Eco-Friendly, High-Performance Humidity Sensor Using Purple Sweet-Potato Peel for Multipurpose Applications
Abstract
1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Sensor Fabrication
2.3. Characterizations
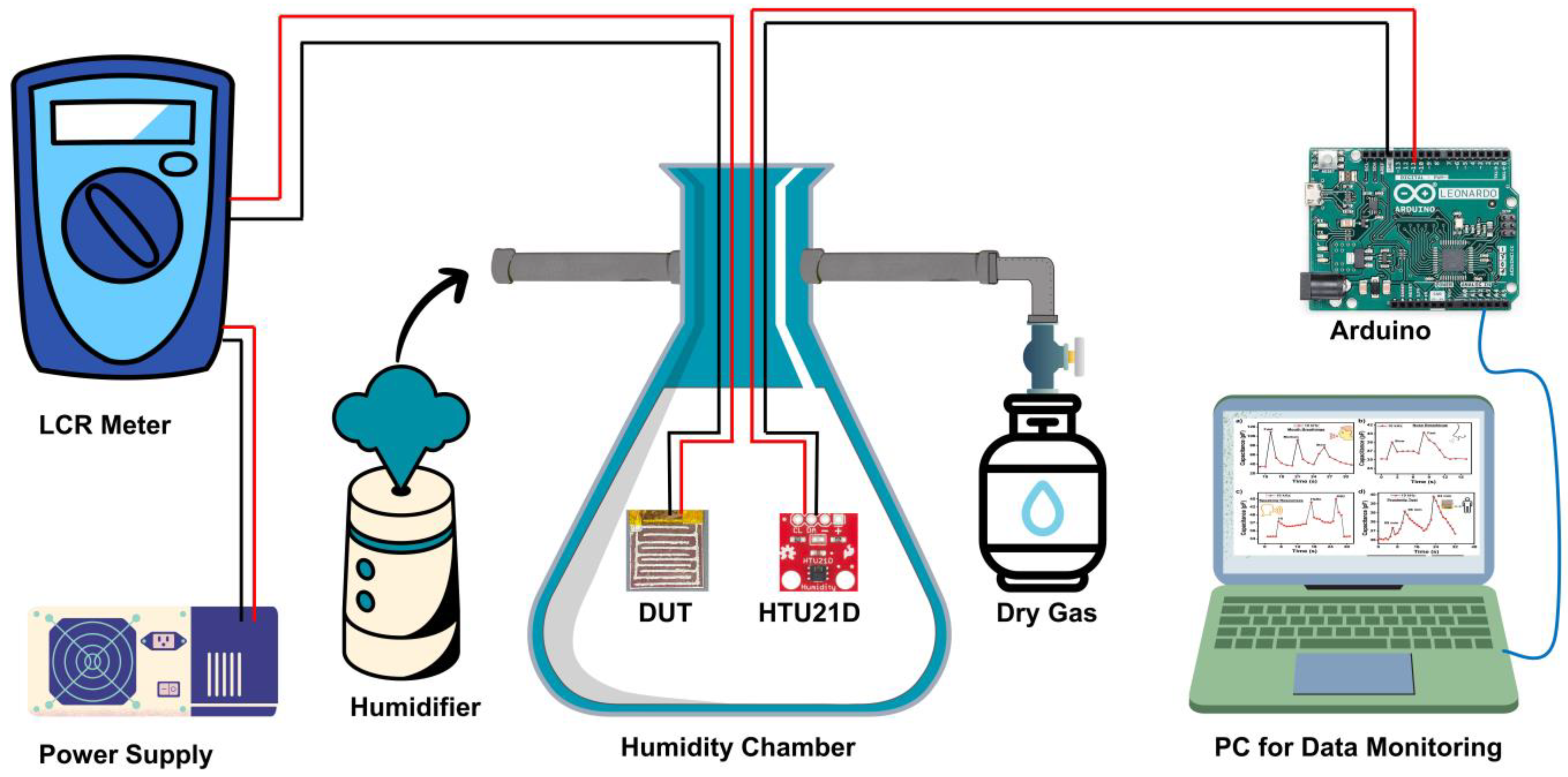
2.4. Sensor Evaluation
3. Results and Discussion
3.1. Characterization of Purple Sweet Potato Peel (PSPP)
3.2. Conduction Mechanism of SPPHS
3.3. Applications of SPPHS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, G.; Wei, Q.; Zhu, Y. Emerging Wearable Sensors for Plant Health Monitoring. Adv. Funct. Mater. 2021, 31, 2106475. [Google Scholar] [CrossRef]
- Khan, M.; Rehman, M.M.; Khan, S.A.; Saqib, M.; Kim, W.Y. Characterization and performance evaluation of fully biocompatible gelatin-based humidity sensor for health and environmental monitoring. Front. Mater. 2023, 10, 1233136. [Google Scholar] [CrossRef]
- Rehman, H.M.M.U.; Prasanna, A.P.S.; Rehman, M.M.; Khan, M.; Kim, S.-J.; Kim, W.Y. Edible rice paper-based multifunctional humidity sensor powered by triboelectricity. Sustain. Mater. Technol. 2023, 36, e00596. [Google Scholar] [CrossRef]
- Khan, S.A.; Saqib, M.; Rehman, M.M.; Mutee Ur Rehman, H.M.; Rahman, S.A.; Yang, Y.; Kim, S.; Kim, W.-Y. A Full-Range Flexible and Printed Humidity Sensor Based on a Solution-Processed P(VDF-TrFE)/Graphene-Flower Composite. Nanomaterials 2021, 11, 1915. [Google Scholar] [CrossRef] [PubMed]
- Saqib, M.; Ali Khan, S.; Mutee Ur Rehman, H.M.; Yang, Y.; Kim, S.; Rehman, M.M.; Young Kim, W. High-Performance Humidity Sensor Based on the Graphene Flower/Zinc Oxide Composite. Nanomaterials 2021, 11, 242. [Google Scholar] [CrossRef]
- Sehrawat, D.; Gill, N.S. Smart Sensors: Analysis of Different Types of IoT Sensors. In Proceedings of the 2019 3rd International Conference on Trends in Electronics and Informatics (ICOEI), Tirunelveli, India, 23–25 April 2019; pp. 523–528. [Google Scholar]
- Crowe, E.; Scott, C.; Cameron, S.; Cundell, J.H.; Davis, J. Developing Wound Moisture Sensors: Opportunities and Challenges for Laser-Induced Graphene-Based Materials. J. Compos. Sci. 2022, 6, 176. [Google Scholar] [CrossRef]
- Soukup, R.; Hamacek, A.; Mracek, L.; Reboun, J. Textile based temperature and humidity sensor elements for healthcare applications. In Proceedings of the 2014 37th International Spring Seminar on Electronics Technology, Dresden, Germany, 7–11 May 2014; pp. 407–411. [Google Scholar]
- Singh, S.; Deb, J.; Sarkar, U.; Sharma, S. MoS2 /MoO3 Nanocomposite for Selective NH3 Detection in a Humid Environment. ACS Sustain. Chem. Eng. 2021, 9, 7328–7340. [Google Scholar] [CrossRef]
- Patel, G.M.; Shah, V.R.; Bhatt, G.J.; Deota, P.T. Humidity nanosensors for smart manufacturing. In Nanosensors for Smart Manufacturing; Elsevier: Amsterdam, The Netherlands, 2021; pp. 555–580. [Google Scholar]
- Arshaka, K.; Twomey, K.; Egan, D. A Ceramic Thick Film Humidity Sensor Based on MnZn Ferrite. Sensors 2002, 2, 50. [Google Scholar] [CrossRef]
- Farahani, H.; Wagiran, R.; Hamidon, M. Humidity Sensors Principle, Mechanism, and Fabrication Technologies: A Comprehensive Review. Sensors 2014, 14, 7881. [Google Scholar] [CrossRef]
- Rahman, S.A.; Khan, S.A.; Rehman, M.M.; Kim, W.-Y. Highly Sensitive and Stable Humidity Sensor Based on the Bi-Layered PVA/Graphene Flower Composite Film. Nanomaterials 2022, 12, 1026. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, F.; Raza, Z.A.; Batool, S.R.; Zahid, M.; Onder, O.C.; Rafique, A.; Nazeer, M.A. Preparation, properties, and applications of gelatin-based hydrogels (GHs) in the environmental, technological, and biomedical sectors. Int. J. Biol. Macromol. 2022, 218, 601–633. [Google Scholar] [CrossRef] [PubMed]
- Kibungu, C.; Kondiah, P.P.D.; Kumar, P.; Choonara, Y.E. This Review Recent Advances in Chitosan and Alginate-Based Hydrogels for Wound Healing Application. Front. Mater. 2021, 8, 681960. [Google Scholar] [CrossRef]
- Liu, X.; Fu, T.; Ward, J.; Gao, H.; Yin, B.; Woodard, T.; Lovley, D.R.; Yao, J. Multifunctional Protein Nanowire Humidity Sensors for Green Wearable Electronics. Adv. Electron. Mater. 2020, 6, 2000721. [Google Scholar] [CrossRef]
- Wang, W.; Yang, S.; Ding, K.; Jiao, L.; Yan, J.; Zhao, W.; Ma, Y.; Wang, T.; Cheng, B.; Ni, Y. Biomaterials- and biostructures Inspired high-performance flexible stretchable strain sensors: A review. Chem. Eng. J. 2021, 425, 129949. [Google Scholar] [CrossRef]
- Anisimov, Y.A.; Evitts, R.W.; Cree, D.E.; Wilson, L.D. Polyaniline/Biopolymer Composite Systems for Humidity Sensor Applications: A Review. Polymers 2021, 13, 2722. [Google Scholar] [CrossRef] [PubMed]
- Khor, L.Q.; Cheong, K.Y. Aloe vera gel as natural organic dielectric in electronic application. J. Mater. Sci. Mater. Electron. 2013, 24, 2646–2652. [Google Scholar] [CrossRef]
- Perrone, G.S.; Leisk, G.G.; Lo, T.J.; Moreau, J.E.; Haas, D.S.; Papenburg, B.J.; Golden, E.B.; Partlow, B.P.; Fox, S.E.; Ibrahim, A.M.S.; et al. The use of silk-based devices for fracture fixation. Nat. Commun. 2014, 5, 3385. [Google Scholar] [CrossRef]
- Mutee ur Rehman, H.M.; Khan, M.; Rehman, M.M.; Khan, S.A.; Kim, W.Y. High-performance humidity sensor for multipurpose applications by recycling of potato peel bio-waste. Sens. Actuators A Phys. 2022, 343, 113662. [Google Scholar] [CrossRef]
- Zhang, Q.; Han, K.; Li, S.; Li, M.; Li, J.; Ren, K. Synthesis of garlic skin-derived 3D hierarchical porous carbon for high-performance supercapacitors. Nanoscale 2018, 10, 2427–2437. [Google Scholar] [CrossRef]
- Sun, B.; Zhu, S.; Mao, S.; Zheng, P.; Xia, Y.; Yang, F.; Lei, M.; Zhao, Y. From dead leaves to sustainable organic resistive switching memory. J. Colloid Interface Sci. 2018, 513, 774–778. [Google Scholar] [CrossRef]
- Immonen, K.; Lyytikäinen, J.; Keränen, J.; Eiroma, K.; Suhonen, M.; Vikman, M.; Leminen, V.; Välimäki, M.; Hakola, L. Potential of Commercial Wood-Based Materials as PCB Substrate. Materials 2022, 15, 2679. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.-L.; Pang, Y.-X.; Huang, P.; Wang, Y.-L.; Zhang, X.-R.; Deng, H.-T.; Zhang, X.-S. Silk Fibroin-Based Wearable All-Fiber Multifunctional Sensor for Smart Clothing. Adv. Fiber Mater. 2022, 4, 873–884. [Google Scholar] [CrossRef]
- Mandal, S.; Mandal, A.; Jana, G.; Mallik, S.; Roy, S.; Ghosh, A.; Chattaraj, P.K.; Goswami, D.K. Low Operating Voltage Organic Field-Effect Transistors with Gelatin as a Moisture-Induced Ionic Dielectric Layer: The Issues of High Carrier Mobility. ACS Appl. Mater. Interfaces 2020, 12, 19727–19736. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Hou, Z.; Wu, K.; Zhao, H.; Liu, S.; Fei, T.; Zhang, T. Flexible humidity sensor based on modified cellulose paper. Sens. Actuators B Chem. 2021, 339, 129879. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, W. Research Progress on Humidity-Sensing Properties of Cu-Based Humidity Sensors: A Review. J. Sens. 2022, 2022, 7749890. [Google Scholar] [CrossRef]
- Martino, J.M.; Fontenele, R.S.; Ferreira, F.A.; Ribeiro, S.G.; Di Feo, L.D.V. First Report of Sweet potato leaf curl Georgia virus in Sweet Potato in Argentina. Plant Dis. 2017, 101, 513. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhu, M.; Gao, S. Genetic and Genomic Research on Sweet Potato for Sustainable Food and Nutritional Security. Genes 2022, 13, 1833. [Google Scholar] [CrossRef]
- Tang, C.; Lu, Y.; Jiang, B.; Chen, J.; Mo, X.; Yang, Y.; Wang, Z. Energy, Economic, and Environmental Assessment of Sweet Potato Production on Plantations of Various Sizes in South China. Agronomy 2022, 12, 1290. [Google Scholar] [CrossRef]
- Li, M.; Chen, X.L.; Zhang, D.F.; Wang, W.Y.; Wang, W.J. Humidity sensitive properties of pure and Mg-doped CaCu3Ti4O12. Sens. Actuators B Chem. 2010, 147, 447–452. [Google Scholar] [CrossRef]
- Tulliani, J.-M.; Bonville, P. Influence of the dopants on the electrical resistance of hematite-based humidity sensors. Ceram. Int. 2005, 31, 507–514. [Google Scholar] [CrossRef]
- Nasution, T.I.; Nainggolan, I.; Dalimunthe, D.; Balyan, M.; Cuana, R.; Khanifah, S. Humidity detection using chitosan film based sensor. IOP Conf. Ser. Mater. Sci. Eng. 2018, 309, 012080. [Google Scholar] [CrossRef]
- Mutee Ur Rehman, H.M.; Rehman, M.M.; Saqib, M.; Ali Khan, S.; Khan, M.; Yang, Y.; Kim, S.; Rahman, S.A.; Kim, W.-Y. Highly Efficient and Wide Range Humidity Response of Biocompatible Egg White Thin Film. Nanomaterials 2021, 11, 1815. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, M.; Zhang, Y.; Zhang, Y.; Liu, K.; Zhang, J.; Yang, J.; Yuan, L. Spider silk-based humidity sensor. Opt. Lett. 2019, 44, 2907. [Google Scholar] [CrossRef]
- Duan, Z.; Zhao, Q.; Wang, S.; Huang, Q.; Yuan, Z.; Zhang, Y.; Jiang, Y.; Tai, H. Halloysite nanotubes: Natural, environmental-friendly and low-cost nanomaterials for high-performance humidity sensor. Sens. Actuators B Chem. 2020, 317, 128204. [Google Scholar] [CrossRef]
- Vivekananthan, V.; Alluri, N.R.; Purusothaman, Y.; Chandrasekhar, A.; Selvarajan, S.; Kim, S.-J. Biocompatible Collagen Nanofibrils: An Approach for Sustainable Energy Harvesting and Battery-Free Humidity Sensor Applications. ACS Appl. Mater. Interfaces 2018, 10, 18650–18656. [Google Scholar] [CrossRef]
- Hamouche, H.; Makhlouf, S.; Chaouchi, A.; Laghrouche, M. Humidity Sensor Based on Keratin bio Polymer Film. Sens. Actuators A Phys. 2018, 282, 132–141. [Google Scholar] [CrossRef]
- Sun, L.; Haidry, A.A.; Li, Z.; Xie, L.; Wang, Z.; Fatima, Q.; Yao, Z. Effective use of biomass ash as an ultra-high humidity sensor. J. Mater. Sci. Mater. Electron. 2018, 29, 18502–18510. [Google Scholar] [CrossRef]
- Duan, Z.; Jiang, Y.; Zhao, Q.; Wang, S.; Yuan, Z.; Zhang, Y.; Liu, B.; Tai, H. Facile and low-cost fabrication of a humidity sensor using naturally available sepiolite nanofibers. Nanotechnology 2020, 31, 355501. [Google Scholar] [CrossRef]
- Smith, B.C. Fundamentals of Fourier Transform Infrared Spectroscopy, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2011; ISBN 9781420069303. [Google Scholar]
- Asuquo, E.D.; Martin, A.D. Sorption of cadmium (II) ion from aqueous solution onto sweet potato (Ipomoea batatas L.) peel adsorbent: Characterisation, kinetic and isotherm studies. J. Environ. Chem. Eng. 2016, 4, 4207–4228. [Google Scholar] [CrossRef]
- Taiwo, A.E.; Betiku, E. A Modeling Study by Response Surface Methodology on the Culture Parameters Optimization of Citric Acid Bioproduction from Sweet Potato Peel. Ife J. Technol. 2013, 22, 21–25. [Google Scholar]
- Mohanraj, R.; Sivasankar, S. Sweet Potato (Ipomoea batatas [L.] Lam)—A Valuable Medicinal Food: A Review. J. Med. Food 2014, 17, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.K. A comprehensive review of sweet potato (Ipomoea batatas [L.] Lam): Revisiting the associated health benefits. Trends Food Sci. Technol. 2021, 115, 512–529. [Google Scholar] [CrossRef]
- Jiang, T.; Ye, S.; Liao, W.; Wu, M.; He, J.; Mateus, N.; Oliveira, H. The botanical profile, phytochemistry, biological activities and protected-delivery systems for purple sweet potato (Ipomoea batatas (L.) Lam.): An up-to-date review. Food Res. Int. 2022, 161, 111811. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Nie, S.; Zhu, F. Chemical constituents and health effects of sweet potato. Food Res. Int. 2016, 89, 90–116. [Google Scholar] [CrossRef] [PubMed]





| Natural Material | Stability Range | Response/Recovery Time | Fabrication Technology | Sensitivity | Stability |
|---|---|---|---|---|---|
| Chitosan [34] | 18–70%RH | _ | Solution casting method | _ | 5 min |
| Potato peel [21] | 10–90%RH | 8/12 s | Peeling | 70 kΩ/%RH | One week |
| Egg white [35] | 10–85%RH | 1.2–1.7 s | Spin coating/thermal evaporation | 50 kΩ/%RH | 36 h |
| Silk [36] | 40–99%RH | 37/- s | _ | 0.99 nm/%RH | Two weeks |
| Halloysite nanotubes [37] | 0–91.5%RH | 0.7/57.5 s | Coated with paint brush | _ | 35 days |
| Collagen [38] | 50–90 % R.H | _ | _ | 0.1287 µA/%RH | 5 min |
| Wool [39] | 16–82%RH | 36/55 s | _ | 855.66 % | _ |
| Biomass ash [40] | 15–90%RH | 2/10 s | Screen printing/drop-casting | 1 × 106 | 1–8 weeks |
| Sepiolite [41] | 10.9–91.5%RH | 528/26 s | Screen printing | _ | 10 cycles |
| Purple Sweet Potato Peel [This work] | 0–85%RH | 1/2 s | Peeling | 183.23 pF/%RH | 25 days |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, S.A.; Khan, S.A.; Iqbal, S.; Rehman, M.M.; Kim, W.Y. Eco-Friendly, High-Performance Humidity Sensor Using Purple Sweet-Potato Peel for Multipurpose Applications. Chemosensors 2023, 11, 457. https://doi.org/10.3390/chemosensors11080457
Rahman SA, Khan SA, Iqbal S, Rehman MM, Kim WY. Eco-Friendly, High-Performance Humidity Sensor Using Purple Sweet-Potato Peel for Multipurpose Applications. Chemosensors. 2023; 11(8):457. https://doi.org/10.3390/chemosensors11080457
Chicago/Turabian StyleRahman, Sheik Abdur, Shenawar Ali Khan, Shahzad Iqbal, Muhammad Muqeet Rehman, and Woo Young Kim. 2023. "Eco-Friendly, High-Performance Humidity Sensor Using Purple Sweet-Potato Peel for Multipurpose Applications" Chemosensors 11, no. 8: 457. https://doi.org/10.3390/chemosensors11080457
APA StyleRahman, S. A., Khan, S. A., Iqbal, S., Rehman, M. M., & Kim, W. Y. (2023). Eco-Friendly, High-Performance Humidity Sensor Using Purple Sweet-Potato Peel for Multipurpose Applications. Chemosensors, 11(8), 457. https://doi.org/10.3390/chemosensors11080457








