Discrimination of Diabetes Mellitus Patients and Healthy Individuals Based on Volatile Organic Compounds (VOCs): Analysis of Exhaled Breath and Urine Samples by Using E-Nose and VE-Tongue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Breath and Urine Samples Collection
2.2. Samples Analysis
2.2.1. E-Nose System for Breath Analysis
2.2.2. VE-Tongue Unit for Urine Analysis
2.3. Data Processing
2.3.1. E-Nose Measurements
- ΔG = (GS − G0): The difference of the stabilized conductance (GS) and the initial conductance (G0);
- AUC: The area under the sensor response curve calculated by a trapezoidal method. The selected area was in the range of 1 to 9 min of the measurement time.
2.3.2. VE-Tongue Measurements
- Imax: Maximum electrical current;
- AUC: Area between the oxidation and reduction phases in the voltammograms. The trapezoidal technique was used to calculate this area.
2.3.3. Chemometric Techniques
2.3.4. Multisensory Data Fusion Approach
3. Results and Discussion
3.1. E-Nose Breath Analysis
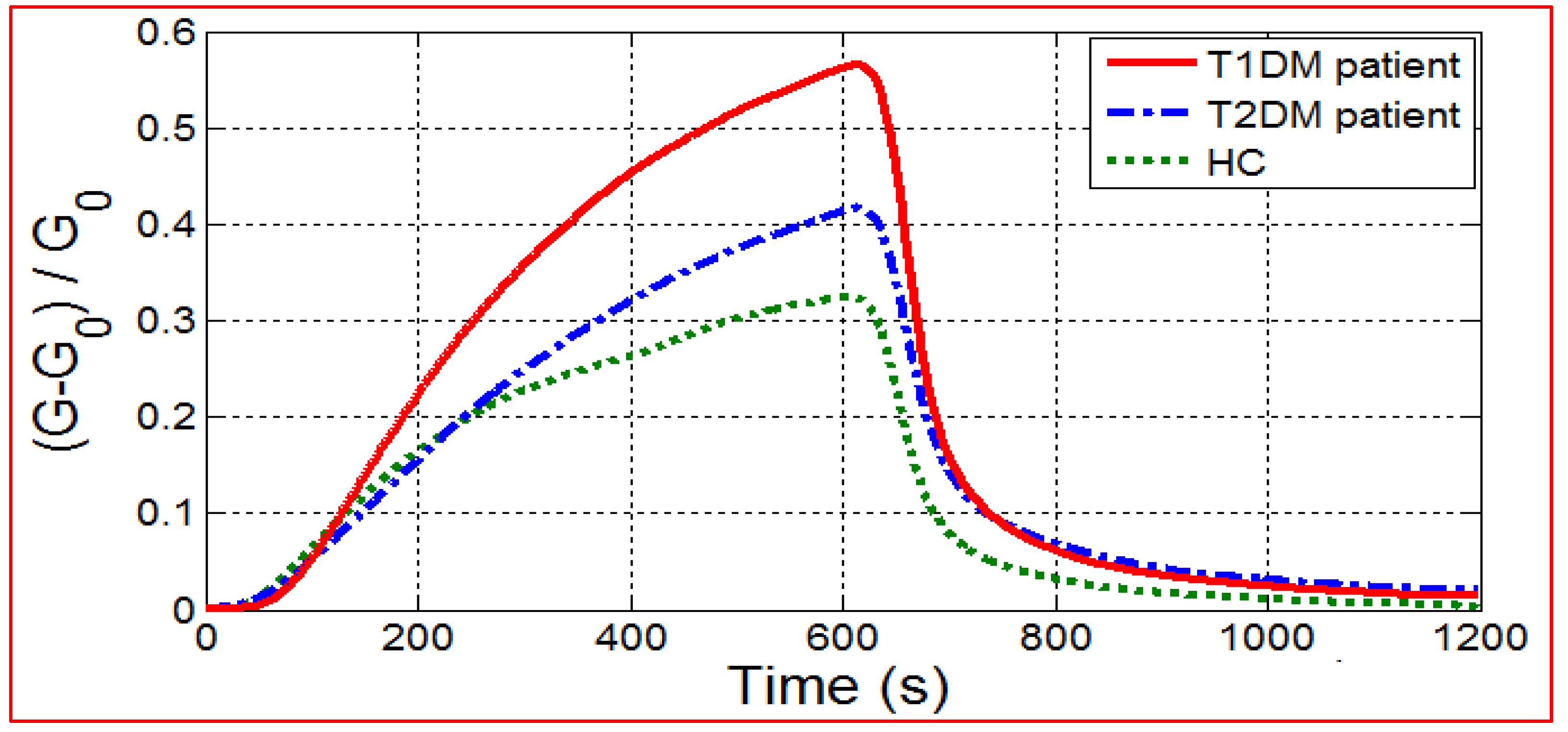
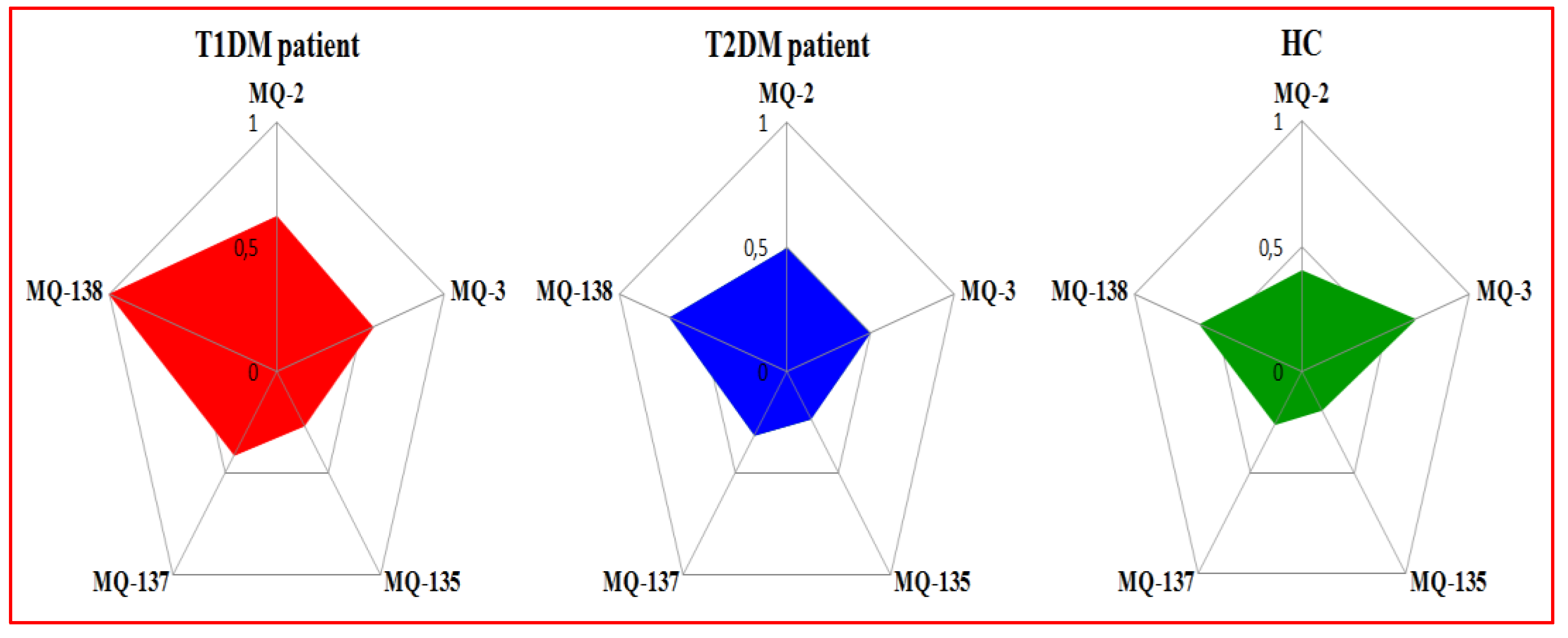
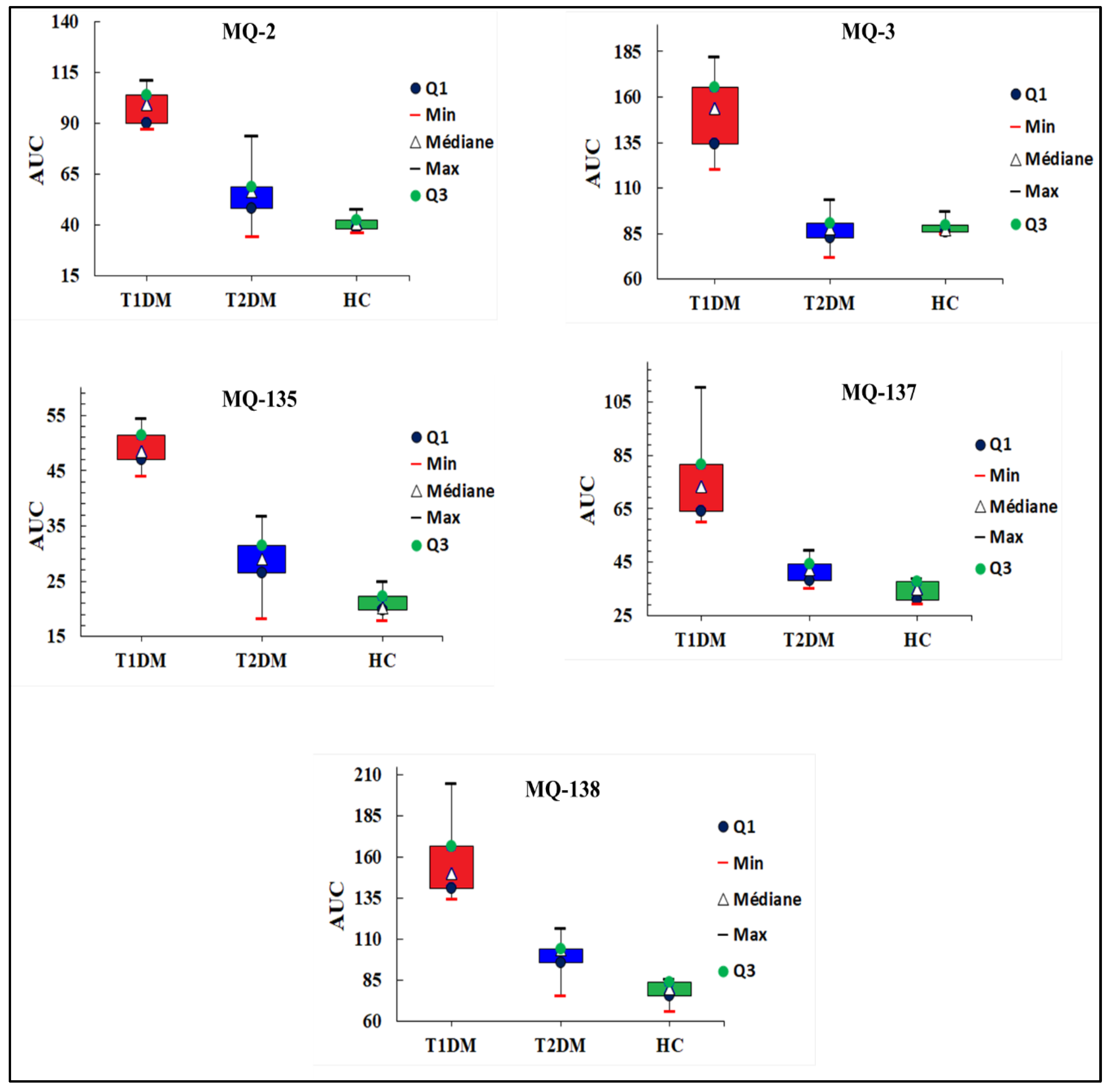
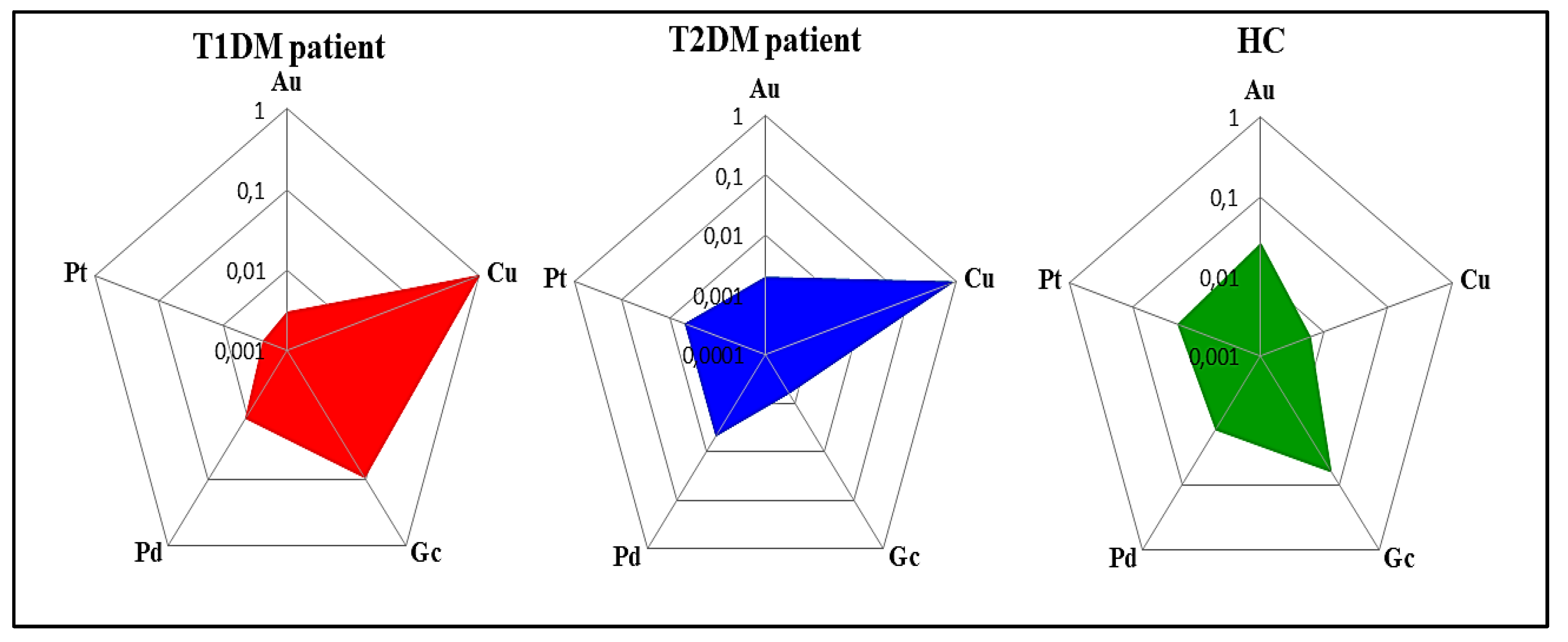
3.1.1. E-Nose Responses
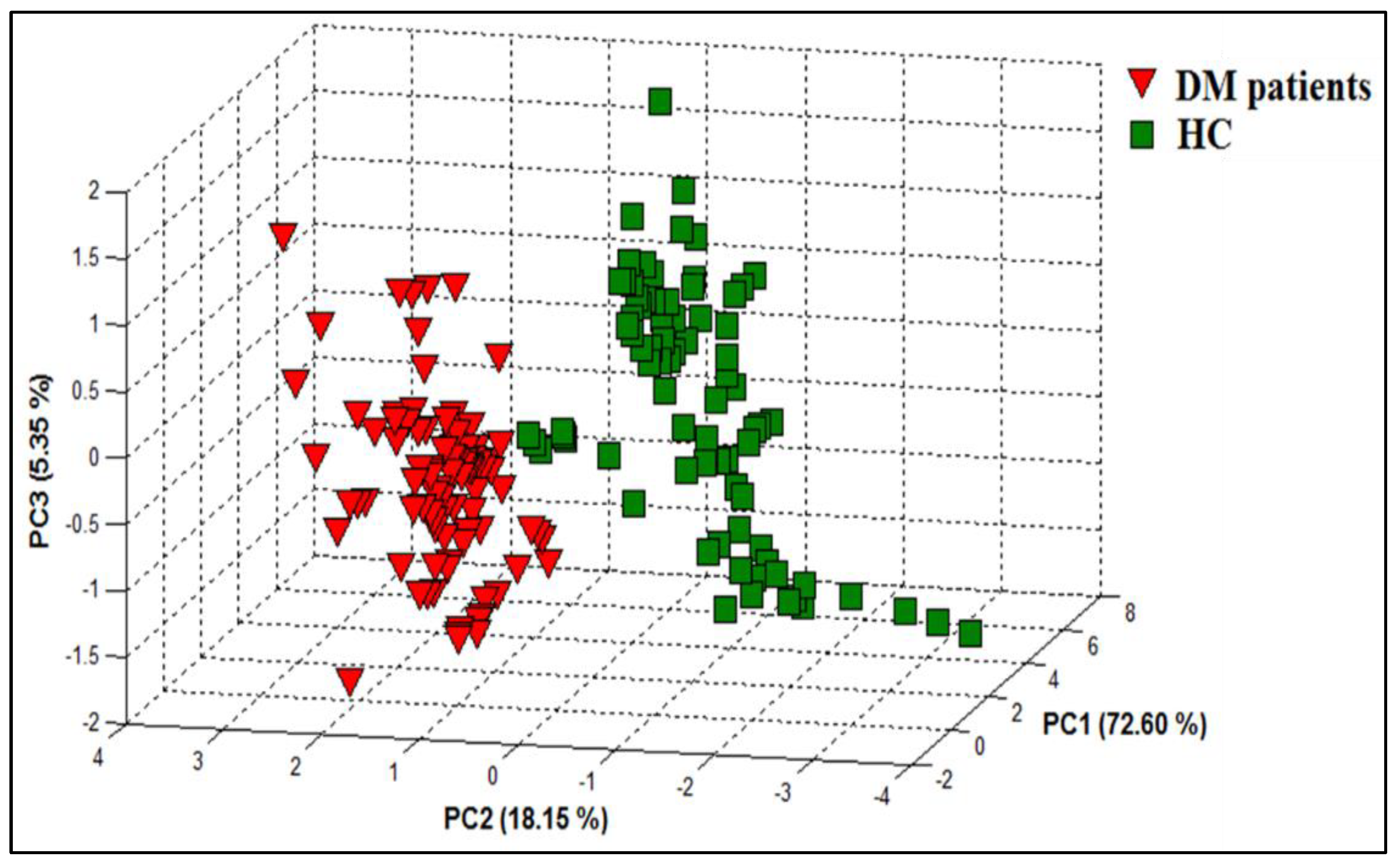
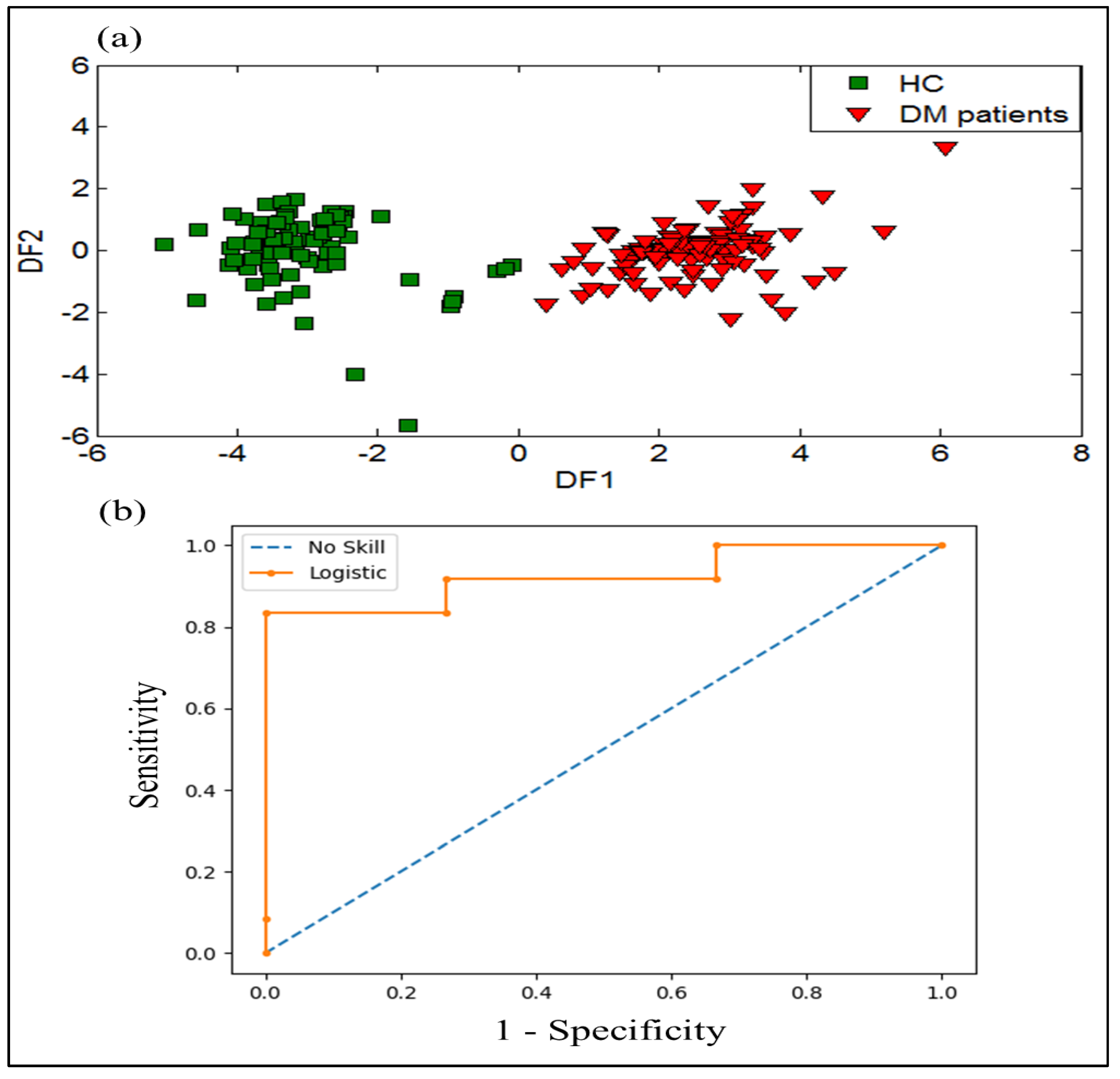
3.1.2. PCA Discrimination Results of Breath Samples from DM and HC
3.1.3. DFA Discrimination Results of Breath Samples from DM and HC
3.1.4. SVM Results of Breath Samples from DM and HC
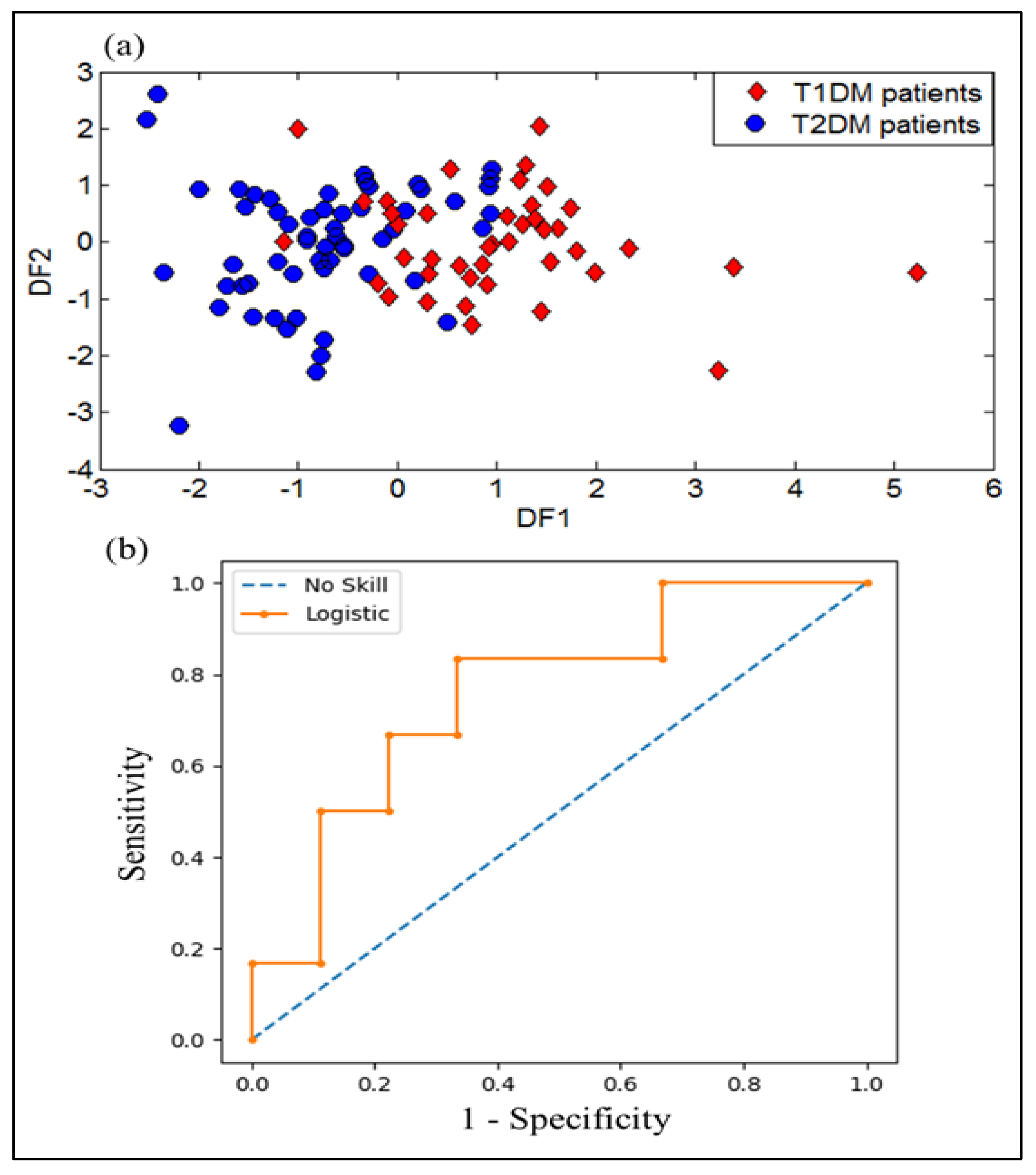
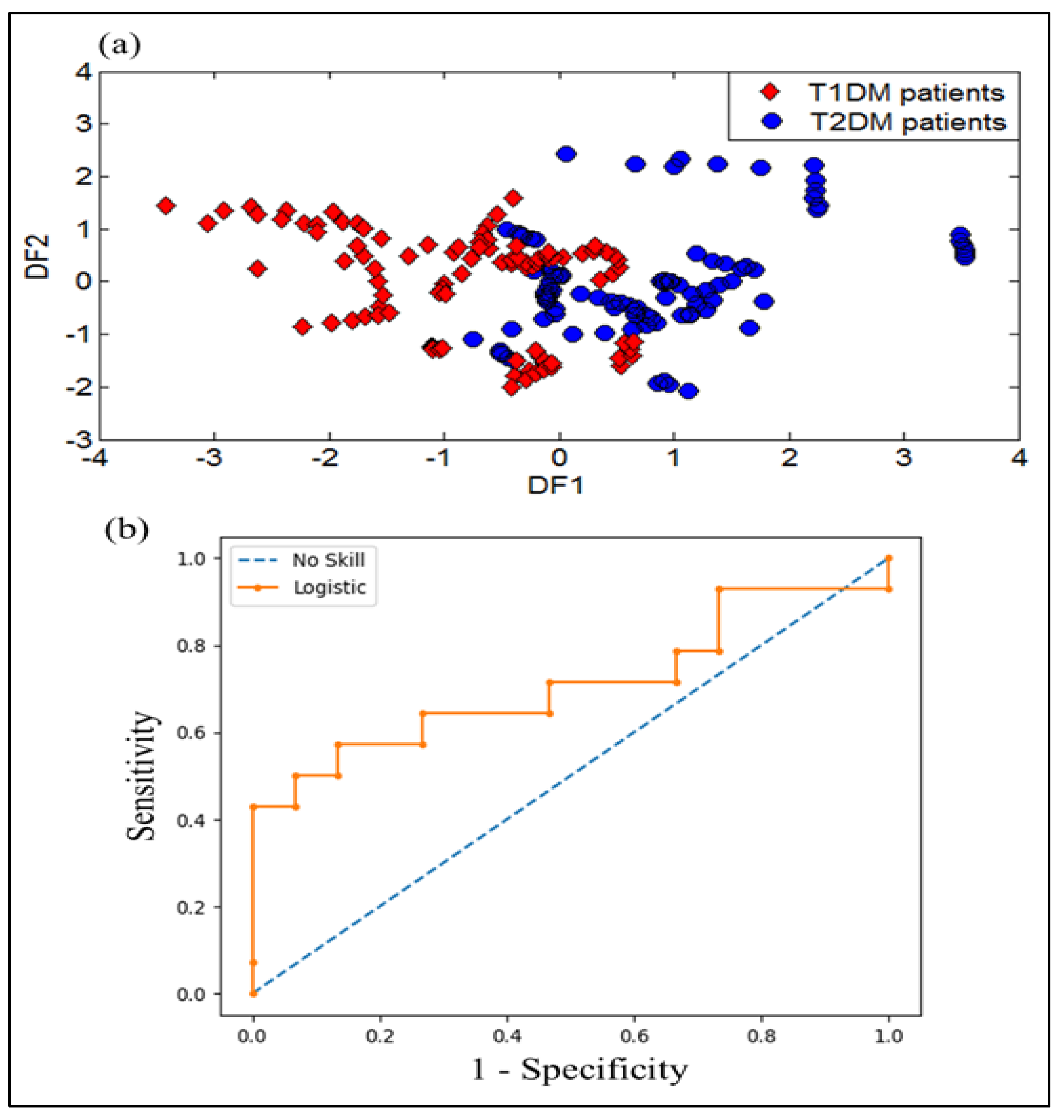
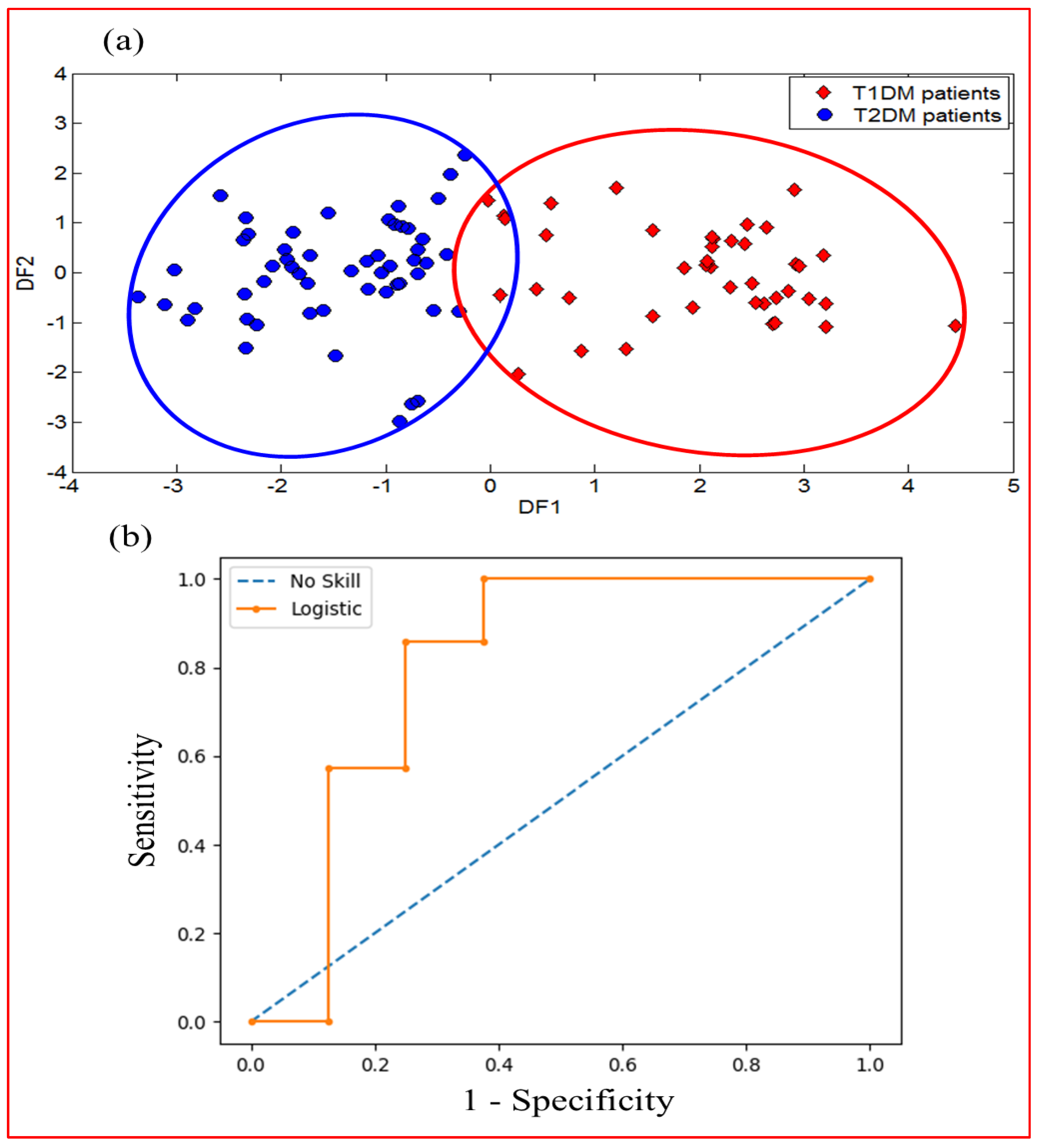
3.1.5. DFA Discrimination Results of Breath Samples from T1DM and T2DM
3.1.6. SVM Results of Breath Samples from T1DM and T2DM
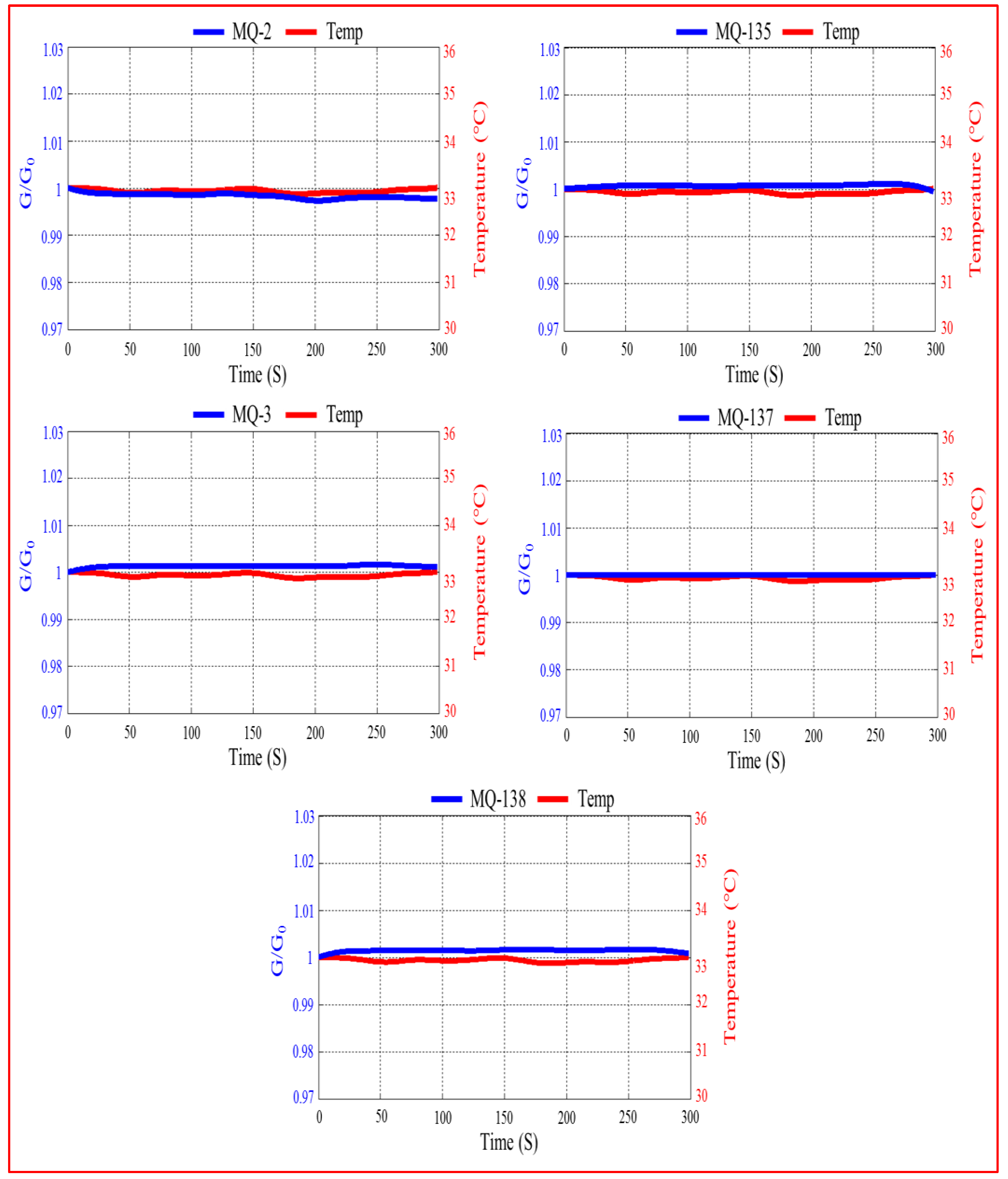
3.1.7. Temperature Effect on the Sensor’s Responses
3.2. Urine Sample Analysis by VE-Tongue
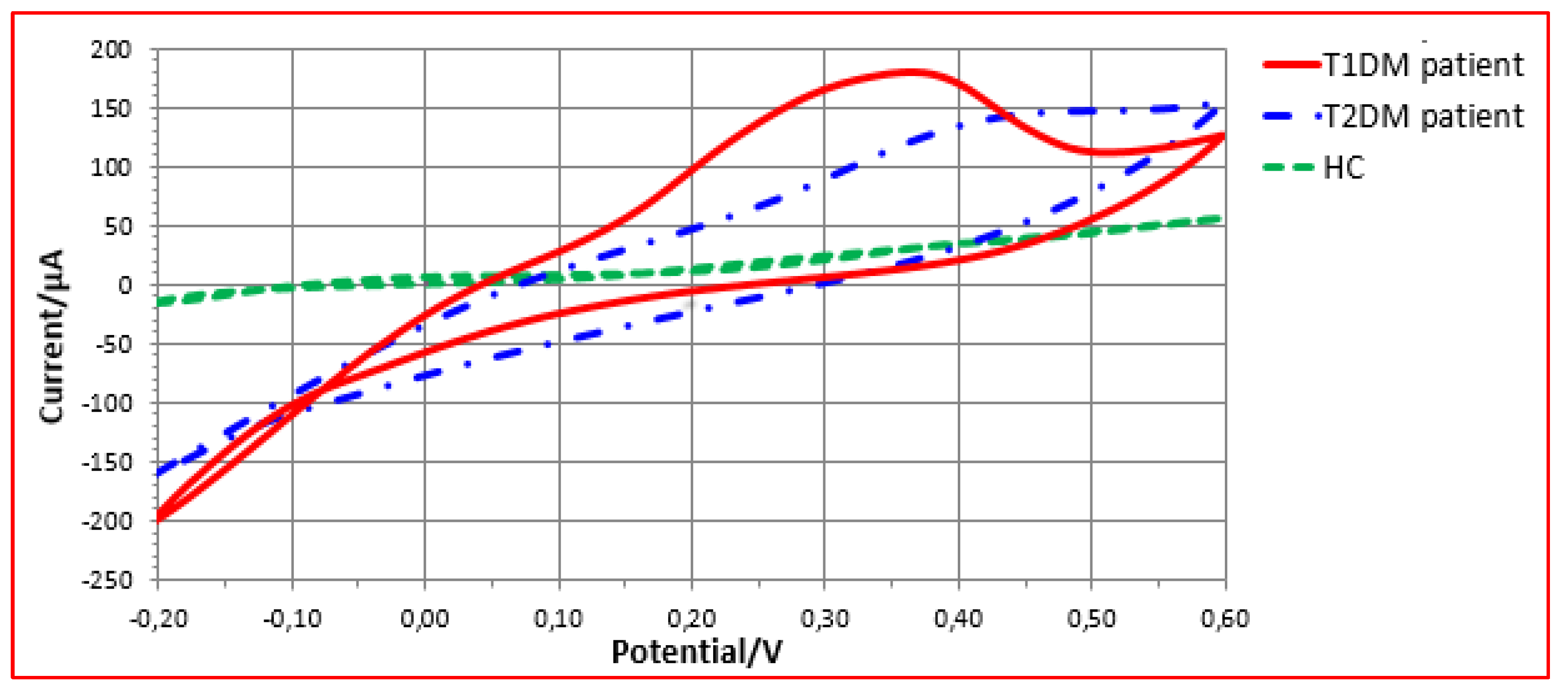
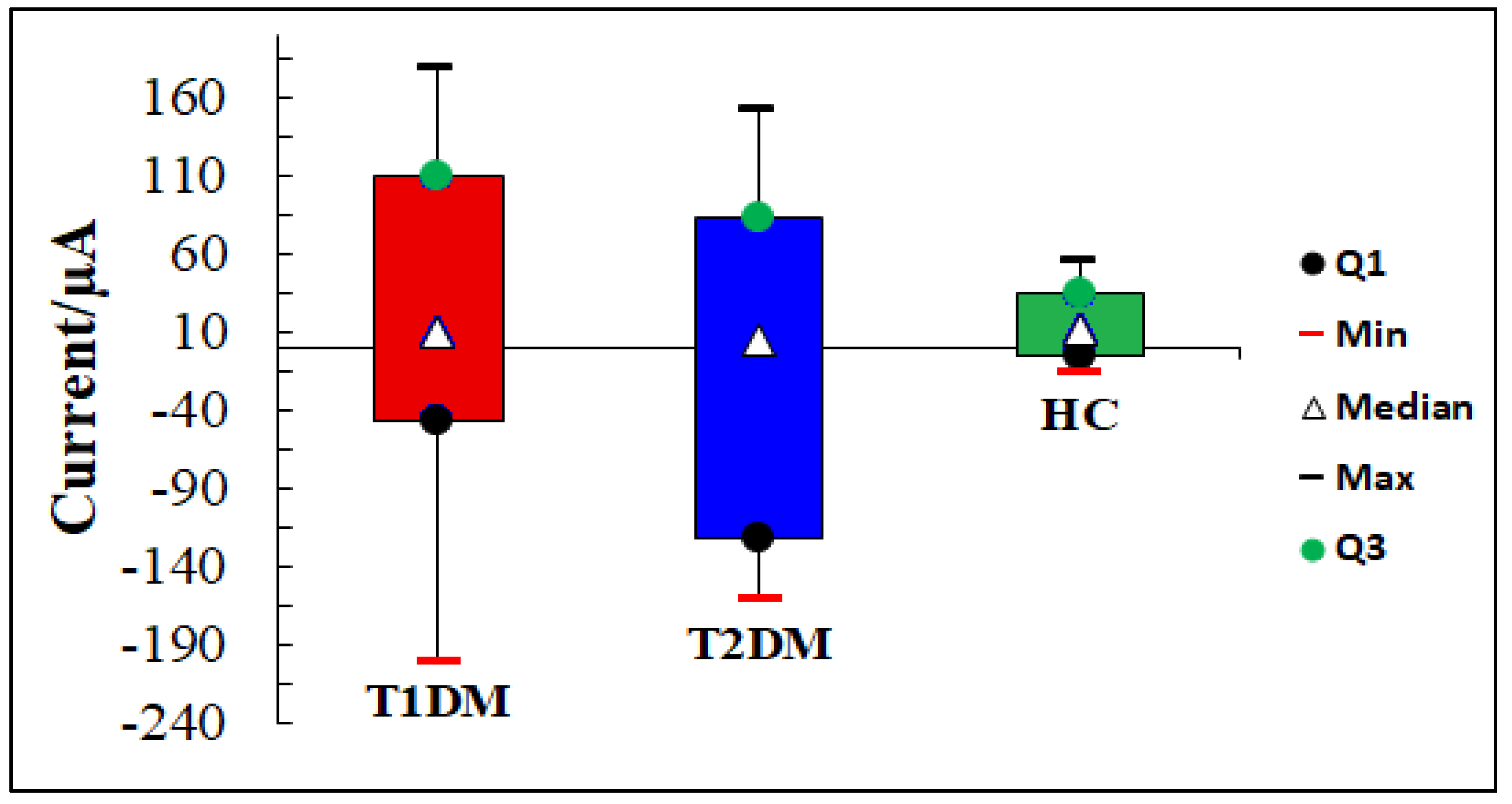
3.2.1. VE-Tongue Responses
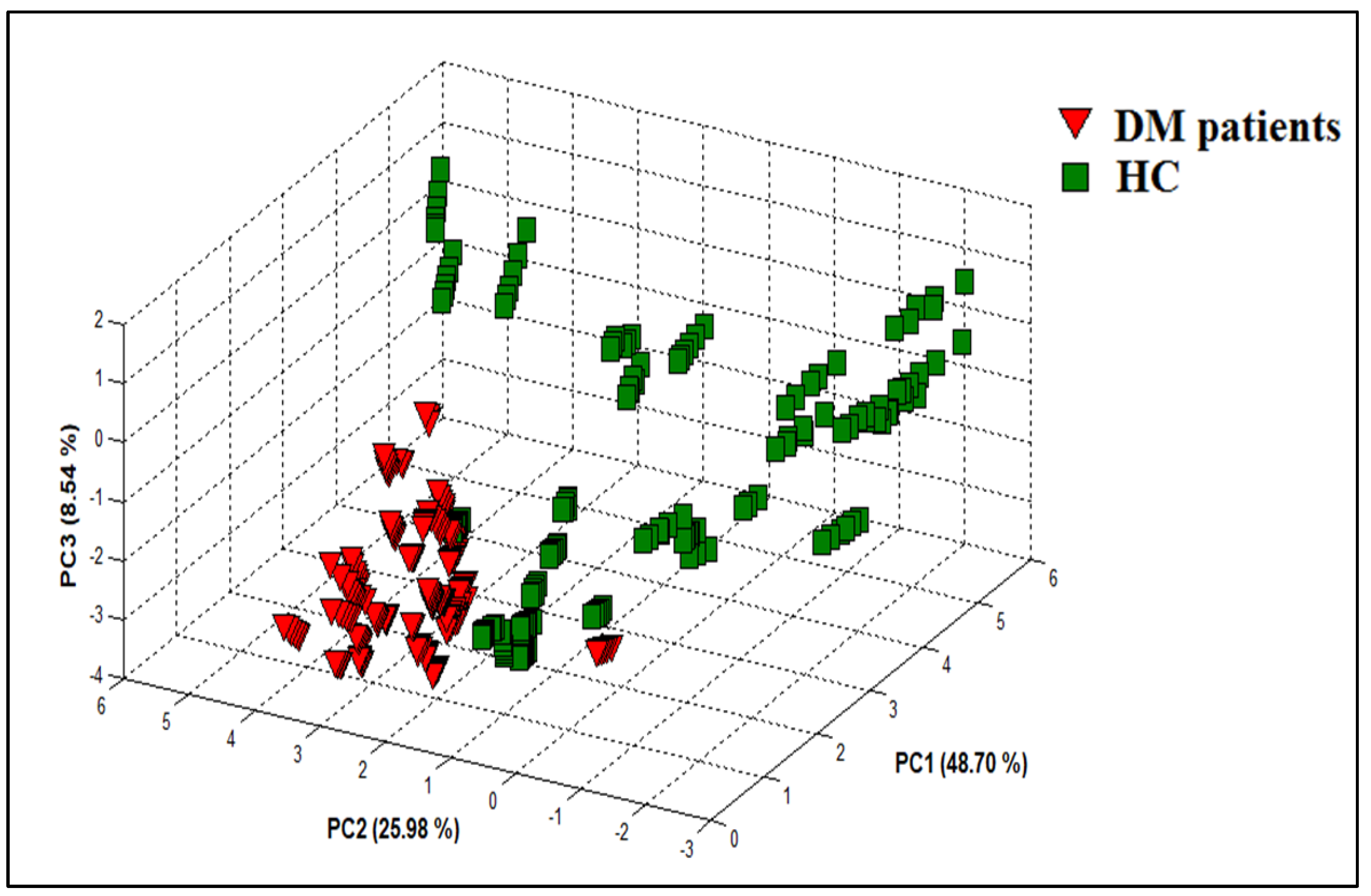
3.2.2. PCA Discrimination Results of Urine Samples from DM and HC
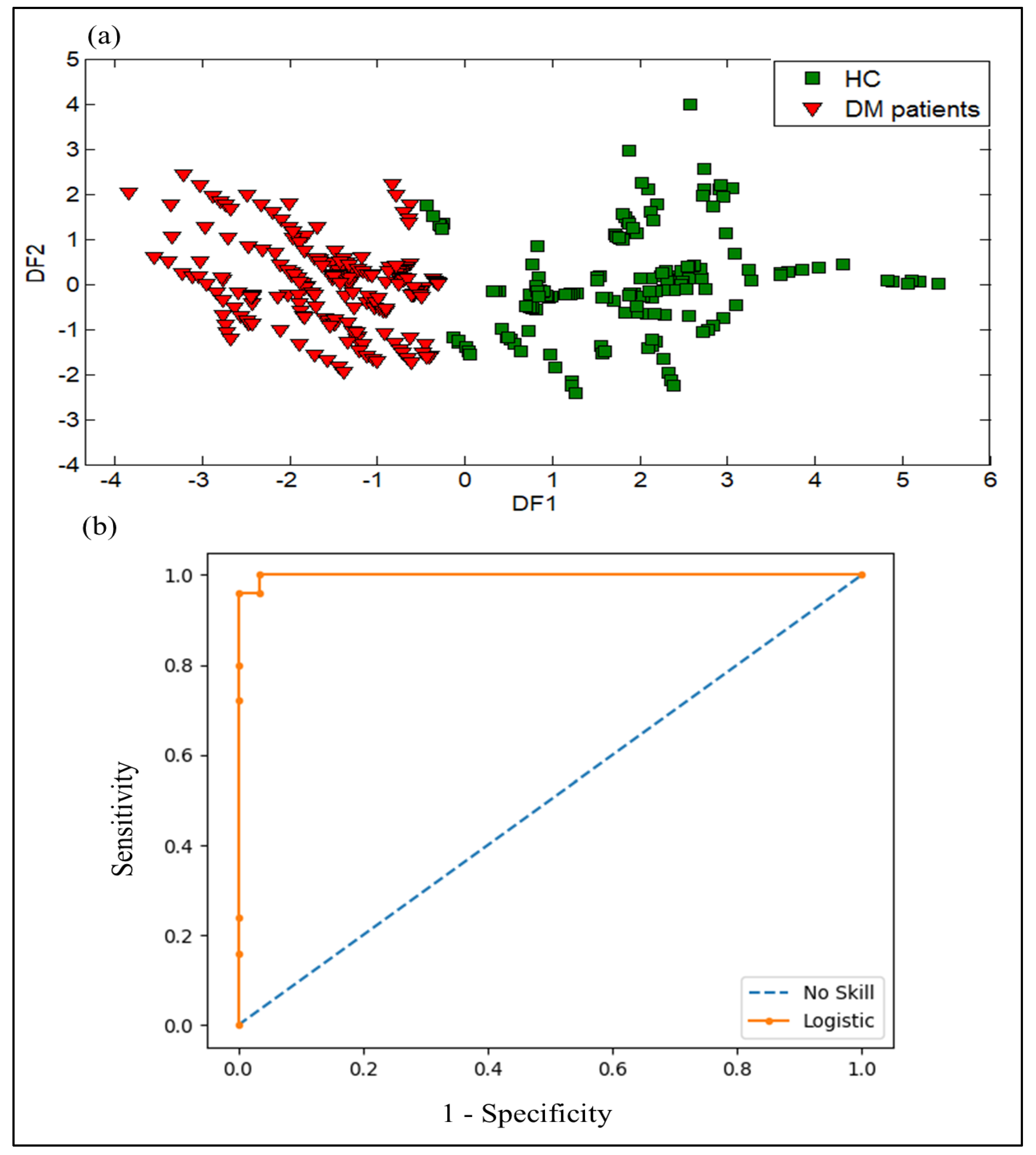
3.2.3. DFA Discrimination Results of Urine Samples from DM and HC
3.2.4. SVM Results of Urine Samples from DM and HC
3.2.5. DFA Discrimination Results and Performance Evaluation Results of VE-Tongue System by the ROC of Urine Samples from T1DM and T2DM
3.2.6. SVM Results of Urine Samples from T1DM and T2DM
3.3. Data Fusion Results of the E-Nose and VE-Tongue Systems
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chetoui, A.; Kaoutar, K.; El Kardoudi, A.; Boutahar, K.; Chigr, F.; Najimi, M. Epidemiology of diabetes in Morocco: Review of data, analysis and perspectives. Int. J. Sci. Eng. Res. 2018, 9, 1310–1316. [Google Scholar]
- Metwally, A.M.; Soliman, M.; Abdelmohsen, A.M.; Kandeel, W.A.; Saber, M.; Elmosalami, D.M.; Asem, N.; Fathy, A.M. Effect of counteracting lifestyle barriers through health education in Egyptian type 2 diabetic patients. J. Med. Sci. 2019, 7, 2886. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37, S81–S90. [Google Scholar] [CrossRef] [Green Version]
- Lekha, S.; Suchetha, M. Recent advancements and future prospects on E-Nose sensors technology and machine learning approaches for non-invasive diabetes diagnosis: A Review. IEEE Rev. Biomed. Eng. 2020, 14, 127–138. [Google Scholar] [CrossRef]
- Mollah, Z.U.A.; Pai, S.; Moore, C.; O’sullivan, B.J.; Harrison, M.J.; Peng, J.; Phillips, K.; Prins, J.B.; Cardinal, J.; Thomas, R. Abnormal NF-κB function characterizes human type 1 diabetes dendritic cells and monocytes. J. Immunol. 2008, 180, 3166–3175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, M.A.; Eisenbarth, G.S. Type 1 diabetes: New perspectives on disease pathogenesis and treatment. Lancet 2001, 358, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Blaha, M.; Spear, C.; Nicholson, W.; Radhika, A.; Shiota, M.; Charron, M.J.; Wright, C.V.E.; Powers, A.C. Reduced PDX-1 expression impairs islet response to insulin resistance and worsens glucose homeostasis. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E707–E714. [Google Scholar]
- Turner, C. Potential of breath and skin analysis for monitoring blood glucose concentration in diabetes. Expert. Rev. Mol. Diagn. 2011, 11, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Vaks, V.L.; Domracheva, E.G.; Sobakinskaya, E.A.; Chernyaeva, M.B. Exhaled breath analysis: Physical methods, instruments, and medical diagnostics. Phys. Usp. 2014, 57, 684. [Google Scholar] [CrossRef]
- Solovieva, S.; Karnaukh, M.; Panchuk, V.; Andreev, E.; Kartsova, L.; Bessonova, E.; Kartsova, L.; Bessonova, E.; Legin, A.; Wang, P.; et al. Potentiometric multisensor system as a possible simple tool for non-invasive prostate cancer diagnostics through urine analysis. Sens. Actuators B Chem. 2019, 289, 42–47. [Google Scholar] [CrossRef]
- Lubes, G.; Goodarzi, M. GC–MS based metabolomics used for the identification of cancer volatile organic compounds as biomarkers. J. Pharm. Biomed. Anal. 2018, 147, 313–322. [Google Scholar] [CrossRef]
- Garg, V.; Kumar, M.; Mahapatra, H.S.; Chitkara, A.; Gadpayle, A.K.; Sekhar, V. Novel urinary biomarkers in pre-diabetic nephropathy. Clin. Exp. Nephrol. 2015, 19, 895–900. [Google Scholar] [CrossRef]
- Trefz, P.; Schmidt, S.C.; Sukul, P.; Schubert, J.K.; Miekisch, W.; Fischer, D.C. Non-invasive assessment of metabolic adaptation in paediatric patients suffering from type 1 diabetes mellitus. J. Clin. Med. 2019, 8, 1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greiter, M.B.; Keck, L.; Siegmund, T.; Hoeschen, C.; Oeh, U.; Paretzke, H.G. Differences in exhaled gas profiles between patients with type 2 diabetes and healthy controls. Diabetes Technol. Ther. 2010, 12, 455–463. [Google Scholar] [CrossRef]
- Krilaviciute, A.; Stock, C.; Leja, M.; Brenner, H. Potential of non-invasive breath tests for preselecting individuals for invasive gastric cancer screening endoscopy. J. Breath. Res. 2018, 12, 036009. [Google Scholar] [CrossRef]
- Markar, S.R.; Lagergren, J.; Hanna, G.B. Research protocol for a diagnostic study of non-invasive exhaled breath analysis for the prediction of oesophago-gastric cancer. BMJ Open 2016, 6, e009139. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Wang, Q.; Li, W.; Zhao, Z.; Yuan, X.; Huang, Y.; Duan, Y. Discovery of potential biomarkers in exhaled breath for diagnosis of type 2 diabetes mellitus based on GC-MS with metabolomics. RSC Adv. 2014, 4, 25430–25439. [Google Scholar] [CrossRef]
- Blake, R.S.; Monks, P.S.; Ellis, A.M. Proton-transfer reaction mass spectrometry. Chem. Rev. 2009, 109, 861–896. [Google Scholar] [CrossRef] [PubMed]
- Westhoff, M.; Litterst, P.; Freitag, L.; Urfer, W.; Bader, S.; Baumbach, J.I. Ion mobility spectrometry for the detection of volatile organic compounds in exhaled breath of patients with lung cancer: Results of a pilot study. Thorax 2009, 64, 744–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armenta, S.; Alcala, M.; Blanco, M. A review of recent, unconventional applications of ion mobility spectrometry (IMS). Anal. Chim. Acta 2011, 703, 114–123. [Google Scholar] [CrossRef]
- Scotter, J.M.; Langford, V.S.; Wilson, P.F.; McEwan, M.J.; Chambers, S.T. Real-time detection of common microbial volatile organic compounds from medically important fungi by Selected Ion Flow Tube-Mass Spectrometry (SIFT-MS). J. Microbiol. Methods. 2005, 63, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Saidi, T.; Moufid, M.; de Jesus Beleño-Saenz, K.; Welearegay, T.G.; El Bari, N.; Lisset, A.; Mogollon, J.; Ionescu, R.; Bourkadi, J.E.; Benamor, J.; et al. Non-invasive prediction of lung cancer histological types through exhaled breath analysis by UV-irradiated electronic nose and GC/QTOF/MS. Sens. Actuators B Chem. 2020, 311, 127932. [Google Scholar] [CrossRef]
- Fitzgerald, J.; Fenniri, H. Cutting edge methods for non-invasive disease diagnosis using e-tongue and e-nose devices. Biosensors 2017, 7, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dragonieri, S.; Schot, R.; Mertens, B.J.; Le Cessie, S.; Gauw, S.A.; Spanevello, A.; Resta, O.; Willard, N.P.; Vink, T.J.; Rabe, K.F.; et al. An electronic nose in the discrimination of patients with asthma and controls. J. Allergy Clin. Immunol. 2007, 120, 856–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saidi, T.; Moufid, M.; Zaim, O.; El Bari, N.; Bouchikhi, B. Voltammetric electronic tongue combined with chemometric techniques for direct identification of creatinine level in human urine. Measurement 2018, 115, 178–184. [Google Scholar] [CrossRef]
- Sarno, R.; Sabilla, S.I.; Wijaya, D.R. Electronic Nose for Detecting Multilevel Diabetes using Optimized Deep Neural Network. Eng. Lett. 2020, 28, 31–42. [Google Scholar]
- Maheswari, V.; Kumar, A.; Joshi, M.D. Evaluating the Potential Use of Electronic Tongue for Diabetic Patient. Int. J. Contemp. Res. Eng. Technol. 2020, 10, 46–49. [Google Scholar]
- Zaim, O.; Diouf, A.; El Bari, N.; Lagdali, N.; Benelbarhdadi, I.; Ajana, F.Z.; Llobet, E.; Bouchikhi, B. Comparative analysis of volatile organic compounds of breath and urine for distinguishing patients with liver cirrhosis from healthy controls by using electronic nose and voltammetric electronic tongue. Anal. Chim. Acta 2021, 1184, 339028. [Google Scholar] [CrossRef]
- He, Y.; Bai, X.; Xiao, Q.; Liu, F.; Zhou, L.; Zhang, C. Detection of adulteration in food based on nondestructive analysis techniques: A review. Crit. Rev. Food Sci. Nutr. 2021, 61, 2351–2371. [Google Scholar] [CrossRef]
- Saidi, T.; Zaim, O.; Moufid, M.; El Bari, N.; Ionescu, R.; Bouchikhi, B. Exhaled breath analysis using electronic nose and gas chromatography–mass spectrometry for non-invasive diagnosis of chronic kidney disease, diabetes mellitus and healthy subjects. Sens. Actuators B Chem. 2018, 257, 178–188. [Google Scholar] [CrossRef]
- Bougrini, M.; Tahri, K.; Haddi, Z.; El Bari, N.; Llobet, E.; Jaffrezic-Renault, N.; Bouchikhi, B. Aging time and brand determination of pasteurized milk using a multisensor e-nose combined with a voltammetric e-tongue. Mater. Sci. Eng. 2014, C 45, 348–358. [Google Scholar] [CrossRef]
- Ahmadou, D.; Laref, R.; Losson, E.; Siadat, M. Reduction of drift impact in gas sensor response to improve quantitative odor analysis. In Proceedings of the IEEE International Conference on Industrial Technology (ICIT), Toronto, ON, Canada, 22–25 March 2017; pp. 928–933. [Google Scholar]
- Zaim, O.; Saidi, T.; El Bari, N.; Bouchikhi, B. Assessment of “breath print” in patients with chronic kidney disease during dialysis by non-invasive breath screening of exhaled volatile compounds using an electronic nose. In Proceedings of the IEEE International Symposium on Olfaction and Electronic Nose (ISOEN), Fukuoka, Japan, 26–29 May 2019; pp. 1–4. [Google Scholar]
- Dragonieri, S.; Annema, J.T.; Schot, R.; van der Schee, M.P.; Spanevello, A.; Carratú, P.; Resta, O.; Rabe, K.F.; Sterk, P.J. An electronic nose in the discrimination of patients with non-small cell lung cancer and COPD. Lung Cancer 2009, 64, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Divya, J.; Vijendra, S. Feature selection and classification system for chronic diseases prediction review. Egypt. Inform. J. 2018, 19, 179–189. [Google Scholar]
- Pechenizkiy, M.; Tsymbal, A.; Puuronen, S. PCA-based feature transformation for classification: Issues in medical diagnostics. In Proceedings of the 17th IEEE Symposium on Computer-Based Medical Systems, Bethesda, MD, USA, 25 June 2004; pp. 535–540. [Google Scholar]
- Bougrini, M.; Tahri, K.; Haddi, Z.; Saidi, T.; El Bari, N.; Bouchikhi, B. Detection of adulteration in argan oil by using an electronic nose and a voltammetric electronic tongue. J. Sens. 2014, 2014, 245831. [Google Scholar] [CrossRef] [Green Version]
- Fawcett, T. An introduction to ROC analysis. Pattern Recognit. Lett. 2006, 27, 861–874. [Google Scholar] [CrossRef]
- Kumar, R.; Indrayan, A. Receiver operating characteristic (ROC) curve for medical researchers. Indian Pediatr. 2011, 48, 277–287. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Haddi, Z.; Amari, A.; Bouchikhi, B.; Mimendia, A.; Cetó, X.; del Valle, M. Hybrid electronic tongue based on multisensor data fusion for discrimination of beers. Sens. Actuators B Chem. 2013, 177, 989–996. [Google Scholar] [CrossRef] [Green Version]
- Haddi, Z.; Mabrouk, S.; Bougrini, M.; Tahri, K.; Sghaier, K.; Barhoumi, H.; El Bari, N.; Maaref, A.; Jaffrezic-Renault, N.; Bouchikhi, B. E-Nose and e-Tongue combination for improved recognition of fruit juice samples. Food Chem. 2014, 150, 246–253. [Google Scholar] [CrossRef]
- Zhu, W.; Mai, Z.; Qin, J.; Duan, X.; Liu, Y. Difference in 24-Hour Urine Composition between Diabetic and Non-Diabetic Adults without Nephrolithiasis. PLoS ONE 2016, 11, e0150006. [Google Scholar] [CrossRef]
- Cameron, M.A.; Maalouf, N.M.; Beverley, A.H.; Moe, O.W.; Sakhaee, K. Urine composition in type 2 diabetes: Predisposition to uric acid nephrolithiasis. J. Am. Soc. Nephrol. 2006, 17, 1422–1428. [Google Scholar] [CrossRef] [Green Version]
| Volunteers | ||||
|---|---|---|---|---|
| Groups | Number | Age Range (Years) Age ± SD * | Male, Number (%) | Smoking Habit + |
| Type 1 diabetes (T1DM) | 14 | 32–67 48 ± 8 | 6 (42%) | 14 NS |
| Type 2 diabetes (T2DM) | 18 | 34–75 59 ± 9 | 2 (11%) | 18 NS |
| Healthy controls (HC) | 28 | 25–64 40 ± 13 | 18 (64%) | |
| 4 S, 24 NS | ||||
| Actual | Predicted | |
|---|---|---|
| DM Patients | HC | |
| DM patients | 96 | 0 |
| HC | 1 | 83 |
| Actual | Predicted | |
|---|---|---|
| T1DM | T2DM | |
| T1DM | 33 | 9 |
| T2DM | 8 | 46 |
| Actual | Predicted | |
|---|---|---|
| DM Patients | HC | |
| DM patients | 189 | 3 |
| HC | 0 | 168 |
| Actual | Predicted | |
|---|---|---|
| T1DM | T2DM | |
| T1DM | 64 | 20 |
| T2DM | 26 | 82 |
| Actual | Predicted | |
|---|---|---|
| T1DM | T2DM | |
| T1DM | 41 | 1 |
| T2DM | 5 | 49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaim, O.; Bouchikhi, B.; Motia, S.; Abelló, S.; Llobet, E.; El Bari, N. Discrimination of Diabetes Mellitus Patients and Healthy Individuals Based on Volatile Organic Compounds (VOCs): Analysis of Exhaled Breath and Urine Samples by Using E-Nose and VE-Tongue. Chemosensors 2023, 11, 350. https://doi.org/10.3390/chemosensors11060350
Zaim O, Bouchikhi B, Motia S, Abelló S, Llobet E, El Bari N. Discrimination of Diabetes Mellitus Patients and Healthy Individuals Based on Volatile Organic Compounds (VOCs): Analysis of Exhaled Breath and Urine Samples by Using E-Nose and VE-Tongue. Chemosensors. 2023; 11(6):350. https://doi.org/10.3390/chemosensors11060350
Chicago/Turabian StyleZaim, Omar, Benachir Bouchikhi, Soukaina Motia, Sònia Abelló, Eduard Llobet, and Nezha El Bari. 2023. "Discrimination of Diabetes Mellitus Patients and Healthy Individuals Based on Volatile Organic Compounds (VOCs): Analysis of Exhaled Breath and Urine Samples by Using E-Nose and VE-Tongue" Chemosensors 11, no. 6: 350. https://doi.org/10.3390/chemosensors11060350
APA StyleZaim, O., Bouchikhi, B., Motia, S., Abelló, S., Llobet, E., & El Bari, N. (2023). Discrimination of Diabetes Mellitus Patients and Healthy Individuals Based on Volatile Organic Compounds (VOCs): Analysis of Exhaled Breath and Urine Samples by Using E-Nose and VE-Tongue. Chemosensors, 11(6), 350. https://doi.org/10.3390/chemosensors11060350







