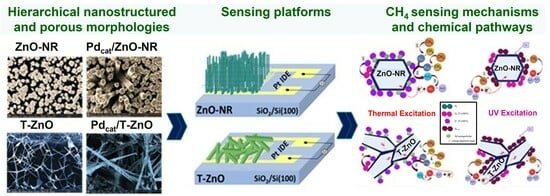
Surface-Catalyzed Zinc Oxide Nanorods and Interconnected Tetrapods as Efficient Methane Gas Sensing Platforms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of ZnO-NR Array Thin Films
2.2. Preparation of Interconnected T-ZnO Films
2.3. Morphological Characterization and Methane Sensing Measurements
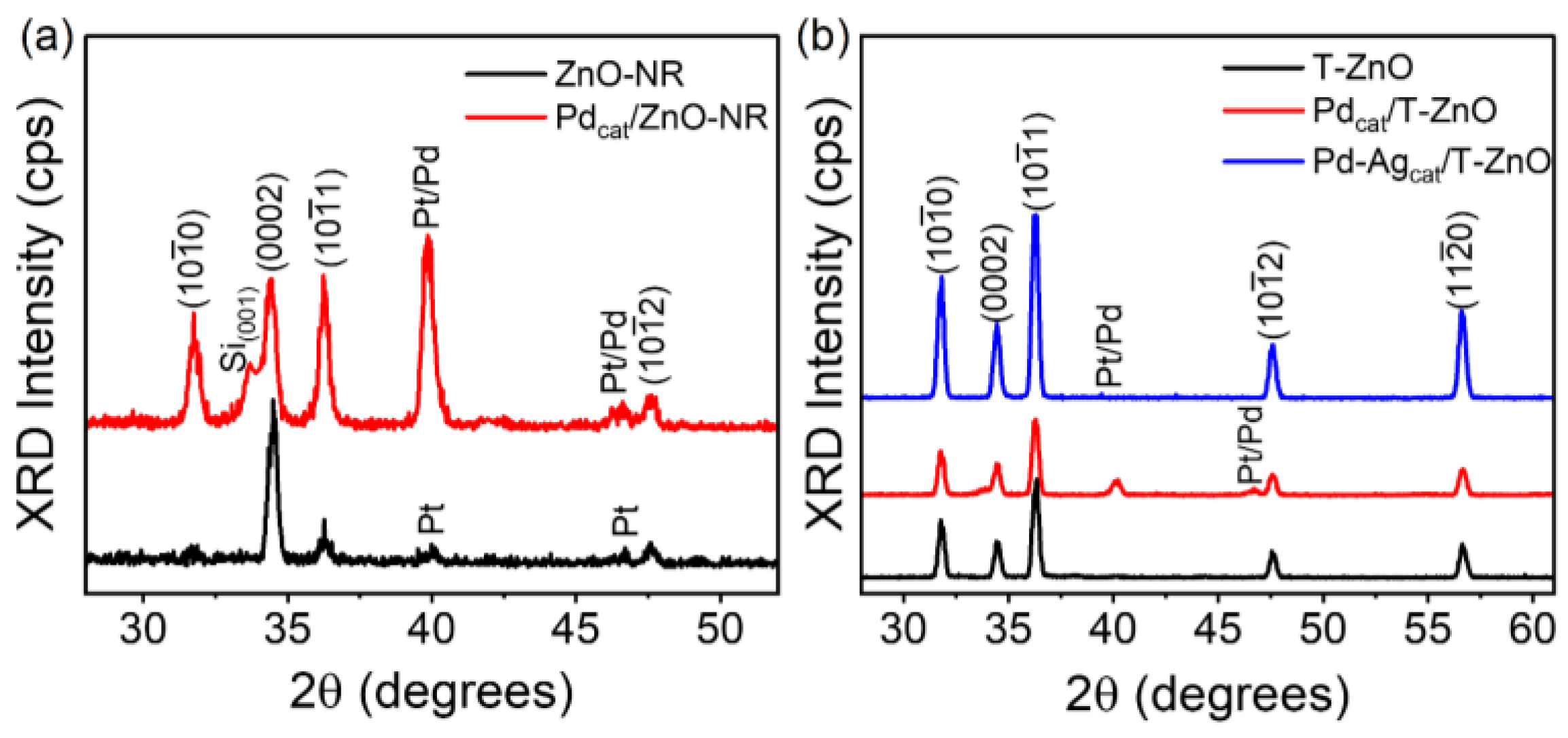
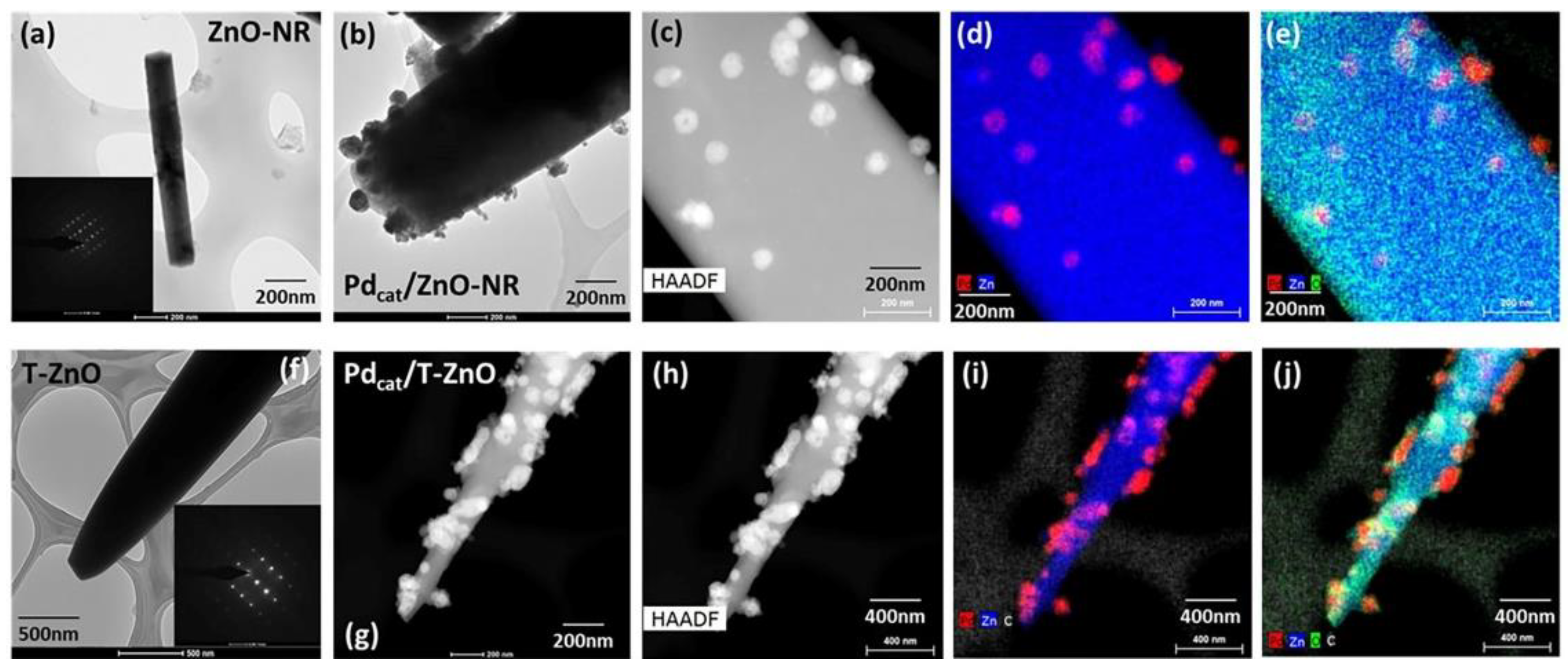
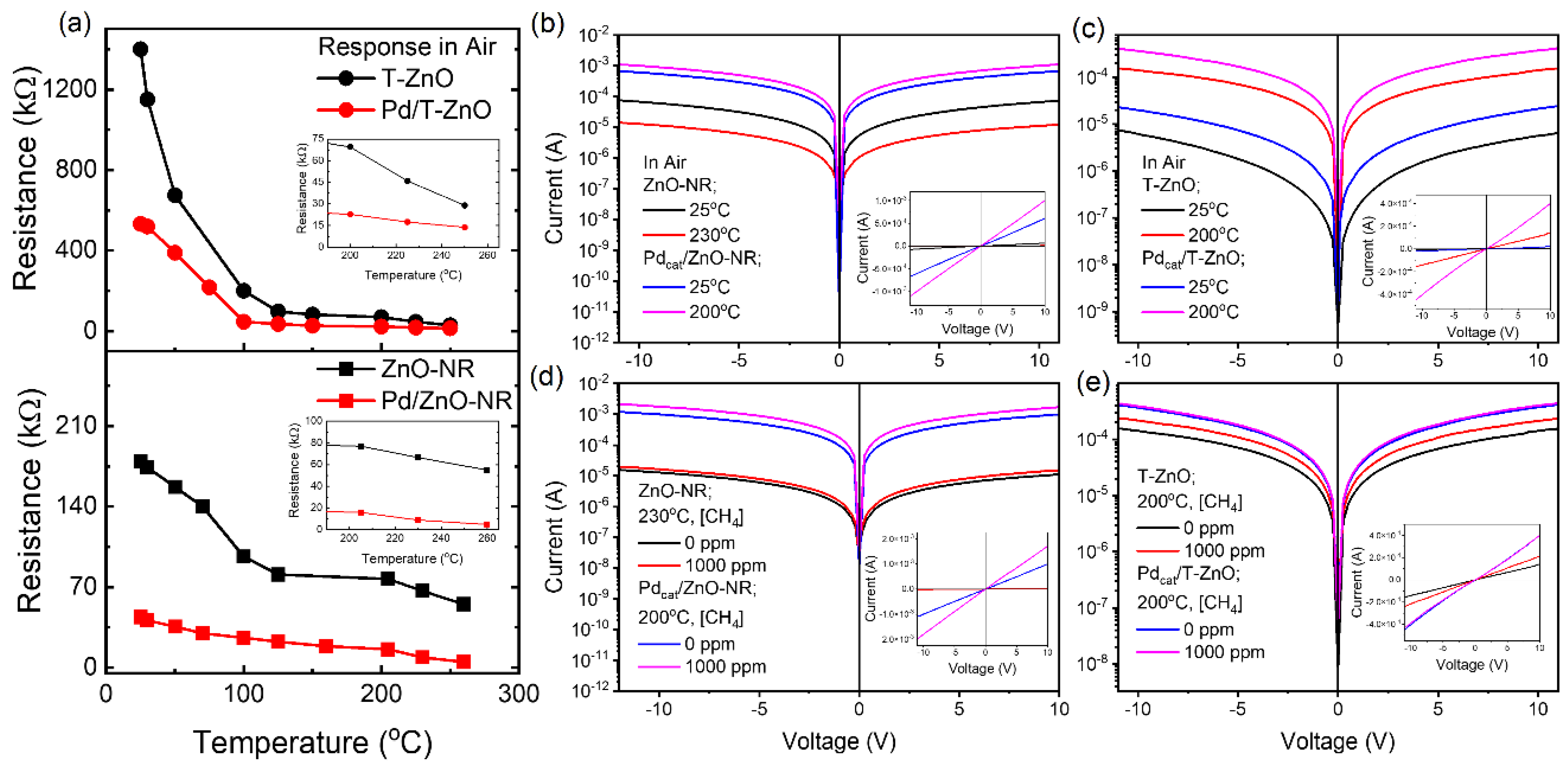
3. Results and Discussion
3.1. Structural, Optical, and Electrical Properties Characterization
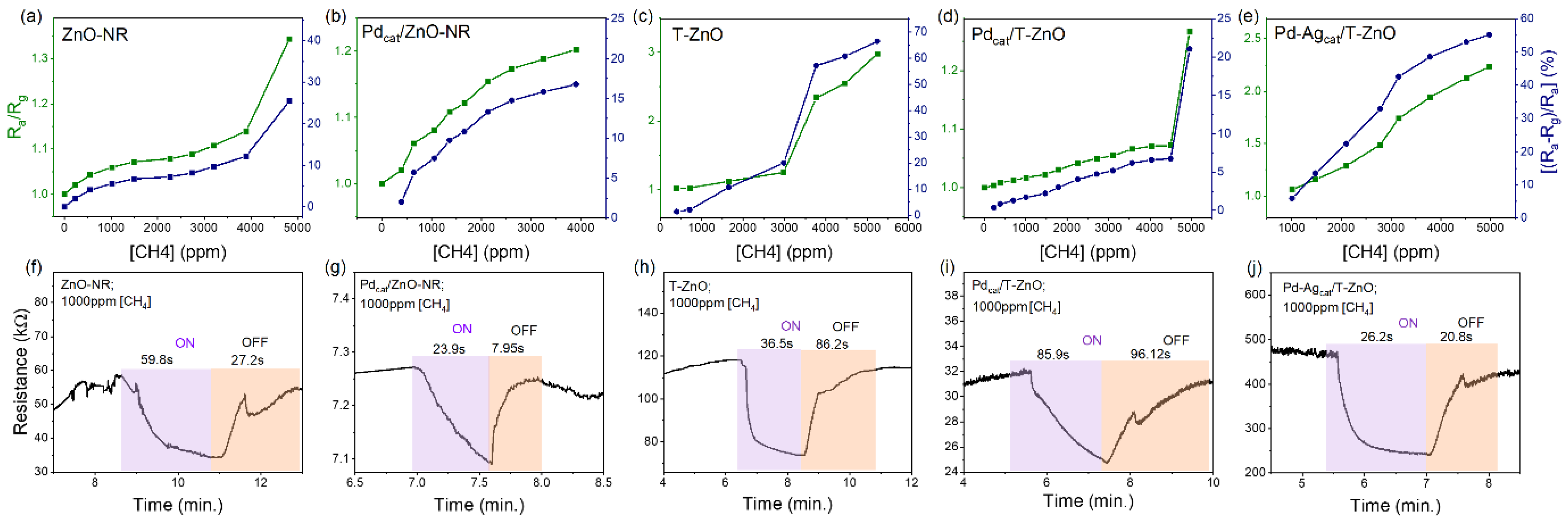
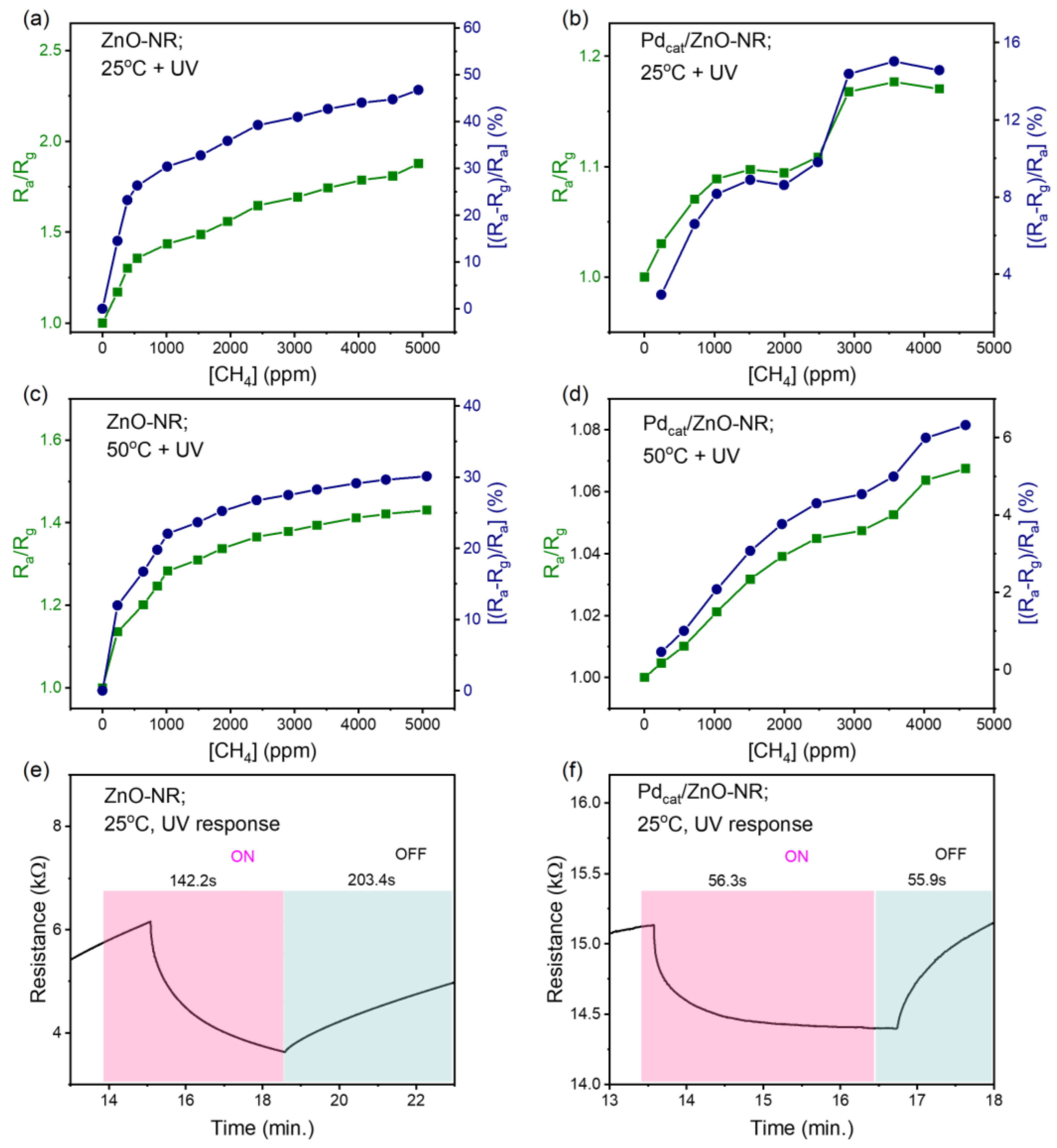
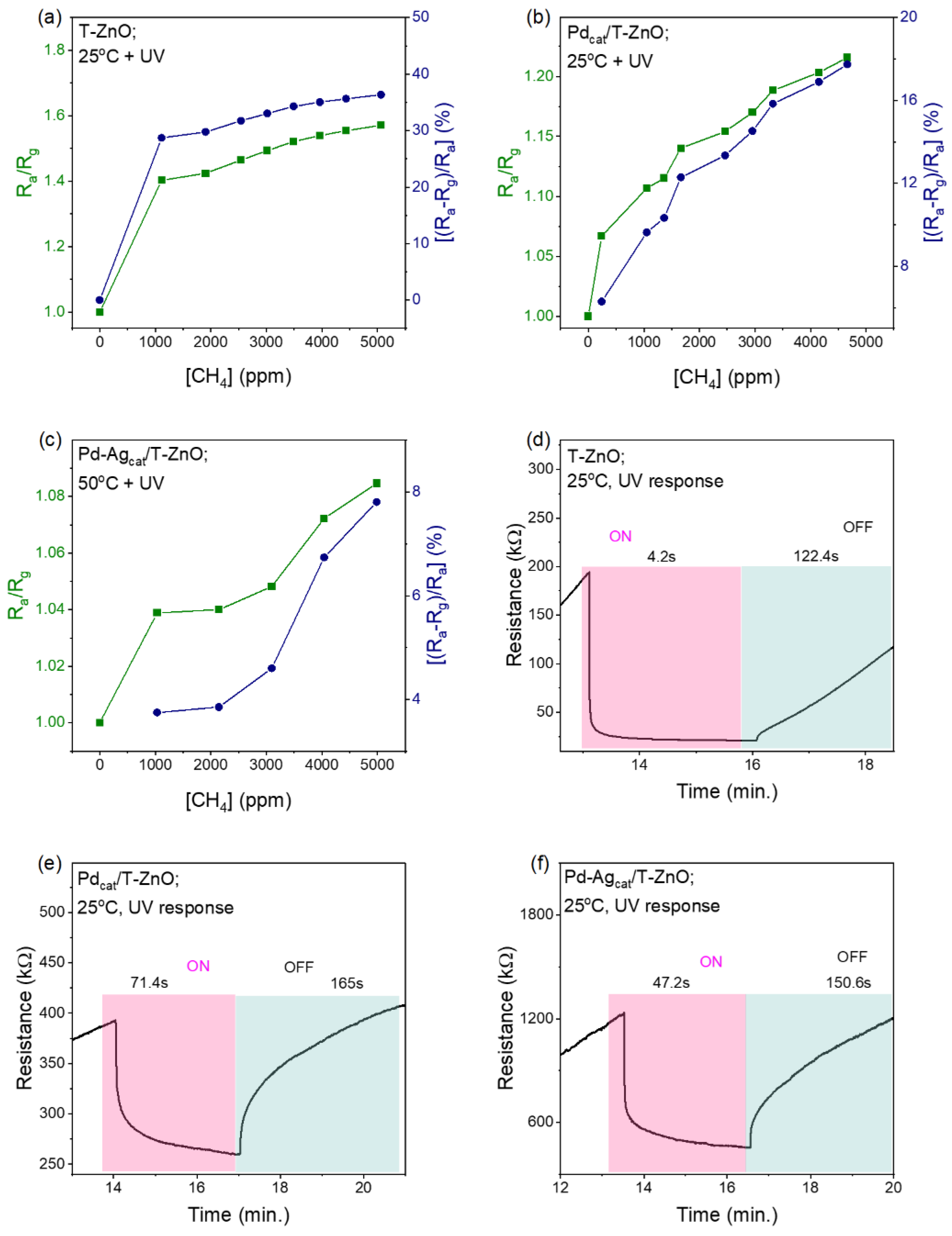
3.2. Methane Gas Sensing Performance of ZnO-NR and Pd-Functionalized Sensors
3.3. Performance of T-ZnO and Pd Functionalized Sensors
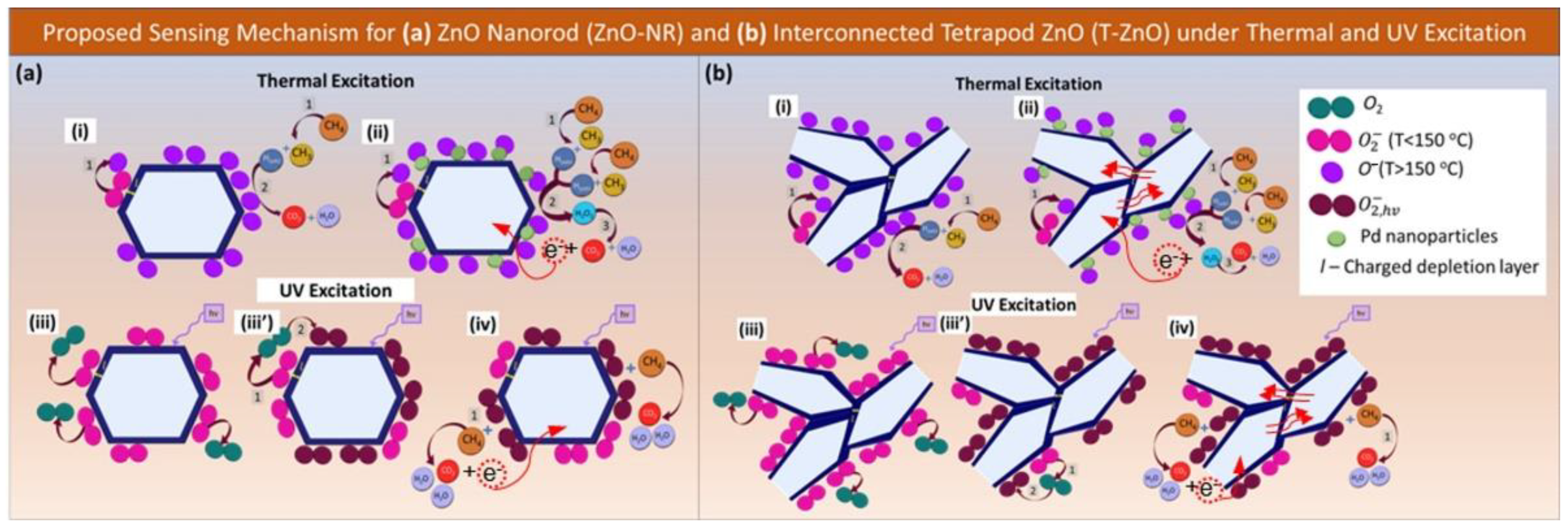
3.4. Proposed Sensing Mechanism and Chemical Pathways
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baceninaite, D.; Dzermeikaite, K.; Antanaitis, R. Global Warming and Dairy Cattle: How to Control and Reduce Methane Emission. Animals 2022, 12, 2687. [Google Scholar] [CrossRef] [PubMed]
- Neethirajan, S. Transforming the Adaptation Physiology of Farm Animals through Sensors. Animals 2020, 10, 1512. [Google Scholar] [CrossRef]
- U.S Environmental Protection Agency. Overview of Greenhouse Gases. 2022. Available online: Epa.gov/ghgemissions/overview-greenhouse-gases (accessed on 1 January 2020).
- Santhanam, K.; Ahamed, N. Greenhouse Gas Sensors Fabricated with New Materials for Climatic Usage: A Review. ChemEngineering 2018, 2, 38. [Google Scholar] [CrossRef]
- Rojas-Downing, M.M.; Nejadhashemi, A.P.; Harrigan, T.; Woznicki, S.A. Climate change and livestock: Impacts, adaptation, and mitigation. Clim. Risk Manag. 2017, 16, 145–163. [Google Scholar] [CrossRef]
- Rezaeipour, A.; Dehghani, S.; Hoghoghifard, S. VOC Sensors Based on Nanoparticles and Nanorods of Nickel Ferrite. IEEE Sens. J. 2022, 22, 16464–16471. [Google Scholar] [CrossRef]
- Nikolic, M.V.; Milovanovic, V.; Vasiljevic, Z.Z.; Stamenkovic, Z. Semiconductor Gas Sensors: Materials, Technology, Design, and Application. Sensors 2020, 20, 6694. [Google Scholar] [CrossRef] [PubMed]
- Bulemo, P.M.; Cheong, J.Y. Review on Porosity Control in Nanostructured Semiconducting Metal Oxides and Its Influence on Chemiresistive Gas Sensing. ACS Appl. Nano Mater. 2023, 6, 1027–1049. [Google Scholar] [CrossRef]
- Bhati, V.S.; Hojamberdiev, M.; Kumar, M. Enhanced sensing performance of ZnO nanostructures-based gas sensors: A review. Energy Rep. 2020, 6, 46–62. [Google Scholar] [CrossRef]
- Yamazoe, N.; Fuchigami, J.; Kishikawa, M.; Seiyama, T. Interactions of Tin Oxide Surface with O2, H2O, and H2. Surf. Sci. 1979, 86, 335–344. [Google Scholar] [CrossRef]
- Gupta, S.; Knoepfel, A.; Zou, H.; Ding, Y. Investigations of methane gas sensor based on biasing operation of n-ZnO nanorods/p-Si assembled diode and Pd functionalized Schottky junctions. Sens. Actuators B. Chem. 2023, 392, 134030:1–134030:18. [Google Scholar] [CrossRef]
- Mondal, B.; Dutta, L.; Roychaudhury, C.; Mohanta, D.; Mukherjee, N.; Saha, H. Effect of Annealing Temperature on the Morphology and Sensitivity of the Zinc Oxide Nanorods-Based Methane Sensor. Acta Metall. Sin. 2014, 27, 593–600. [Google Scholar] [CrossRef]
- Yamazoe, N. New approaches for improving semiconductor gas sensors. Sens. Actuators B 1991, 5, 7–19. [Google Scholar] [CrossRef]
- Feng, Z.; Gao, C.; Ma, X.; Zhan, J. Well-dispersed Pd nanoparticles on porous ZnO nanoplates via surface ion exchange for chlorobenzene-selective sensor. RSC Adv. 2019, 9, 42351–42359. [Google Scholar] [CrossRef]
- Young, S.-J.; Liu, Y.-H. Pd Nanoparticle Adsorption ZnO Nanorods for Enhancing Photodetector UV-Sensing Performance. IEEE J. Electron Devices Soc. 2021, 9, 265–270. [Google Scholar] [CrossRef]
- Vayssieres, L. Growth of arrayed nanorods and nanowires of ZnO from aqueous solutions. Adv. Mater. 2003, 15, 464–466. [Google Scholar] [CrossRef]
- Wang, Z.L.; Zou, H.; Wang, L. Nanowires and Nanotubes. In Encyclopedia of Applied Physics; Wiley-VCH GmbH: Weinheim, Germany, 2022. [Google Scholar] [CrossRef]
- Song, Y.G.; Kim, G.S.; Ju, B.-K.; Kang, C.-Y. Design of Semiconducting Gas Sensors for Room-Temperature Operation. J. Sens. Sci. Technol. 2020, 29, 1–6. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, L.; Sun, S.; Wang, J.; Yan, W. One-Dimensional Nanomaterials in Resistive Gas Sensor: From Material Design to Application. Chemosensors 2021, 9, 198. [Google Scholar] [CrossRef]
- Fan, S.-W.; Srivastava, A.K.; Dravid, V.P. UV-activated room-temperature gas sensing mechanism of polycrystalline ZnO. Appl. Phys. Lett. 2009, 95, 142106. [Google Scholar] [CrossRef]
- Su, X.; Duan, G.; Xu, Z.; Zhou, F.; Cai, W. Structure, and thickness-dependent gas sensing responses to NO2 under UV irradiation for the multilayered ZnO micro/nanostructured porous, thin films. J. Colloid Interface Sci. 2017, 503, 150–158. [Google Scholar] [CrossRef]
- Kumar, R.; Liu, X.; Zhang, J.; Kumar, M. Room-Temperature Gas Sensors under Photoactivation: From Metal Oxides to 2D Materials. Nano Micro. Lett. 2020, 12, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Mishra, Y.K.; Modi, G.; Cretu, V.; Postica, V.; Lupan, O.; Reimer, T.; Paulowicz, I.; Hrkac, V.; Benecke, W.; Kienle, L.; et al. Direct Growth of Freestanding ZnO Tetrapod Networks for Multifunctional Applications in Photocatalysis, UV Photodetection, and Gas Sensing. ACS Appl. Mater. Interfaces 2015, 7, 14303–14316. [Google Scholar] [CrossRef] [PubMed]
- Mishra, Y.K.; Adelung, R. ZnO tetrapod materials for functional applications. Mater. Today 2018, 21, 631–651. [Google Scholar] [CrossRef]
- Knoepfel, A.; Liu, N.; Hou, Y.; Sujani, S.; dos Reis, B.R.; White, R.; Wang, K.; Poudel, B.; Gupta, S.; Priya, S. Development of tetrapod zinc oxide-based UV sensor for precision livestock farming and productivity. Biosensors 2022, 12, 837. [Google Scholar] [CrossRef] [PubMed]
- Saboor, F.H.; Ueda, T.; Kamada, K.; Hyodo, T.; Mortazavi, Y.; Khodadadi, A.A.; Shimizu, Y. Enhanced NO2 gas sensing performance of bare and Pd-loaded SnO2 thick film sensors under UV-light irradiation at room temperature. Sens. Actuators Part B 2016, 223, 429–439. [Google Scholar] [CrossRef]
- Aghagoli, Z.; Ardyanian, M. Synthesis and study of the structure, magnetic, optical and methane gas sensing properties of cobalt doped zinc oxide microstructures. J. Mater. Sci. Mater. Electron. 2018, 29, 7130–7141. [Google Scholar] [CrossRef]
- Wang, J.; Hu, C.; Xia, Y.; Zhang, B. Mesoporous ZnO nanosheets with rich surface oxygen vacancies for UV-activated methane gas sensing at room temperature. Sens. Actuators B Chem. 2021, 333, 129547. [Google Scholar] [CrossRef]
- Wyckoff, R.W.G. Crystal Structures, 2nd ed.; Interscience Publishers: Geneva, Switzerland, 1963. [Google Scholar]
- Swanson, H.E.; Eleanor, T. Standard X-ray Diffraction Powder Patterns; Commerce, D.O., Ed.; NIST: Gaithersburg, MD, USA, 1953; Volume 1. [Google Scholar]
- Kittel, C. Introduction to Solid State Physics; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Cullity, B.D. Elements of X-ray Diffraction, 3rd ed.; Prentice Hall: Hoboken, NJ, USA, 2001. [Google Scholar]
- Foo, K.L.; Kashif, M.; Hashim, U.; Liu, W.W. Effect of different solvents on the structural and optical properties of zinc oxide thin films for optoelectronic applications. Ceram. Int. 2014, 40, 753–761. [Google Scholar] [CrossRef]
- Yogamalar, R.; Srinivasan, R.; Vinu, A.; Ariga, K.; Bose, A.C. X-ray peak broadening analysis in ZnO nanoparticles. Solid State Commun. 2009, 149, 1919–1923. [Google Scholar] [CrossRef]
- Koida, T.; Chichibu, S.; Uedono, A.; Sota, T.; Tsukazaki, A.; Kawasaki, M. Radiative and nonradiative excitonic transitions in nonpolar (1120) and polar (0001) and (0001) ZnO epilayers. Appl. Phys. Lett. 2004, 84, 1079–1081. [Google Scholar] [CrossRef]
- Deepak, F.L.; Casillas-Garcia, G.; Esparza, R.; Barron, H.; Jose-Yacaman, M. New insights into the structure of PdAu nanoparticles as revealed by aberration-corrected STEM. J. Crys. Growth 2011, 325, 60–67. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, J.; Wei, Q.; Yu, P.; Zhang, S.; Xu, Y.; Dong, Y.; Ni, Y.; Ao, J.; Xia, Y. Visible Light-Induced Room-Temperature Formaldehyde Gas Sensor Based on Porous Three-Dimensional ZnO Nanorod Clusters with Rich Oxygen Vacancies. ACS Omega 2022, 7, 22861–22871. [Google Scholar] [CrossRef] [PubMed]
- Edvinsson, T. Optical quantum confinement and photocatalytic properties in two-, one- and zero-dimensional nanostructures. R. Soc. Open Sci. 2018, 5, 180387–180392. [Google Scholar] [CrossRef]
- Bedia, A.; Bedia, F.Z.; Aillerie, M.; Maloufi, N.; Benyoucef, B. Morphological and Optical Properties of ZnO Thin Films Prepared by Spray Pyrolysis on Glass Substrates at Various Temperatures for Integration in Solar Cell. Energy Procedia 2015, 74, 529–538. [Google Scholar] [CrossRef]
- Saad, R.; Gamal, A.; Zayed, M.; Ahmed, A.M.; Shaban, M.; BinSabt, M.; Rabia, M.; Hamdy, H. Fabrication of ZnO/CNTs for Application in CO2 Sensor at Room Temperature. Nanomaterials 2021, 11, 3087. [Google Scholar] [CrossRef] [PubMed]
- Varshni, Y.P. Temperature dependence of the energy gap in semiconductors. Physica 1967, 34, 149–156. [Google Scholar] [CrossRef]
- Gür, E.; Tüzemen, S.; Kili, B.; Coşkun, C. High-temperature Schottky diode characteristics of bulk ZnO. J. Phys. Condens. Matter 2007, 19, 196206–196211. [Google Scholar] [CrossRef]
- Sze, S.M. Physics of Semiconductor Devices, 2nd ed.; Wiley: New York, NY, USA, 1981. [Google Scholar]
- Somvanshi, D.; Jit, S. Mean Barrier Height and Richardson Constant for Pd/ZnO Thin Film-Based Schottky Diodes Grown on n-Si Substrates by Thermal Evaporation Method. IEEE Electron Dev. Lett. 2013, 34, 1238–1240. [Google Scholar] [CrossRef]
- Cheung, S.K.; Cheung, N.W. Extraction of Schottky diode parameters from forward current-voltage characteristics. Appl. Phys. Lett. 1986, 49, 85–87. [Google Scholar] [CrossRef]
- Liangruska, M.; Laomettachit, T.; Siriwong, C. Enhancing gas sensing properties of novel palladium-decorated zinc oxide surface: A first-principles study. Mater. Res. Express 2021, 8, 45004. [Google Scholar] [CrossRef]
- Musa, I.; Qamhieh, N.; Mahmoud, S.T. Synthesis and length dependent photoluminescence property of zinc oxide nanorods. Results Phys. 2017, 7, 3552–3556. [Google Scholar] [CrossRef]
- Melnick, D.A. Zinc Oxide Photoconduction, an Oxygen Adsorption Process. J. Chem. Phys. 1957, 26, 1136–1146. [Google Scholar] [CrossRef]
- Kumar, M.; Singh Bhati, V.; Ranwa, S.; Singh, J.; Kumar, M. Pd/ZnO nanorods based sensor for highly selective detection of extremely low concentration hydrogen. Sci. Rep. 2017, 7, 236–241. [Google Scholar] [CrossRef]
- Chen, R.; Wang, J.; Luo, S.; Xiang, L.; Li, W.; Xie, D. Unraveling photoexcited electron transfer pathway of oxygen vacancy-enriched ZnO/Pd hybrid toward visible light-enhanced methane detection at a relatively low temperature. Appl. Catal. B Environ. 2020, 264, 11855. [Google Scholar] [CrossRef]
- Look, D.C.; Hemsky, J.W.; Sizelove, J.R. Residual native shallow donor in ZnO. Phys. Rev. Lett. 1999, 82, 2552–2555. [Google Scholar] [CrossRef]
- Janotti, A.; de Walle, C.G.V. Native point defects in ZnO. Phys. Rev. B 2007, 76, 165202. [Google Scholar] [CrossRef]
- Xu, L.; Wu, X.C.; Zhu, J.J. Green preparation and catalytic application of Pd nanoparticles. Nanotechnology 2008, 19, 305603–305609. [Google Scholar] [CrossRef] [PubMed]
- Karnati, P. Design, Fabrication and Characterization of Core-Shell Nanowires for Resistive Type Gas Sensing; Ohio State University: Columbus, OH, USA, 2021. [Google Scholar]
- Wang, Y.; Meng, X.; Yao, M.; Sun, G.; Zhang, Z. Enhanced CH4 sensing properties of Pd modified ZnO nanosheets. Ceram. Int. 2019, 45, 13150–13157. [Google Scholar] [CrossRef]
- Ghosh, S.; Roychaudhuri, C.; Bhattacharya, R.; Saha, H.; Mukherjee, N. Palladium-silver-activated ZnO surface: Highly selective methane sensor at reasonably low operating temperature. ACS Appl. Mater. Interfaces 2014, 6, 3879–3887. [Google Scholar] [CrossRef]
- Cheng, J.; Hu, D.; Yao, A.; Gao, Y.; Asadi, H. A computational study on the Pd-decorated ZnO nanocluster for H2 gas sensing: A comparison with experimental results. Phys. E 2020, 124, 114237. [Google Scholar] [CrossRef]
- Zhang, S.; Li, H.; Zhang, N.; Zhao, X.; Zhang, Z.; Wang, Y. Self-sacrificial templated formation of ZnO with decoration of catalysts for regulating CO and CH4 sensitive detection. Sens. Actuators B Chem. 2021, 330–337, 129286. [Google Scholar] [CrossRef]
- Soci, C.; Zhang, A.; Xiang, B.; Dayeh, S.A.; Aplin, D.P.R.; Park, J.; Bao, X.Y.; Lo, Y.H.; Wang, D. ZnO Nanowire UV Photodetectors with High Internal Gain. Nano Lett. 2007, 7, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Pierret, R.F. Semiconductor Fundamentals; Addison-Wesley: Boston, MA, USA, 1988. [Google Scholar]
- Procek, M.; Stolarczyk, A.; Pustelny, T. Impact of Temperature and UV Irradiation on Dynamics of NO2 Sensors Based on ZnO Nanostructures. Nanomaterials 2017, 7, 312. [Google Scholar] [CrossRef]



| Sample | Response * | Sensitivity ^ (%) | Response Time (s) | Recovery Time (s) | |
|---|---|---|---|---|---|
| ZnO-NR | 200 °C | 1.34 | 25.5 | 41.7 | 151 |
| 90 °C | 1.02 | 2.08 | 30.4 | 65 | |
| UV at 25 °C | 1.88 | 46.6 | - | - | |
| UV at 50 °C | 1.43 | 30.18 | - | - | |
| Pdcat/ZnO-NR | 200 °C | 1.2 | 16.7 | 23.9 | 7.95 |
| 70 °C | 1.00 | 0.6 | 33.9 | 139 | |
| UV at 25 °C | 1.17 | 14.6 | - | - | |
| UV at 50 °C | 1.07 | 6.33 | - | - | |
| T-ZnO | 200 °C | 2.98 | 66.4 | 36.5 | 86.2 |
| 70 °C | 1.12 | 10.3 | 72.2 | 156.8 | |
| UV at 25 °C | 1.57 | 36.3 | - | - | |
| Pdcat/T-ZnO | 230 °C | 1.27 | 21.06 | 85.9 | 96.1 |
| 70 °C | 1.05 | 4.5 | 105 | 34.6 | |
| UV at 25 °C | 1.22 | 17.74 | - | - | |
| Pd-Agcat/T-ZnO | 200 °C | 2.23 | 55.2 | 26.2 | 20.8 |
| 70 °C | 1.06 | 5.25 | 67.8 | 40.9 | |
| ** UV at 50 °C | 1.08 | 7.8 | - | - | |
| Pd dotscat/T-ZnO | 200 °C | 50.6 | 98 | 19.1 | 57.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knoepfel, A.; Poudel, B.; Gupta, S. Surface-Catalyzed Zinc Oxide Nanorods and Interconnected Tetrapods as Efficient Methane Gas Sensing Platforms. Chemosensors 2023, 11, 506. https://doi.org/10.3390/chemosensors11090506
Knoepfel A, Poudel B, Gupta S. Surface-Catalyzed Zinc Oxide Nanorods and Interconnected Tetrapods as Efficient Methane Gas Sensing Platforms. Chemosensors. 2023; 11(9):506. https://doi.org/10.3390/chemosensors11090506
Chicago/Turabian StyleKnoepfel, Abbey, Bed Poudel, and Sanju Gupta. 2023. "Surface-Catalyzed Zinc Oxide Nanorods and Interconnected Tetrapods as Efficient Methane Gas Sensing Platforms" Chemosensors 11, no. 9: 506. https://doi.org/10.3390/chemosensors11090506
APA StyleKnoepfel, A., Poudel, B., & Gupta, S. (2023). Surface-Catalyzed Zinc Oxide Nanorods and Interconnected Tetrapods as Efficient Methane Gas Sensing Platforms. Chemosensors, 11(9), 506. https://doi.org/10.3390/chemosensors11090506