Haptoglobin Electrochemical Diagnostic Method for Subclinical Mastitis Detection in Bovine Milk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Instrumentation
2.3. Synthesis and Functionalization of the Polyaniline Coated Magnetic Nanoparticles
2.4. Biofunctionalization of the Polyaniline Coated Magnetic Nanoparticles
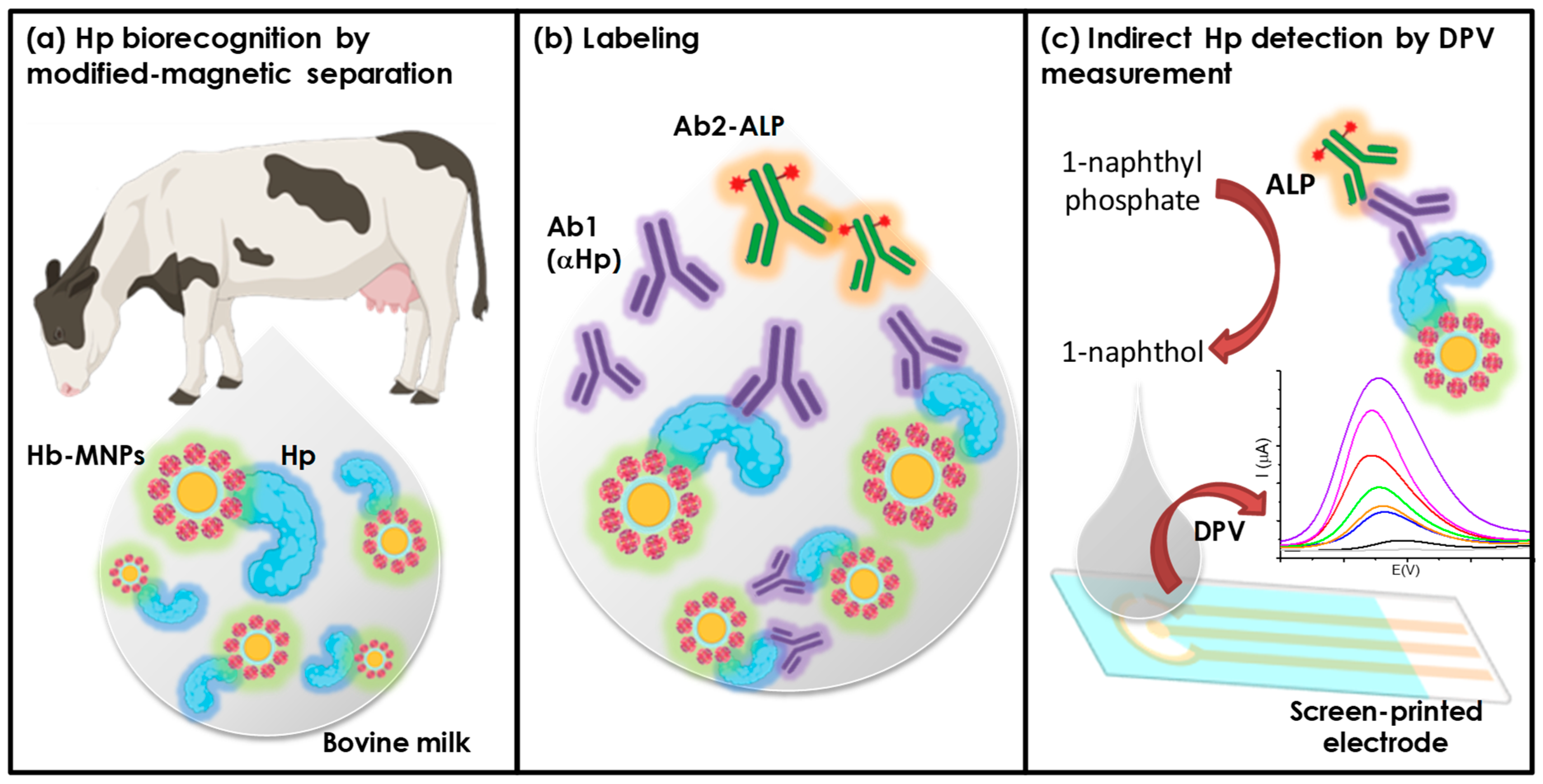
2.5. Electrochemical Determination of Haptoglobin Using MNPs@Chi/PANI-Hb
2.6. Sample Preparation
2.7. Bovine Haptoglobin Determination by ELISA Kit
3. Results and Discussion
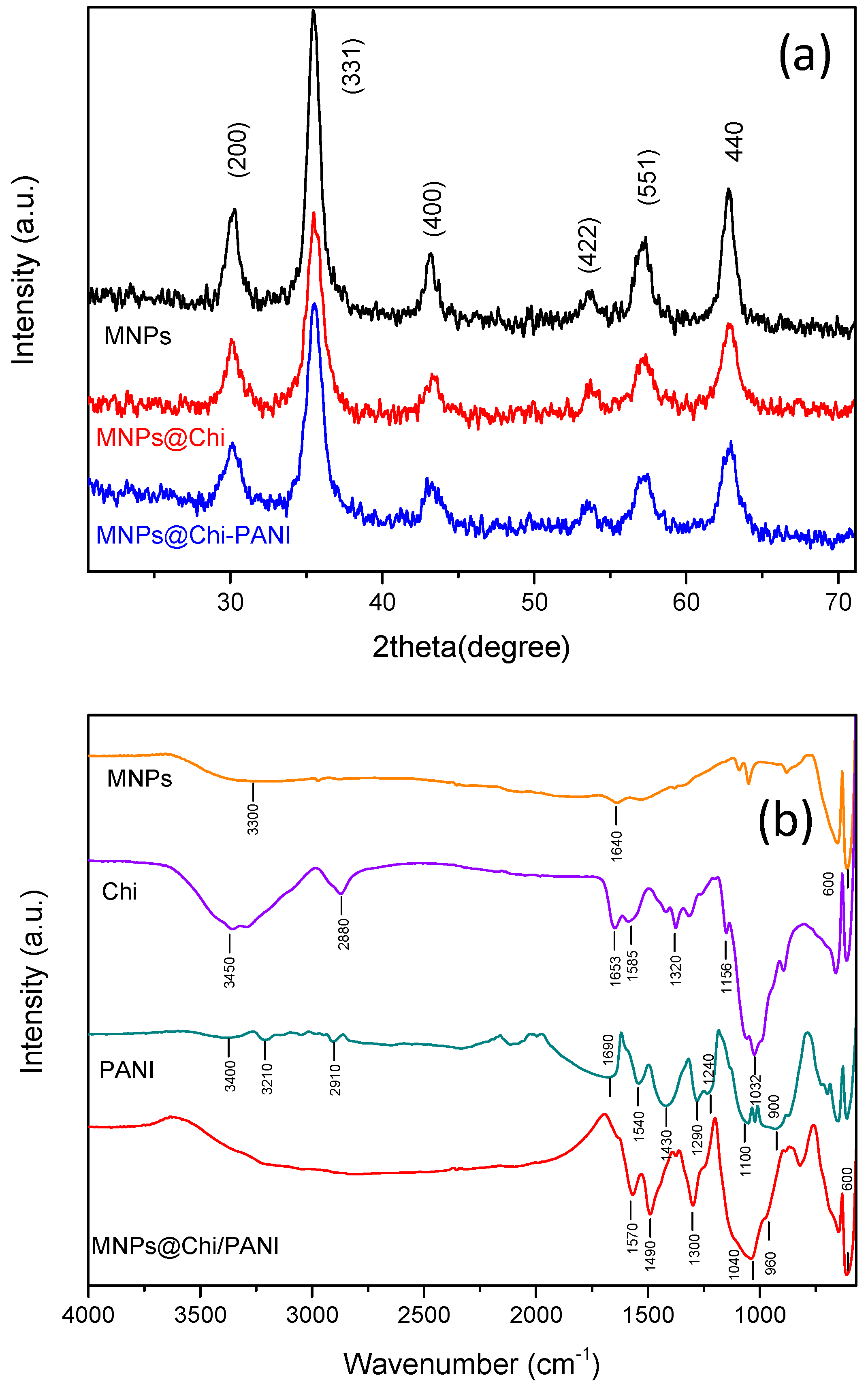
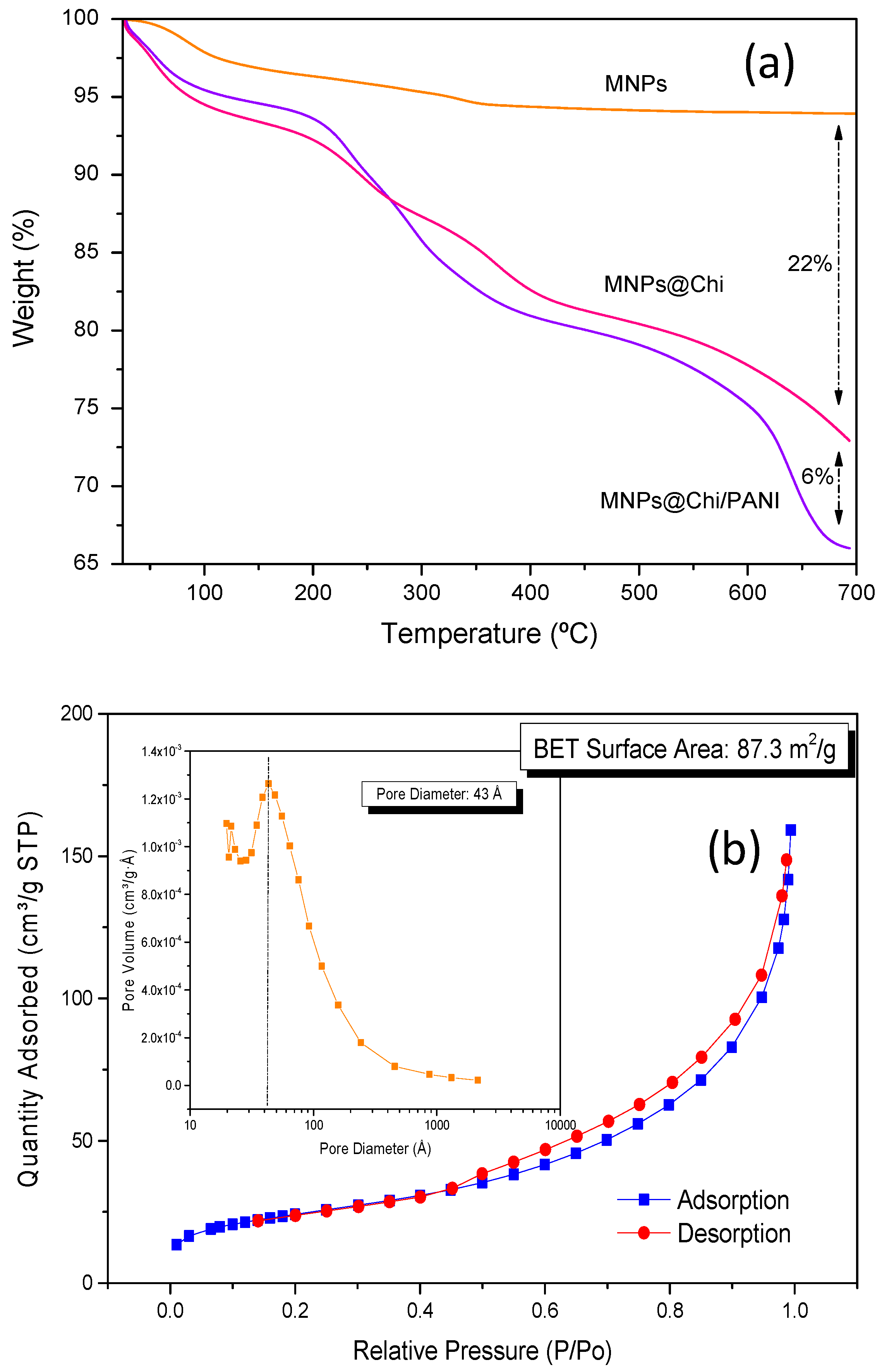
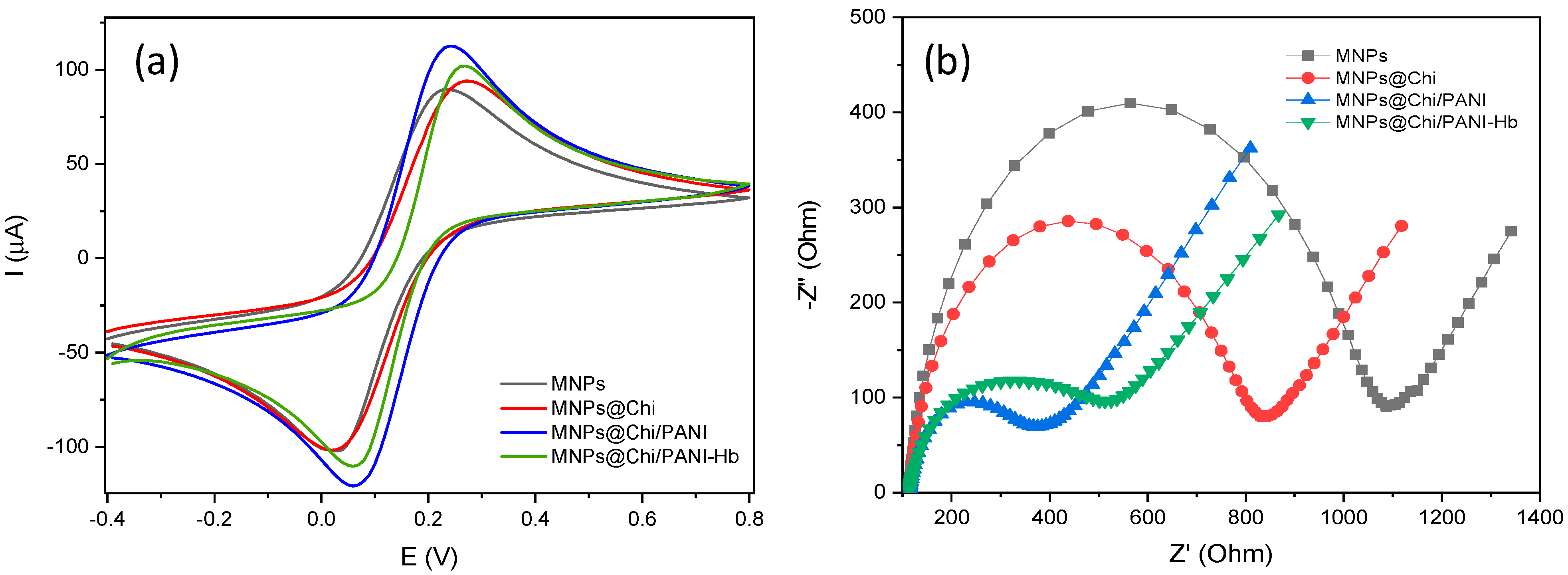
3.1. Characterization of MNPs@Chi/PANI Core–Shell Nanoparticles
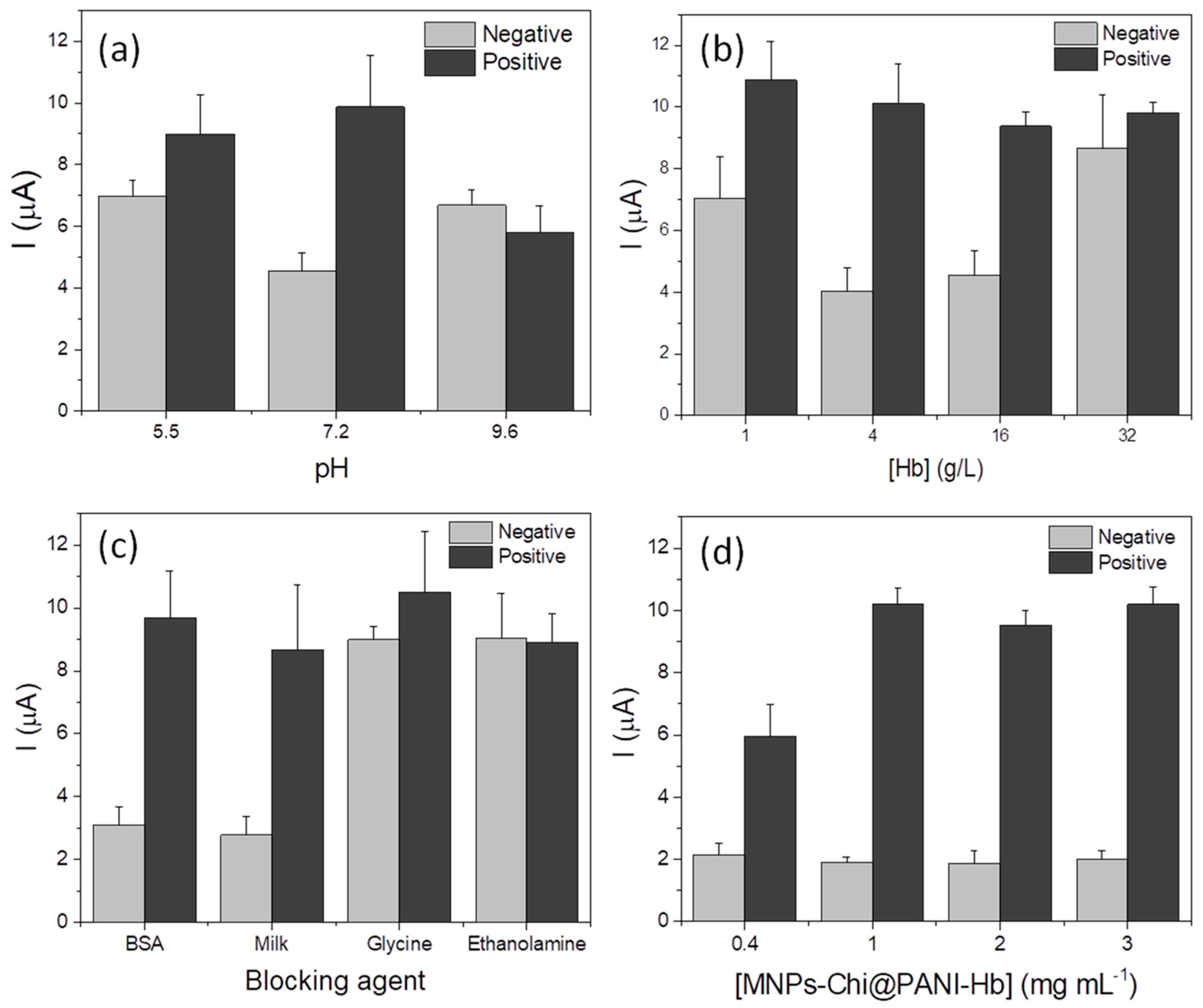
3.2. Optimization of Biofunctionalization of the MNPs@Chi/PANI with Hemoglobin
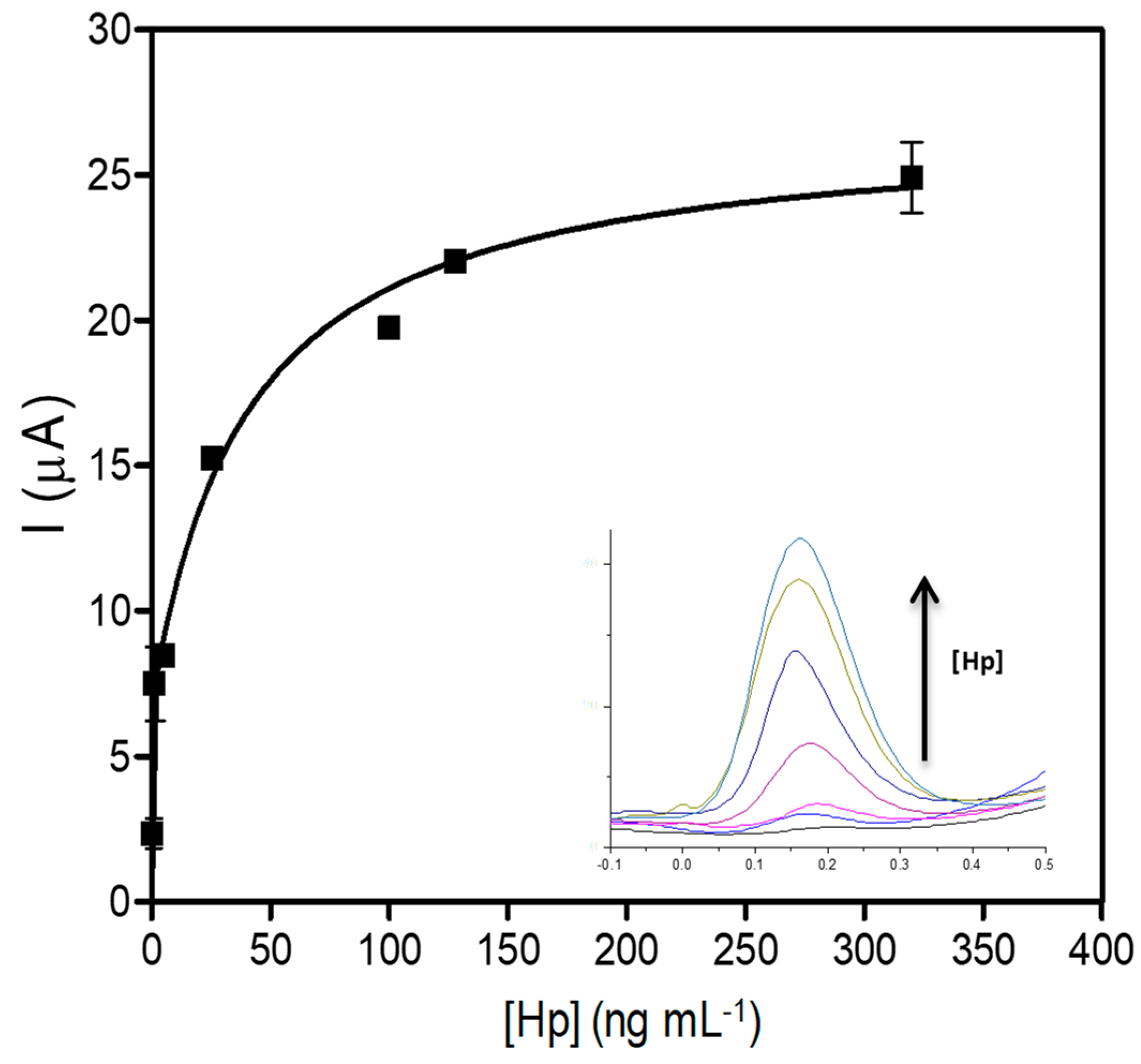
3.3. Calibration Curve for the Electrochemical Hp Detection
3.4. Matrix Effect of Milk on Bioassay Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petrovski, K.R.; Trajcev, M.; Buneski, G. A review of the factors affecting the costs of bovine mastitis. J. S. Afr. Vet. Assoc. 2006, 77, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Seegers, H.; Fourichon, C.; Beaudeau, F. Production effects related to mastitis and mastitis economics in dairy cattle herds. Vet. Res. 2003, 34, 475–491. [Google Scholar] [CrossRef] [Green Version]
- Abb-Schwedler, K.; Maeschli, A.; Boss, R.; Graber, H.U.; Steiner, A.; Klocke, P. Feeding mastitis milk to organic dairy calves: Effect on health and performance during suckling and on udder health at first calving. BMC Vet. Res. 2014, 10, 267. [Google Scholar] [CrossRef]
- Neculai-Valeanu, A.S.; Ariton, A.M. Udder health monitoring for prevention of bovine mastitis and improvement of milk quality. Bioengineering 2022, 9, 608. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, F.J.S.; Santman-Berends, I.M.G.A.; Lam, T.J.G.M.; Hogeveen, H. Failure and preventive costs of mastitis on Dutch dairy farms. J. Dairy Sci. 2016, 99, 8365–8374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reshi, A.A.; Husain, I.; Bhat, S.A.; Rehman, M.U.; Razak, R.; Bilal, S.; Mir, M.u.R. BOVINE MASTITIS AS AN EVOLVING DISEASE AND ITS IMPACT ON THE DAIRY INDUSTRY. Int. J. Curr. Res. Rev. 2015, 7, 48–55. [Google Scholar]
- Halasa, T.; Huijps, K.; Østerås, O.; Hogeveen, H. Economic effects of bovine mastitis and mastitis management: A review. Vet. Q. 2007, 29, 18–31. [Google Scholar] [CrossRef]
- FAO. Impact of Mastitis in Small Scale Dairy Production Systems; FAO: Rome, Italy, 2014. [Google Scholar]
- Corbellini, C.N. La mastitis bovina y su impacto sobre la calidad de la leche. In Seminario Internacional de Competitividad en Leche y Carne (3: Argentina); Memorias; INTA: Castelar, Argentina, 2002; pp. 251–263. [Google Scholar]
- Bedolla, C.; Castañeda, V.; Wolter, W. Métodos de detección de la mastitis bovina (Methods of detection of the bovine mastitis). Redvet 2007, 9. [Google Scholar]
- Hussein, H.A.; El-Razik, K.A.E.-H.A.; Gomaa, A.M.; Elbayoumy, M.K.; Abdelrahman, K.A.; Hosein, H.I. Milk amyloid A as a biomarker for diagnosis of subclinical mastitis in cattle. Vet. World 2018, 11, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, A.; Reis, E.; Ferraz, P.; Barbari, M.; Santos, G.; Cruz, M.; Silva, G.; Silva, A. Infrared thermography as a technique for detecting subclinical bovine mastitis. Arq. Bras. Med. Veterinária Zootec. 2023, 74, 992–998. [Google Scholar] [CrossRef]
- Addis, M.F.; Tedde, V.; Puggioni, G.M.G.; Pisanu, S.; Casula, A.; Locatelli, C.; Rota, N.; Bronzo, V.; Moroni, P.; Uzzau, S. Evaluation of milk cathelicidin for detection of bovine mastitis. J. Dairy Sci. 2016, 99, 8250–8258. [Google Scholar] [CrossRef] [Green Version]
- Mansor, R.; Mullen, W.; Albalat, A.; Zerefos, P.; Mischak, H.; Barrett, D.C.; Biggs, A.; Eckersall, P.D. A peptidomic approach to biomarker discovery for bovine mastitis. J. Proteom. 2013, 85, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Åkerstedt, M.; Waller, K.P.; Larsen, L.B.; Forsbäck, L.; Sternesjö, Å. Relationship between haptoglobin and serum amyloid A in milk and milk quality. Int. Dairy J. 2008, 18, 669–674. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, S.; Dhama, K.; Tiwari, R.; Iqbal Yatoo, M.; Khurana, S.K.; Khandia, R.; Munjal, A.; Munuswamy, P.; Kumar, M.A.; Singh, M. Technological interventions and advances in the diagnosis of intramammary infections in animals with emphasis on bovine population—A review. Vet. Q. 2019, 39, 76–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leroux, C.; Pawlowski, K.; Billa, P.-A.; Pires, J.A.; Faulconnier, Y. Milk fat globules as a source of microRNAs for mastitis detection. Livest. Sci. 2022, 263, 104997. [Google Scholar] [CrossRef]
- Martins, S.A.; Martins, V.C.; Cardoso, F.A.; Germano, J.; Rodrigues, M.; Duarte, C.; Bexiga, R.; Cardoso, S.; Freitas, P.P. Biosensors for on-farm diagnosis of mastitis. Front. Bioeng. Biotechnol. 2019, 7, 186. [Google Scholar] [CrossRef]
- Engler, R. Acute-phase proteins in inflammation. Comptes Rendus Seances Soc. Biol. Ses Fil. 1995, 189, 563–578. [Google Scholar]
- Jain, S.; Gautam, V.; Naseem, S. Acute-phase proteins: As diagnostic tool. J. Pharm. Bioallied Sci. 2011, 3, 118. [Google Scholar] [CrossRef]
- Tothova, C.; Nagy, O.; KOVAC, G. Acute phase proteins and their use in the diagnosis of diseases in ruminants: A review. Vet. Med. 2014, 59, 163. [Google Scholar] [CrossRef] [Green Version]
- Eckersall, P.; Conner, J. Bovine and canine acute phase proteins. Vet. Res. Commun. 1988, 12, 169–178. [Google Scholar] [CrossRef]
- Grönlund, U.; Hultén, C.; Eckersall, P.D.; Hogarth, C.; Waller, K.P. Haptoglobin and serum amyloid A in milk and serum during acute and chronic experimentally induced Staphylococcus aureus mastitis. J. Dairy Res. 2003, 70, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, M.; Sugiyama, M.; Ito, T.; Tsukano, K.; Oikawa, S.; Suzuki, K. Diagnostic utility of measuring serum amyloid A with a latex agglutination turbidimetric immunoassay in bovine mastitis: Comparison with haptoglobin and alpha 1 acid glycoprotein. J. Vet. Med. Sci. 2021, 83, 329–332. [Google Scholar] [CrossRef]
- Bassols, A.; Robles-Guirado, J.A.; Arroyo, L.; Soler, L.; García, N.; Pato, R.; Peña, R.; Saco, Y.; Armengol, R.; Lampreave, F. Validation of new automated turbidimetric immunoassays for the measurement of haptoglobin and inter-α-trypsin inhibitor heavy chain H4 specific for the bovine species. Vet. Clin. Pathol. 2023, 52, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Åkerstedt, M.; Björck, L.; Waller, K.P.; Sternesjö, Å. Biosensor assay for determination of haptoglobin in bovine milk. J. Dairy Res. 2006, 73, 299–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nirala, N.R.; Harel, Y.; Lellouche, J.-P.; Shtenberg, G. Ultrasensitive haptoglobin biomarker detection based on amplified chemiluminescence of magnetite nanoparticles. J. Nanobiotechnol. 2020, 18, 6. [Google Scholar] [CrossRef] [Green Version]
- Nirala, N.R.; Pinker, N.; Desitti, C.; Shtenberg, G. Milk haptoglobin detection based on enhanced chemiluminescence of gold nanoparticles. Talanta 2019, 197, 257–263. [Google Scholar] [CrossRef]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763. [Google Scholar] [CrossRef]
- Razavi, N.; Es’haghi, Z. Employ of magnetic polyaniline coated chitosan nanocomposite for extraction and determination of phthalate esters in diapers and wipes using gas chromatography. Microchem. J. 2018, 142, 359–366. [Google Scholar] [CrossRef]
- Jiang, X.; Cheng, J.; Zhou, H.; Li, F.; Wu, W.; Ding, K. Polyaniline-coated chitosan-functionalized magnetic nanoparticles: Preparation for the extraction and analysis of endocrine-disrupting phenols in environmental water and juice samples. Talanta 2015, 141, 239–246. [Google Scholar] [CrossRef]
- Jang, J.; Ha, J.; Lim, B. Synthesis and characterization of monodisperse silica–polyaniline core–shell nanoparticles. Chem. Commun. 2006, 15, 1622–1624. [Google Scholar] [CrossRef] [Green Version]
- Martín, M.; González Orive, A.; Lorenzo-Luis, P.; Hernández Creus, A.; González-Mora, J.L.; Salazar, P. Quinone-Rich Poly(dopamine) Magnetic Nanoparticles for Biosensor Applications. ChemPhysChem 2014, 15, 3742–3752. [Google Scholar] [CrossRef] [PubMed]
- Prodan, A.M.; Iconaru, S.L.; Chifiriuc, C.M.; Bleotu, C.; Ciobanu, C.S.; Motelica-Heino, M.; Sizaret, S.; Predoi, D. Magnetic Properties and Biological Activity Evaluation of Iron Oxide Nanoparticles. J. Nanomater. 2013, 2013, 893970. [Google Scholar] [CrossRef] [Green Version]
- Esyanti, R.R.; Zaskia, H.; Amalia, A.; Nugrahapraja, D.H. Chitosan Nanoparticle-Based Coating as Post-harvest Technology in Banana. J. Phys. Conf. Ser. 2019, 1204, 012109. [Google Scholar] [CrossRef]
- Nirmala, R.; Il, B.W.; Navamathavan, R.; El-Newehy, M.H.; Kim, H.Y. Preparation and characterizations of anisotropic chitosan nanofibers via electrospinning. Macromol. Res. 2011, 19, 345. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, S.; Wu, Q.; Ran, J.; Zhang, W.; Wu, S. Studies of Fe3O4-chitosan nanoparticles prepared by co-precipitation under the magnetic field for lipase immobilization. Catal. Commun. 2011, 12, 717–720. [Google Scholar] [CrossRef]
- Zulfikar, M.A.; Afrita, S.; Wahyuningrum, D.; Ledyastuti, M. Preparation of Fe3O4-chitosan hybrid nano-particles used for humic acid adsorption. Environ. Nanotechnol. Monit. Manag. 2016, 6, 64–75. [Google Scholar] [CrossRef]
- Davodi, B.; Jahangiri, M.; Ghorbani, M. Magnetic Fe3O4@ polydopamine biopolymer: Synthesis, characterization and fabrication of promising nanocomposite. J. Vinyl Addit. Technol. 2019, 25, 41–47. [Google Scholar] [CrossRef]
- Ziegler-Borowska, M.; Chełminiak, D.; Kaczmarek, H. Thermal stability of magnetic nanoparticles coated by blends of modified chitosan and poly(quaternary ammonium) salt. J. Therm. Anal. Calorim. 2015, 119, 499–506. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Xiao, L.; Qin, C. The Characterization and Thermal Investigation of Chitosan-Fe3O4 Nanoparticles Synthesized Via A Novel One-step Modifying Process. J. Macromol. Sci. Part A 2010, 48, 57–64. [Google Scholar] [CrossRef]
- Unsoy, G.; Yalcin, S.; Khodadust, R.; Gunduz, G.; Gunduz, U. Synthesis optimization and characterization of chitosan-coated iron oxide nanoparticles produced for biomedical applications. J. Nanopart. Res. 2012, 14, 964. [Google Scholar] [CrossRef]
- Yavuz, A.G.; Uygun, A.; Bhethanabotla, V.R. Substituted polyaniline/chitosan composites: Synthesis and characterization. Carbohydr. Polym. 2009, 75, 448–453. [Google Scholar] [CrossRef]
- Alothman, Z.A. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef] [Green Version]
- Schneider, P. Adsorption isotherms of microporous-mesoporous solids revisited. Appl. Catal. A Gen. 1995, 129, 157–165. [Google Scholar] [CrossRef]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques 2004, 37, 790–802. [Google Scholar] [CrossRef]
- Carinelli, S.; Xufré, C.; Martí, M.; Pividori, M.I. Interferon gamma transcript detection on T cells based on magnetic actuation and multiplex double-tagging electrochemical genosensing. Biosens. Bioelectron. 2018, 117, 183–190. [Google Scholar] [CrossRef]
- Masdor, N.A. Determination of the detection limit using the four-parameter logistic model for the double-antibody sandwich ELISA for the rapid detection of Bacillus cereus in food. J. Environ. Microbiol. Toxicol. 2017, 5, 12–13. [Google Scholar] [CrossRef]
- Brady, N.; O’Reilly, E.L.; McComb, C.; Macrae, A.I.; Eckersall, P.D. An immunoturbidimetric assay for bovine haptoglobin. Comp. Clin. Pathol. 2019, 28, 21–27. [Google Scholar] [CrossRef]
- Mohamad, A.; Keasberry, N.A.; Ahmed, M.U. Enzyme-free gold-silver core-shell nanozyme immunosensor for the detection of haptoglobin. Anal. Sci. 2018, 34, 1257–1263. [Google Scholar] [CrossRef] [Green Version]
- Weng, X.; Ahmed, S.R.; Neethirajan, S. A nanocomposite-based biosensor for bovine haptoglobin on a 3D paper-based analytical device. Sens. Actuators B Chem. 2018, 265, 242–248. [Google Scholar] [CrossRef]
- Botía, M.; López-Arjona, M.; Escribano, D.; Contreras-Aguilar, M.; Vallejo-Mateo, P.; Cerón, J.; Martínez-Subiela, S. Measurement of haptoglobin in saliva of cows: Validation of an assay and a pilot study of its potential application. Res. Vet. Sci. 2023, 158, 44–49. [Google Scholar] [CrossRef]
- N Sonuc, M.; K Sezgintürk, M. A Disposable, highly sensitive biosensing system: Determination of haptoglobin as a significant acute phase biomarker. Curr. Anal. Chem. 2016, 12, 43–53. [Google Scholar] [CrossRef]
- Carinelli, S.; Fernandez, I.; González-Mora, J.L.; Salazar-Carballo, P.A. Hemoglobin-modified nanoparticles for electrochemical determination of haptoglobin: Application in bovine mastitis diagnosis. Microchem. J. 2022, 179, 107528. [Google Scholar] [CrossRef]
- Tan, X.; Ding, S.Q.; Hu, Y.X.; Li, J.J.; Zhou, J.Y. Development of an immunosensor assay for detection of haptoglobin in mastitic milk. Vet. Clin. Pathol. 2012, 41, 575–581. [Google Scholar] [CrossRef]
- Quy, D.V.; Hieu, N.M.; Tra, P.T.; Nam, N.H.; Hai, N.H.; Thai Son, N.; Nghia, P.T.; Anh, N.T.V.; Hong, T.T.; Luong, N.H. Synthesis of Silica-Coated Magnetic Nanoparticles and Application in the Detection of Pathogenic Viruses. J. Nanomater. 2013, 2013, 603940. [Google Scholar] [CrossRef] [Green Version]
- Martín, M.; Salazar, P.; Campuzano, S.; Villalonga, R.; Pingarrón, J.M.; González-Mora, J.L. Amperometric magnetobiosensors using poly(dopamine)-modified Fe3O4 magnetic nanoparticles for the detection of phenolic compounds. Anal. Methods 2015, 7, 8801–8808. [Google Scholar] [CrossRef] [Green Version]
- Petcharoen, K.; Sirivat, A. Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B 2012, 177, 421–427. [Google Scholar] [CrossRef]
- De Queiroz Antonino, R.S.C.M.; Lia Fook, B.R.P.; de Oliveira Lima, V.A.; de Farias Rached, R.Í.; Lima, E.P.N.; da Silva Lima, R.J.; Peniche Covas, C.A.; Lia Fook, M.V. Preparation and Characterization of Chitosan Obtained from Shells of Shrimp (Litopenaeus vannamei Boone). Mar. Drugs 2017, 15, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.; Peng, S.; Jiang, W. Electrochemical Properties of PANI as Single Electrode of Electrochemical Capacitors in Acid Electrolytes. Sci. World J. 2013, 2013, 940153. [Google Scholar] [CrossRef] [PubMed]
- Trchová, M.; Šeděnková, I.; Tobolková, E.; Stejskal, J. FTIR spectroscopic and conductivity study of the thermal degradation of polyaniline films. Polym. Degrad. Stab. 2004, 86, 179–185. [Google Scholar] [CrossRef]
- Malorny, B.; Hoorfar, J.; Bunge, C.; Helmuth, R. Multicenter validation of the analytical accuracy of Salmonella PCR: Towards an international standard. Appl. Environ. Microbiol. 2003, 69, 290–296. [Google Scholar] [CrossRef] [Green Version]


| Assay Format | Detection System | Bioreceptor | Detection Range/LOD | Matrix/Sample | Description | Ref. |
|---|---|---|---|---|---|---|
| Bioassay based on magnetite nanoparticles | Chemiluminescence | Haemoglobin | Range: 1 pg mL−1 to 1 µg mL−1; LOD: 0.89 pg mL−1 | Milk matrices |
| [27] |
| Antibody-modified latex microparticles (immunoturbidimetry) | Turbidimetric detection | Hp-specific antibody | Range: 0.250 to 0.016 mg mL−1; LOD: 0.005 mg mL−1, LOQ: 0.007 mg mL−1. | Serum from Holstein cows |
| [25] |
| Automated species-specific immunoturbidimetric (IT) | Immunoturbidimetric | Hp-specific antibody | Range: 0.2 to 1.5 g L−1; LOD: 0.018 mg mL−1, LOQ: 0.033 g L−1. | Spiked serum samples |
| [47] |
| AlphaLISA technology, based on amplified luminescence by the proximity of donor and acceptor beads. | Luminescent amplification | Hp-specific antibody | Range: 8 to 533 ng mL−1; LOQ: 7.9 ng mL−1. The LOD could not be calculated since all values obtained were zero. | Bovine saliva cow saliva, and simple saliva samples were taken from Holstein–Friesen multiparous cows from a farm |
| [50] |
| Paper-based immunochromatographic assay | Colour intensity | Hp-specific antibody | Range: 10 to 900 μg mL−1; LOD: 28 μg mL−1. | Spiked |
| [49] |
| ITO-based anti-Hp biosensor | Impedimetric biosensor | Hp-specific antibody | Range: 0.2 to 1 fg mL−1; LOD: 0.001 fg mL−1. | Artificial serum samples |
| [51] |
| Enzyme-mimic NP immunoassay, enzyme-free gold–silver core–shell nanozyme immunosensor | Colorimetric | Hp-specific antibody | Range: 100 to 1000 pg mL−1; LOD: 100 pg mL−1. | Artificial and spiked serum samples |
| [48] |
| Electrochemical Hp biosensor based on MNPs-Chi@PANI-Hb | Differential pulse voltammetry | Haemoglobin | Range: 0.001 to 0.32 μg mL−1; LOD: 31 ng mL−1. | Spiked milk and real milk sample |
| This work |
| Samples | Hp (ELISA) (μg mL−1) * | Biosensor Classification |
|---|---|---|
| 1 | 21.3 ± 0.9 | Unacceptable |
| 2 | 50 ± 1 | Unacceptable |
| 3 | 46.9 ± 0.3 | Unacceptable |
| 4 | 7.42 ± 0.08 | Acceptable |
| 5 | 7.6 ± 0.3 | Acceptable |
| 6 | 40.9 ± 0.1 | Ambiguous |
| 7 | 47 ± 1 | Unacceptable |
| 8 | 9.0 ± 0.2 | Acceptable |
| 9 | 0.13 ± 0.01 | Acceptable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carinelli, S.; Fernández, I.; González-Mora, J.L.; Salazar-Carballo, P.A. Haptoglobin Electrochemical Diagnostic Method for Subclinical Mastitis Detection in Bovine Milk. Chemosensors 2023, 11, 378. https://doi.org/10.3390/chemosensors11070378
Carinelli S, Fernández I, González-Mora JL, Salazar-Carballo PA. Haptoglobin Electrochemical Diagnostic Method for Subclinical Mastitis Detection in Bovine Milk. Chemosensors. 2023; 11(7):378. https://doi.org/10.3390/chemosensors11070378
Chicago/Turabian StyleCarinelli, Soledad, Iñigo Fernández, José Luis González-Mora, and Pedro A. Salazar-Carballo. 2023. "Haptoglobin Electrochemical Diagnostic Method for Subclinical Mastitis Detection in Bovine Milk" Chemosensors 11, no. 7: 378. https://doi.org/10.3390/chemosensors11070378








