Development of Chemiluminescent ELISA for Detection of Diisobutyl Phthalate in Water, Lettuce and Aquatic Organisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
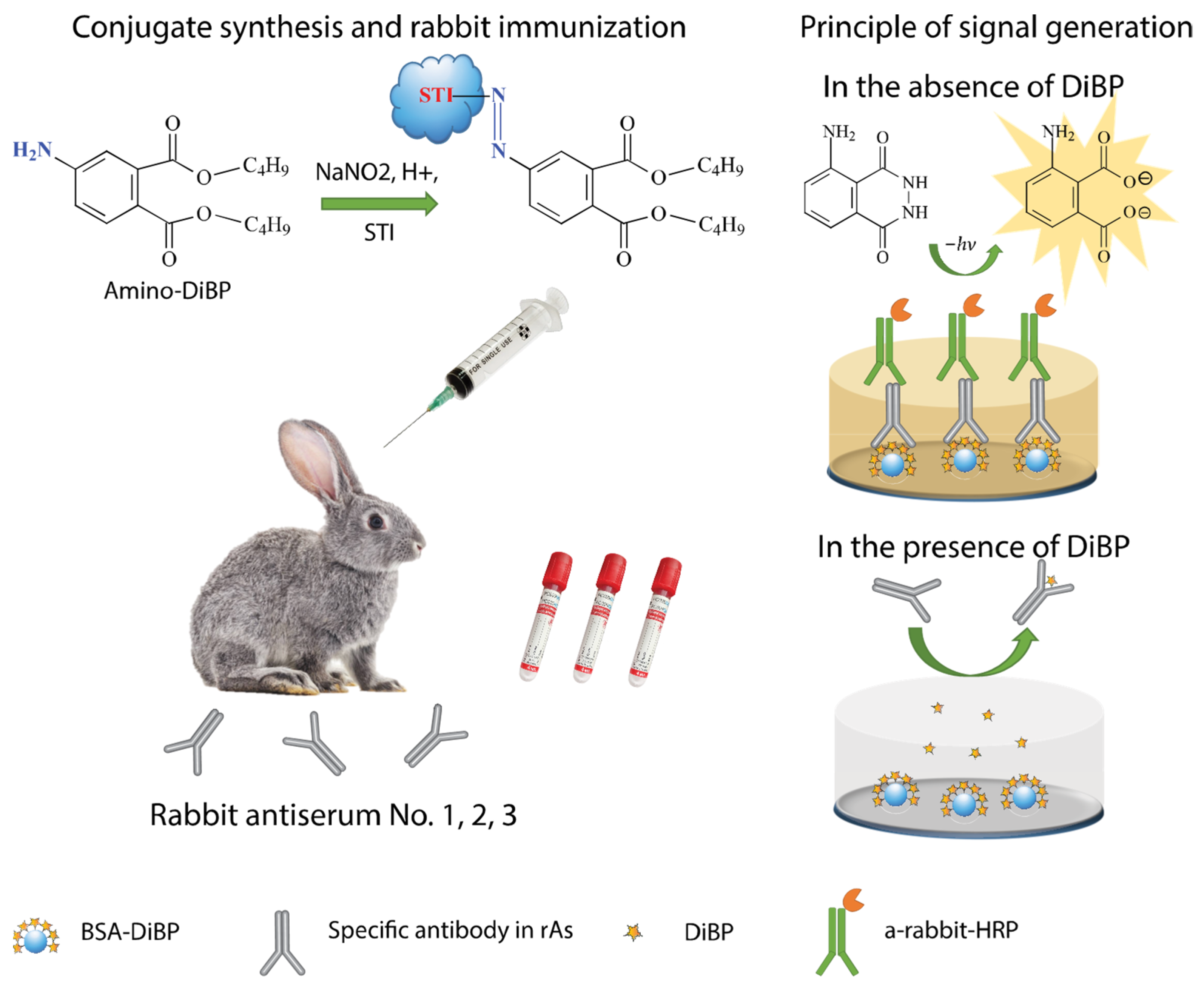
2.2.1. Synthesis of the Amino Derivative of DiBP
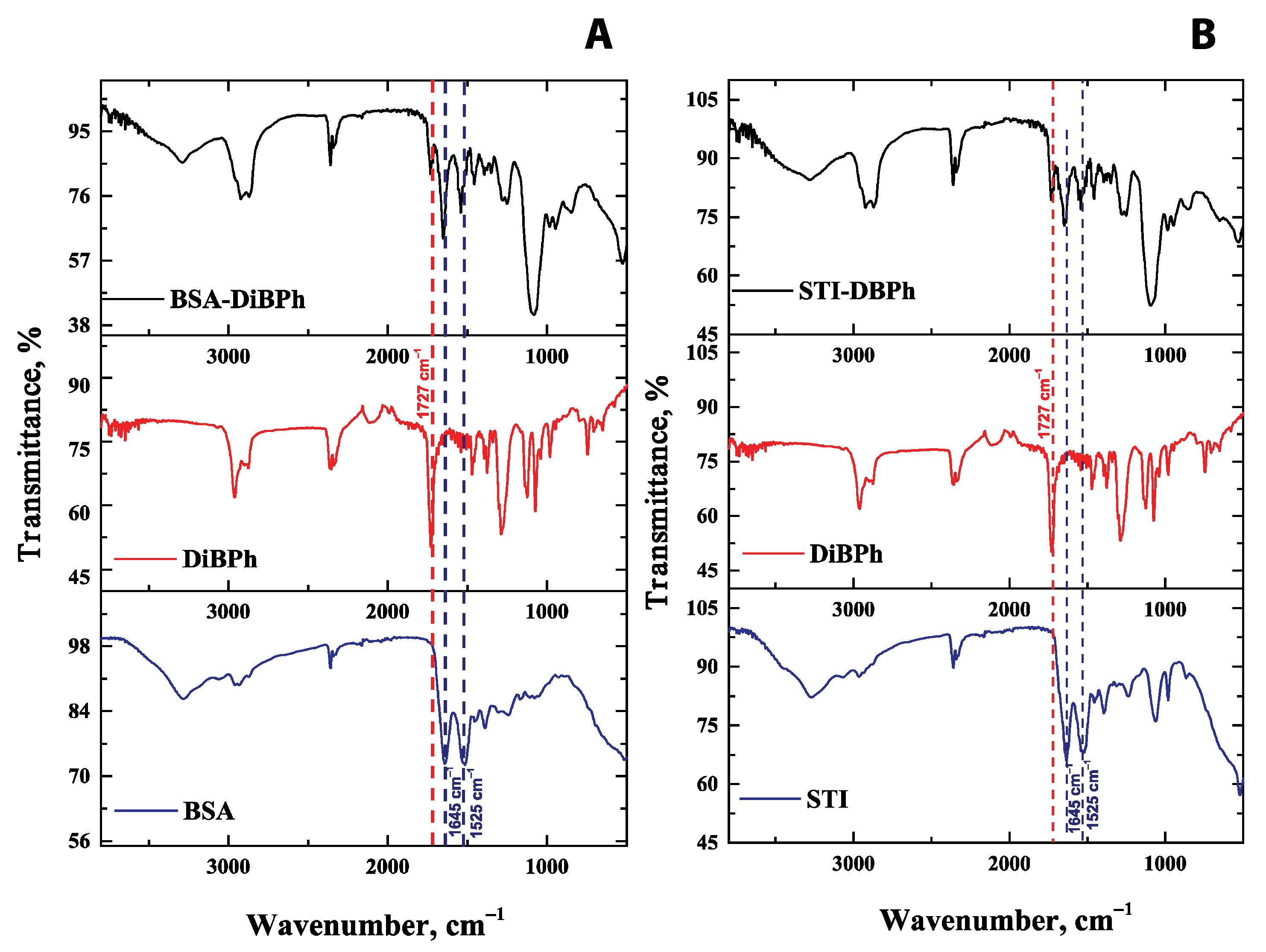
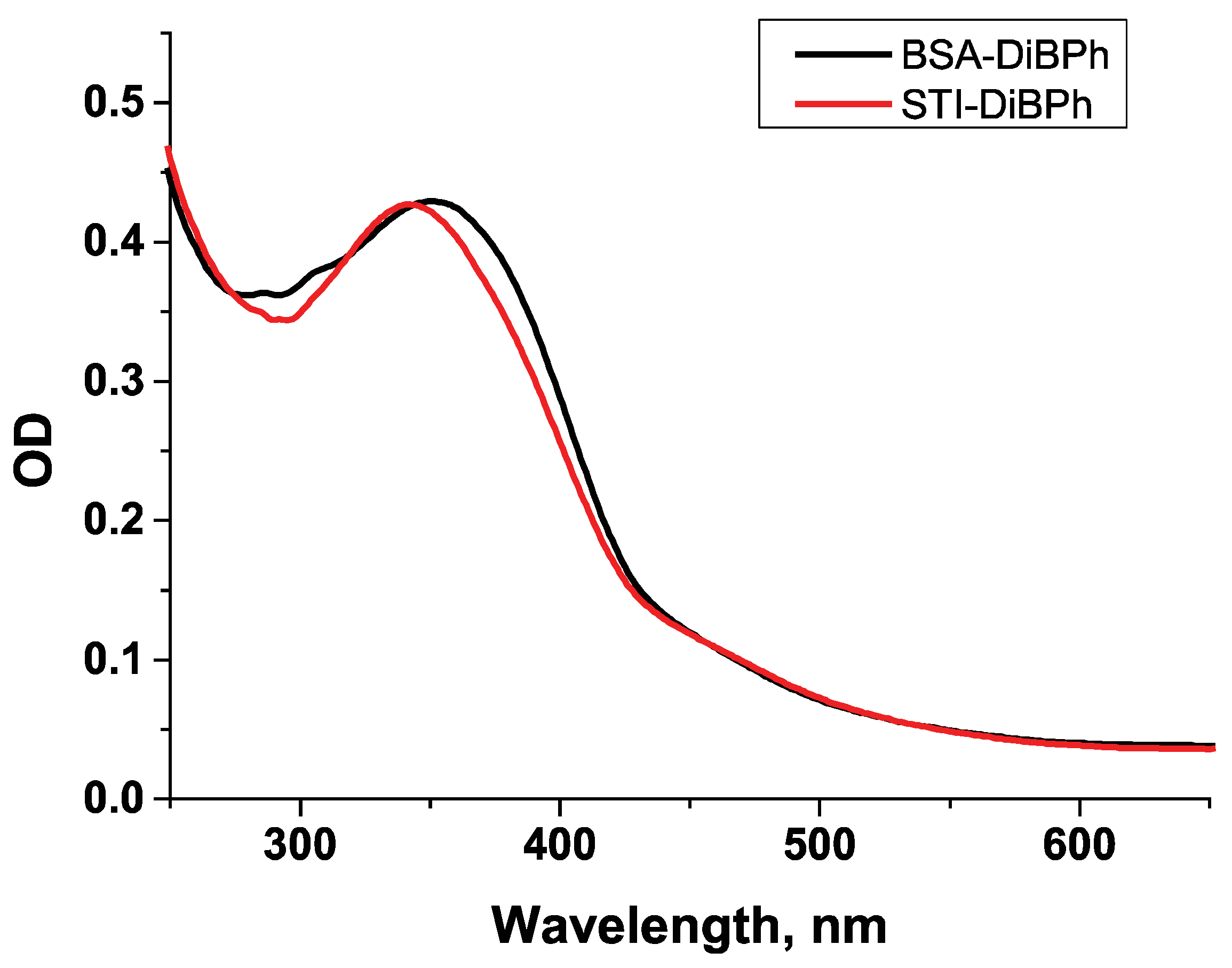
2.2.2. Synthesis of Conjugates of DiBP with Carrier Proteins
2.2.3. Rabbit Immunization
2.2.4. Determination of Antibodies by ELISA
2.2.5. Chemiluminescent ELISA (CL-ELISA)
2.2.6. ELISA Data Processing
2.2.7. Collection and Preparation of the Probes
3. Results and Discussion
3.1. Rabbit Immunization and Antisera Obtaining
3.2. Characterization of Antisera by Colorimetric ELISA
3.3. The Use of Methanol in the Working Buffer during Competitive Interaction in Chemiluminescent ELISA
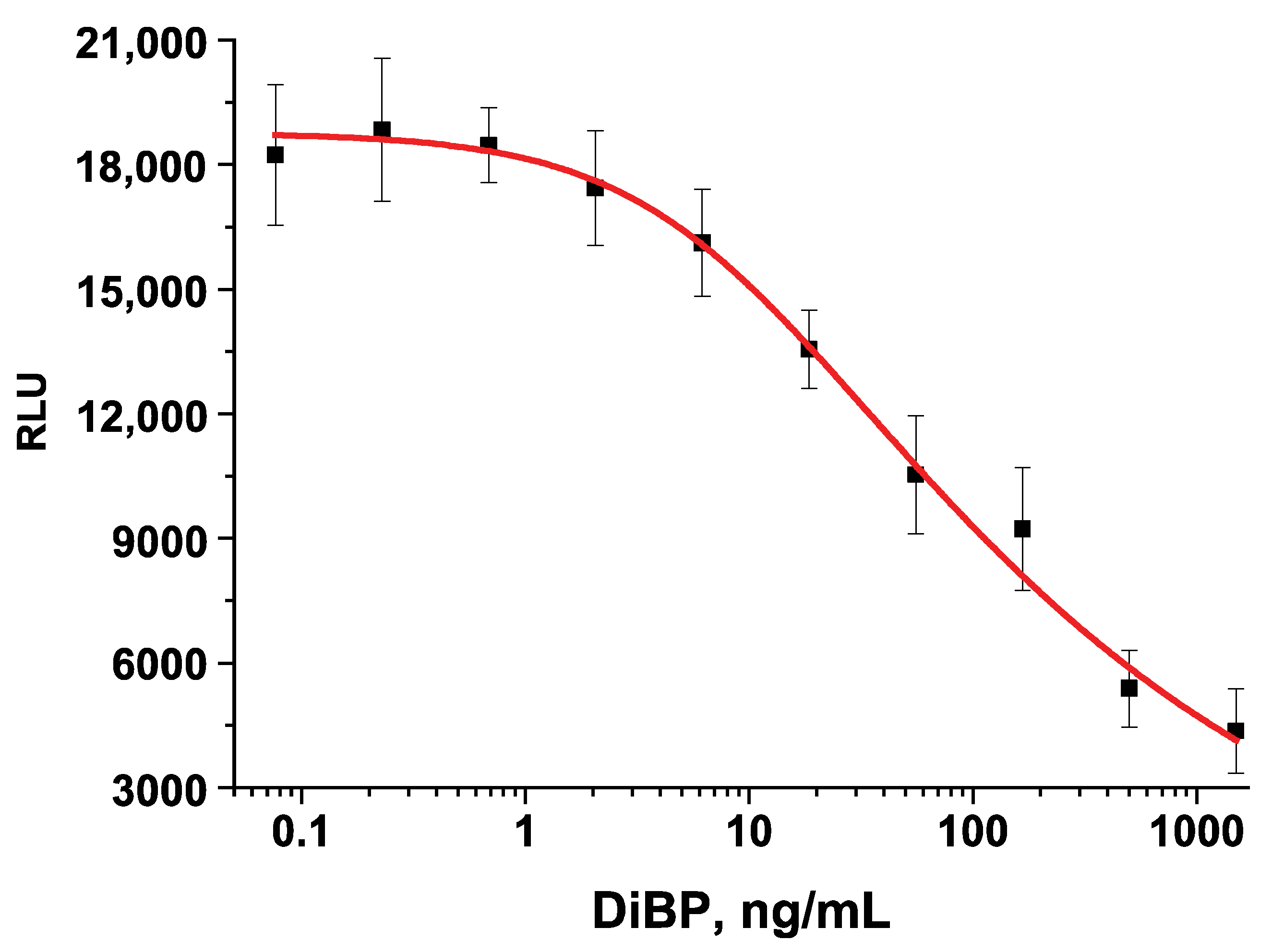
3.4. Analytical Parameters of CL-ELISA
3.5. Analysis of Samples Obtained by Various Extraction Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rubio, L.; Valverde-Som, L.; Sarabia, L.; Ortiz, M. The behaviour of Tenax as food simulant in the migration of polymer additives from food contact materials by means of gas chromatography/mass spectrometry and PARAFAC. J. Chromatogr. A 2019, 1589, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Bononi, M.; Tateo, F. Identification of diisobutyl phthalate (DIBP) suspected as possible contaminant in recycled cellulose for take-away pizza boxes. Packag. Technol. Sci. Int. J. 2009, 22, 53–58. [Google Scholar] [CrossRef]
- Benson, R. Hazard to the developing male reproductive system from cumulative exposure to phthalate esters—Dibutyl phthalate, diisobutyl phthalate, butylbenzyl phthalate, diethylhexyl phthalate, dipentyl phthalate, and diisononyl phthalate. Regul. Toxicol. Pharmacol. 2009, 53, 90–101. [Google Scholar] [CrossRef]
- Latini, G.; Del Vecchio, A.; Massaro, M.; Verrotti, A.; De Felice, C. Phthalate exposure and male infertility. Toxicology 2006, 226, 90–98. [Google Scholar] [CrossRef]
- Giribabu, N.; Reddy, P.S. Protection of male reproductive toxicity in rats exposed to di-n-butyl phthalate during embryonic development by testosterone. Biomed. Pharmacother. 2017, 87, 355–365. [Google Scholar] [CrossRef]
- Wang, S.; Wang, S.S.; Wu, D.; Lin, Y.; Ku, C.; Wu, C.; Chai, C.; Lee, J.; Tsai, E.; Lin, C.-L. Androgen receptor-mediated apoptosis in bovine testicular induced pluripotent stem cells in response to phthalate esters. Cell Death Dis. 2013, 4, e907. [Google Scholar] [CrossRef] [Green Version]
- Geueke, B.; Inostroza, P.A.; Maffini, M.; Backhaus, T.; Carney-Almroth, B.; Groh, K.J.; Muncke, J. Prioritization Approaches for Hazardous Chemicals Associated with Plastic Packaging; Food Packaging Forum: Zurich, Switzerland, 2018. [Google Scholar]
- Maffini, M.V.; Geueke, B.; Groh, K.; Carney Almroth, B.; Muncke, J. Role of epidemiology in risk assessment: A case study of five ortho-phthalates. Environ. Health 2021, 20, 114. [Google Scholar] [CrossRef]
- Yost, E.E.; Euling, S.Y.; Weaver, J.A.; Beverly, B.E.J.; Keshava, N.; Mudipalli, A.; Arzuaga, X.; Blessinger, T.; Dishaw, L.; Hotchkiss, A.; et al. Hazards of diisobutyl phthalate (DIBP) exposure: A systematic review of animal toxicology studies. Environ. Int. 2019, 125, 579–594. [Google Scholar] [CrossRef]
- Hlisníková, H.; Kolena, B.; Šidlovská, M.; Mlynček, M.; Petrovičová, I. Urinary Phthalate Biomarkers during Pregnancy, and Maternal Endocrine Parameters in Association with Anthropometric Parameters of Newborns. Children 2022, 9, 413. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Leung, A.O.W.; Chu, L.H.; Wong, M.H. Phthalates contamination in China: Status, trends and human exposure-with an emphasis on oral intake. Environ. Pollut. 2018, 238, 771–782. [Google Scholar] [CrossRef]
- Guo, Y.; Kannan, K. Challenges encountered in the analysis of phthalate esters in foodstuffs and other biological matrices. Anal. Bioanal. Chem. 2012, 404, 2539–2554. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Serrano, M.; Borrull, F.; Marcé, R.M.; Pocurull, E. Phthalate esters in marine ecosystems: Analytical methods, occurrence and distribution. TrAC Trends Anal. Chem. 2022, 151, 116598. [Google Scholar] [CrossRef]
- Cui, X.; Wu, P.; Lai, D.; Zheng, S.; Chen, Y.; Eremin, S.A.; Peng, W.; Zhao, S. Development of a highly specific fluorescence immunoassay for detection of diisobutyl phthalate in edible oil samples. J. Agric. Food Chem. 2015, 63, 9372–9378. [Google Scholar] [CrossRef] [PubMed]
- Vavrouš, A.; Ševčík, V.C.; Dvořáková, M.T.; Čabala, R.R.; Moulisová, A.; Vrbík, K. Easy and Inexpensive Method for Multiclass Analysis of 41 Food Contact Related Contaminants in Fatty Food by Liquid Chromatography–Tandem Mass Spectrometry. J. Agric. Food Chem. 2019, 67, 10968–10976. [Google Scholar] [CrossRef]
- Li, L.; Zhang, M. Development of immunoassays for the determination of phthalates. Food Agric. Immunol. 2020, 31, 303–316. [Google Scholar] [CrossRef]
- Chen, Y.; He, Q.; Shen, D.; Jiang, Z.; Eremin, S.A.; Zhao, S. Fluorescence polarization immunoassay based on a new monoclonal antibody for the detection of the Diisobutyl phthalate in Yoghurt. Food Control 2019, 105, 38–44. [Google Scholar] [CrossRef]
- Chen, Y.; Cui, X.; Wu, P.; Jiang, Z.; Jiao, L.; Hu, Q.; Eremin, S.A.; Zhao, S. Development of a homologous fluorescence polarization immunoassay for diisobutyl phthalate in romaine lettuce. Food Anal. Methods 2017, 10, 449–458. [Google Scholar] [CrossRef]
- Zhai, R.; Chen, G.; Liu, G.; Huang, X.; Xu, X.; Li, L.; Zhang, Y.; Xu, D.; El-Aty, A.M.A. Comparison of Chemiluminescence Enzyme Immunoassay (Cl-ELISA) with Colorimetric Enzyme Immunoassay (Co-ELISA) for Imidacloprid Detection in Vegetables. Foods 2023, 12, 196. [Google Scholar] [CrossRef]
- Darwish, I.A.; Alzoman, N.Z.; Khalil, N.N. A Novel Highly Sensitive Chemiluminescence Enzyme Immunoassay with Signal Enhancement Using Horseradish Peroxidase-Luminol-Hydrogen Peroxide Reaction for the Quantitation of Monoclonal Antibodies Used for Cancer Immunotherapy. Chemosensors 2023, 11, 245. [Google Scholar] [CrossRef]
- Yu, F.-Y.; Gribas, A.V.; Vdovenko, M.M.; Sakharov, I.Y. Development of ultrasensitive direct chemiluminescent enzyme immunoassay for determination of aflatoxin B1 in food products. Talanta 2013, 107, 25–29. [Google Scholar] [CrossRef]
- Kurseev, S.A.; Solovjev, A.M.; Neumann, M.M.; Medvedko, A.V.; Sakharov, I.Y. Chemiluminescent and colorimetric aptamer-based assays of human α-thrombin. Anal. Lett. 2020, 53, 140–151. [Google Scholar] [CrossRef]
- Sakharov, I.Y.; Berlina, A.N.; Zherdev, A.V.; Dzantiev, B.B. Advantages of soybean peroxidase over horseradish peroxidase as the enzyme label in chemiluminescent enzyme-linked immunosorbent assay of sulfamethoxypyridazine. J. Agric. Food Chem. 2010, 58, 3284–3289. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Dong, Y.; Wang, Y.; Song, Z.; Meng, M.; Eremin, S.A.; Deng, C.; Yin, Y.; Xi, R. Quantification of Diethyl Phthalate by a Rapid and Homogenous Fluorescence Polarization Immunoassay. Anal. Lett. 2015, 48, 2843–2855. [Google Scholar] [CrossRef]
- Berlina, A.N.; Ragozina, M.Y.; Komova, N.S.; Serebrennikova, K.V.; Zherdev, A.V.; Dzantiev, B.B. Development of Lateral Flow Test-System for the Immunoassay of Dibutyl Phthalate in Natural Waters. Biosensors 2022, 12, 1002. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, C.-H.; Zheng, H.; Zhang, H.-B. Characterization and comparison of fumonisin B1-protein conjugates by six methods. Int. J. Mol. Sci. 2011, 13, 84–96. [Google Scholar] [CrossRef] [Green Version]
- Mi, J.; Dong, X.; Zhang, X.; Li, C.; Wang, J.; Mujtaba, M.G.; Zhang, S.; Wen, K.; Yu, X.; Wang, Z. Novel hapten design, antibody recognition mechanism study, and a highly sensitive immunoassay for diethylstilbestrol in shrimp. Anal. Bioanal. Chem. 2019, 411, 5255–5265. [Google Scholar] [CrossRef]
- Kuang, H.; Xu, L.; Cui, G.; Ma, W.; Xu, C. Development of determination of di-n-octyl phthalate (DOP) residue by an indirect enzyme-linked immunosorbent assay. Food Agric. Immunol. 2010, 21, 265–277. [Google Scholar] [CrossRef]
- Nevanen, T.K.; Söderholm, L.; Kukkonen, K.; Suortti, T.; Teerinen, T.; Linder, M.; Söderlund, H.; Teeri, T.T. Efficient enantioselective separation of drug enantiomers by immobilised antibody fragments. J. Chromatogr. A 2001, 925, 89–97. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, F.; Chen, F.; Yang, T. Development of a sensitive monoclonal antibody-based ELISA for the detection of clenbuterol in animal tissues. Food Agric. Immunol. 2009, 20, 333–344. [Google Scholar] [CrossRef] [Green Version]
- Berlina, A.N.; Zherdev, A.V.; Xu, C.; Eremin, S.A.; Dzantiev, B.B. Development of lateral flow immunoassay for rapid control and quantification of the presence of the colorant Sudan I in spices and seafood. Food Control 2017, 73, 247–253. [Google Scholar] [CrossRef]
- Kuang, H.; Liu, L.; Xu, L.; Ma, W.; Guo, L.; Wang, L.; Xu, C. Development of an enzyme-linked immunosorbent assay for dibutyl phthalate in liquor. Sensors 2013, 13, 8331–8339. [Google Scholar] [CrossRef] [Green Version]
- Komova, N.S.; Serebrennikova, K.V.; Berlina, A.N.; Zherdev, A.V.; Dzantiev, B.B. Low-Tech Test for Mercury Detection: A New Option for Water Quality Assessment. Chemosensors 2022, 10, 413. [Google Scholar] [CrossRef]
- Castro-Jiménez, J.; Ratola, N. An innovative approach for the simultaneous quantitative screening of organic plastic additives in complex matrices in marine coastal areas. Environ. Sci. Pollut. Res. 2020, 27, 11450–11457. [Google Scholar] [CrossRef] [PubMed]
- Lo Brutto, S.; Iaciofano, D.; Lo Turco, V.; Potortì, A.G.; Rando, R.; Arizza, V.; Di Stefano, V. First assessment of plasticizers in marine coastal litter-feeder fauna in the Mediterranean Sea. Toxics 2021, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Savinova, O.; Shabaev, A.; Glazunova, O.; Eremin, S.; Fedorova, T. Biodestruction of phthalic acid esters by white rot fungi. Appl. Biochem. Microbiol. 2022, 58, 598–612. [Google Scholar] [CrossRef]
- Russo, M.V.; Avino, P.; Perugini, L.; Notardonato, I. Extraction and GC-MS analysis of phthalate esters in food matrices: A review. Rsc. Adv. 2015, 5, 37023–37043. [Google Scholar] [CrossRef]
- Vinod, V.; Harathi, P. A critical review on extraction and analytical methods of phthalates in water and beverages. J. Chromatogr. A 2022, 1675, 463175. [Google Scholar]
- Das, M.T.; Ghosh, P.; Thakur, I.S. Intake estimates of phthalate esters for South Delhi population based on exposure media assessment. Environ. Pollut. 2014, 189, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wang, W.; Jiang, H.; Wang, Z.; Wang, Z.; Guo, P.; Sun, S.; Ding, S. Indirect competitive enzyme-linked immunosorbent assay for the detection of dibutyl phthalate in white wine, compared with GC-MS. Food Anal. Methods 2014, 7, 1619–1626. [Google Scholar] [CrossRef]
- Hendrickson, O.D.; Zvereva, E.A.; Zherdev, A.V.; Dzantiev, B.B. Cascade-enhanced lateral flow immunoassay for sensitive detection of okadaic acid in seawater, fish, and seafood. Foods 2022, 11, 1691. [Google Scholar] [CrossRef]
- Hendrickson, O.D.; Zvereva, E.A.; Solopova, O.N.; Zherdev, A.V.; Sveshnikov, P.G.; Eremin, S.A.; Dzantiev, B.B. Double immunochromatographic test system for sensitive detection of phycotoxins domoic acid and okadaic acid in seawater and seafood. Micromachines 2022, 13, 1506. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Zhu, F.; Xu, F.; Yang, J.; Liu, C.; Sun, Y.; Wang, H.; Lei, H.; Shen, Y. Indirect competitive chemiluminescent enzyme immunoassay method for determination of dimethyl phthalate in soy sauce and liquor. Chin. J. Anal. Chem. 2015, 43, 1027–1032. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, Y.; Liu, S.; Cong, Y.; Liu, B.; Wang, L. Rapid monitoring of dipropyl phthalate in food samples using a chemiluminescent enzyme immunoassay. Food Anal. Methods 2012, 5, 1105–1113. [Google Scholar] [CrossRef]
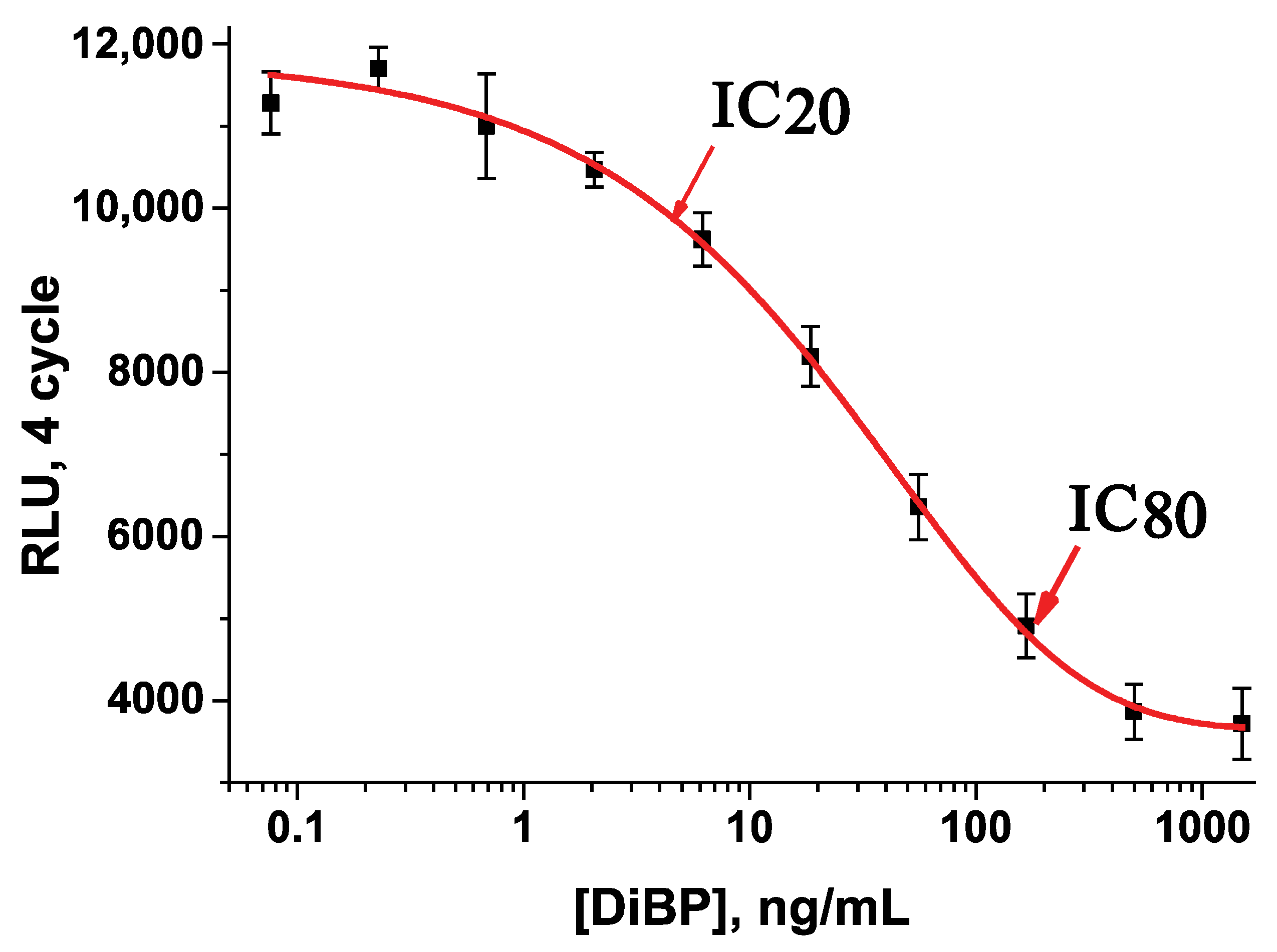
| rAs Number | IC10, ng/mL | IC20, ng/mL | IC80, ng/mL |
|---|---|---|---|
| No. 1 | 4.8 | 14.3 | 593.7 |
| No. 2 | 5.2 | 12.6 | 271.6 |
| No. 3 | 3.3 | 7.9 | 161.6 |
| Measurement | IC10, ng/mL | IC20, ng/mL | IC80, ng/mL | IC50, ng/mL |
|---|---|---|---|---|
| PBST + 10% CH3OH | ||||
| Cycle 2 (280 sec. є) | 0.9 | 3.0 | 164.2 | 22.3 |
| Cycle 3 (430 sec. є) | 1.2 | 3.7 | 174.8 | 25.3 |
| Cycle 4 (570 sec. є) | 1.8 | 5.0 | 170.8 | 29.2 |
| PBST | ||||
| Cycle 2 (280 sec. є) | 2.1 | 6.1 | 248.9 | 38.9 |
| Cycle 3 (430 sec. є) | 2.2 | 6.4 | 235.3 | 38.7 |
| Cycle 4 (570 sec. є) | 2.7 | 7.3 | 220.1 | 40.1 |
| Sample | Added DiBP, ng/mL | Found DiBP, ng/mL | Recovery, % | CV, % |
|---|---|---|---|---|
| Spring water | 10 | 11.3 | 113.4 | 3.4 |
| 30 | 23.9 | 79.5 | 4.5 | |
| 90 | 89.0 | 98.8 | 1.8 | |
| Waterfall water | 10 | 10.3 | 103.0 | 6.8 |
| 30 | 32.9 | 109.5 | 5.9 | |
| 90 | 92.6 | 102.9 | 3.8 | |
| Romaine lettuce | 10 | 7.8 | 78.4 | 3.2 |
| 30 | 29.9 | 99.7 | 6.9 | |
| 90 | 94.7 | 105.2 | 4.3 | |
| Iceberg lettuce | 10 | 9.8 | 97.7 | 5.4 |
| 30 | 36.7 | 122.3 | 8.2 | |
| 90 | 105.0 | 116.7 | 2.9 | |
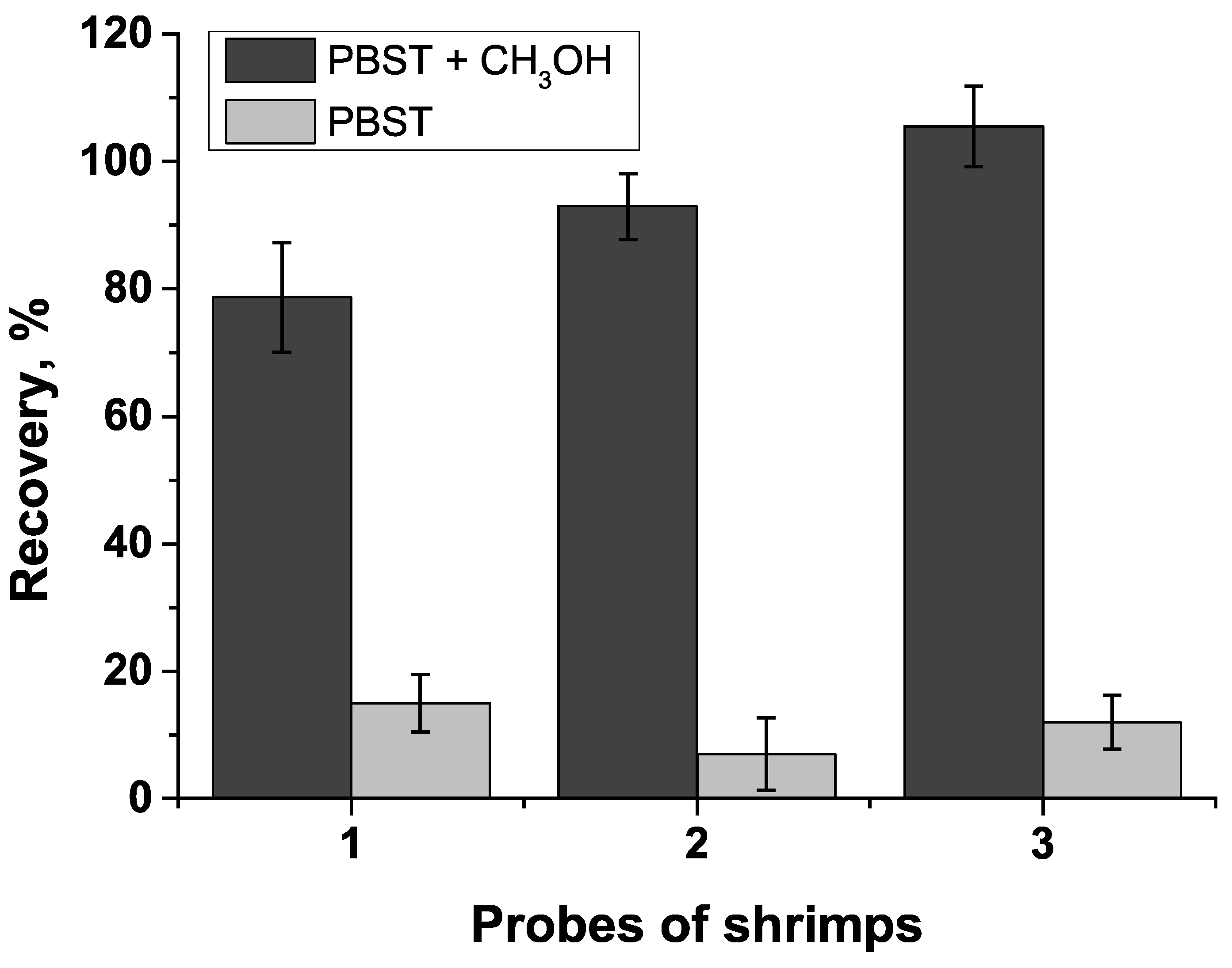
| Shrimps | 10 | 7.9 | 78.7 | 5.4 |
| 30 | 27.9 | 92.97 | 8.0 | |
| 90 | 95.0 | 105.5 | 6.1 | |
| Oysters * | 10 | 6.7 | 134.2 * | 7.4 |
| 30 | 13.0 | 88.0 * | 6.5 | |
| 90 | 40.9 | 90.89 * | 5.0 | |
| Squid | 10 | 9.6 | 95.5 | 2.6 |
| 18.5 | 20.9 | 112.7 | 6.5 | |
| 55.5 | 43.4 | 78.2 | 5.5 | |
| Octopus | 6.2 | 6.6 | 106.8 | 3.2 |
| 18.5 | 11.1 | 60.2 | 8.5 | |
| 55.5 | 47.7 | 86.0 | 4.6 | |
| Mussels | 6.2 | 7.9 | 126.9 | 2.3 |
| 18.5 | 14.7 | 79.2 | 4.5 | |
| 55.5 | 42.7 | 76.9 | 8.9 |
| Sample | Assay Type | LOD | Working Range | Type of Ab | Selectivity (% of Cross-Reaction) | Ref. |
|---|---|---|---|---|---|---|
| Yoghurt | FPIA | 0.82 ng/mL | 1.16–74.97 ng/mL | mAb | DiBP (100%) | [17] |
| Romaine lettuce | FPIA | 1.77 ng/mL | 8.82–2152.84 ng/mL | pAb | DiBP (100%) DCHP (7.37%) | [18] |
| Edible oil | Fluorescent immunoassay | 5.82 ng/mL | pAb | DiBP (100%) DiBAP (12.6%) DBP (0.95%) | [14] | |
| Romaine lettuce aquatic organisms natural water | CL-ELISA | 1.8 ng/mL | 5.0–170.8 ng/mL | pAb | DiBP (100%) DBP (12.4%) | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berlina, A.N.; Ragozina, M.Y.; Gusev, D.I.; Zherdev, A.V.; Dzantiev, B.B. Development of Chemiluminescent ELISA for Detection of Diisobutyl Phthalate in Water, Lettuce and Aquatic Organisms. Chemosensors 2023, 11, 393. https://doi.org/10.3390/chemosensors11070393
Berlina AN, Ragozina MY, Gusev DI, Zherdev AV, Dzantiev BB. Development of Chemiluminescent ELISA for Detection of Diisobutyl Phthalate in Water, Lettuce and Aquatic Organisms. Chemosensors. 2023; 11(7):393. https://doi.org/10.3390/chemosensors11070393
Chicago/Turabian StyleBerlina, Anna N., Maria Y. Ragozina, Daniil I. Gusev, Anatoly V. Zherdev, and Boris B. Dzantiev. 2023. "Development of Chemiluminescent ELISA for Detection of Diisobutyl Phthalate in Water, Lettuce and Aquatic Organisms" Chemosensors 11, no. 7: 393. https://doi.org/10.3390/chemosensors11070393
APA StyleBerlina, A. N., Ragozina, M. Y., Gusev, D. I., Zherdev, A. V., & Dzantiev, B. B. (2023). Development of Chemiluminescent ELISA for Detection of Diisobutyl Phthalate in Water, Lettuce and Aquatic Organisms. Chemosensors, 11(7), 393. https://doi.org/10.3390/chemosensors11070393









