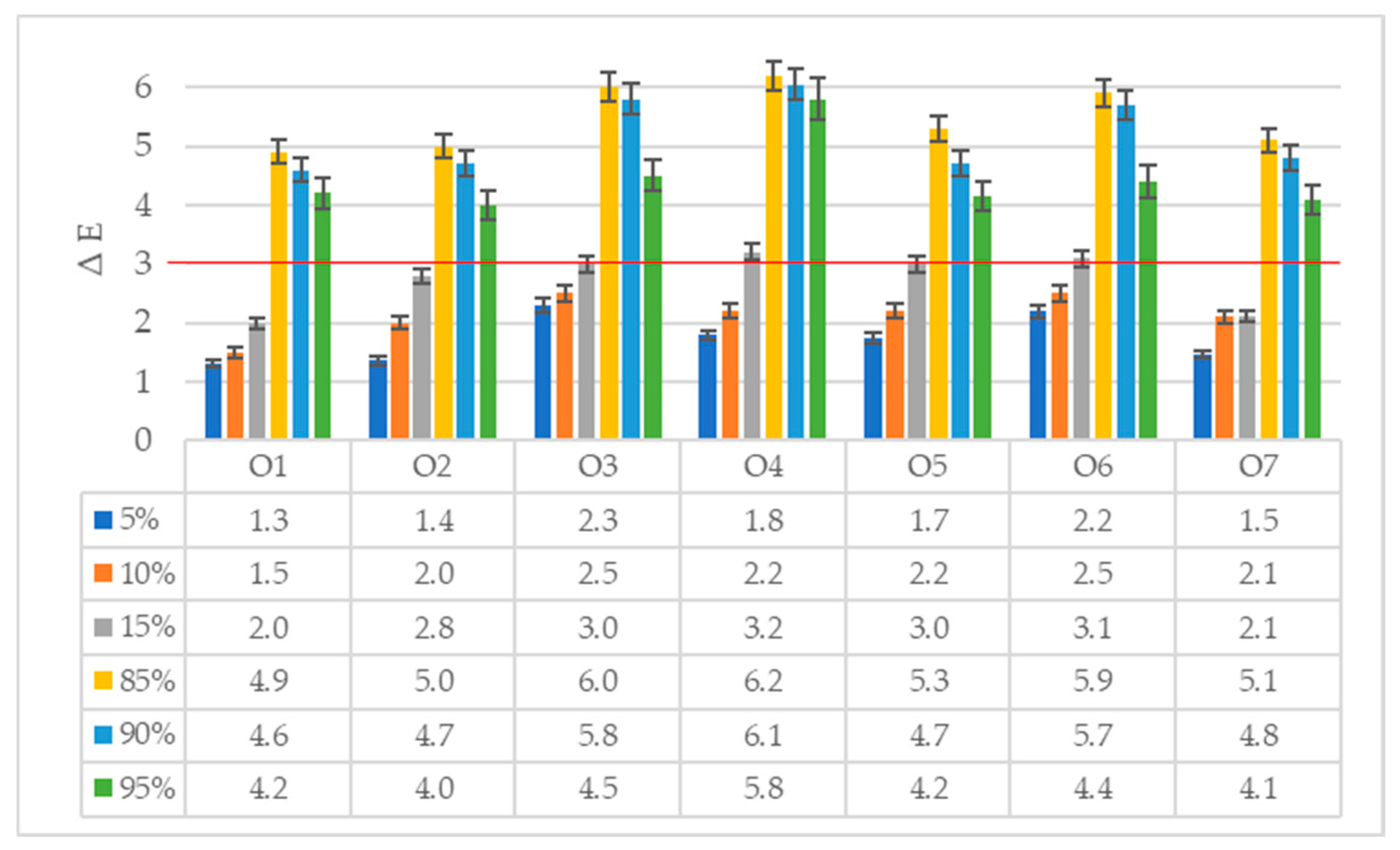
Color Measurement of Oil Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klepo, T.; Benčić, Đ. Utjecaj genotipa na kemijski sastav maslinovog ulja. Glas. Zašt. Bilja 2014, 37, 44–53. [Google Scholar]
- Aparicio, R.; Hardwood, J. (Eds.) Preface. In Handbook of Olive Oil: Analysis and Properties, 2nd ed.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Petir, M. Patvorine Maslinova Ulja na Tržištu EU-a—Parlamentarno Pitanje. 2019. Available online: https://www.europarl.europa.eu/doceo/document/E-8-2019-001332_HR.html (accessed on 10 June 2023).
- Coff, C.; Barling, D.; Korthals, M.; Nielsen, T. (Eds.) Ethical Traceability and Communicating Food, 15th ed.; The International Library of Environmental, Agricultural and Food Ethics; Springer: Dordrecht, The Netherlands, 2008; pp. 1–18. [Google Scholar]
- Barjol, J.L. Introduction. In Handbook of Olive Oil: Analysis and Properties, 2nd ed.; Aparicio, R., Hardwood, J., Eds.; Springer: New York, NY, USA, 2013; pp. 1–15. [Google Scholar]
- Christy, A.A.; Kasemsumran, S.; Du, Y.; Ozaki, Y. The Detection and Quantification of Adulteration in Olive Oil by Near-Infrared Spectroscopy and Chemometrics. Anal. Sci. 2004, 20, 935–940. [Google Scholar] [CrossRef]
- Aparicio, R.; Conte, L.S.; Fiebig, H.J. Olive Oil Authentication. In Handbook of Olive Oil: Analysis and Properties, 2nd ed.; Aparicio, R., Hardwood, J., Eds.; Springer: New York, NY, USA, 2013; pp. 590–641. [Google Scholar]
- Ferragut, V. The Toxic Oil Syndrome in Spain. In Case Studies in Food Safety and Environmental Health, 1st ed.; Ho, P., Cortez Vieira, M.M., Eds.; Springer: New York, NY, USA, 2007; pp. 43–51. [Google Scholar]
- Meenu, M.; Cai, Q.; Xu, B. A critical review on analytical techniques to detect adulteration of extra virgin olive oil. Trends Food Sci. Technol. 2019, 91, 391–408. [Google Scholar] [CrossRef]
- Mikulec, V.; Adamović, P.; Cvetković, Ž.; Ivešić, M.; Gajdoš Kljusurić, J. Green Techniques for Detecting Microplastics in Marine with Emphasis on FTIR and NIR Spectroscopy—Short Review. Processes 2023, 11, 2360. [Google Scholar] [CrossRef]
- Osborne, B.G. Near-Infrared Spectroscopy in Food Analysis. In Encyclopedia of Analytical Chemistry, 1st ed.; Meyers, R.A., McGorrin, R.J., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006. [Google Scholar] [CrossRef]
- Folli, G.S.; Santos, L.P.; Santos, F.D.; Cunha, P.H.P.; Schaffel, I.F.; Borghi, F.T.; Barros, A.H.A.S.; Pires, A.A.; Ribeiro, A.V.F.N.; Romao, W.; et al. Food analysis by portable NIR spectrometer. Food Chem. Adv. 2022, 1, 100074. [Google Scholar] [CrossRef]
- Amirvaresi, A.; Parastar, H. Miniaturized NIR spectroscopy and chemometrics: A smart combination to solve food authentication challenges. Front. Anal. Sci. 2023, 3, 1118590. [Google Scholar] [CrossRef]
- Arroyo-Cerezo, A.; Yang, X.; Jiménez-Carvelo, A.M.; Pellegrino, M.; Felicita Savino, A.F.; Berzaghi, P. Assessment of extra virgin olive oil quality by miniaturized near infrared instruments in a rapid and non-destructive procedure. Food Chem. 2024, 430, 137043. [Google Scholar] [CrossRef]
- Strgar Kurečić, M. Osnove o Boji: Kontrola Boja-od Percepcije do Mjerenja. 2013. Available online: http://repro.grf.unizg.hr/media/download_gallery/OSNOVE%20O%20BOJI.pdf (accessed on 7 June 2023).
- ISO 12966-2:2017; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters. International Organization for Standardization: Geneva, Switzerland, 2017.
- Posom, J.; Sirisomboon, P. Evaluation of the higher heating value, volatile matter, fixed carbon and ash content of ground bamboo using near infrared spectroscopy. J. Near Infrared Spectrosc. 2017, 25, 301–310. [Google Scholar] [CrossRef]
- Wolfrum, E.J.; Payne, C.; Schwartz, A.; Jacobs, J.; Kressin, R.W. A Performance Comparison of Low-Cost Near-Infrared (NIR) Spectrometers to a Conventional Laboratory Spectrometer for Rapid Biomass Compositional Analysis. Bioenerg. Res. 2020, 13, 1121–1129. [Google Scholar] [CrossRef]
- Beć, K.B.; Grabska, J.; Huck, C.W. Miniaturized NIR Spectroscopy in Food Analysis and Quality Control: Promises, Challenges, and Perspectives. Foods 2022, 11, 1465. [Google Scholar] [CrossRef]
- Kljusurić, J.G.; Boban, A.; Mucalo, A.; Budić-Leto, I. Novel Application of NIR Spectroscopy for Non-Destructive Determina-tion of ‘Maraština’Wine Parameters. Foods 2022, 11, 1172. [Google Scholar] [CrossRef] [PubMed]
- Rinnan, Å.; Van Der Berg, F.W.J.; Engelsen, S.B. Review of the most common pre-processing techniques for near-infrared spectra. TrAC Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Mata, M.M.D.; Rocha, P.D.; Farias, I.K.T.; Silva, J.L.B.D.; Medeiros, E.P.; Silva, C.S.; Simões, S.D.S. Distinguishing cotton seed genotypes by means of vibrational spectroscopic methods (NIR and Raman) and chemometrics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 266, 120399. [Google Scholar] [CrossRef] [PubMed]
- Fearn, T. Assessing Calibrations: SEP, RPD, RER and R2. NIR News 2002, 13, 12–13. [Google Scholar] [CrossRef]
- Žanetić, M.; Gugić, M. Zdravstvene vrijednosti maslinovog ulja. Pomol. Croat. 2006, 12, 159–173. [Google Scholar]
- Gandul-Rojas, B.; Gallardo-Guerrero, L.; Roca, M.; Aparicio-Ruiz, R. Chromatographic Methodologies: Compounds for Olive Oil Color Issues. In Handbook of Olive Oil: Analysis and Properties, 2nd ed.; Aparicio, R., Hardwood, J., Eds.; Springer: New York, NY, USA, 2013; pp. 220–254. [Google Scholar]
- Li, Q.; Wang, M.; Belén Fernández, M.; Sagymbek, A.; Dong, Y.; Gao, Y.; Yu, X. Indication of the color change on the oxidation properties of fragrant rapeseed oil during shelf storage. Food Chem. X 2023, 20, 100908. [Google Scholar] [CrossRef] [PubMed]
- Gandul-Rojas, B.; Cepero, M.R.L.; Mínguez-Mosquera, M.I. Use of chlorophyll and carotenoid pigment composition to determine authenticity of virgin olive oil. J. Am. Oil Chem. Soc. 2000, 77, 853–858. [Google Scholar] [CrossRef]
- Üçüncüoğlu, D.; Sivri-Özay, D. Geographical origin impact on volatile composition and some quality parameters of virgin olive oils extracted from the “Ayvalık” variety. Heliyon 2020, 6, e04919. [Google Scholar] [CrossRef] [PubMed]
- Jabeur, H.; Zribi, A.; Bouaziz, M. Changes in chemical and sensory characteristics of Chemlali extra-virgin olive oil as depending on filtration. Eur. J. Lipid Sci. Technol. 2016, 119, 1500602. [Google Scholar] [CrossRef]
- Sar, T.; Ozturk, M.; Taherzadeh, M.J.; Ferreira, J.A. New Insights on Protein Recovery from Olive Oil Mill Wastewater through Bioconversion with Edible Filamentous Fungi. Processes 2020, 8, 1210. [Google Scholar] [CrossRef]
- Yu, X.; Yang, C.; Du, S.; Gao, J. A New Method for Determining Free Fatty Acid Content in Edible Oils by Using Electrical Conductivity. Food Anal. Methods 2012, 5, 1453–1458. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Q.; Yu, X.; Chen, X.; Wang, Y. A novel method for determining peroxide value of edible oils using electrical conductivity. Food Control 2014, 39, 198–203. [Google Scholar] [CrossRef]
- Turgut, A.; Tavman, I.; Tavman, S. Measurement of Thermal Conductivity of Edible Oils Using Transient Hot Wire Method. Int. J. Food Prop. 2009, 12, 741–747. [Google Scholar] [CrossRef]
- Ilak Peršurić, A.S. Segmenting Olive Oil Consumers Based on Consumption and Preferences toward Extrinsic, Intrinsic and Sensorial Attributes of Olive Oil. Sustainability 2020, 12, 6379. [Google Scholar] [CrossRef]
- Sands, S. Delta E: A Key to Understanding Lightfastness Readings. 2016. Available online: https://justpaint.org/delta-e/ (accessed on 7 June 2024).
- Škevin, D.; Kraljić, K.; Miletić, L.; Obranović, M.; Neđeral, S.; Petričević, S. Adulteration of Oblica Virgin Olive Oil with Edible Sunflower and Refined Olive Pomace Oil. CJFTBN 2011, 6, 117–122. [Google Scholar]
- Downey, G.; McIntyre, P.; Davies, A.N. Detecting and quantifying sunflower oil adulteration in extra virgin olive oils from the eastern mediterranean by visible and near-infrared spectroscopy. J. Agric. Food Chem. 2002, 50, 5520–5525. [Google Scholar] [CrossRef] [PubMed]
- Christopoulou, E.; Lazaraki, M.; Komaitis, M.; Kaselimis, K. Effectiveness of determinations of fatty acids and triglycerides for the detection of adulteration of olive oils with vegetable oils. Food Chem. 2004, 84, 463–474. [Google Scholar] [CrossRef]
- Kazazić, S.; Gajdoš Kljusurić, J.; Radeljević, B.; Plavljanić, D.; Špoljarić, J.; Ljubić, T.; Bilić, B.; Mikulec, N. Comparison of GC and NIR spectra as a rapid tool for food fraud detection—The case of butter adulteration with different fats. J. Food Process. Preserv. 2021, 45, e15732. [Google Scholar] [CrossRef]
- Nutrizio, M.; Gajdoš Kljusurić, J.; Marijanović, Z.; Dubrović, I.; Viskić, M.; Mikolaj, E.; Chemat, F.; Režek Jambrak, A. The Potential of High Voltage Discharges for Green Solvent Extraction of Bioactive Compounds and Aromas from Rosemary (Rosmarinus officinalis L.)—Computational Simulation and Experimental Methods. Molecules 2020, 25, 3711. [Google Scholar] [CrossRef]
- Barbin, D.F.; De Souza Madureira Felicio, A.L.; Sun, D.W.; Nixdorf, S.L.; Hirooka, E.Y. Application of infrared spectral techniques on quality and compositional attributes of coffee: An overview. Food Res. Int. 2014, 61, 23–32. [Google Scholar] [CrossRef]
- García Martín, J.F. Potential of Near-Infrared Spectroscopy for the Determination of Olive Oil Quality. Sensors 2022, 22, 2831. [Google Scholar] [CrossRef] [PubMed]
- Inarejos-García, A.M.; Gómez-Alonso, S.; Fregapane, G.; Salvador, M.D. Evaluation of minor components, sensory characteristics and quality of virgin olive oil by near infrared (NIR) spectroscopy. Food Res. Int. 2013, 50, 250–258. [Google Scholar] [CrossRef]
- Cascant, M.M.; Breil, C.; Fabiano-Tixier, A.S.; Chemat, F.; Garrigues, S.; De la Guardia, M. Determination of fatty acids and lipid classes in salmon oil by near infrared spectroscopy. Food Chem. 2018, 239, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, İ.S.; Dağ, Ç.; Özinanç, G.; Suçsoran, Ö.; Ertaş, E.; Bekiroğlu, S. Quantification of sterols and fatty acids of extra virgin olive oils by FT-NIR spectroscopy and multivariate statistical analyses. LWT Food Sci. Technol. 2018, 91, 125–132. [Google Scholar] [CrossRef]
- De Pascali, S.A.; Gambacorta, L.; Oswald, I.P.; Del Coco, L.; Solfrizzo, M.; Fanizzi, F.P. 1H NMR and MVA metabolomic profiles of urines from piglets fed with boluses contaminated with a mixture of five mycotoxins. Biochem. Biophys. Rep. 2017, 11, 9–18. [Google Scholar] [CrossRef]
- Abu-Khalaf, N.; Hmidat, M. Visible/Near Infrared (VIS/NIR) spectroscopy as an optical sensor for evaluating olive oil quality. Comput. Electron. Agric. 2020, 173, 105445. [Google Scholar] [CrossRef]
| Olive Oil (Industrial Production) | Olive Oil (Small Producers) | Sunflower Oil | Mediterranean Oil (OSm) (Industrial, 10% Olive Oil) | |
|---|---|---|---|---|
| Energy (kcal/kJ) | 899/3696 | 884/3695 | 828/3404 | 828/3404 |
| Fats (g) | 99.9 | 100 | 92 | 92 |
| SFA (g) | 15.4 | 14 | 11 | 11 |
| Vitamin E (mg) | 17 | 17 | 46 | 37 |
| Oil Samples | Color Parameters | Conductivity | TDS | ||||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | C* | h* | (µS/cm) | (mg/L) | |
| O1 | 40.8 ± 0.1 c,a | −0.7 ± 0 a | 7.5 ± 0 a | 7.6 ± 0 a | 84.9 ± 0 a | 1094 ± 8.7 a | 547 ± 4.4 a |
| O2 | 40.9 ± 0.1 a | −0.4 ± 0 b | 7.5 ± 0 a | 7.5 ± 0 a | 86.9 ± 0.1 b | 1083.3 ± 1.5 a | 541.3 ± 0.6 a |
| O3 | 41.2 ± 0.1 b | −0.5 ± 0 b | 7.8 ± 0 b | 7.8 ± 0 b | 86.4 ± 0 b | 1478.7 ± 29 b | 739 ± 14.8 b |
| O4 | 39.8 ± 0.1 c | −0.7 ± 0 a | 5.8 ± 0 c | 5.8 ± 0 c | 83.2 ± 0 c | 2766.7 ± 282.9 b,c | 1382.7 ± 140 b,c |
| O5 | 40.7 ± 0.1 c,a | −0.6 ± 0 b | 7 ± 0 b | 7.1 ± 0 c | 85.2 ± 0.1 b | 3033.3 ± 49.3 c | 1517 ± 22.7 c |
| O6 | 40.3 ± 0.1 c | −0.6 ± 0 b | 6.4 ± 0.1 c | 6.4 ± 0.1 c | 84.8 ± 0 a | 1091 ± 10.4 a | 545.7 ± 4.9 a |
| O7 | 39.8 ± 0.1 c | −0.6 ± 0 b | 7 ± 0 b | 7.1 ± 0 c | 83.2 ± 0.1 c | 1504.9 ± 33 b | 747 ± 17.2 b |
| S | 42.5 ± 0 d | −0.2 ± 0 c | 3.2 ± 0 d | 3.3 ± 0 d | 84.6 ± 0 c | 2266.7 ± 20.8 c | 1133.7 ± 8.3 b |
| OSm1 | 42.3 ± 0 d | −0.3 ± 0 d | 5.4 ± 0 c | 5.4 ± 0 c | 87.9 ± 0 b | 1056.3 ± 4 d | 528 ± 1.7 b |
| OSm2 | 42.2 ± 0 d | −0.3 ± 0 d | 5.4 ± 0 c | 5.4 ± 0 c | 88.2 ± 0.2 b | 1469 ± 0 b | 735 ± 0 b |
| Fatty Acid | Olive Oil 100% | Share of Added Sunflower Oil | Sunflower Oil 100% | |||||
|---|---|---|---|---|---|---|---|---|
| 5% | 10% | 15% | 85% | 90% | 95% | |||
| Long-chain fatty acids (LCFAs) | ||||||||
| Saturated LCFA | ||||||||
| C 14:0 | 0.1 ± 0 | 0.1 ± 0 | 0.1 ± 0 | 0.1 ± 0 | 0.1 ± 0 | 0.1 ± 0 | 0.1 ± 0 | 0.1 ± 0 |
| C 16:0 | 11.5 ±0.35 $ | 11.27 ± 0.34 | 11.04 ± 0.33 | 10.81 ± 0.32 #,$ | 7.59 ± 0.23 # | 7.36 ± 0.22 # | 7.13 ± 0.21 # | 6.9 ± 0.23 # |
| C 17:0 | 0.1 ± 0 $ | 0.1 ± 0 | 0.09 ± 0 | 0.09 ± 0 | 0.02 ± 0 | 0.01 ± 0 | 0.01 ± 0 | 0 ± 0 # |
| C 18:0 | 3.1 ± 0.09 | 3.13 ± 0.09 | 3.16 ± 0.09 | 3.19 ± 0.1 | 3.61 ± 0.11 | 3.64 ± 0.11 | 3.67 ± 0.11 | 3.7 ± 0.11 |
| C 20:0 | 0.4 ± 0.01 | 0.4 ± 0.01 | 0.39 ± 0.01 | 0.39 ± 0.01 | 0.32 ± 0.01 | 0.31 ± 0.01 | 0.31 ± 0.01 | 0.3 ± 0.01 |
| Monounsaturated LCFA | ||||||||
| C 16:1 | 1 ± 0.03 $ | 0.96 ± 0.03 | 0.92 ± 0.03 | 0.88 ± 0.03 $ | 0.32 ± 0.01 #,$ | 0.28 ±0.01 #,$ | 0.24 ± 0.01 # | 0.2 ± 0.01 # |
| C 17:1 | 0.1 ± 0 $ | 0.1 ± 0 | 0.09 ± 0 | 0.09 ± 0 | 0.02 ± 0 | 0.01 ± 0 | 0.01 ± 0 | 0. ± 0 # |
| C 18:1 | 76.8 ± 2.3 $ | 74.53 ± 2.24 $ | 72.25 ± 2.17 $ | 69.98 ± 2.1 #,$ | 38.13 ± 1.14 | 35.85 ± 1.08 | 33.58 ± 1.01 | 31.3 ± 1.07 # |
| C 20:1 | 0.3 ± 0.01 | 0.3 ± 0.01 | 0.29 ± 0.01 | 0.29 ± 0.01 | 0.22 ± 0.01 | 0.21 ± 0.01 | 0.21 ± 0.01 | 0.2 ± 0.01 |
| Polyunsaturated LCFA | ||||||||
| C 18:2 | 5.1 ± 0.15 $ | 7.66 ± 0.23 | 10.21 ± 0.31 #,$ | 12.77 ± 0.38 #,$ | 48.54 ± 1.46 | 51.09 ± 1.53 | 53.65 ± 1.61 | 56.2 ± 1.69 # |
| C 18:3 | 0.6 ± 0.02 $ | 0.58 ± 0.02 $ | 0.55 ± 0.02 $ | 0.53 ± 0.02 $ | 0.18 ± 0.01 #,$ | 0.15 ± 0 # | 0.13 ± 0 # | 0.1 ± 0.01 # |
| Very-long-chain fatty acids | ||||||||
| C 22:0 | 0.1 ± 0 $ | 0.14 ± 0 | 0.17 ± 0.01 | 0.21 ± 0.01 #,$ | 0.7 ± 0.02 # | 0.73 ± 0.02 # | 0.77 ± 0.02 # | 0.8 ± 0.02 # |
| C 24:0 | 0.1 ± 0 $ | 0.11 ± 0 $ | 0.12 ± 0 $ | 0.13 ± 0 $ | 0.27 ± 0.01 # | 0.28 ± 0.01 # | 0.29 ± 0.01 # | 0.3 ± 0.01 # |
| SFA | 15.4 ± 0.46 | 15.24 ± 0.46 | 15.07 ± 0.45 | 14.91 ± 0.45 | 12.6 ± 0.38 | 12.43 ± 0.37 | 12.27 ± 0.37 | 12.1 ± 0.36 |
| MUFA | 78.2 ±2.35 $ | 75.88 ± 2.28 $ | 73.55 ± 2.21 $ | 71.23± 2.14 $ | 38.68 ± 1.16 # | 36.35 ±1.09 # | 34.03 ± 1.02 # | 31.7 ± 0.98 # |
| PUFA | 5.7 ± 0.17 $ | 8.23 ± 0.25 $ | 10.76 ± 0.32 #,$ | 13.29 ± 0.4 #,$ | 48.71 ± 1.46 #,$ | 51.24 ±1.54 # | 53.77 ±1.61 # | 56.3 ± 1.69 # |
| MUFA/SFA | 5.08 ±0.15 $ | 4.98 ± 0.15 $ | 4.88 ± 0.15 $ | 4.78 ± 0.14 $ | 3.07 ± 0.09 # | 2.92 ± 0.09 # | 2.77 ± 0.08 # | 2.62 ± 0.09 # |
| Precision Parameters for Different Devices | NIR Spectra Wavelengths | ||
|---|---|---|---|
| ≈1210 nm | ≈1400 nm | ≈1650 nm | |
| Repeatability | |||
| Portable NIR | 1.7 × 10−2 | 2.1 × 10−2 | 1.8 × 10−2 |
| Benchtop NIR | 1.1 × 10−2 | 1.8 × 10−3 | 4.7 × 10−3 |
| Reproducibility | |||
| Portable NIR | 2.5 × 10−3 | 6.7 × 10−3 | 6.7 × 10−3 |
| Benchtop NIR | 1.8 × 10−3 | 1.2 × 10−3 | 1.9 × 10−3 |
| Benchtop NIR | Micro NIR | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2p | R2v | SEV | RMSEV | RPD | R2p | R2v | SEV | RMSEV | RPD | |
| Level of adulteration | ||||||||||
| as % | 0.9999 | 0.9992 | 15.6620 | 12.5151 | 8.6 | 0.9941 | 0.9843 | 16.4432 | 13.2211 | 8.2 |
| Color attributes | ||||||||||
| L* | 0.9950 | 0.9151 | 0.0205 | 0.1431 | 6.9 | 0.7233 | 0.6601 | 0.1542 | 0.3927 | 2.0 |
| a* | 0.9989 | 0.9514 | 0.0269 | 0.1639 | 7.6 | 0.8930 | 0.8458 | 0.1958 | 0.4425 | 3.1 |
| b* | 0.9998 | 0.9242 | 0.2018 | 0.4493 | 7.0 | 0.8354 | 0.7487 | 0.6689 | 0.8178 | 4.2 |
| Fatty acids | ||||||||||
| C 16:0 | 0.9999 | 0.9987 | 0.0051 | 0.0717 | 8.5 | 0.9924 | 0.9860 | 0.4072 | 0.6382 | 8.3 |
| C 16:1 | 0.9999 | 0.9989 | 0.0001 | 0.0117 | 8.5 | 0.9940 | 0.9866 | 0.0140 | 0.1184 | 8.3 |
| C 17:0 | 0.9999 | 0.9902 | 0.0001 | 0.0042 | 8.4 | 0.9663 | 0.9207 | 0.0002 | 0.0146 | 7.0 |
| C 17:1 | 0.9999 | 0.9902 | 0.0001 | 0.0042 | 8.4 | 0.9663 | 0.9207 | 0.0002 | 0.0146 | 7.0 |
| C 18:0 | 0.9999 | 0.9985 | 0.0001 | 0.0101 | 8.5 | 0.9917 | 0.9862 | 0.0069 | 0.0830 | 8.3 |
| C 18:1 | 0.9999 | 0.9992 | 0.3185 | 0.564 | 8.5 | 0.9942 | 0.9841 | 0.3342 | 0.6221 | 8.2 |
| C 18:2 | 0.9999 | 0.9992 | 0.4085 | 0.6392 | 8.5 | 0.9941 | 0.9743 | 0.5410 | 0.7190 | 8.0 |
| C 18:3 | 0.9999 | 0.9979 | 0.0001 | 0.0099 | 8.5 | 0.9897 | 0.9762 | 0.0048 | 0.0690 | 8.1 |
| C 20:0 | 0.9999 | 0.9989 | 0.00001 | 0.0015 | 8.5 | 0.9940 | 0.9866 | 0.0002 | 0.0148 | 8.3 |
| C 20:1 | 0.9999 | 0.9989 | 0.00001 | 0.0015 | 8.5 | 0.9940 | 0.9866 | 0.0002 | 0.0148 | 8.3 |
| C 22:0 | 0.9999 | 0.9989 | 0.00001 | 0.0104 | 8.5 | 0.9928 | 0.9859 | 0.0095 | 0.0973 | 8.3 |
| C 24:0 | 0.9999 | 0.984 | 0.0001 | 0.0107 | 8.2 | 0.9476 | 0.9333 | 0.0010 | 0.0312 | 7.2 |
| SFA | 0.9999 | 0.9989 | 0.022 | 0.047 | 8.5 | 0.9936 | 0.9756 | 0.2114 | 0.4598 | 8.0 |
| MUFA | 0.9999 | 0.9992 | 0.3306 | 0.575 | 8.5 | 0.9942 | 0.9841 | 43.0000 | 6.6000 | 8.2 |
| PUFA | 0.9999 | 0.9992 | 0.3984 | 0.6312 | 8.5 | 0.9941 | 0.9742 | 50.0000 | 7.1200 | 8.0 |
| MUFA/SFA | 0.9999 | 0.999 | 0.0011 | 0.0335 | 8.5 | 0.9928 | 0.9810 | 0.1228 | 0.3500 | 8.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klinar, M.; Benković, M.; Jurina, T.; Jurinjak Tušek, A.; Valinger, D.; Tarandek, S.M.; Prskalo, A.; Tonković, J.; Gajdoš Kljusurić, J. Fast Monitoring of Quality and Adulteration of Blended Sunflower/Olive Oils Applying Near-Infrared Spectroscopy. Chemosensors 2024, 12, 150. https://doi.org/10.3390/chemosensors12080150
Klinar M, Benković M, Jurina T, Jurinjak Tušek A, Valinger D, Tarandek SM, Prskalo A, Tonković J, Gajdoš Kljusurić J. Fast Monitoring of Quality and Adulteration of Blended Sunflower/Olive Oils Applying Near-Infrared Spectroscopy. Chemosensors. 2024; 12(8):150. https://doi.org/10.3390/chemosensors12080150
Chicago/Turabian StyleKlinar, Magdalena, Maja Benković, Tamara Jurina, Ana Jurinjak Tušek, Davor Valinger, Sandra Maričić Tarandek, Anamaria Prskalo, Juraj Tonković, and Jasenka Gajdoš Kljusurić. 2024. "Fast Monitoring of Quality and Adulteration of Blended Sunflower/Olive Oils Applying Near-Infrared Spectroscopy" Chemosensors 12, no. 8: 150. https://doi.org/10.3390/chemosensors12080150
APA StyleKlinar, M., Benković, M., Jurina, T., Jurinjak Tušek, A., Valinger, D., Tarandek, S. M., Prskalo, A., Tonković, J., & Gajdoš Kljusurić, J. (2024). Fast Monitoring of Quality and Adulteration of Blended Sunflower/Olive Oils Applying Near-Infrared Spectroscopy. Chemosensors, 12(8), 150. https://doi.org/10.3390/chemosensors12080150









