Application of a Novel Disposable Flow Cell for Spectroscopic Bioprocess Monitoring
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biomass Monitoring Using NIR Scattering Flow Cell
2.2. Fed-Batch Cultivation with Komagataella phaffii
2.3. Perfusion Cultivation with CHO Cells
2.4. UV-Vis Measurements Using Absorption Flow Cell
2.5. Protein A Capture Step
2.6. EC-HPLC
2.7. Antibody Aggregation by Freeze–Thawing
2.8. Spectral Data Post-Processing and Analysis
3. Results and Discussion
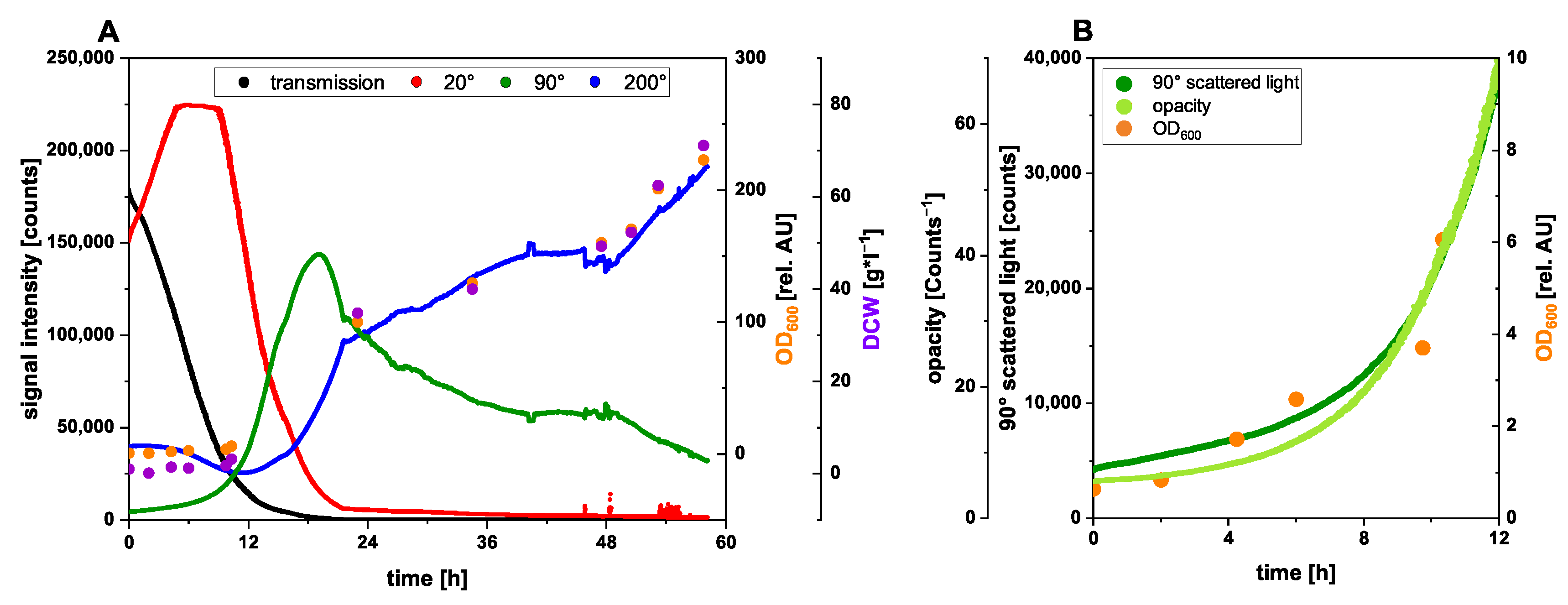
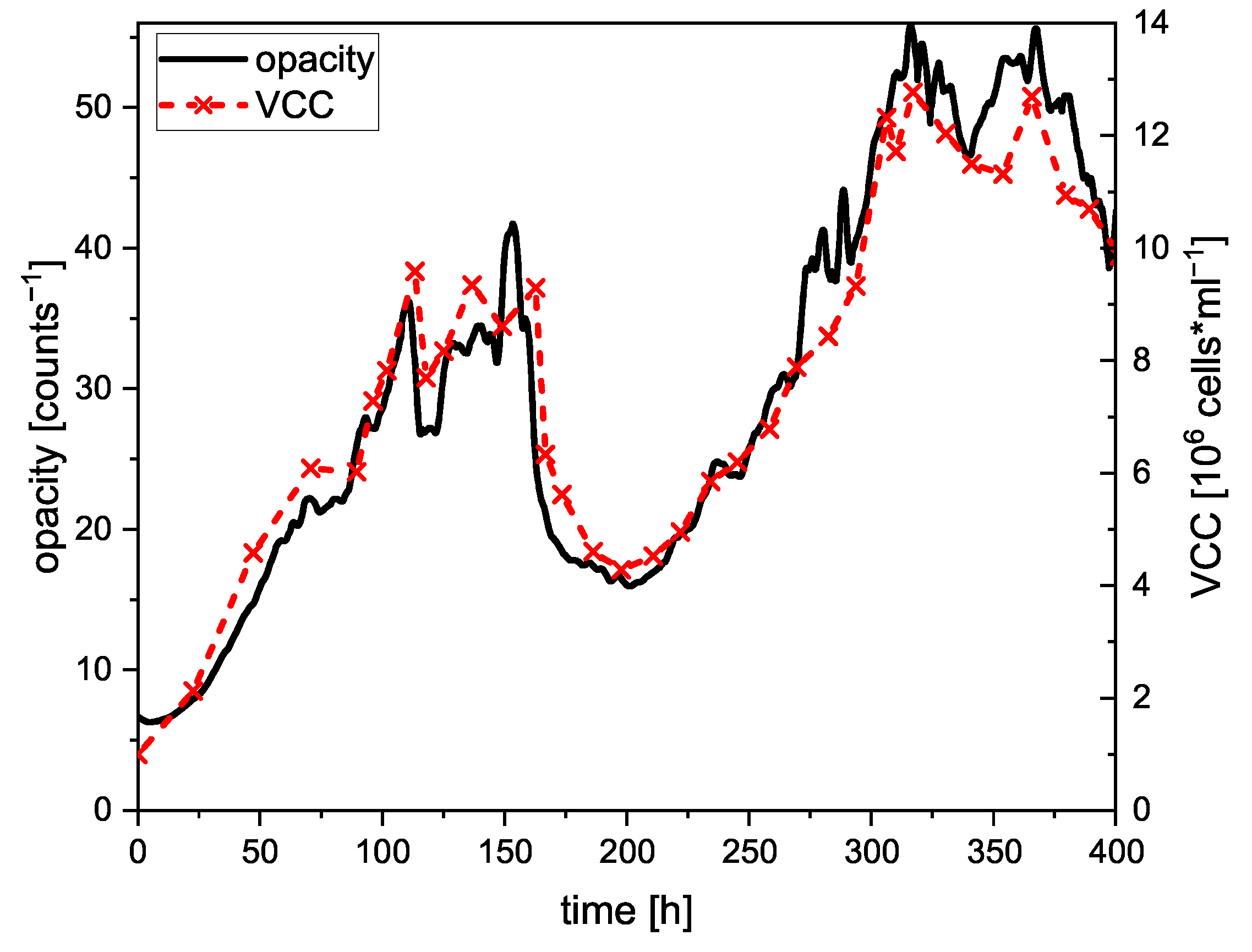
3.1. NIR Scattering for Biomass Monitoring
3.1.1. Biomass Monitoring during Komagataella phaffii High-Cell-Density Fed-Batch Cultivation
3.1.2. Long-Term Turbidity Measurement during CHO Perfusion Cultivation
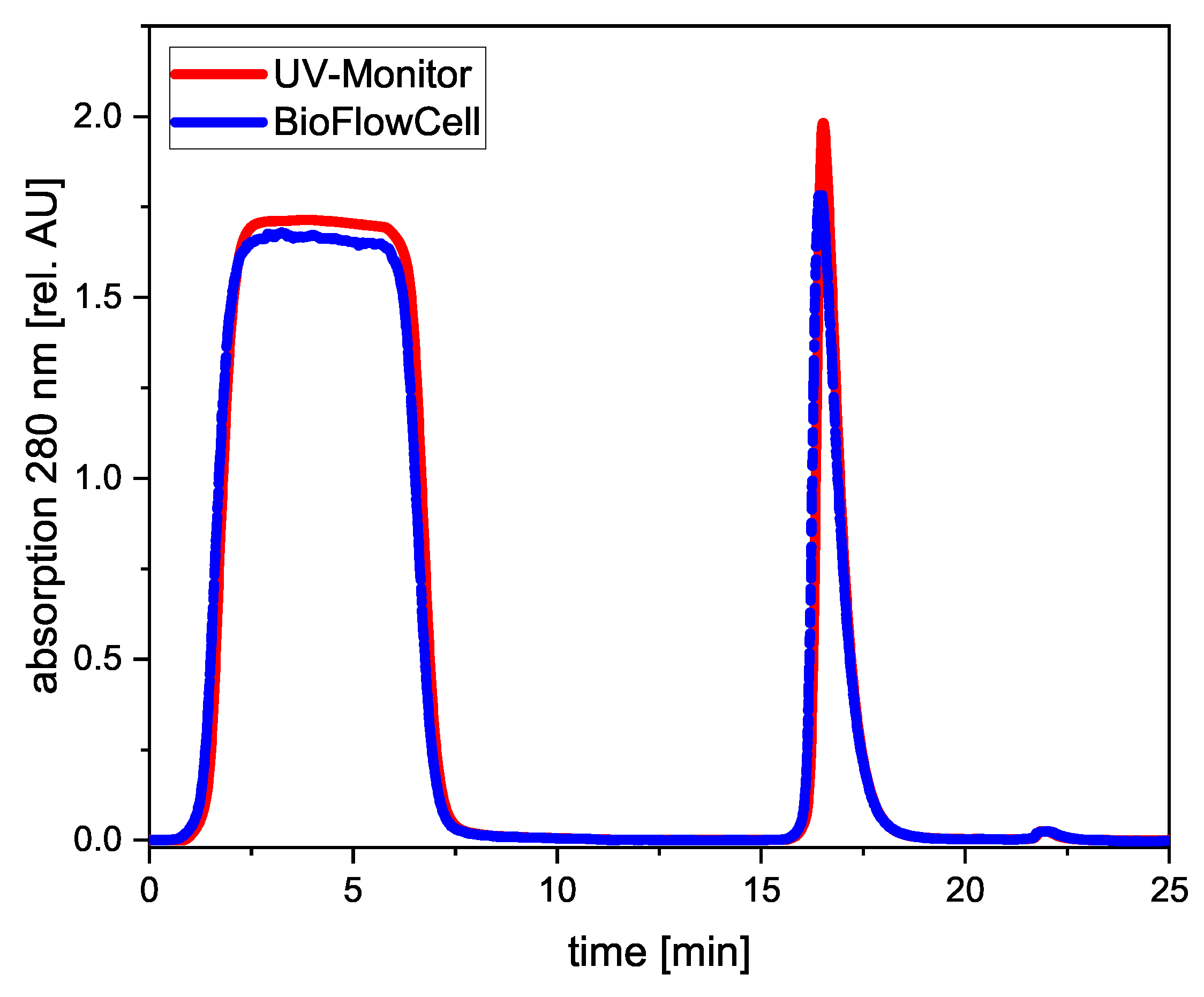
3.2. UV-Vis Spectroscopy during Purification of a Monoclonal Antibody
3.2.1. Protein A Capture Step
3.2.2. Detection of Antibody Aggregates
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FDA. Guidance for Industry Guidance for Industry PAT—A Framework for Innovative Pharmaceutical; FDA: Silver Spring, MD, USA, 2004; p. 19.
- Gerzon, G.; Sheng, Y.; Kirkitadze, M. Process Analytical Technologies—Advances in bioprocess integration and future perspectives. J. Pharm. Biomed. Anal. 2022, 207, 114379. [Google Scholar] [CrossRef] [PubMed]
- Kessler, D.P. (Ed.) Prozessanalytik: Strategien und Fallbeispiele aus der Industriellen Praxis; Wiley-VCH Verlag: Weinheim, Germany, 2006. [Google Scholar]
- Gillespie, C.; Wasalathanthri, D.P.; Ritz, D.B.; Zhou, G.; Davis, K.A.; Wucherpfennig, T.; Hazelwood, N. Systematic assessment of process analytical technologies for biologics. Biotechnol. Bioeng. 2022, 119, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Wasalathanthri, D.P.; Patel, B.A. The Role of Process Analytical Technology (PAT) in Biologics Development. In Continuous Pharmaceutical Processing and Process Analytical Technology; CRC Press: Boca Raton, FL, USA, 2023; pp. 339–354. [Google Scholar]
- Brunner, V.; Siegl, M.; Geier, D.; Becker, T. Challenges in the Development of Soft Sensors for Bioprocesses: A Critical Review. Front. Bioeng. Biotechnol. 2021, 9, 722202. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Rani, A.; Khan, J.; Pant, B.N.; Pandey, G. Applications of Biotechnology in Pharmaceutical Product Analysis. In Concepts in Pharmaceutical Biotechnology and Drug Development; Interdisciplinary Biotechnological Advances; Bose, S., Shukla, A.C., Baig, M.R., Banerjee, S., Eds.; Springer: Singapore, 2024. [Google Scholar]
- Rodríguez-Duran, L.V.; Torres-Mancera, M.T.; Trujillo-Roldán, M.A.; Valdez-Cruz, N.A.; Favela-Torres, E.; Saucedo-Castañeda, G. Standard instruments for bioprocess analysis and control. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 593–626. [Google Scholar]
- Mitra, S.; Murthy, G.S. Bioreactor control systems in the biopharmaceutical industry: A critical perspective. Syst. Microbiol. Biomanuf. 2022, 2, 91–112. [Google Scholar] [CrossRef] [PubMed]
- Steinwedel, T.; Dahlmann, K.; Solle, D.; Scheper, T.; Reardon, K.F.; Lammers, F. Sensors for Disposable Bioreactor Systems. In Single-Use Technology in Biopharmaceutical Manufacture; Wiley Online Library: Hoboken, NJ, USA, 2019; pp. 69–82. [Google Scholar]
- Lopes, A.G. Single-use in the biopharmaceutical industry: A review of current technology impact, challenges and limitations. Food Bioprod. Process. 2015, 93, 98–114. [Google Scholar] [CrossRef]
- Reardon, K.F. Practical monitoring technologies for cells and substrates in biomanufacturing. Curr. Opin. Biotechnol. 2021, 71, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, N.D.; Lopes, J.A.; Almeida, C.F.; Sarraguça, M.C.; Pinheiro, H.M. Bioreactor monitoring with spectroscopy and chemometrics: A review. Anal. Bioanal. Chem. 2012, 404, 1211–1237. [Google Scholar] [CrossRef] [PubMed]
- Furey, J.; Clark, K.; Card, C. Adoption of single-use sensors for bioprocess operations. BioProcess Int. 2011, 9, 36–42. [Google Scholar]
- Bareither, R.; Pollard, D. A review of advanced small-scale parallel bioreactor technology for accelerated process development: Current state and future need. Biotechnol. Prog. 2011, 27, 2–14. [Google Scholar] [CrossRef]
- Raithel, P.; Steinwedel, T.; Belz, M.; Solle, D. Disposable flowcell for spectroscopic analysis in bioprocesses. In Proceedings of the Optical Fibers and Sensors for Medical Diagnostics, Treatment and Environmental Applications XXI, Online, 6–12 March 2021; Volume 11635, pp. 157–164. [Google Scholar]
- Thiel, P.; Steinwedel, T.; Raithel, P.; Belz, M.; Solle, D. Development of a novel disposable flowcell for spectroscopic bioprocess monitoring. Chemosensors 2024. [Google Scholar]
- Benavides, M.; Mailier, J.; Hantson, A.L.; Muñoz, G.; Vargas, A.; Van Impe, J.; Vande Wouwer, A. Design and test of a low-cost RGB sensor for online measurement of microalgae concentration within a photo-bioreactor. Sensors 2015, 15, 4766–4780. [Google Scholar] [CrossRef] [PubMed]
- Schellenberg, J.; Dehne, M.; Lange, F.; Scheper, T.; Solle, D.; Bahnemann, J. Establishment of a Perfusion Process with Antibody-Producing CHO Cells Using a 3D-Printed Microfluidic Spiral Separator with Web-Based Flow Control. Bioengineering 2023, 10, 656. [Google Scholar] [CrossRef] [PubMed]
- Gellermann, P.; Schneider-Barthold, C.; Bolten, S.N.; Overfelt, E.; Scheper, T.; Pepelanova, I. Production of a Recombinant Non-Hydroxylated Gelatin Mimetic in Pichia pastoris for Biomedical Applications. J. Funct. Biomater. 2019, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Kortmann, C.; Habib, T.; Heuer, C.; Solle, D.; Bahnemann, J. A Novel 3D-Printed and Miniaturized Periodic Counter Current Chromatography System for Continuous Purification of Monoclonal Antibodies. Micromachines 2024, 15, 382. [Google Scholar] [CrossRef] [PubMed]
- Brämer, C.; Tünnermann, L.; Gonzalez Salcedo, A.; Reif, O.W.; Solle, D.; Scheper, T.; Beutel, S. Membrane Adsorber for the Fast Purification of a Monoclonal Antibody Using Protein A Chromatography. Membranes 2019, 9, 159. [Google Scholar] [CrossRef] [PubMed]
- Wolfrum, E.J.; Payne, C.; Schwartz, A.; Jacobs, J.; Kressin, R.W. A performance comparison of low-cost near-infrared (NIR) spectrometers to a conventional laboratory spectrometer for rapid biomass compositional analysis. BioEnergy Res. 2020, 13, 1121–1129. [Google Scholar] [CrossRef]
- Hawe, A.; Friess, W.; Sutter, M.; Jiskoot, W. Online fluorescent dye detection method for the characterization of immunoglobulin G aggregation by size exclusion chromatography and asymmetrical flow field flow fractionation. Anal. Biochem. 2008, 378, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Burastero, O.; Draper-Barr, G.; Raynal, B.; Chevreuil, M.; Engl, P.; Garcia Alai, M. Raynals, an online tool for the analysis of dynamic light scattering. Acta Crystallogr. Sect. D Struct. Biol. 2023, 79, 673–683. [Google Scholar] [CrossRef] [PubMed]


| Aggregate peak | |||||
| Sample | Retention [min] | Area [mAU2] | Area [%] | Height [mAU] | Height [%] |
| Control | 7.45 | 30,630 | 2.17 | 786 | 2.14 |
| 10 × | 7.49 | 31,015 | 2.54 | 766 | 2.37 |
| 50 × | 7.53 | 61,289 | 5.51 | 1422 | 4.77 |
| mAb peak | |||||
| Sample | Retention [min] | Area [mAU2] | Area [%] | Height [mAU] | Height [%] |
| Control | 8.56 | 1,379,056 | 97.83 | 35999 | 97.86 |
| 10 × | 8.58 | 1,191,014 | 97.46 | 31572 | 97.63 |
| 50 × | 8.593 | 1,051,363 | 94.49 | 28415 | 95.23 |
| Sample | 280 nm [AU] | 350 nm [AU] | AI |
|---|---|---|---|
| Control | 0.286 | 0.001 | 0.211 |
| 10 × FT | 0.284 | 0.020 | 7.587 |
| 15 × FT | 0.304 | 0.024 | 8.661 |
| 30 × FT | 0.313 | 0.052 | 20.064 |
| 40 × FT | 0.305 | 0.047 | 18.344 |
| 50 × FT | 0.298 | 0.050 | 20.314 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steinwedel, T.; Raithel, P.; Schellenberg, J.; Kortmann, C.; Gellermann, P.; Belz, M.; Solle, D. Application of a Novel Disposable Flow Cell for Spectroscopic Bioprocess Monitoring. Chemosensors 2024, 12, 202. https://doi.org/10.3390/chemosensors12100202
Steinwedel T, Raithel P, Schellenberg J, Kortmann C, Gellermann P, Belz M, Solle D. Application of a Novel Disposable Flow Cell for Spectroscopic Bioprocess Monitoring. Chemosensors. 2024; 12(10):202. https://doi.org/10.3390/chemosensors12100202
Chicago/Turabian StyleSteinwedel, Tobias, Philipp Raithel, Jana Schellenberg, Carlotta Kortmann, Pia Gellermann, Mathias Belz, and Dörte Solle. 2024. "Application of a Novel Disposable Flow Cell for Spectroscopic Bioprocess Monitoring" Chemosensors 12, no. 10: 202. https://doi.org/10.3390/chemosensors12100202





