A Fluorescence Strategy Based on Guanidinylated Carbon Dots and FAM-Labeled ssDNA for Facile Detection of Lipopolysaccharide
Abstract
1. Introduction
2. Experimental Section
2.1. Synthesis of GQ-CDs
2.2. Establish the Quenching System of GQ-CDs and FAM-DNA (FAM-DNA/GQ-CDs)
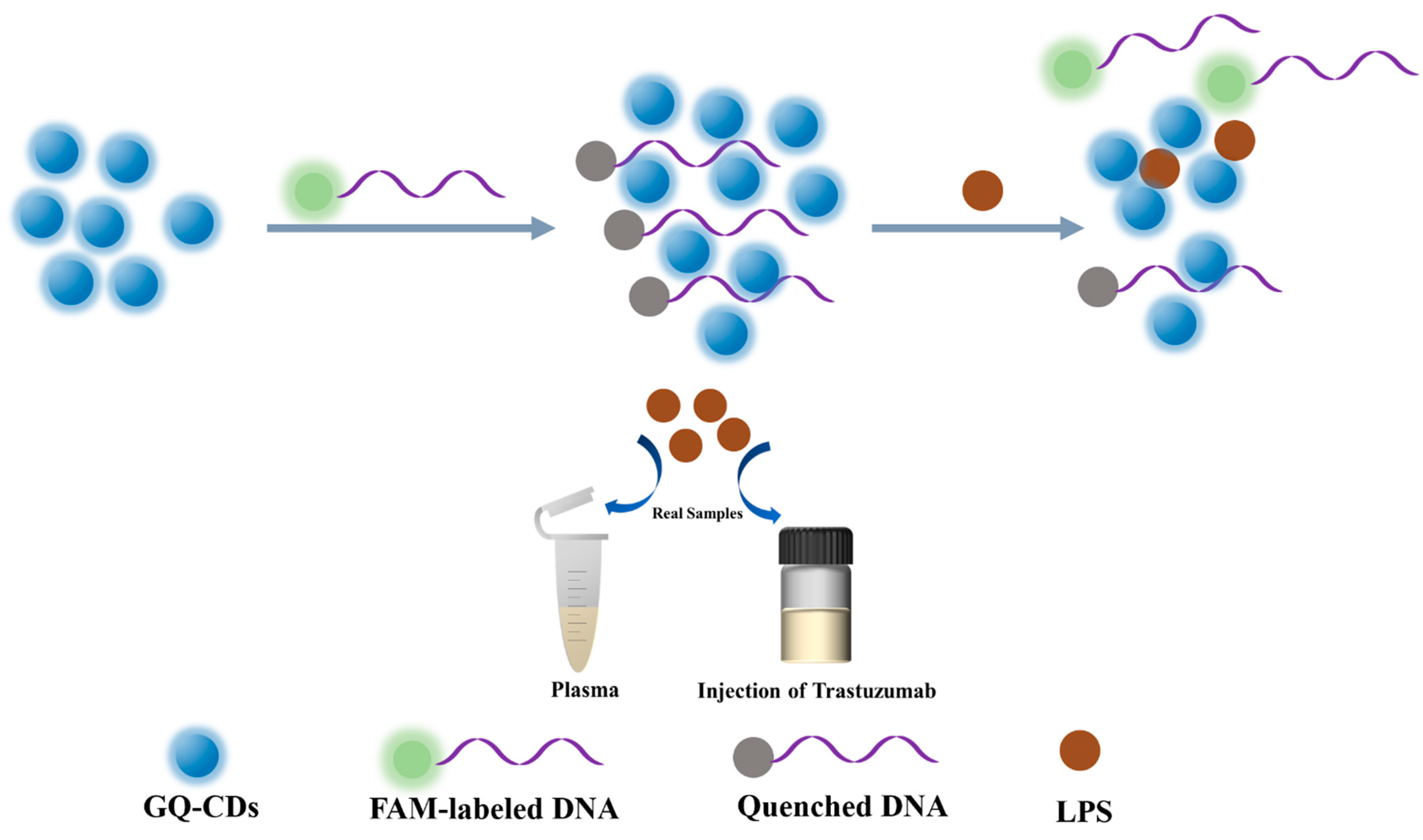
2.3. Feasibility of the GQ-CDs/FAM-DNA for LPS Detection
2.4. Analytical Performance of This Method for Detecting LPS
2.5. Detection of LPS Content in Real Samples
3. Results and Discussion
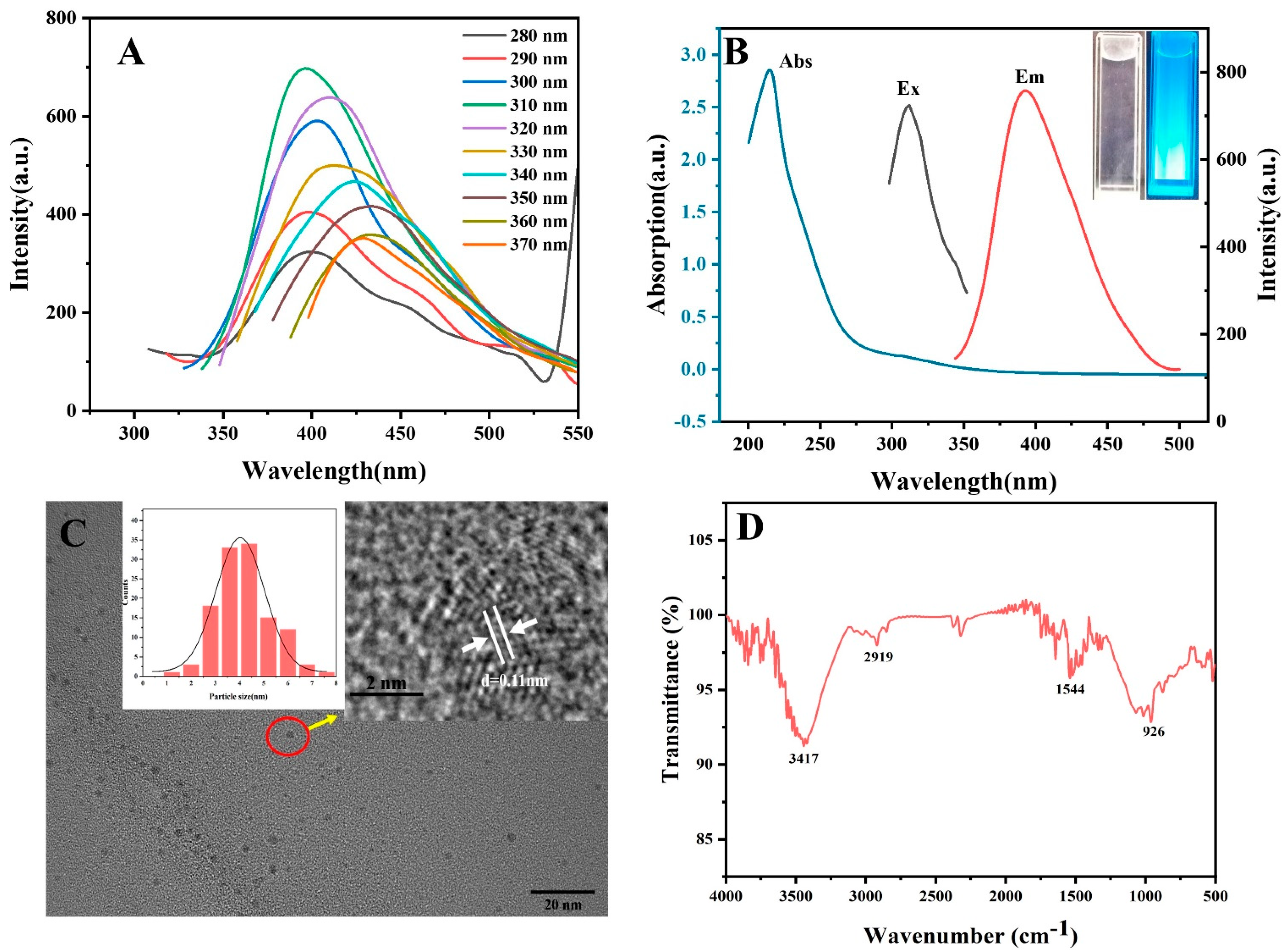
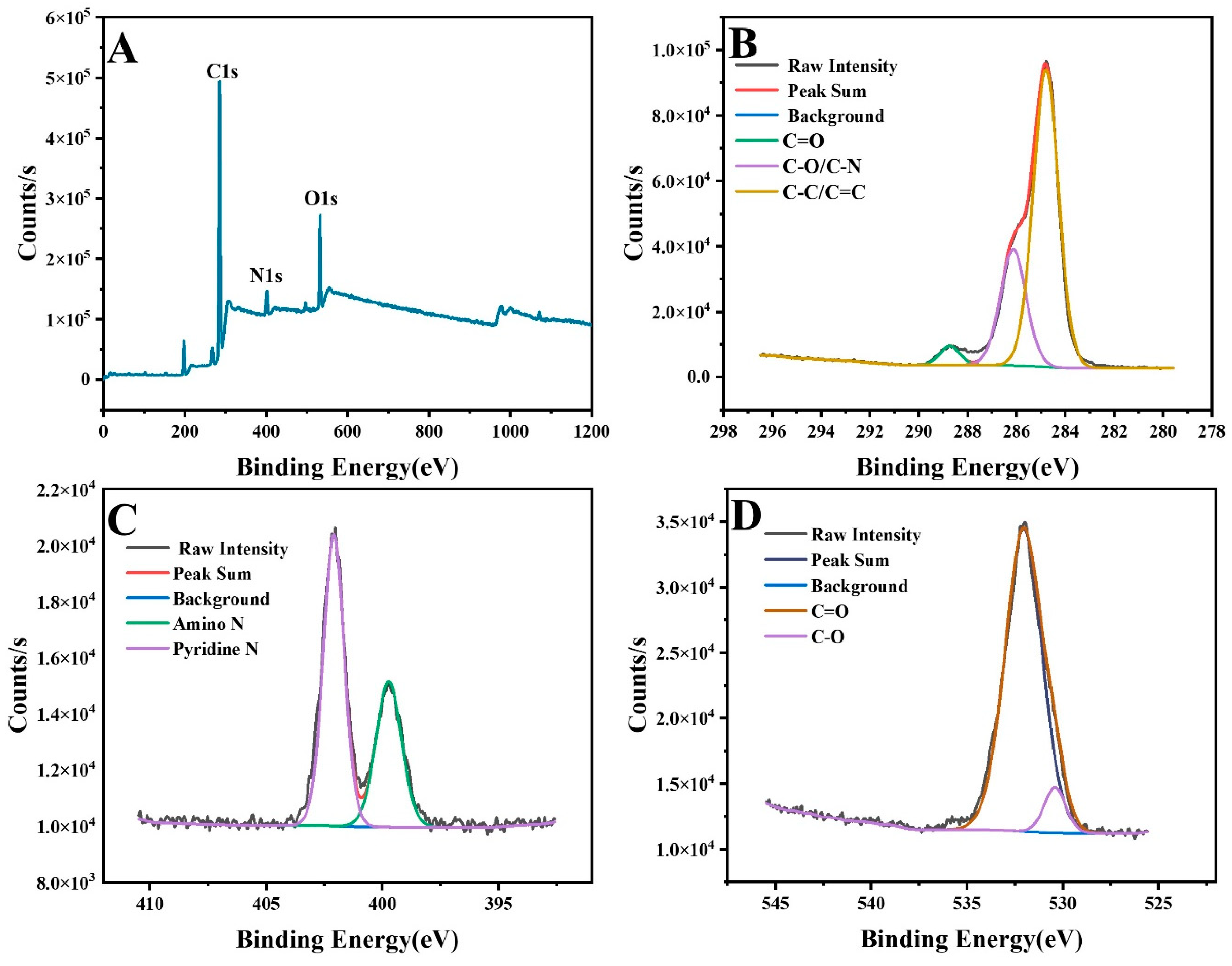
3.1. Characterization of GQ-CDs
3.2. Establishment and Properties of GQ-CDs/FAM-DNA System
3.3. The Feasibility and the Mechanism of the Detection Strategy
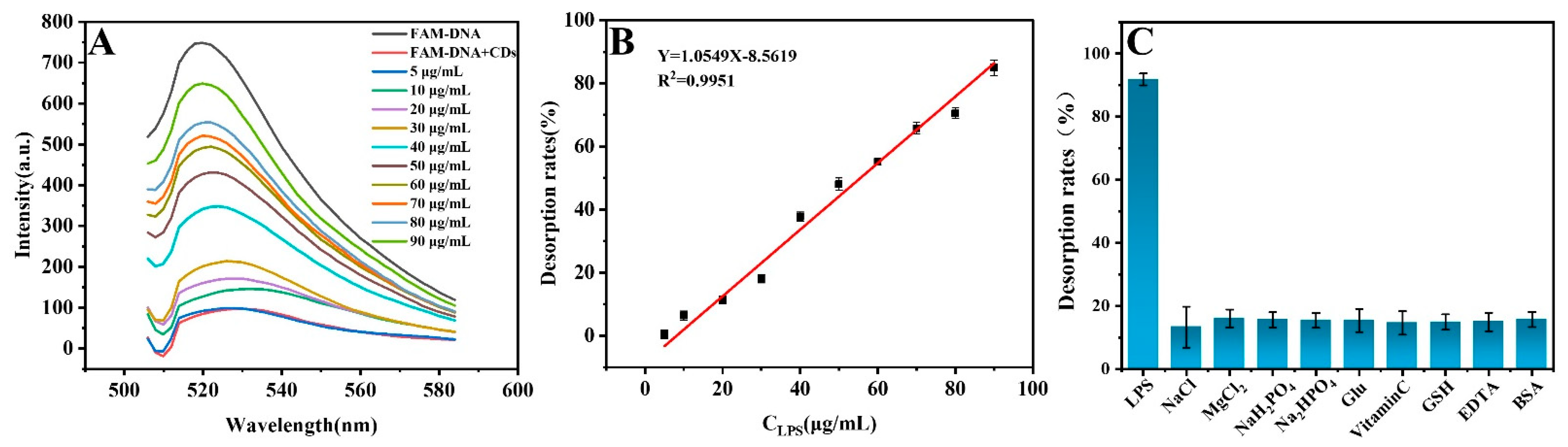
3.4. Evaluating the Analytical Performance
3.5. Detection of Actual Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.Y.; Quinn, P.J. Lipopolysaccharide: Biosynthetic pathway and structure modification. Prog. Lipid Res. 2010, 49, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Sperandeo, P.; Martorana, A.M.; Polissi, A. The lipopolysaccharide transport (Lpt) machinery: A nonconventional transporter for lipopolysaccharide assembly at the outer membrane of Gram-negative bacteria. J. Biol. Chem. 2017, 292, 17981–17990. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.S. Hit ‘em Where It Hurts: Gram-Negative Bacterial Lipopolysaccharide as a Vaccine Target. Microbiol. Mol. Biol. Rev. 2023, 87, e00045-22. [Google Scholar] [CrossRef]
- Ma, F.B.; Li, Z.; Cao, J.; Kong, X.Q.; Gong, G.P. A TGFBR2/SMAD2/DNMT1/miR-145 negative regulatory loop is responsible for LPS-induced sepsis. Biomed. Pharmacother. 2019, 112, 108626. [Google Scholar] [CrossRef]
- Ji, Z.R.; Xue, W.L.; Zhang, L.; Schisandrin, B. Attenuates Inflammation in LPS-Induced Sepsis Through miR-17-5p Downregulating TLR4. Inflammation 2019, 42, 731–739. [Google Scholar] [CrossRef]
- Solati, S.; Zhang, T.; Timman, S. The monocyte activation test detects potentiated cytokine release resulting from the synergistic effect of endotoxin and non-endotoxin pyrogens. Innate Immun. 2022, 28, 130–137. [Google Scholar] [CrossRef] [PubMed]
- An, Z.F.; Jang, C.H. Simple and Label-Free Liquid Crystal-based Optical Sensor for Highly Sensitive and Selective Endotoxin Detection by Aptamer Binding and Separation. ChemistrySelect 2019, 4, 1416–1422. [Google Scholar] [CrossRef]
- Yamashita, K.; Takahashi, D.; Yamamoto, Y.; Kiyomoto, S.; Shibata, T.; Kawabata, S. Effects of Ca2+ ions on the horseshoe crab coagulation cascade triggered by lipopolysaccharide. J. Biochem. 2023, 174, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Ruiz, Z.; Menéndez-Sardiñas, D.E.; Pacios-Michelena, A.; Gabilondo-Ramírez, T.; Montero-Alejo, V.; Perdomo-Morales, R. Soluble β-(1,3)-glucans enhance LPS-induced response in the monocyte activation test, but inhibit LPS-mediated febrile response in rabbits: Implications for pyrogenicity tests. Eur. J. Pharm. Sci. 2016, 81, 18–26. [Google Scholar] [CrossRef]
- Stang, K.; Fennrich, S.; Krajewski, S.; Stoppelkamp, S.; Burgener, I.A.; Wendel, H.; Post, M. Highly sensitive pyrogen detection on medical devices by the monocyte activation test. J. Mater. Sci. Mater. Med. 2014, 25, 1065–1075. [Google Scholar] [CrossRef]
- Cui, F.C.; Zhu, P.; Ji, J.; Blaženović, I.; Gholami, M.; Zhang, Y.Z.; Sun, X.L. Gas chromatography-mass spectrometry metabolomic study of lipopolysaccharides toxicity on rat basophilic leukemia cells. Chem.-Biol. Interact. 2018, 281, 81–88. [Google Scholar] [CrossRef]
- de Barros, J.P.P.; Gautier, T.; Sali, W.; Adrie, C.; Choubley, H.; Charron, E.; Lalande, C.; Guern, N.L.; Deckert, V.; Monchi, M.; et al. Quantitative lipopolysaccharide analysis using HPLC/MS/MS and its combination with the limulus amebocyte lysate assay. J. Lipid Res. 2015, 56, 1363–1369. [Google Scholar] [CrossRef]
- Fung, F.M.; Su, M.; Feng, H.T.; Li, S.F.Y. Extraction, separation and characterization of endotoxins in water samples using solid phase extraction and capillary electrophoresis-laser induced fluorescence. Sci. Rep. 2017, 7, 10774. [Google Scholar] [CrossRef]
- Fux, A.C.; Melo, C.C.; Michelini, S.; Swartzwelter, B.J.; Neusch, A.; Italiani, P.; Himly, M. Heterogeneity of Lipopolysaccharide as Source of Variability in Bioassays and LPS-Binding Proteins as Remedy. Int. J. Mol. Sci. 2023, 24, 8395. [Google Scholar] [CrossRef]
- Ye, H.; Zhou, Y.; Ma, P.F.; Guo, Y.X.; Wang, Z.P. Analysis of the anti-inflammatory effect of the aptamer LA27 and its binding mechanism. Int. J. Biol. Macromol. 2020, 165, 308–313. [Google Scholar] [CrossRef]
- Zhu, L.J.; Li, S.T.; Shao, X.L.; Feng, Y.X.; Xie, P.Y.; Luo, Y.B.; Huang, K.L.; Xu, W.T. Colorimetric detection and typing of E-coli lipopolysaccharides based on adual aptamer-functionalized gold nanoparticle probe. Microchim. Acta 2019, 186, 111. [Google Scholar] [CrossRef]
- Niu, J.X.; Hu, X.M.; Ouyang, W.; Chen, Y.; Liu, S.W.; Han, J.; Liu, L.H. Femtomolar Detection of Lipopolysaccharide in Injectables and Serum Samples Using Aptamer-Coupled Reduced Graphene Oxide in a Continuous Injection-Electrostacking Biochip. Anal. Chem. 2019, 91, 2360–2367. [Google Scholar] [CrossRef]
- Yang, X.Y.; Li, J.Y.; Tan, X.H.; Yang, X.J.; Song, P.; Ming, D.M.; Yang, D.Q. Ratiometric fluorescence probe integrated with smartphone for visually detecting lipopolysaccharide. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 286, 121961. [Google Scholar] [CrossRef]
- Adeola, A.O.; Clermont-Paquette, A.; Piekny, A.; Naccache, R. Advances in the design and use of carbon dots for analytical and biomedical applications. Nanotechnology 2024, 35, 012001. [Google Scholar] [CrossRef]
- Mohiuddin, I.; Singh, R.; Kaur, V. A Review of Sensing Applications of Molecularly Imprinted Fluorescent Carbon Dots for Food and Biological Sample Analysis. Crit. Rev. Anal. Chem. 2023, 1–22. [Google Scholar] [CrossRef]
- Cai, R.Y.; Miao, C.F.; Zhang, L.; Zhou, Y.; Liu, Y.B.; Zheng, C.; Han, W.D.; Huang, Z.J.; Zhou, X.; Weng, S.H. Determination and the pharmacokinetic study of tigecycline by fluorescence strategy with F, N codoping carbon dots as probe. Sens. Actuators B Chem. 2022, 361, 131721. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Chen, Y.P.; Zhang, M.H.; Xu, L.L.; Xu, Y.C.; Chen, L.; Yin, X.Y.; Yang, S.Y.; Weng, S.H.; Zheng, Y.J.; et al. Fluorine-doped carbon dots (F-CDs) adsorbing DNA via hydrophobic interaction play dual-role of quenching carrier and signal reference for ratiometric fluorescence strategy to detect microRNA. Sens. Actuators B Chem. 2024, 403, 135180. [Google Scholar] [CrossRef]
- Deng, X.Q.; Zhang, M.H.; Wang, Y.; Li, C.P.; Zhang, X.T.; Weng, S.H.; Li, Y.M. Carbon dots with selective fluorescence response to hydroxyl radical for sensitive detection of bleomycin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 306, 123582. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.F.; Guo, X.Z.; Zhang, X.T.; Lin, Y.N.; Han, W.D.; Huang, Z.J.; Weng, S.H. Ratiometric fluorescence assay based on carbon dots and Cu2+-catalyzed oxidation of O-Phenylenediamine for the effective detection of deferasirox. RSC Adv. 2021, 11, 34525–34532. [Google Scholar] [CrossRef]
- Han, W.D.; Miao, C.F.; Zhang, X.T.; Lin, Y.N.; Hao, X.L.; Huang, Z.J.; Weng, S.H.; Lin, X.H.; Guo, X.Z.; Huang, J.Y. A signal-off fluorescent strategy for deferasirox effective detection using carbon dots as probe and Cu2+ as medium. Anal. Chim. Acta 2021, 1179, 338853. [Google Scholar] [CrossRef]
- Guo, R.B.; Chen, B.; Li, F.L.; Weng, S.H.; Zheng, Z.F.; Chen, M.; Wu, W.; Lin, X.H.; Yang, C.Y. Positive carbon dots with dual roles of nanoquencher and reference signal for the ratiometric fluorescence sensing of DNA. Sens. Actuators B Chem. 2018, 264, 193–201. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Xue, Z.Q.; Hao, X.L.; Miao, C.F.; Zhang, J.Z.; Zheng, Y.J.; Zheng, Z.F.; Lin, X.H.; Weng, S.H. Ratiometric fluorescence sensor based on carbon dots as internal reference signal and T7 exonuclease-assisted signal amplification strategy for microRNA-21 detection. Anal. Chim. Acta 2020, 1103, 212–219. [Google Scholar] [CrossRef]
- Zhang, X.T.; Wu, P.P.; Hao, X.L.; Liu, J.M.; Huang, Z.J.; Weng, S.H.; Chen, W.F.; Huang, L.L.; Huang, J.Y. Quaternization carbon dots with enhanced antimicrobial ability to Gram-negative bacteria for the treatment of acute peritonitis caused by E. coli. J. Mater. Chem. B 2023, 11, 7696–7706. [Google Scholar] [CrossRef]
- Hao, X.L.; Huang, L.L.; Zhao, C.F.; Chen, S.N.; Lin, W.J.; Lin, Y.N.; Zhang, L.R.; Sun, A.A.; Miao, C.F.; Lin, X.H.; et al. Antibacterial activity of positively charged carbon quantum dots without detectable resistance for wound healing with mixed bacteria infection. Mat. Sci. Eng. C-Mater. 2021, 123, 111971. [Google Scholar] [CrossRef]
- Zhao, C.F.; Wang, X.W.; Wu, L.N.; Wu, W.; Zheng, Y.J.; Lin, L.Q.; Weng, S.H.; Lin, X.H. Nitrogen-doped carbon quantum dots as an antimicrobial agent against Staphylococcus for the treatment of infected wounds. Colloids Surf. B Biointerfaces 2019, 179, 17–27. [Google Scholar] [CrossRef]
- Zhang, X.T.; Bai, X.X.; Deng, X.Q.; Peng, K.; Zheng, Z.F.; Xiao, J.C.; Zhang, R.; Huang, Z.J.; Huang, J.Y.; Chen, M.; et al. Long-term antibacterial activity of guanidinium carbon dots without detectable resistance for the effective treatment of pneumonia caused by Gram-negative bacteria. Carbon 2023, 213, 118229. [Google Scholar] [CrossRef]
- Zu, F.L.; Yan, F.Y.; Bai, Z.J.; Xu, J.X.; Wang, Y.Y.; Huang, Y.C.; Zhou, X.W. The quenching of the fluorescence of carbon dots: A review on mechanisms and applications. Microchim. Acta 2017, 184, 1899–1914. [Google Scholar] [CrossRef]
- Jian, Z.W.; Wang, H.; Liu, M.L.; Chen, S.Y.; Wang, Z.H.; Qian, W.; Luo, G.X.; Xia, H.S. Polyurethane-modified graphene oxide composite bilayer wound dressing with long-lasting antibacterial effect. Mater. Sci. Eng. C-Mater. 2020, 111, 110833. [Google Scholar] [CrossRef]
- Ye, S.J.; Wei, D.F.; Xu, X.; Guan, Y.; Zheng, A. Surface antimicrobial modification of polyamide by poly(hexamethylene guanidine) hydrochloride. Polym. Advan. Technol. 2020, 31, 1847–1856. [Google Scholar] [CrossRef]
- Zhu, Y.W.; Xu, C.; Zhang, N.; Ding, X.K.; Yu, B.R.; Xu, F.J. Polycationic Synergistic Antibacterial Agents with Multiple Functional Components for Efficient Anti-Infective Therapy. Adv. Funct. Mater. 2018, 28, 1706709. [Google Scholar] [CrossRef]
- Wang, Y.J.; Wang, L.; Wang, H.Y. Investigation on the Relationship Between Carbon Cores and Fluorescence Moieties by Measurement of Fluorescence Anisotropy of CDs with Different Sizes. Chem. Res. Chin. Univ. 2020, 36, 894–900. [Google Scholar] [CrossRef]
- Wu, J.; Zawistowski, A.; Ehrmann, M.; Yi, T.; Schmuck, C. Peptide Functionalized Polydiacetylene Liposomes Act as Fluorescent Turn-On Sensor for Bacterial Lipopolysaccharide. J. Am. Chem. Soc. 2011, 133, 9720–9723. [Google Scholar] [CrossRef]
- Gopal, J.; Abdelhamid, H.N.; Hua, P.Y.; Wu, H.F. Chitosan Nanomagnets for Effective Extraction and Sensitive Mass Spectrometric Detection of Pathogenic Bacterial Endotoxin from Human Urine. J. Mater. Chem. B 2013, 1, 2463–2475. [Google Scholar] [CrossRef]
- Paul, I.E.; Raichur, A.M.; Chandrasekaran, N.; Mukherjee, A. Fluorometric Sensing of Endotoxin Based on Aggregation of CTAB Capped Gold Nanospheres. J. Lumin. 2016, 178, 106–114. [Google Scholar] [CrossRef]
- Thakur, M.; Dan, A. Poly-L-lysine-Functionalized Green-Light-Emitting Carbon Dots as a Fluorescence Turn-on Sensor for Ultrasensitive Detection of Endotoxin. ACS Appl. Bio Mater. 2021, 4, 3410–3422. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Z.; Li, J.; Pan, G.; Wang, J.; Wang, Y.; Peng, K.; Zhang, X.; Huang, Z.; Weng, S. A Fluorescence Strategy Based on Guanidinylated Carbon Dots and FAM-Labeled ssDNA for Facile Detection of Lipopolysaccharide. Chemosensors 2024, 12, 201. https://doi.org/10.3390/chemosensors12100201
Zheng Z, Li J, Pan G, Wang J, Wang Y, Peng K, Zhang X, Huang Z, Weng S. A Fluorescence Strategy Based on Guanidinylated Carbon Dots and FAM-Labeled ssDNA for Facile Detection of Lipopolysaccharide. Chemosensors. 2024; 12(10):201. https://doi.org/10.3390/chemosensors12100201
Chicago/Turabian StyleZheng, Zongfu, Junrong Li, Gengping Pan, Jing Wang, Yao Wang, Kai Peng, Xintian Zhang, Zhengjun Huang, and Shaohuang Weng. 2024. "A Fluorescence Strategy Based on Guanidinylated Carbon Dots and FAM-Labeled ssDNA for Facile Detection of Lipopolysaccharide" Chemosensors 12, no. 10: 201. https://doi.org/10.3390/chemosensors12100201
APA StyleZheng, Z., Li, J., Pan, G., Wang, J., Wang, Y., Peng, K., Zhang, X., Huang, Z., & Weng, S. (2024). A Fluorescence Strategy Based on Guanidinylated Carbon Dots and FAM-Labeled ssDNA for Facile Detection of Lipopolysaccharide. Chemosensors, 12(10), 201. https://doi.org/10.3390/chemosensors12100201




