Electrochemical Perovskite-Based Sensors for the Detection of Relevant Biomarkers for Human Kidney Health
Abstract
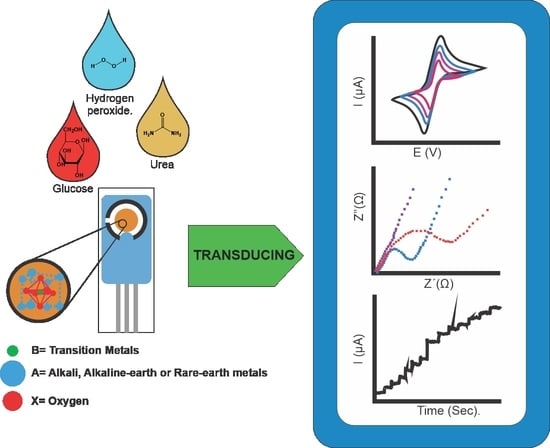
:1. Introduction
2. Perovskite’s Overview
3. Application of Perovskites in the Sensing of NPNC Biomarkers
3.1. Sensing of Glucose
3.2. Sensing of Urea
3.3. Sensing of Hydrogen Peroxide (H2O2)

4. Perspectives and Current Challenges
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz, D.N.; Bagshaw, S.M.; Maisel, A.; Lewington, A.; Thadhani, R.; Chakravarthi, R.; Murray, P.T.; Mehta, R.L.; Chawla, L.S. Use of Biomarkers to Assess Prognosis and Guide Management of Patients with Acute Kidney Injury. Contrib. Nephrol. 2013, 182, 45–64. [Google Scholar] [CrossRef] [PubMed]
- Berl, T. American Society of Nephrology Renal Research Report. J. Am. Soc. Nephrol. 2005, 16, 1886–1903. [Google Scholar] [CrossRef]
- Hojs, N.V.; Bevc, S.; Ekart, R.; Hojs, R. Oxidative Stress Markers in Chronic Kidney Disease with Emphasis on Diabetic Nephropathy. Antioxidants 2020, 9, 925. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Li, Y.; Li, S.; Hu, X.; Wei, Q.; Dong, Z. Glucose Metabolism in Acute Kidney Injury and Kidney Repair. Front. Med. 2021, 8, 744122. [Google Scholar] [CrossRef] [PubMed]
- Geleta, G.S. A Colorimetric Aptasensor Based on Two Dimensional (2D) Nanomaterial and Gold Nanoparticles for Detection of Toxic Heavy Metal Ions: A Review. Food Chem. Adv. 2023, 2, 100184. [Google Scholar] [CrossRef]
- Kailasa, S.K.; Vajubhai, G.N.; Koduru, J.R.; Park, T.J. Recent Progress of Nanomaterials for Colorimetric and Fluorescence Sensing of Reactive Oxygen Species in Biological and Environmental Samples. Trends Environ. Anal. Chem. 2023, 37, e00196. [Google Scholar] [CrossRef]
- Yang, L.; Janie, E.; Huang, T.; GHzen, J.; Kissinger, P.T.; Vreeke, M.; Heller, A. Applications of “Wired” Peroxidase Electrodes for Peroxide Determination in Liquid Chromatography Coupled to Oxidase Immobilized Enzyme Reactors. Anal. Chem. 1995, 67, 1326–1331. [Google Scholar] [CrossRef]
- Turner, A.; Karube, I.; Wilson, G.S. Biosensors: Fundamentals and Applications; Oxford University Press: New York, NY, USA, 2019; ISBN 9783110641080. [Google Scholar]
- Narang, J.; Pundir, C.S. Biosensors: An Introductory Textbook; Pan Standford Publishing Pte, Ltd.: Singapore, 2017; ISBN 978-1-315-15652-1. [Google Scholar]
- Zhang, X.; Shen, L.; Wang, M.; Siqin, G.; Tong, Z.; Xu, R.; Zhang, D.; Ma, J.; Liu, L. A Novel Glucose Biosensor Constructed by Glucose Oxidase Immobilized with Methylene Blue Intercalated Layered Lanthanum Niobic Acid Nanocomposite. Mater. Lett. 2014, 135, 39–42. [Google Scholar] [CrossRef]
- Cai, B.; Zhao, M.; Ma, Y.; Ye, Z.; Huang, J. Bio-Inspired Formation of Mesoporous LiNbO3 Nanotubes and Application for Glucose Biosensor. Electrochim. Acta 2014, 147, 176–182. [Google Scholar] [CrossRef]
- Park, S.; Boo, H.; Chung, T.D. Electrochemical Non-Enzymatic Glucose Sensors. Anal. Chim. Acta 2006, 556, 46–57. [Google Scholar] [CrossRef]
- Chen, L.; Yang, J.; Chen, W.; Sun, S.; Tang, H.; Li, Y. Perovskite Mesoporous LaFeO3 with Peroxidase-like Activity for Colorimetric Detection of Gallic Acid. Sens. Actuators B Chem. 2020, 321, 128642. [Google Scholar] [CrossRef]
- Xu, W.; Li, F.; Cai, Z.; Wang, Y.; Luo, F.; Chen, X. An Ultrasensitive and Reversible Fluorescence Sensor of Humidity Using Perovskite CH3NH3PbBr3. J. Mater. Chem. C 2016, 4, 9651–9655. [Google Scholar] [CrossRef]
- George, J.; Halali, V.V.; Sanjayan, C.G.; Suvina, V.; Sakar, M.; Balakrishna, R.G. Perovskite Nanomaterials as Optical and Electrochemical Sensors. Inorg. Chem. Front. 2020, 7, 2702–2725. [Google Scholar] [CrossRef]
- Cardoso, A.G.; Viltres, H.; Ortega, G.A.; Phung, V.; Grewal, R.; Mozaffari, H.; Ahmed, S.R.; Rajabzadeh, A.R.; Srinivasan, S. Electrochemical Sensing of Analytes in Saliva: Challenges, Progress, and Perspectives. TrAC-Trends Anal. Chem. 2023, 160, 116965. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Lu, W.; Yuan, Q.; Zheng, Y.; Yao, B. A Thin Film Polyethylene Terephthalate (PET) Electrochemical Sensor for Detection of Glucose in Sweat. Talanta 2019, 198, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, W.; Zhang, L.; Yang, J.; Yao, Z.P.; He, Y.; Li, Y. Integrated Hand-Held Electrochemical Sensor for Multicomponent Detection in Urine. Biosens. Bioelectron. 2021, 193, 113534. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.R.; Chuang, H.C.; Tripathi, A.; Wang, Y.L.; Ko, M.L.; Chuang, C.C.; Chen, J.C. High-Sensitivity and Trace-Amount Specimen Electrochemical Sensors for Exploring the Levels of β-Amyloid in Human Blood and Tears. Anal. Chem. 2021, 93, 8099–8106. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yang, J.; Dong, Q.; Zhou, F.; Wang, Q.; Wang, Z.; Huang, K.; Yu, H.; Xiong, X. One-Step Synthesis of CuO Nanoparticles Based on Flame Synthesis: As a Highly Effective Non-Enzymatic Sensor for Glucose, Hydrogen Peroxide and Formaldehyde. J. Electroanal. Chem. 2021, 881, 114965. [Google Scholar] [CrossRef]
- Shimizu, Y.; Komatsu, H.; Michishita, S.; Miura, N.; Yamazo, N. Sensing Characteristics of Hydrogen Peroxide Sensor Using Carbon-Based Electrode Loaded with Perovskite-Type Oxide. Sens. Actuators B Chem. 1996, 34, 493–498. [Google Scholar] [CrossRef]
- Li, Z.; Meng, M.; Li, Q.; Xie, Y.; Hu, T.; Zhang, J. Fe-Substituted Nanometric La0.9K0.1CoxFexO 3-δ Perovskite Catalysts Used for Soot Combustion, NOx Storage and Simultaneous Catalytic Removal of Soot and NOx. Chem. Eng. J. 2010, 164, 98–105. [Google Scholar] [CrossRef]
- Li, C.L.; Jiang, B.S.; Fanchiang, W.L.; Lin, Y.C. The Effect of Pd Content in LaMnO3 for Methanol Partial Oxidation. Catal. Commun. 2011, 16, 165–169. [Google Scholar] [CrossRef]
- Li, C.L.; Wang, C.L.; Lin, Y.C. Pd-Integrated Lanthanum-Transition Metal Perovskites for Methanol Partial Oxidation. Catal. Today 2011, 174, 135–140. [Google Scholar] [CrossRef]
- Attfield, J.P. “A” Cation Control of Perovskite Properties. Cryst. Eng. 2002, 5, 427–438. [Google Scholar] [CrossRef]
- King, G.; Woodward, P.M. Cation Ordering in Perovskites. J. Mater. Chem. 2010, 20, 5785–5796. [Google Scholar] [CrossRef]
- Garibay-Alvarado, J.A.; Farías, R.; Reyes-López, S.Y. Sol-Gel and Electrospinning Synthesis of Lithium Niobate-Silica Nanofibers. Coatings 2019, 9, 212. [Google Scholar] [CrossRef]
- Kobylyanskaya, S.D.; Gavrilenko, O.N.; Belous, A.G. Synthesis of Nanosized (Li,La){Ti,Nb,Ta}O3 Particles Using the Sol-Gel Method. Russ. J. Inorg. Chem. 2013, 58, 637–643. [Google Scholar] [CrossRef]
- Masloboeva, S.M.; Palatnikov, M.N.; Arutyunyan, L.G. Sol–Gel Synthesis of a Zn-Doped Lithium Tantalate Growth Charge. Inorg. Mater. 2020, 56, 270–276. [Google Scholar] [CrossRef]
- Mudra, E.; Brunckova, H.; Streckova, M.; Sopcak, T.; Sebek, M.; Durisin, J.; Girman, V.; Dusza, J. Preparation and Characterization of Ceramic Nanofibers Based on Lanthanum Tantalates. J. Sol-Gel Sci. Technol. 2016, 78, 322–330. [Google Scholar] [CrossRef]
- Wang, B.; Gu, S.; Ding, Y.; Chu, Y.; Zhang, Z.; Ba, X.; Zhang, Q.; Li, X. A Novel Route to Prepare LaNiO3 Perovskite-Type Oxide Nanofibers by Electrospinning for Glucose and Hydrogen Peroxide Sensing. Analyst 2013, 138, 362–367. [Google Scholar] [CrossRef]
- Luque, G.L.; Ferreyra, N.F.; Leyva, A.G.; Rivas, G.A. Characterization of Carbon Paste Electrodes Modified with Manganese Based Perovskites-Type Oxides from the Amperometric Determination of Hydrogen Peroxide. Sens. Actuators B Chem. 2009, 142, 331–336. [Google Scholar] [CrossRef]
- Xu, X.; Zhong, Y.; Shao, Z. Double Perovskites in Catalysis, Electrocatalysis, and Photo(Electro)Catalysis. Trends Chem. 2019, 1, 410–424. [Google Scholar] [CrossRef]
- Grimaud, A.; May, K.J.; Carlton, C.E.; Lee, Y.L.; Risch, M.; Hong, W.T.; Zhou, J.; Shao-Horn, Y. Double Perovskites as a Family of Highly Active Catalysts for Oxygen Evolution in Alkaline Solution. Nat. Commun. 2013, 4, 2439. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Wang, S.; Jacobson, A.J.; Reimus, L.; Brodersen, P.; Mims, C.A. Rapid Oxygen Ion Diffusion and Surface Exchange Kinetics in PrBaCo 2O5+x with a Perovskite Related Structure and Ordered a Cations. J. Mater. Chem. 2007, 17, 2500–2505. [Google Scholar] [CrossRef]
- Chen, D.; Ran, R.; Zhang, K.; Wang, J.; Shao, Z. Intermediate-Temperature Electrochemical Performance of a Polycrystalline PrBaCo2O5+δ Cathode on Samarium-Doped Ceria Electrolyte. J. Power Sources 2009, 188, 96–105. [Google Scholar] [CrossRef]
- Shellaiah, M.; Sun, K.W. Review on Sensing Applications of Perovskite Nanomaterials. Chemosensors 2020, 8, 55. [Google Scholar] [CrossRef]
- Wang, W.; Pang, S.; Su, Y.; Shen, X.; Wang, Y.; Xu, K.; Xi, X.; Xiang, J. The Effect of Calcium on the Properties of SmBa1−xCaxCoCuO5+δ as a Cathode Material for Intermediate-Temperature Solid Oxide Fuel Cells. J. Eur. Ceram. Soc. 2017, 37, 1557–1562. [Google Scholar] [CrossRef]
- Yoo, S.; Jun, A.; Ju, Y.W.; Odkhuu, D.; Hyodo, J.; Jeong, H.Y.; Park, N.; Shin, J.; Ishihara, T.; Kim, G. Development of Double-Perovskite Compounds as Cathode Materials for Low-Temperature Solid Oxide Fuel Cells. Angew. Chemie-Int. Ed. 2014, 53, 13064–13067. [Google Scholar] [CrossRef]
- West, M.; Manthiram, A. Layered LnBa1-XSrxCoCuO5+δ (Ln = Nd and Gd) Perovskite Cathodes for Intermediate Temperature Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2013, 38, 3364–3372. [Google Scholar] [CrossRef]
- Jun, A.; Kim, J.; Shin, J.; Kim, G. Optimization of Sr Content in Layered SmBa 1-XSr XCo 2O 5+δ Perovskite Cathodes for Intermediate-Temperature Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2012, 37, 18381–18388. [Google Scholar] [CrossRef]
- He, J.; Xu, X.; Li, M.; Zhou, S.; Zhou, W. Recent Advances in Perovskite Oxides for Non-Enzymatic Electrochemical Sensors: A Review. Anal. Chim. Acta 2023, 1251, 341007. [Google Scholar] [CrossRef]
- Burke, L.D.; Lyons, M.E.G. Electrochemistry of Hydrous Oxide Films. Mod. Asp. Electrochem. 1986, 18, 169–248. [Google Scholar] [CrossRef]
- Mefford, J.T.; Rong, X.; Abakumov, A.M.; Hardin, W.G.; Dai, S.; Kolpak, A.M.; Johnston, K.P.; Stevenson, K.J. Water Electrolysis on La1-XSrxCoO3-δ Perovskite Electrocatalysts. Nat. Commun. 2016, 7, 11053. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhu, Y.; Wang, Y.; Yuan, G.; Liu, J.M. A Review of Flexible Perovskite Oxide Ferroelectric Films and Their Application. J. Mater. 2020, 6, 1–16. [Google Scholar] [CrossRef]
- Rørvik, P.M.; Grande, T.; Einarsrud, M.A. One-Dimensional Nanostructures of Ferroelectric Perovskites. Adv. Mater. 2011, 23, 4007–4034. [Google Scholar] [CrossRef] [PubMed]
- Bhaumik, S.; Ray, S.; Batabyal, S.K. Recent Advances of Lead-Free Metal Halide Perovskite Single Crystals and Nanocrystals: Synthesis, Crystal Structure, Optical Properties, and Their Diverse Applications. Mater. Today Chem. 2020, 18, 100363. [Google Scholar] [CrossRef]
- Adinolfi, V.; Peng, W.; Walters, G.; Bakr, O.M.; Sargent, E.H. The Electrical and Optical Properties of Organometal Halide Perovskites Relevant to Optoelectronic Performance. Adv. Mater. 2018, 30, 1700764. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Liu, Q.; Lu, S.; Chen, G.; Gao, S.; Lu, W.; Sun, X. High-Performance Non-Enzymatic Glucose Detection: Using a Conductive Ni-MOF as an Electrocatalyst. J. Mater. Chem. B 2020, 8, 5411–5415. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.C.; Lyons, C. Electrode Systems for Continuous Monitoring in Cardiovascular Surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical Glucose Biosensors. Electrochem. Sensors, Biosens. their Biomed. Appl. 2008, 108, 814–825. [Google Scholar] [CrossRef]
- Rocchitta, G.; Spanu, A.; Babudieri, S.; Latte, G.; Madeddu, G.; Galleri, G.; Nuvoli, S.; Bagella, P.; Demartis, M.I.; Fiore, V.; et al. Enzyme Biosensors for Biomedical Applications: Strategies for Safeguarding Analytical Performances in Biological Fluids. Sensors 2016, 16, 780. [Google Scholar] [CrossRef] [PubMed]
- Dzyadevych, S.V.; Arkhypova, V.N.; Soldatkin, A.P.; El’skaya, A.V.; Martelet, C.; Jaffrezic-Renault, N. Amperometric Enzyme Biosensors: Past, Present and Future. Itbm-Rbm 2008, 29, 171–180. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Luo, L.; Ding, Y.; Liu, X. Preparation of Perovskite-Type Composite Oxide LaNi0.5Ti0.5O3-NiFe2O4 and Its Application in Glucose Biosensor. J. Electroanal. Chem. 2010, 642, 35–40. [Google Scholar] [CrossRef]
- George, G.; Ede, S.R.; Luo, Z. Fundamentals of Perovskite Oxides: Synthesis, Structure, Properties and Applications; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Boubezari, I.; Zazoua, A.; Errachid, A.; Jaffrezic-Renault, N. Sensitive Electrochemical Detection of Bioactive Molecules (Hydrogen Peroxide, Glucose, Dopamine) with Perovskites-Based Sensors. Chemosensors 2021, 9, 289. [Google Scholar] [CrossRef]
- Natali Sora, I.; Caronna, T.; Fontana, F.; De Julián Fernández, C.; Caneschi, A.; Green, M. Crystal Structures and Magnetic Properties of Strontium and Copper Doped Lanthanum Ferrites. J. Solid State Chem. 2012, 191, 33–39. [Google Scholar] [CrossRef]
- Atta, N.F.; Galal, A.; El-Ads, E.H. Effect of B-Site Doping on Sr2PdO3 Perovskite Catalyst Activity for Non-Enzymatic Determination of Glucose in Biological Fluids. J. Electroanal. Chem. 2019, 852, 113523. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Zhong, H.; Li, X.M.; Jia, F.F.; Shi, Y.X.; Zhang, W.G.; Cheng, Z.P.; Zhang, L.L.; Wang, J.K. Perovskite LaTiO3-Ag0.2 Nanomaterials for Nonenzymatic Glucose Sensor with High Performance. Biosens. Bioelectron. 2013, 48, 56–60. [Google Scholar] [CrossRef]
- Sivakumar, M.; Pandi, K.; Chen, S.M.; Cheng, Y.H.; Sakthivel, M. Facile Synthesis of Perovskite-Type NdNiO3 Nanoparticles for an Effective Electrochemical Non-Enzymatic Glucose Biosensor. New J. Chem. 2017, 41, 11201–11207. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Luo, L.; Ding, Y.; Liu, X.; Huang, A. A Novel Sensitive Nonenzymatic Glucose Sensor Based on Perovskite LaNi 0.5Ti0.5O3-Modified Carbon Paste Electrode. Sens. Actuators B Chem. 2010, 151, 65–70. [Google Scholar] [CrossRef]
- Liotta, L.F.; Puleo, F.; LaParola, V.; Leonardi, S.G.; Donato, N.; Aloisio, D.; Neri, G. La0.6Sr0.4FeO3-δ and La0.6Sr0.4Co0.2Fe0.8O3-δ Perovskite Materials for H2O2 and Glucose Electrochemical Sensors. Electroanalysis 2015, 27, 684–692. [Google Scholar] [CrossRef]
- El-Ads, E.H.; Galal, A.; Atta, N.F. The Effect of A-Site Doping in a Strontium Palladium Perovskite and Its Applications for Non-Enzymatic Glucose Sensing. RSC Adv. 2016, 6, 16183–16196. [Google Scholar] [CrossRef]
- He, J.; Sunarso, J.; Zhu, Y.; Zhong, Y.; Miao, J.; Zhou, W.; Shao, Z. High-Performance Non-Enzymatic Perovskite Sensor for Hydrogen Peroxide and Glucose Electrochemical Detection. Sens. Actuators B Chem. 2017, 244, 482–491. [Google Scholar] [CrossRef]
- Pelucarte, K.D.; Hatchell, T.A.; George, G.; Ede, S.R.; Adhikari, M.; Lin, Y.; Wen, J.; Luo, Z.; Han, S. Electrospun Porous La-Sr-Co-Ni-O Nanofibers for Highly Sensitive Non-Enzymatic Glucose Detection. Mater. Adv. 2022, 3, 2096–2103. [Google Scholar] [CrossRef]
- Rajaji, U.; Ganesh, P.S.; Chen, S.M.; Govindasamy, M.; Kim, S.Y.; Alshgari, R.A.; Shimoga, G. Deep Eutectic Solvents Synthesis of Perovskite Type Cerium Aluminate Embedded Carbon Nitride Catalyst: High-Sensitive Amperometric Platform for Sensing of Glucose in Biological Fluids. J. Ind. Eng. Chem. 2021, 102, 312–320. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, L.; Wu, J.; Li, N.; He, N.; Zhao, H.; Wu, Q.; Li, X. Perovskite-SrTiO3/TiO2/PDA as Photoelectrochemical Glucose Biosensor. Ceram. Int. 2021, 47, 29807–29814. [Google Scholar] [CrossRef]
- Gao, M.; Li, J.; Qiu, L.; Xia, X.; Cheng, X.; Xu, F.; Xu, G.; Wei, F.; Yang, J.; Hu, Q.; et al. Glucose and PH Responsive Fluorescence Detection System Based on Simple Synthesis of Silicon-Coated Perovskite Quantum Dots. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2023, 289, 122212. [Google Scholar] [CrossRef]
- Niu, X.; Gao, H.; Du, J. CsPbBr3Perovskite Nanocrystals Decorated with Cu Nanoclusters for Ratiometric Detection of Glucose. ACS Appl. Nano Mater. 2022, 5, 2350–2357. [Google Scholar] [CrossRef]
- Jia, F.F.; Zhong, H.; Zhang, W.G.; Li, X.R.; Wang, G.Y.; Song, J.; Cheng, Z.P.; Yin, J.Z.; Guo, L.P. A Novel Nonenzymatic ECL Glucose Sensor Based on Perovskite LaTiO3-Ag0.1 Nanomaterials. Sens. Actuators B Chem. 2015, 212, 174–182. [Google Scholar] [CrossRef]
- Encyclopaedia Britannica. Urea. Chemical Compound. Encyclopaedia Britannica 2023. Available online: https://www.britannica.com/science/urea (accessed on 11 August 2023).
- Sha, R.; Komori, K.; Badhulika, S. Graphene–Polyaniline Composite Based Ultra-Sensitive Electrochemical Sensor for Non-Enzymatic Detection of Urea. Electrochim. Acta 2017, 233, 44–51. [Google Scholar] [CrossRef]
- Mondal, S.; Sangaranarayanan, M.V. A Novel Non-Enzymatic Sensor for Urea Using a Polypyrrole-Coated Platinum Electrode. Sens. Actuators B Chem. 2013, 177, 478–486. [Google Scholar] [CrossRef]
- Mozaffari, S.A.; Rahmanian, R.; Abedi, M.; Amoli, H.S. Urea Impedimetric Biosensor Based on Reactive RF Magnetron Sputtered Zinc Oxide Nanoporous Transducer. Electrochim. Acta 2014, 146, 538–547. [Google Scholar] [CrossRef]
- Tran, T.Q.N.; Yoon, S.W.; Park, B.J.; Yoon, H.H. CeO2-Modified LaNi0.6Fe0.4O3 Perovskite and MWCNT Nanocomposite for Electrocatalytic Oxidation and Detection of Urea. J. Electroanal. Chem. 2018, 818, 76–83. [Google Scholar] [CrossRef]
- Ezhilan, M.; Gumpu, M.B.; Ramachandra, B.L.; Nesakumar, N.; Babu, K.J.; Krishnan, U.M.; Rayappan, J.B.B. Design and Development of Electrochemical Biosensor for the Simultaneous Detection of Melamine and Urea in Adulterated Milk Samples. Sens. Actuators B Chem. 2017, 238, 1283–1292. [Google Scholar] [CrossRef]
- Kumar, V.; Chopra, A.; Arora, S.; Yadav, S.; Kumar, S.; Kaur, I. Amperometric Sensing of Urea Using Edge Activated Graphene Nanoplatelets. RSC Adv. 2015, 5, 13278–13284. [Google Scholar] [CrossRef]
- Verma, R.; Gupta, B.D. A Novel Approach for Simultaneous Sensing of Urea and Glucose by SPR Based Optical Fiber Multianalyte Sensor. Analyst 2014, 139, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.; Pradhan, S.; Pramanik, S.; Bandyopadhyay, R.; Das, D.K.; Pramanik, P. Efficient Electrochemical Detection of Guanine, Uric Acid and Their Mixture by Composite of Nano-Particles of Lanthanides Ortho-Ferrite XFeO3 (X = La, Gd, Pr, Dy, Sm, Ce and Tb). J. Electroanal. Chem. 2018, 830–831, 95–105. [Google Scholar] [CrossRef]
- Kumar, Y.; Singh, P.P.; Pramanik, P.; Das, D. Electrochemical Determination of Guanine and Uric Acid Using NdFeO3 Nps Modified Graphite Paste Electrode. J. Sci. Ind. Res. 2019, 78, 177–181. [Google Scholar]
- Durai, L.; Badhulika, S. One Pot Hydrothermal Synthesis of Large Area Nano Cube like ZnSnO3 Perovskite for Simultaneous Sensing of Uric Acid and Dopamine Using Differential Pulse Voltammetry. IEEE Sens. J. 2020, 20, 13212–13219. [Google Scholar] [CrossRef]
- Ahmad, K.; Kumar, P.; Mobin, S.M. Hydrothermally Grown Novel Pyramids of the CaTiO3perovskite as an Efficient Electrode Modifier for Sensing Applications. Mater. Adv. 2020, 1, 2003–2009. [Google Scholar] [CrossRef]
- Soundharraj, P.; Jagannathan, M.; Dhinasekaran, D.; Thiruvarasu, P. Fluorescent Zinc Titanate as an Effective Sensing Platform for Urea Detection. Mater. Today Proc. 2021, 50, 101–106. [Google Scholar] [CrossRef]
- Atta, N.F.; Ali, S.M.; El-Ads, E.H.; Galal, A. The Electrochemistry and Determination of Some Neurotransmitters at SrPdO 3 Modified Graphite Electrode. J. Electrochem. Soc. 2013, 160, G3144–G3151. [Google Scholar] [CrossRef]
- Atta, N.F.; Ali, S.M.; El-Ads, E.H.; Galal, A. Nano-Perovskite Carbon Paste Composite Electrode for the Simultaneous Determination of Dopamine, Ascorbic Acid and Uric Acid. Electrochim. Acta 2014, 128, 16–24. [Google Scholar] [CrossRef]
- Marinho, H.S.; Real, C.; Cyrne, L.; Soares, H.; Antunes, F. Hydrogen Peroxide Sensing, Signaling and Regulation of Transcription Factors. Redox Biol. 2014, 2, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.; Hofferber, E.; Stapleton, J.A.; Iverson, N.M. Hydrogen Peroxide Sensors for Biomedical Applications. Chemosensors 2019, 7, 64. [Google Scholar] [CrossRef]
- Pravda, J. Hydrogen Peroxide and Disease: Towards a Unified System of Pathogenesis and Therapeutics. Mol. Med. 2020, 26, 1–10. [Google Scholar] [CrossRef]
- Li, W.; Yin, X.; Yan, Y.; Liu, C.; Li, G. Kurarinone Attenuates Hydrogen Peroxide-Induced Oxidative Stress and Apoptosis through Activating the PI3K/Akt Signaling by Upregulating IGF1 Expression in Human Ovarian Granulosa Cells. Environ. Toxicol. 2023, 38, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Song, K.E.; Hwang, H.R.; Hong, E.S.H.; Konvalina, P.; Jun, W.J.; Jung, J.W.; Shim, S. Hydrogen Peroxide Ameliorates the Adversities of Drought Stress during Germination and Seedling Growth in Sorghum (Sorghum Bicolor L.). Agronomy 2023, 13, 330. [Google Scholar] [CrossRef]
- Ahmad, T.; Iqbal, A.; Halim, S.A.; Uddin, J.; Khan, A.; El Deeb, S.; Al-Harrasi, A. Recent Advances in Electrochemical Sensing of Hydrogen Peroxide (H2O2) Released from Cancer Cells. Nanomaterials 2022, 12, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Talachi, N.; Afzal, E.; Nouri, M.; Abroun, S.; Zarrabi, M.; Jahandar, H. Protective Effect of Human Amniotic Membrane Extract against Hydrogen Peroxide-induced Oxidative Damage in Human Dermal Fibroblasts. Int. J. Cosmet. Sci. 2023, 45, 73–82. [Google Scholar] [CrossRef]
- Hadi, F.; Maqbool, T.; Shakoori, T.A.; Muhammad, T.; Awan, S.J.; Akhtar, S. Role of Oxidative Stress and Inflammatory Markers in Osteoporosis. Int. J. Appl. Exp. Biol. 2022, 2, 27–32. [Google Scholar] [CrossRef]
- Xing, L.; Zhang, W.; Fu, L.; Lorenzo, J.M.; Hao, Y. Fabrication and Application of Electrochemical Sensor for Analyzing Hydrogen Peroxide in Food System and Biological Samples. Food Chem. 2022, 385, 132555. [Google Scholar] [CrossRef]
- Khan, F.; Shekhar, C.; Mondal, T.; Sabapathy, M. Removal of Industrial Dye and Pharmaceutical Product Using the Nano and Micron-Sized PS Rough Particles Studded with Pt Nanoparticles. arXiv 2023, arXiv:2301.03891. [Google Scholar]
- dos Santos Pereira, T.; Mauruto de Oliveira, G.C.; Santos, F.A.; Raymundo-Pereira, P.A.; Oliveira, O.N.; Janegitz, B.C. Use of Zein Microspheres to Anchor Carbon Black and Hemoglobin in Electrochemical Biosensors to Detect Hydrogen Peroxide in Cosmetic Products, Food and Biological Fluids. Talanta 2019, 194, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Albanese, L.; Meneguzzo, F.; Pagliaro, M. Hydrogen Peroxide: A Key Chemical for Today’s Sustainable Development. ChemSusChem 2016, 9, 3374–3381. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, H.; Maleki, F.; Khaaki, P.; Kadhom, M.; Kudaibergenov, N.; Khataee, A. Electrochemical-Based Sensing Platforms for Detection of Glucose and H2O2 by Porous Metal–Organic Frameworks: A Review of Status and Prospects. Biosensors 2023, 13, 347. [Google Scholar] [CrossRef] [PubMed]
- Maduraiveeran, G. Nanomaterials-Based Portable Electrochemical Sensing and Biosensing Systems for Clinical and Biomedical Applications. J. Anal. Sci. Technol. 2022, 13, 1–13. [Google Scholar] [CrossRef]
- Dhara, K.; Mahapatra, D.R. Recent Advances in Electrochemical Nonenzymatic Hydrogen Peroxide Sensors Based on Nanomaterials: A Review. J. Mater. Sci. 2019, 54, 12319–12357. [Google Scholar] [CrossRef]
- Soleymani, M.; Moheb, A.; Babakhani, D. Hydrogen Peroxide Decomposition over Nanosized La1-XCaXMnO3 (0 ≤ X ≤ 0.6) Perovskite Oxides. Chem. Eng. Technol. 2011, 34, 49–55. [Google Scholar] [CrossRef]
- Navrotsky, A. Thermochemistry of Perovskite-Related Oxides with High Oxidation States: Superconductors, Sensors, Fuel Cell Materials. Pure Appl. Chem. 1994, 66, 1759–1764. [Google Scholar] [CrossRef]
- Wang, G.; Bao, Y.; Tian, Y.; Xia, J.; Cao, D. Electrocatalytic Activity of Perovskite La1-XSrxMnO3 towards Hydrogen Peroxide Reduction in Alkaline Medium. J. Power Sources 2010, 195, 6463–6467. [Google Scholar] [CrossRef]
- Dong, W.; Zhao, G.; Song, B.; Xu, G.; Zhou, J.; Han, G. Surfactant-Free Fabrication of CaTiO3 Butterfly-like Dendrite via a Simple One-Step Hydrothermal Route. CrystEngComm 2012, 14, 6990–6997. [Google Scholar] [CrossRef]
- Kimijima, T.; Kanie, K.; Nakaya, M.; Muramatsu, A. Hydrothermal Synthesis of Size- and Shape-Controlled CaTiO3 Fine Particles and Their Photocatalytic Activity. CrystEngComm 2014, 16, 5591–5597. [Google Scholar] [CrossRef]
- Yang, X.; Fu, J.; Jin, C.; Chen, J.; Liang, C.; Wu, M.; Zhou, W. Formation Mechanism of CaTiO3 Hollow Crystals with Different Microstructures. J. Am. Chem. Soc. 2010, 132, 14279–14287. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Guo, Z.; Chen, Y.; Hao, J.; Liu, W. In Situ Hydrothermal Synthesis of Nanolamellate CaTiO3 with Controllable Structures and Wettability. Inorg. Chem. 2007, 46, 7707–7709. [Google Scholar] [CrossRef]
- Mahmood, K.; Khalid, A.; Mehran, M.T. MAPbI3 Microneedle-Arrays for Perovskite Photovoltaic Application. Nanoscale Adv. 2019, 1, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Deganello, F.; Liotta, L.F.; Leonardi, S.G.; Neri, G. Electrochemical Properties of Ce-Doped SrFeO3 Perovskites-Modified Electrodes towards Hydrogen Peroxide Oxidation. Electrochim. Acta 2016, 190, 939–947. [Google Scholar] [CrossRef]
- He, J.; Zhou, W.; Sunarso, J.; Xu, X.; Zhong, Y.; Shao, Z.; Chen, X.; Zhu, H. 3D Ordered Macroporous SmCoO3 Perovskite for Highly Active and Selective Hydrogen Peroxide Detection. Electrochim. Acta 2018, 260, 372–383. [Google Scholar] [CrossRef]
- Wang, X.T.; Li, B.; Kong, D.R.; Zhang, Z.Y.; Zhang, X.F.; Deng, Z.P.; Huo, L.H.; Gao, S. T- and T′-Type Layered Perovskite Ln2CuO4 Nanocrystals for Enhanced Sensing Detection of Hydrogen Peroxide. J. Alloys Compd. 2022, 911, 165037. [Google Scholar] [CrossRef]
- Qu, X.; Zhao, S.; Gao, P.; Qian, X.; Lu, S.; Duan, F.; Zhu, H.; Du, M. Ultrasensitive Hydrogen Peroxide Electrochemical Sensor Based on Dual-Phase Perovskite Oxide Tubular Nanofiber. New J. Chem. 2023, 47, 1540–1547. [Google Scholar] [CrossRef]
- Rao, C.N.R. Perovskites. In Encyclopedia of Physical Science and Technology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 707–714. ISBN 9780122274107. [Google Scholar]
- Hammad, A.B.A.; Magar, H.S.; Mansour, A.M.; Hassan, R.Y.A.; Nahrawy, A.M.E. Construction and Characterization of Nano-Oval BaTi0.7Fe0.3O3@NiFe2O4 Nanocomposites as an Effective Platform for the Determination of H2O2. Sci. Rep. 2023, 13, 9048. [Google Scholar] [CrossRef]
- Chakraborty, T.; Das, M.; Lin, C.Y.; Lei, K.F.; Kao, C.H. Highly Sensitive and Selective Electrochemical Detection of Lipocalin 2 by NiO Nanoparticles/Perovskite CeCuOx Based Immunosensor to Diagnose Renal Failure. Anal. Chim. Acta 2022, 1205, 339754. [Google Scholar] [CrossRef]
- Manna, S.; Kumar, S.; Sharma, A.; Sahoo, S.; Dey, M.K.; Mishra, P.K.; Satpati, A.K. RGO/ReO3 Nano Composite Modified Electrode for the Ultra-Sensitive Determination of Dopamine and Uric Acid. Biosens. Bioelectron. X 2022, 11, 100156. [Google Scholar] [CrossRef]
- Tamilalagan, E.; Muthumariappan, A.; Chen, T.-W.; Chen, S.-M.; Maheshwaran, S.; Huang, P.-J. A Highly Selective Enzyme-Free Amperometric Detection of Glucose Using Perovskite-Type Lanthanum Cobaltite (LaCoO3). J. Electrochem. Soc. 2021, 168, 086501. [Google Scholar] [CrossRef]
- Boubezari, I.; Zazoua, A.; Bessueille, F.; Errachid, A.; Jaffrezic-Renault, N. Design of a New Non-Enzymatic Sensor Based on a Substituted A2BO4+δ Perovskite for the Voltammetric Detection of Glucose. Electroanalysis 2020, 32, 1642–1650. [Google Scholar] [CrossRef]
- El-Ads, E.H.; Galal, A.; Atta, N.F. Electrochemistry of Glucose at Gold Nanoparticles Modified Graphite/SrPdO3 Electrode-Towards a Novel Non-Enzymatic Glucose Sensor. J. Electroanal. Chem. 2015, 749, 42–52. [Google Scholar] [CrossRef]
- Xu, D.; Luo, L.; Ding, Y.; Xu, P. Sensitive Electrochemical Detection of Glucose Based on Electrospun La0.88Sr0.12MnO3 Naonofibers Modified Electrode. Anal. Biochem. 2015, 489, 38–43. [Google Scholar] [CrossRef] [PubMed]




| Perovskite Structure | Analyte | LOD (µM) | LROD (µM) | Interference | Year | Ref. |
|---|---|---|---|---|---|---|
| LaCuO3, LaSrCuO4, LaBaCuO4, NaCuO2, KCuO2. | H2O2 | - | - | - | 1994 | [22] |
| La0.6Ca0.4Ni0.7Fe0.3O3 | H2O2 | 0.5 | 0.1–1 | - | 1996 | [28] |
| La0.66Sr0.33MnO3 | H2O2 | - | - | - | 2009 | [21] |
| La1−xSrxMnO3 | H2O2 | - | - | - | 2010 | [23] |
| La1−xCaxCoO3 | H2O2 | - | - | - | 2012 | [19] |
| LaNiTiO3 | H2O2 | 0.01 | 0.05–1000 | AA, DA, and UA | 2013 | [27] |
| LaNiO3 | H2O2 | 0.32 | 0.05–1000 | AA, DA, and UA | 2013 | [24] |
| KNbO3 | H2O2 | 12 | - | - | 2014 | [26] |
| La0.6Sr0.4Co0.2Fe0.8O3−δ | H2O2 | 5 | 0–3000 | AA, DA, and UA | 2015 | [29] |
| La0.7Sr0.3Mn0.75Co0.25O3 | H2O2 | 0.17 | 0.5–1000 | AA, DA, and UA | 2015 | [25] |
| Ce doped SrFeO3 | H2O2 | 10 | 0–500 | Glu, CA, Na, NO2, Ca, N, Cu, Fe. | 2016 | [30] |
| LaNi0.6Co0.4O3 | H2O2 | 0.05 | 0.2–3350 | AA, DA, and UA | 2017 | [20] |
| SmCoO3 | H2O2 | 0.004 | 0.1–10,000 | Glu, AA, DA, and UA | 2018 | [31] |
| LaNiO3 | H2O2 | 0.035 | 0.2–50, 50–3240 | Suc, Glu, Fru, Ara, Urea, CA, L-Cys, Arg, APAP, and His. | 2022 | [34] |
| PrBaCo2O6 (PBC), PrBaCo0.9Ni0.1O6−δ (PBCN) | H2O2 | (0.17), (0.34) | 5–6950, 5–6380 | - | 2022 | [35] |
| La2CuO4 Sm2CuO4 | H2O2 | (0.160), (0.410) | 0.50–15.87 15.87–23,350 1.24–37.20 37.20–35,440 | Glu, AA, UA, L-Cys, and TrTrp | 2022 | [32] |
| La0.9Sr0.1NiO3 | H2O2 | 0.018 | 1~7000 | - | 2023 | [33] |
| LaNi0.6Fe0.4O-CeO2 | Urea | 1 | 25 to 670 | Creatinine, AA, Glu, Na+, Mg+, SO4−2 | 2018 | [75] |
| XFeO3 X=La, Ni, Fe, Ce | Uric acid | For La = 0.2 | 0.5 to 120 | - | 2018 | [79] |
| NdFeO3 | Uric acid | 0.35 | 1 to 120 | - | 2019 | [80] |
| ZnSnO3 | Uric acid | 0.550 | 1 to 5 | Ca2+, Cl−, Na+, K+, Glu, and AA | 2020 | [81] |
| CaTiO3 | Urea | 1.6 | 50 to 450 | AA, H2O2, UA, catechol, resorcinol, phenol, DA, and Glu | 2020 | [82] |
| ZnTiO3 | Urea | 76.16 | 30 to 150 | - | 2021 | [83] |
| NiO/CeCuOx | Lipocalin | 4.23 ng mL−1 | 25 to 400 ng mL−1 | Crt, AA, human serum albumin (HSA), and hemoglobin (Hb) | 2021 | [115] |
| ReO3 | Uric acid | 0.06 | 1–10.5 | Glu, Fe3+, Cys, Pb2+, Cd2+, and Cu2+ | 2022 | [116] |
| La0.6Sr0.4CoO3 | Glucose | 0.05 | 2–3350 | DA, UA, and AA | 2017 | [64] |
| LaSrCoNiO | Glucose | 83 | 100–1000 | Glu, Fru, Lac, Gal, Man, DA, and AA | 2022 | [65] |
| CsPbBr3 | Glucose | 0.8 | 2.0 to 170.0 | Glu, Gly, Val, Glut, Cys, Mal, Lac,Man, Fru, and Suc | 2022 | [69] |
| CeAlO3 | Glucose | 0.00086 | 0.01–1034.5 | NO3, NO2, H2O2, FA, UA, AA, PA, DA, Epi, CPF, and NFA | 2021 | [66] |
| LaCoO3 | Glucose | 0.0016 | 0.01–407.2 | - | 2021 | [117] |
| SrTiO3 | Glucose | - | 0–32,000 | NaCl, DA, UA, AA, Suc | 2021 | [67] |
| Pr1.92Ba0.08Ni0.95Zn0.05O4+δ | Glucose | 0.5 | 1.5 μM–7000 | - | 2020 | [118] |
| Sr2PdO3 | Glucose | 0.0021 | 0.2–100 | UA, AA, and DA | 2019 | [58] |
| NdNiO3 | Glucose | 0.3 | 0.5 to 4600 | AA, DA, UA, Chol, Lac, Fru, Suc, NaCl, KCl, NO2, and Na2SO4 | 2017 | [60] |
| CaTiO3 | Glucose | 2.3 | 0.7 to 1490 | UA and AA | 2017 | [69] |
| Sr1.7Ca0.3PdO3 | Glucose | 0.0845 | 5 to 5600 | - | 2016 | |
| LaTiO3 | Glucose | 0.0000025 | 0.01 to 1000 | Gly, Fruc, Mal, UU, AA, DP, Cu2+, Na+, Fe3+, Mn2+, and SO42− | 2015 | [70] |
| SrPdO3 | Glucose | 10.1 | 100–6000 | UA, AA, DA, and PA | 2015 | [119] |
| La0.88Sr0.12MnO3 | Glucose | - | 0.05–100 | AA, UA, and DA | 2015 | [120] |
| LaxSr1−xCoyFe1−yO3−δ | Glucose | 5 | 0–200 | AA, UA, and DA | 2014 | [62] |
| LiNbO3 | Glucose | 10 | 300 to 3300 | UA and AA | 2014 | [11] |
| LaNb2O7 | Glucose | 20 | 25 to 2830 | - | 2014 | [10] |
| LaTiO3 | Glucose | 0.21 | 2.5 to 4000 | AA, UA, and DA | 2013 | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piñón-Balderrama, C.I.; Leyva-Porras, C.; Conejo-Dávila, A.S.; Estrada-Monje, A.; Maldonado-Orozco, M.C.; Reyes-López, S.Y.; Zaragoza-Contreras, E.A. Electrochemical Perovskite-Based Sensors for the Detection of Relevant Biomarkers for Human Kidney Health. Chemosensors 2023, 11, 507. https://doi.org/10.3390/chemosensors11090507
Piñón-Balderrama CI, Leyva-Porras C, Conejo-Dávila AS, Estrada-Monje A, Maldonado-Orozco MC, Reyes-López SY, Zaragoza-Contreras EA. Electrochemical Perovskite-Based Sensors for the Detection of Relevant Biomarkers for Human Kidney Health. Chemosensors. 2023; 11(9):507. https://doi.org/10.3390/chemosensors11090507
Chicago/Turabian StylePiñón-Balderrama, Claudia Ivone, César Leyva-Porras, Alain Salvador Conejo-Dávila, Anayansi Estrada-Monje, María Cristina Maldonado-Orozco, Simón Yobanny Reyes-López, and Erasto Armando Zaragoza-Contreras. 2023. "Electrochemical Perovskite-Based Sensors for the Detection of Relevant Biomarkers for Human Kidney Health" Chemosensors 11, no. 9: 507. https://doi.org/10.3390/chemosensors11090507