Sustainable Biopolymer-Based Electrochemical Sensors for Trace Heavy Metal Determination in Water: A Comprehensive Review
Abstract
1. Introduction
- Proteins (e.g., gelatin and collagen), known for their high mechanical strength;
- Poly(lactic acid) and poly(hydroxyalkanoates);
- Natural rubber latex, offering flexibility and conductivity;
- Polysaccharides (e.g., cellulose, chitosan, lignin), valued for their biocompatibility, biodegradability, and versatile functional groups [5].
2. Heavy Metals
2.1. Heavy Metals Definition and Sources
2.2. Toxicological Effects
| Metal | WHO (mg L−1) | Toxicity | Anthropogenic Sources | Effects | Refs. | |
|---|---|---|---|---|---|---|
| Tolerable Daily Intake (mg/per day) | Lethal Dose mg kg−1 Body Weight | |||||
| Lead (Pb) | 0.05 | 0.025–0.052 | 94–158 | PVC pipes in sanitation, agriculture, recycled PVC, lead paint, jewelry, lead batteries, lunch boxes. | Alzheimer’s disease and senile dementia, damage to the nervous system, also leads to neurodegenerative diseases, lower IQ, kidney damage, reduced bone growth, behavioral problems, digestive problems, urinary insufficiency. | [11,14,15,16] |
| Cadmium (Cd) | 0.005 | 0.018–0.052 | 4.4–6.2 | Paints, pigments, batteries, plastics rubbers, engraving process, photoconductors, and photovoltaic cells. | Renal toxicity, hypertension, weight loss, fatigue, microcytic hypochromic anemia, lymphocytosis, pulmonary fibrosis, lung cancer. | [15,17] |
| Mercury (Hg) | 0.001 | 0.03 | 5.1–10.0 | Combustion of coal, municipal solid waste incineration, and volcanic emissions. | Impaired neurologic development, effects on digestive system, immune system, lungs, kidneys, skin and eyes, hypertension. | [18,19] |
| Arsenic (Ar) | 0.05 | 0.03 | 41 | Wooden electricity poles that are treated with arsenic-based preservatives, pesticides, fertilizers. | Affects the cardiovascular system, pulmonary diseases, gastrointestinal tract, genitourinary system, hematopoietic system, dermatology, fetal and teratogenic diseases, anorexia, brown pigmentation, hyperpigmentation, local edema, and skin cancer. | |
| Chromium (Cr) | 0.05 | 0.013–0.099 | - | Leather industry, tanning, and chrome plating industries. | Gastrointestinal diseases, hepatic encephalopathy, respiratory and cardiovascular problems, renal and endocrine systems defects, hematological, ocular problems. | [15,20] |
| Silver (Ag) | 0.1 | - | - | Refining of copper, gold, nickel, zinc, jewelry, and electroplating industries. | Argyria, gastroenteritis, neuronal disorders, mental fatigue, rheumatism, knotting of cartilage, cytopathological effects in fibroblast, keratinocytes, and mast cells. | [4,17] |
| Zinc (Zn) | 5 | 15–16.2 | 1–25.3 | Soldering, cosmetics and pigments, respiratory disorders, metal fume fever, bronchiolar. | leucocytes, neuronal disorder, prostate cancer risks, macular degeneration, and impotence. | [9,15] |
| Copper (Cu) | 1.3 | 10 | 4.0–7.2 | Fertilizers, tanning, and photovoltaic cells. | Adrenocortical hyperactivity, allergies, anemia, alopecia, arthritis, autism, cystic fibrosis, diabetes, hemorrhaging, and kidney disorders. | [20,21] |
| Nickel (Ni) | 0.07 | 0.089–0.231 | - | Coal burning, diesel and fuel oil burning, tobacco smoking, wind dust, volcanic activity, garbage burning, cheap jewelry, stainless steel appliances. | Dermatitis, pulmonary fibrosis, asthma, respiratory and cardiovascular diseases, immune system failure, carcinogenic, DNA damage. | [22,23] |
2.3. Conventional Methods for Heavy Metal Detection
2.4. Electrochemical Sensors
2.5. Electrochemical Methods of Detection
3. Polymers and Biopolymers as Sensing Layers
3.1. Definition and Characteristics
- Biopolymers produced from renewable (biological) and biodegradable raw materials.
- Biopolymers produced from sustainable (biological), non-biodegradable raw materials.
- Biodegradable biopolymers based on fossil fuels [30].
3.2. Synthetic Biopolymers
- Non-biodegradable synthetic biopolymers, which resist environmental degradation and contribute to waste (e.g., polyamide, polyvinylchloride, polypropylene)
- Biodegradable synthetic biopolymers, which break down when exposed to environmental factors, such as poly(glycolic acid), poly(lactic acid), polycaprolactone, and polyhydroxy butyrate [2].
3.3. Natural Biopolymers (Bio-Sourced Polymers)
- Polynucleotides: polymers composed of nucleotide monomers (e.g., RNA, DNA);
- Polypeptides: polymers made of amino acids (e.g., proteins);
- Polysaccharides: polymers made of carbohydrates (e.g., cellulose, hemicellulose, pectin) [33].
3.4. Properties of Bio−Sourced Polymers
- Biocompatibility: ability to interact harmoniously with biological systems.
- High adsorption capacity: enhanced ability to absorb or adsorb molecules.
- Hydrophilicity: affinity for water, which can improve performance in sensing applications.
- Relative thermostability: ability to withstand moderate thermal variations [35].
4. Application of Biopolymers for the Removal of Heavy Metals from Water
5. Applications of Bio-Sourced Polymers in Electrochemical Sensing of Heavy Metals
5.1. Cellulose and Cellulose Composite-Based Sensors
5.2. Alginate-Based Sensors
5.3. Chitosan-Based Sensors
5.4. Polyphenols as Sensing Platforms
5.5. Other Biopolymers in Heavy Metal Detection
5.6. Discussion: Pros and Cons of Using Biopolymers in Heavy Metal Detection
6. Future Research Directions
- Exploring new methods for biopolymer production using biological methods such as enzymatic or bacterial production that can give reproducible biopolymers.
- Enhancing biopolymer functionalization by exploring more efficient methods of functionalizing biopolymers to improve their metal ion binding capacities. This could include advanced chemical modification techniques and the development of new biopolymer–nanomaterial composites.
- Sensor stability and durability: Long-term sensor stability remains a challenge, and future research should aim to enhance the durability of biopolymer-based sensors under different environmental conditions, ensuring consistent performance in real-world applications by developing cross-linking techniques or nanocomposite formulations. These modifications should enhance the mechanical and chemical stability of biopolymer sensors under prolonged exposure to challenging environments (pH fluctuations, temperature variations, salinity) and evaluate the degradation rates of biopolymer-based sensors, optimizing their durability while maintaining biodegradability.
- Real-time sensing and multi-metal detection: Current sensors often target specific metals, but future research should aim to develop sensors capable of simultaneously detecting multiple heavy metals in real-time.
- Exploring wireless sensor networks and portable detection systems can provide real-time data transmission for the remote monitoring of aquatic environments. This would provide a more comprehensive solution for environmental monitoring.
- Developing new biopolymer sources: Exploring less common biopolymer sources could lead to materials with novel properties that enhance sensor performance, and future research could explore the following:
- ○
- Investigating biopolymers from algae, fungi, or microorganisms may offer unique structural advantages or metal-binding capacities.
- ○
- Using genetic engineering or synthetic biology to design biopolymers with optimized electrochemical properties and metal ion selectivity.
- ○
- Assessing the environmental impact of harvesting new biopolymer sources to ensure they align with sustainability goals.
- Scalability and commercialization: Although biopolymer-based sensors show great potential in laboratory settings, their scalability for mass production and commercialization remains challenging. Further research should focus on cost-effective manufacturing processes and material sourcing to facilitate the widespread adoption of these sensors.
- Integrating biopolymer-based sensors with internet of things (IoT) and wireless technologies significantly enhances their capabilities for environmental monitoring. Connecting these sensors to IoT systems makes continuous, real-time monitoring of heavy metal concentrations in water and soil possible. Wireless modules enable remote data transmission to cloud platforms, facilitating a faster response to pollution events and informed decision-making. These sensors’ lightweight and flexible nature allows their deployment in remote and inaccessible areas, while wireless connectivity ensures data transmission without direct physical access. This enables the creation of comprehensive contamination maps, supporting large-scale environmental assessment and remediation efforts. Additionally, integrating low-cost, printed biopolymer sensors with IoT systems reduces infrastructure costs and enables large-scale, dense sensor networks for improved spatial resolution in environmental data.
- Biopolymer-based sensors are generally low-power, making them compatible with energy-efficient wireless protocols like LoRaWAN and NB-IoT. When combined with solar-powered or other renewable energy sources, these sensors form sustainable, self-sufficient monitoring systems that can be well-suited for long-term environmental monitoring. This also helps reduce the ecological impact of continuous heavy metal monitoring operations.
7. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, C.; Liu, G.; Tan, Q.; Gao, M.; Chen, G.; Huang, X.; Xu, X.; Li, L.; Wang, J.; Zhang, Y. Polysaccharide-Based Biopolymer Hydrogels for Heavy Metal Detection and Adsorption. J. Adv. Res. 2023, 44, 53–70. [Google Scholar] [CrossRef]
- Bilge, S.; Karadurmus, L.; Sınağ, A.; Ozkan, S.A. Green Synthesis and Characterization of Carbon-Based Materials for Sensitive Detection of Heavy Metal Ions. TrAC Trends Anal. Chem. 2021, 145, 116473. [Google Scholar] [CrossRef]
- Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V.H. Heavy Metal Water Pollution: A Fresh Look about Hazards, Novel and Conventional Remediation Methods. Environ. Technol. Innov. 2021, 22, 101504. [Google Scholar] [CrossRef]
- Langari, M.M.; Antxustegi, M.M.; Labidi, J. Nanocellulose-Based Sensing Platforms for Heavy Metal Ions Detection: A Comprehensive Review. Chemosphere 2022, 302, 134823. [Google Scholar] [CrossRef]
- Imre, B.; Pukánszky, B. Compatibilization in Bio-Based and Biodegradable Polymer Blends. Eur. Polym. J. 2013, 49, 1215–1233. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, N.; Li, Y.; Ren, B.; Ding, X.; Bian, H.; Yao, X. Total Concentrations and Sources of Heavy Metal Pollution in Global River and Lake Water Bodies from 1972 to 2017. Glob. Ecol. Conserv. 2020, 22, e00925. [Google Scholar] [CrossRef]
- Fouda-Mbanga, B.; Prabakaran, E.; Pillay, K. Carbohydrate Biopolymers, Lignin Based Adsorbents for Removal of Heavy Metals (Cd2+, Pb2+, Zn2+) from Wastewater, Regeneration and Reuse for Spent Adsorbents Including Latent Fingerprint Detection: A Review. Biotechnol. Rep. 2021, 30, e00609. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chemother. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Vareda, J.P.; Valente, A.J.; Durães, L. Assessment of Heavy Metal Pollution from Anthropogenic Activities and Remediation Strategies: A Review. J. Environ. Manag. 2019, 246, 101–118. [Google Scholar] [CrossRef]
- Vélez-Pérez, L.; Ramirez-Nava, J.; Hernández-Flores, G.; Talavera-Mendoza, O.; Escamilla-Alvarado, C.; Poggi-Varaldo, H.; Solorza-Feria, O.; López-Díaz, J. Industrial Acid Mine Drainage and Municipal Wastewater Co-Treatment by Dual-Chamber Microbial Fuel Cells. Int. J. Hydrogen Energy 2020, 45, 13757–13766. [Google Scholar] [CrossRef]
- Birn, A.-E.; Shipton, L.; Schrecker, T. Canadian Mining and Ill Health in Latin America: A Call to Action. Can. J. Public Health 2018, 109, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Malik, L.A.; Bashir, A.; Qureashi, A.; Pandith, A.H. Detection and Removal of Heavy Metal Ions: A Review. Environ. Chem. Lett. 2019, 17, 1495–1521. [Google Scholar] [CrossRef]
- Ding, R.; Cheong, Y.H.; Ahamed, A.; Lisak, G. Heavy Metals Detection with Paper-Based Electrochemical Sensors. Anal. Chem. 2021, 93, 1880–1888. [Google Scholar] [CrossRef]
- Fu, Z.; Xi, S. The Effects of Heavy Metals on Human Metabolism. Toxicol. Mech. Methods 2020, 30, 167–176. [Google Scholar] [CrossRef]
- Hasanpour, M.; Hatami, M. Application of Three Dimensional Porous Aerogels as Adsorbent for Removal of Heavy Metal Ions from Water/Wastewater: A Review Study. Adv. Colloid Interface Sci. 2020, 284, 102247. [Google Scholar] [CrossRef]
- Qadri, H.; Bhat, R.A.; Mehmood, M.A.; Dar, G.H. Fresh Water Pollution Dynamics and Remediation; Springer: Berlin/Heidelberg, Germany, 2020; ISBN 9811382778. [Google Scholar]
- Gumpu, M.B.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. A Review on Detection of Heavy Metal Ions in Water–an Electrochemical Approach. Sens. Actuators B Chem 2015, 213, 515–533. [Google Scholar] [CrossRef]
- Kinuthia, G.K.; Ngure, V.; Beti, D.; Lugalia, R.; Wangila, A.; Kamau, L. Levels of Heavy Metals in Wastewater and Soil Samples from Open Drainage Channels in Nairobi, Kenya: Community Health Implication. Sci. Rep. 2020, 10, 8434. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, L.; Tian, H.; Lu, A. Universal Preparation of Cellulose-Based Colorimetric Sensor for Heavy Metal Ion Detection. Carbohydr. Polym. 2020, 236, 116037. [Google Scholar] [CrossRef]
- Balusamy, B.; Senthamizhan, A.; Uyar, T. Functionalized Electrospun Nanofibers as a Versatile Platform for Colorimetric Detection of Heavy Metal Ions in Water: A Review. Materials 2020, 13, 2421. [Google Scholar] [CrossRef]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A Review on Heavy Metal Pollution, Toxicity and Remedial Measures: Current Trends and Future Perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Cempel, M.; Nikel, G. Nickel: A Review of Its Sources and Environmental Toxicology. Pol. J. Environ. Stud. 2006, 15, 375–382. [Google Scholar]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Niu, Y.; Xing, L.; Liang, Z.; Song, X.; Ding, M.; Huang, W. Research Progress of the Detection and Analysis Methods of Heavy Metals in Plants. Front. Plant Sci. 2024, 15, 1310328. [Google Scholar] [CrossRef] [PubMed]
- Nadakuditi, A.; Reddy-Vangala, V. An Overview of Analytical Techniques for Heavy Metal Ion Detection and Removal from Industrial Sewage. AiBi Rev. Investig. Adm. E Ing. 2024, 12, 29–37. [Google Scholar] [CrossRef]
- Maciel, J.V.; Durigon, A.M.M.; Souza, M.M.; Quadrado, R.F.; Fajardo, A.R.; Dias, D. Polysaccharides Derived from Natural Sources Applied to the Development of Chemically Modified Electrodes for Environmental Applications: A Review. Trends Environ. Anal. Chem. 2019, 22, e00062. [Google Scholar] [CrossRef]
- Helim, R.; Zazoua, A.; Jaffrezic-Renault, N.; Korri-Youssoufi, H. Label Free Electrochemical Sensors for Pb(II) Detection Based on Hemicellulose Extracted from Opuntia Ficus indica Cactus. Talanta 2023, 265, 124784. [Google Scholar] [CrossRef] [PubMed]
- Ejeian, F.; Etedali, P.; Mansouri-Tehrani, H.-A.; Soozanipour, A.; Low, Z.-X.; Asadnia, M.; Taheri-Kafrani, A.; Razmjou, A. Biosensors for Wastewater Monitoring: A Review. Biosens. Bioelectron. 2018, 118, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yao, Y.; Li, X.; Lu, J.; Zhou, J.; Huang, Z. Comparison of Heavy Metal Removals from Aqueous Solutions by Chemical Precipitation and Characteristics of Precipitates. J. Water Process Eng. 2018, 26, 289–300. [Google Scholar] [CrossRef]
- Abhilash, M.; Thomas, D. Biopolymers for Biocomposites and Chemical Sensor Applications. In Biopolymer Composites in Electronics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 405–435. [Google Scholar]
- Francis, R.; Sasikumar, S.; Gopalan, G.P. Synthesis, Structure, and Properties of Biopolymers (Natural and Synthetic). In Polymer Composites; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 11–107. ISBN 978-3-527-67422-0. [Google Scholar]
- Chaabouni, E.; Gassara, F.; Brar, S.K. Biopolymers Synthesis and Application. In Biotransformation of Waste Biomass into High Value Biochemicals; Brar, S.K., Dhillon, G.S., Soccol, C.R., Eds.; Springer: New York, NY, USA, 2014; pp. 415–443. ISBN 978-1-4614-8005-1. [Google Scholar]
- Prabhu, A.; Crapnell, R.D.; Eersels, K.; van Grinsven, B.; Kunhiraman, A.K.; Singla, P.; McClements, J.; Banks, C.E.; Novakovic, K.; Peeters, M. Reviewing the Use of Chitosan and Polydopamine for Electrochemical Sensing. Curr. Opin. Electrochem. 2022, 32, 100885. [Google Scholar] [CrossRef]
- Sawant, S. Development of Biosensors from Biopolymer Composites. In Biopolymer Composites in Electronics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 353–383. [Google Scholar]
- Ramdzan, N.S.M.; Fen, Y.W.; Anas, N.A.A.; Omar, N.A.S.; Saleviter, S. Development of Biopolymer and Conducting Polymer-Based Optical Sensors for Heavy Metal Ion Detection. Molecules 2020, 25, 2548. [Google Scholar] [CrossRef]
- Feng, K.; Wen, G. Absorbed Pb2+ and Cd2+ Ions in Water by Cross-Linked Starch Xanthate. Int. J. Polym. Sci. 2017, 2017, 6470306. [Google Scholar] [CrossRef]
- Joly, N.; Ghemati, D.; Aliouche, D.; Martin, P. Interaction of Metal Ions with Mono-and Polysaccharides for Wastewater Treatment: A Review. Nat. Prod. Chem. Res. 2020, 8, 373. [Google Scholar]
- Bashir, A.; Malik, L.A.; Ahad, S.; Manzoor, T.; Bhat, M.A.; Dar, G.; Pandith, A.H. Removal of Heavy Metal Ions from Aqueous System by Ion-Exchange and Biosorption Methods. Environ. Chem. Lett. 2019, 17, 729–754. [Google Scholar] [CrossRef]
- Xu, D.; Zhou, B.; Yuan, R. Optimization of Coagulation-Flocculation Treatment of Wastewater Containing Zn (II) and Cr (VI). IOP Publ. 2019, 227, 052049–052055. [Google Scholar] [CrossRef]
- Bolisetty, S.; Peydayesh, M.; Mezzenga, R. Sustainable Technologies for Water Purification from Heavy Metals: Review and Analysis. Chem. Soc. Rev. 2019, 48, 463–487. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y. Cu2+, Cd2+, and Pb2+ Ions Adsorption from Wastewater Using Polysaccharide Hydrogels Made of Oxidized Carboxymethyl Cellulose and Chitosan Grafted with Catechol Groups. Iran. Polym. J. 2024, 33, 57–66. [Google Scholar] [CrossRef]
- Doyo, A.N.; Kumar, R.; Barakat, M.A. Recent Advances in Cellulose, Chitosan, and Alginate Based Biopolymeric Composites for Adsorption of Heavy Metals from Wastewater. J. Taiwan Inst. Chem. Eng. 2023, 151, 105095. [Google Scholar] [CrossRef]
- Garg, S.; Goel, N. Encapsulation of Heavy Metal Ions via Adsorption Using Cellulose/ZnO Composite: First Principles Approach. J. Mol. Graph. Model. 2023, 124, 108566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, Y.; Wang, D.; Yu, D.; Wu, C. Lignin-Based Adsorbents for Heavy Metals. Ind. Crops Prod. 2023, 193, 116119. [Google Scholar] [CrossRef]
- Naseer, A.; Jamshaid, A.; Hamid, A.; Muhammad, N.; Ghauri, M.; Iqbal, J.; Rafiq, S.; Khuram, S.; Shah, N.S. Lignin and Lignin Based Materials for the Removal of Heavy Metals from Waste Water-An Overview. Z. Für Phys. Chem. 2019, 233, 315–345. [Google Scholar] [CrossRef]
- Santoso, S.P.; Kurniawan, A.; Angkawijaya, A.E.; Shuwanto, H.; Warmadewanthi, I.; Hsieh, C.-W.; Hsu, H.-Y.; Soetaredjo, F.E.; Ismadji, S.; Cheng, K.-C. Removal of Heavy Metals from Water by Macro-Mesoporous Calcium Alginate–Exfoliated Clay Composite Sponges. Chem. Eng. J. 2023, 452, 139261. [Google Scholar] [CrossRef]
- Zhang, W.; Duo, H.; Li, S.; An, Y.; Chen, Z.; Liu, Z.; Ren, Y.; Wang, S.; Zhang, X.; Wang, X. An Overview of the Recent Advances in Functionalization Biomass Adsorbents for Toxic Metals Removal. Colloid Interface Sci. Commun. 2020, 38, 100308. [Google Scholar] [CrossRef]
- Na, Y.; Lee, J.; Lee, S.H.; Kumar, P.; Kim, J.H.; Patel, R. Removal of Heavy Metals by Polysaccharide: A Review. Polym. Plast. Technol. Mater. 2020, 59, 1770–1790. [Google Scholar] [CrossRef]
- Wu, S.; Kan, J.; Dai, X.; Shen, X.; Zhang, K.; Zhu, M. Ternary Carboxymethyl Chitosan-Hemicellulose-Nanosized TiO2 Composite as Effective Adsorbent for Removal of Heavy Metal Contaminants from Water. Fibers Polym. 2017, 18, 22–32. [Google Scholar] [CrossRef]
- Luo, X.; Yuan, J.; Liu, Y.; Liu, C.; Zhu, X.; Dai, X.; Ma, Z.; Wang, F. Improved Solid-Phase Synthesis of Phosphorylated Cellulose Microsphere Adsorbents for Highly Effective Pb2+ Removal from Water: Batch and Fixed-Bed Column Performance and Adsorption Mechanism. ACS Sustain. Chem. Eng. 2017, 5, 5108–5117. [Google Scholar] [CrossRef]
- Luo, W.; Bai, Z.; Zhu, Y. Fast Removal of Co( II ) from Aqueous Solution Using Porous Carboxymethyl Chitosan Beads and Its Adsorption Mechanism. RSC Adv. 2018, 8, 13370–13387. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sharma, R.K.; Singh, A.P. Grafting of Cellulose with N-Isopropylacrylamide and Glycidyl Methacrylate for Efficient Removal of Ni (II), Cu (II) and Pd (II) Ions from Aqueous Solution. Sep. Purif. Technol. 2019, 219, 249–259. [Google Scholar] [CrossRef]
- Wang, J.; Liu, M.; Duan, C.; Sun, J.; Xu, Y. Preparation and Characterization of Cellulose-Based Adsorbent and Its Application in Heavy Metal Ions Removal. Carbohydr. Polym. 2019, 206, 837–843. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, X.; Wang, G.; Hao, C.; Li, X.; Li, T. Adsorption Performance of a Polysaccharide Composite Hydrogel Based on Crosslinked Glucan/Chitosan for Heavy Metal Ions. Compos. Part B Eng. 2019, 169, 45–54. [Google Scholar] [CrossRef]
- Lin, Z.; Yang, Y.; Liang, Z.; Zeng, L.; Zhang, A. Preparation of Chitosan/Calcium Alginate/Bentonite Composite Hydrogel and Its Heavy Metal Ions Adsorption Properties. Polymers 2021, 13, 1891. [Google Scholar] [CrossRef]
- Xu, X.; Ouyang, X.; Yang, L.-Y. Adsorption of Pb (II) from Aqueous Solutions Using Crosslinked Carboxylated Chitosan/Carboxylated Nanocellulose Hydrogel Beads. J. Mol. Liq. 2021, 322, 114523. [Google Scholar] [CrossRef]
- Zhao, H.; Ouyang, X.-K.; Yang, L.-Y. Adsorption of Lead Ions from Aqueous Solutions by Porous Cellulose Nanofiber–Sodium Alginate Hydrogel Beads. J. Mol. Liq. 2021, 324, 115122. [Google Scholar] [CrossRef]
- Mahmood-ul-Hassan, M.; Yasin, M.; Yousra, M.; Ahmad, R.; Sarwar, S. Kinetics, Isotherms, and Thermodynamic Studies of Lead, Chromium, and Cadmium Bio-Adsorption from Aqueous Solution onto Picea smithiana Sawdust. Environ. Sci. Pollut. Res. 2018, 25, 12570–12578. [Google Scholar] [CrossRef]
- Zhang, S.; Arkin, K.; Zheng, Y.; Ma, J.; Bei, Y.; Liu, D.; Shang, Q. Preparation of a Composite Material Based on Self-Assembly of Biomass Carbon Dots and Sodium Alginate Hydrogel and Its Green, Efficient and Visual Adsorption Performance for Pb2+. J. Environ. Chem. Eng. 2022, 10, 106921. [Google Scholar] [CrossRef]
- Lian, Y.; Zhang, J.; Li, N.; Ping, Q. Preparation of Hemicellulose-Based Hydrogel and Its Application as an Adsorbent Towards Heavy Metal Ions. BioResources 2018, 13, 3208–3218. [Google Scholar] [CrossRef]
- Qu, J.; Tian, X.; Jiang, Z.; Cao, B.; Akindolie, M.S.; Hu, Q.; Feng, C.; Feng, Y.; Meng, X.; Zhang, Y. Multi-Component Adsorption of Pb (II), Cd (II) and Ni (II) onto Microwave-Functionalized Cellulose: Kinetics, Isotherms, Thermodynamics, Mechanisms and Application for Electroplating Wastewater Purification. J. Hazard. Mater. 2020, 387, 121718. [Google Scholar] [CrossRef]
- Choi, H.Y.; Bae, J.H.; Hasegawa, Y.; An, S.; Kim, I.S.; Lee, H.; Kim, M. Thiol-Functionalized Cellulose Nanofiber Membranes for the Effective Adsorption of Heavy Metal Ions in Water. Carbohydr. Polym. 2020, 234, 115881. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ni, S.; Wang, X.; Zhang, W.; Lagerquist, L.; Qin, M.; Willför, S.; Xu, C.; Fatehi, P. Ultrafast Adsorption of Heavy Metal Ions onto Functionalized Lignin-Based Hybrid Magnetic Nanoparticles. Chem. Eng. J. 2019, 372, 82–91. [Google Scholar] [CrossRef]
- Zhou, F.; Feng, X.; Yu, J.; Jiang, X. High Performance of 3D Porous Graphene/Lignin/Sodium Alginate Composite for Adsorption of Cd(II) and Pb(II). Environ. Sci. Pollut. Res. 2018, 25, 15651–15661. [Google Scholar] [CrossRef] [PubMed]
- Malayoglu, U. Removal of Heavy Metals by Biopolymer (Chitosan)/Nanoclay Composites. Sep. Sci. Technol. 2018, 53, 2741–2749. [Google Scholar] [CrossRef]
- Ijaz, I.; Bukhari, A.; Gilani, E.; Nazir, A.; Zain, H.; Bukhari, A.; Shaheen, A.; Hussain, S.; Imtiaz, A. Functionalization of Chitosan Biopolymer Using Two Dimensional Metal-Organic Frameworks and MXene for Rapid, Efficient, and Selective Removal of Lead (II) and Methyl Blue from Wastewater. Process Biochem. 2023, 129, 257–267. [Google Scholar] [CrossRef]
- Khalil, T.E.; Abdel-Salam, A.H.; Mohamed, L.A.; El-Meligy, E.; El-Dissouky, A. Crosslinked Modified Chitosan Biopolymer for Enhanced Removal of Toxic Cr (VI) from Aqueous Solution. Int. J. Biol. Macromol. 2023, 234, 123719. [Google Scholar] [CrossRef] [PubMed]
- Elgueta, E.; Becerra, Y.; Martínez, A.; Pereira, M.; Carrillo-Varela, I.; Sanhueza, F.; Nuñez, D.; Rivas, B.L. Adsorbents Derived from Xylan Hemicellulose with Removal Properties of Pollutant Metals. Chin. J. Polym. Sci. 2023, 41, 874–886. [Google Scholar] [CrossRef]
- Radotić, K.; Djikanović, D.; Radosavljević, J.S.; Jović-Jovičić, N.; Mojović, Z. Comparative Study of Lignocellulosic Biomass and Its Components as Electrode Modifiers for Detection of Lead and Copper Ions. J. Electroanal. Chem. 2020, 862, 114010. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, N.; Chen, X. Structurally Modified Polysaccharides: Physicochemical Properties, Biological Activities, Structure–Activity Relationship, and Applications. J. Agric. Food Chem. 2024, 72, 3259–3276. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, Y.; Cheng, W.; Chen, W.; Wu, Y.; Yu, H. Cellulose-based Flexible Functional Materials for Emerging Intelligent Electronics. Adv. Mater. 2021, 33, 2000619. [Google Scholar] [CrossRef] [PubMed]
- Swingler, S.; Gupta, A.; Gibson, H.; Kowalczuk, M.; Heaselgrave, W.; Radecka, I. Recent Advances and Applications of Bacterial Cellulose in Biomedicine. Polymers 2021, 13, 412. [Google Scholar] [CrossRef]
- Ning, J.; Luo, X.; Wang, F.; Huang, S.; Wang, J.; Liu, D.; Liu, D.; Chen, D.; Wei, J.; Liu, Y. Synergetic Sensing Effect of Sodium Carboxymethyl Cellulose and Bismuth on Cadmium Detection by Differential Pulse Anodic Stripping Voltammetry. Sensors 2019, 19, 5482. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Hu, X.; Dong, Y.; Mamat, X.; Li, Y.; Wågberg, T.; Hu, G. An Electrochemical Sensor Based on Green γ-AlOOH-Carbonated Bacterial Cellulose Hybrids for Simultaneous Determination Trace Levels of Cd (II) and Pb (II) in Drinking Water. J. Electrochem. Soc. 2018, 165, B328–B334. [Google Scholar] [CrossRef]
- Zinoubi, K.; Majdoub, H.; Barhoumi, H.; Boufi, S.; Jaffrezic-Renault, N. Determination of Trace Heavy Metal Ions by Anodic Stripping Voltammetry Using Nanofibrillated Cellulose Modified Electrode. J. Electroanal. Chem. 2017, 799, 70–77. [Google Scholar] [CrossRef]
- Taheri, M.; Ahour, F.; Keshipour, S. Sensitive and Selective Determination of Cu2+ at D-Penicillamine Functionalized Nano-Cellulose Modified Pencil Graphite Electrode. J. Phys. Chem. Solids 2018, 117, 180–187. [Google Scholar] [CrossRef]
- Priya, T.; Dhanalakshmi, N.; Karthikeyan, V.; Thinakaran, N. Highly Selective Simultaneous Trace Determination of Cd2+ and Pb2+ Using Porous Graphene/Carboxymethyl Cellulose/Fondaparinux Nanocomposite Modified Electrode. J. Electroanal. Chem. 2019, 833, 543–551. [Google Scholar] [CrossRef]
- Teodoro, K.B.; Migliorini, F.L.; Facure, M.H.; Correa, D.S. Conductive Electrospun Nanofibers Containing Cellulose Nanowhiskers and Reduced Graphene Oxide for the Electrochemical Detection of Mercury (II). Carbohydr. Polym. 2019, 207, 747–754. [Google Scholar] [CrossRef]
- Padmalaya, G.; Sreeja, B.; Dinesh Kumar, P.; Radha, S.; Poornima, V.; Arivanandan, M.; Shrestha, S.; Uma, T. A Facile Synthesis of Cellulose Acetate Functionalized Zinc Oxide Nanocomposite for Electrochemical Sensing of Cadmium Ions. J. Inorg. Organomet. Polym. Mater. 2019, 29, 989–999. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Georgouvelas, D.; Edlund, U.; Mathew, A.P. CelloZIFPaper: Cellulose-ZIF Hybrid Paper for Heavy Metal Removal and Electrochemical Sensing. Chem. Eng. J. 2022, 446, 136614. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, J.; Zhu, H.; Yang, T.; Zou, M.; Zhang, M.; Du, M. Facile and Green Fabrication of Size-Controlled AuNPs/CNFs Hybrids for the Highly Sensitive Simultaneous Detection of Heavy Metal Ions. Electrochim. Acta 2016, 196, 422–430. [Google Scholar] [CrossRef]
- Bressi, V.; Celesti, C.; Ferlazzo, A.; Len, T.; Moulaee, K.; Neri, G.; Luque, R.; Espro, C. Waste-Derived Carbon Nanodots for Fluorimetric and Simultaneous Electrochemical Detection of Heavy Metals in Water. Environ. Sci. Nano 2024, 11, 1245–1258. [Google Scholar] [CrossRef]
- Sotolářová, J.; Vinter, Š.; Filip, J. Cellulose Derivatives Crosslinked by Citric Acid on Electrode Surface as a Heavy Metal Absorption/Sensing Matrix. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127242. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.; Zhu, Z. Study on the Blends of Silk Fibroin and Sodium Alginate: Hydrogen Bond Formation, Structure and Properties. Polymer 2019, 163, 144–153. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, M.; Liao, Q.; Wang, L.; Li, H.; Niu, X.; Liu, X.; Wang, K. Integrated Polysaccharides via Amidation for Sensitive Electrochemical Detection of Heavy Metal Ions. J. Mater. Sci. Mater. Electron. 2022, 33, 8140–8150. [Google Scholar] [CrossRef]
- Chrouda, A. A Novel Electrochemical Sensor Based on Sodium Alginate-Decorated Single-Walled Carbon Nanotubes for the Direct Electrocatalysis of Heavy Metals Ions. Polym. Adv. Technol. 2023, 34, 1807–1816. [Google Scholar] [CrossRef]
- Crini, G.; Badot, P.-M.; Roberts, G.A.; Guibal, E. Chitine et Chitosane: Du Biopolymère à l’application; Presses Universitaires de. Franche-Comté: Besançon, France, 2009; p. 209. ISBN 2-84867-249-8. [Google Scholar]
- Jiang, Y.; Wu, J. Recent Development in Chitosan Nanocomposites for Surface-based Biosensor Applications. Electrophoresis 2019, 40, 2084–2097. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Wang, X.; Pathak, P.; Rex, M.M.; Cho, H.J.; Lee, W.H. Enhanced Electrochemical Detection of Multiheavy Metal Ions Using a Biopolymer-Coated Planar Carbon Electrode. IEEE Trans. Instrum. Meas. 2019, 68, 2387–2393. [Google Scholar] [CrossRef]
- Hadnine, S.; Zighed, L.; Abbassi, H.; Rahmouni, S.; Zouaoui, E. Determination of Hg (II) by Thiourea Grafted Chitosan Modified Carbon Paste Electrode: Reversibility and Electrochemical Parameters Studies. Anal. Bioanal. Electrochem. 2019, 11, 1716–1734. [Google Scholar]
- Fort, C.I.; Cotet, L.C.; Vulpoi, A.; Turdean, G.L.; Danciu, V.; Baia, L.; Popescu, I.C. Bismuth Doped Carbon Xerogel Nanocomposite Incorporated in Chitosan Matrix for Ultrasensitive Voltammetric Detection of Pb (II) and Cd (II). Sens. Actuators B Chem. 2015, 220, 712–719. [Google Scholar] [CrossRef]
- Pathak, P.; Hwang, J.-H.; Li, R.H.T.; Rodriguez, K.L.; Rex, M.M.; Lee, W.H.; Cho, H.J. Flexible Copper-Biopolymer Nanocomposite Sensors for Trace Level Lead Detection in Water. Sens. Actuators B Chem. 2021, 344, 130263. [Google Scholar] [CrossRef]
- Zhou, S.-F.; Han, X.-J.; Liu, Y.-Q. SWASV Performance toward Heavy Metal Ions Based on a High-Activity and Simple Magnetic Chitosan Sensing Nanomaterials. J. Alloys Compd. 2016, 684, 1–7. [Google Scholar] [CrossRef]
- Shang, J.; Zhao, M.; Qu, H.; Li, H.; Gao, R.; Chen, S. New Application of Pn Junction in Electrochemical Detection: The Detection of Heavy Metal Ions. J. Electroanal. Chem. 2019, 855, 113624. [Google Scholar] [CrossRef]
- Guo, Z.; Luo, X.; Li, Y.; Zhao, Q.-N.; Li, M.; Zhao, Y.; Sun, T.; Ma, C. Simultaneous Determination of Trace Cd (II), Pb (II) and Cu (II) by Differential Pulse Anodic Stripping Voltammetry Using a Reduced Graphene Oxide-Chitosan/Poly-l-Lysine Nanocomposite Modified Glassy Carbon Electrode. J. Colloid Interface Sci. 2017, 490, 11–22. [Google Scholar] [CrossRef]
- Wei, P.; Zhu, Z.; Song, R.; Li, Z.; Chen, C. An Ion-Imprinted Sensor Based on Chitosan-Graphene Oxide Composite Polymer Modified Glassy Carbon Electrode for Environmental Sensing Application. Electrochim. Acta 2019, 317, 93–101. [Google Scholar] [CrossRef]
- Yin, J.; Zhai, H.; Wang, Y.; Wang, B.; Chu, G.; Guo, Q.; Zhang, Y.; Sun, X.; Guo, Y.; Zhang, Y. COF/MWCNTs/CLS-Based Electrochemical Sensor for Simultaneous and Sensitive Detection of Multiple Heavy Metal Ions. Food Anal. Methods 2022, 15, 3244–3256. [Google Scholar] [CrossRef]
- Xu, T.; Dai, H.; Jin, Y. Electrochemical Sensing of Lead (II) by Differential Pulse Voltammetry Using Conductive Polypyrrole Nanoparticles. Microchim. Acta 2020, 187, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Kushwaha, C.S.; Shukla, S. Potentiometric Detection of Copper Ion Using Chitin Grafted Polyaniline Electrode. Int. J. Biol. Macromol. 2020, 147, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Deng, Y.; Li, T.; Chen, Z.; Chen, H.; Li, S.; Liu, H. Aptamer-Based Electrochemical Biosensor for Mercury Ions Detection Using AuNPs-Modified Glass Carbon Electrode. J. Biomed. Nanotechnol. 2018, 14, 2156–2161. [Google Scholar] [CrossRef]
- Liu, Y.; Lai, Y.; Yang, G.; Tang, C.; Deng, Y.; Li, S.; Wang, Z. Cd-Aptamer Electrochemical Biosensor Based on AuNPs/CS Modified Glass Carbon Electrode. J. Biomed. Nanotechnol. 2017, 13, 1253–1259. [Google Scholar] [CrossRef]
- Mo, Z.; Liu, H.; Hu, R.; Gou, H.; Li, Z.; Guo, R. Amino-Functionalized Graphene/Chitosan Composite as an Enhanced Sensing Platform for Highly Selective Detection of Cu2+. Ionics 2018, 24, 1505–1513. [Google Scholar] [CrossRef]
- Wu, S.; Li, K.; Dai, X.; Zhang, Z.; Ding, F.; Li, S. An Ultrasensitive Electrochemical Platform Based on Imprinted Chitosan/Gold Nanoparticles/Graphene Nanocomposite for Sensing Cadmium (II) Ions. Microchem. J. 2020, 155, 104710. [Google Scholar] [CrossRef]
- Wong, A.; Ferreira, P.A.; Santos, A.M.; Cincotto, F.H.; Silva, R.A.B.; Sotomayor, M.D.P.T. A New Electrochemical Sensor Based on Eco-Friendly Chemistry for the Simultaneous Determination of Toxic Trace Elements. Microchem. J. 2020, 158, 105292. [Google Scholar] [CrossRef]
- Guo, C.; Wang, C.; Sun, H.; Dai, D.; Gao, H. A Simple Electrochemical Sensor Based on rGO/MoS2/CS Modified GCE for Highly Sensitive Detection of Pb( Ii ) in Tobacco Leaves. RSC Adv. 2021, 11, 29590–29597. [Google Scholar] [CrossRef]
- Boultif, W.; Dehchar, C.; Belhocine, Y.; Zouaoui, E.; Rahali, S.; Zouari, S.E.; Sbei, N.; Seydou, M. Chitosan and Metal Oxide Functionalized Chitosan as Efficient Sensors for Lead (II) Detection in Wastewater. Separations 2023, 10, 479. [Google Scholar] [CrossRef]
- Bouraoui, S.; Zazoua, A.; Braiek, M.; Jaffrezic-Renault, N. A New Sensitive and Selective Sensor for Heavy Metal Ions Based on Tannin Extracted from the Skin of Punica granatum L. Int. J. Environ. Anal. Chem. 2016, 96, 739–751. [Google Scholar] [CrossRef]
- Zazoua, A.; Khedimallah, N.; Jaffrezic-Renault, N. Electrochemical Determination of Cadmium, Lead, and Nickel Using a Polyphenol–Polyvinyl Chloride—Boron-Doped Diamond Electrode. Anal. Lett. 2018, 51, 336–347. [Google Scholar] [CrossRef]
- Suherman, A.L.; Kuss, S.; Tanner, E.E.L.; Young, N.P.; Compton, R.G. Electrochemical Hg2+ Detection at Tannic Acid-Gold Nanoparticle Modified Electrodes by Square Wave Voltammetry. Analyst 2018, 143, 2035–2041. [Google Scholar] [CrossRef]
- Gonçalves, S.S.L.; Rudnitskaya, A.; Sales, A.J.M.; Costa, L.M.C.; Evtuguin, D.V. Nanocomposite Polymeric Materials Based on Eucalyptus Lignoboost® Kraft Lignin for Liquid Sensing Applications. Materials 2020, 13, 1637. [Google Scholar] [CrossRef] [PubMed]
- Mahfoud, H.; Morakchi, K.; Hamel, A.; Bendjama, A.; Saifi, H.; Belghiche, R. Electrochemical Characterization of Modified Platinum Electrode by Using Lignin from Stone Olive as Ionophore. J. Iran. Chem. Soc. 2022, 19, 313–318. [Google Scholar] [CrossRef]
- Bao, Q.-X.; Liu, Y.; Liang, Y.-Q.; Weerasooriya, R.; Li, H.; Wu, Y.-C.; Chen, X. Tea Polyphenols Mediated Zero-Valent Iron/Reduced Graphene Oxide Nanocomposites for Electrochemical Determination of Hg2+. J. Electroanal. Chem. 2022, 917, 116428. [Google Scholar] [CrossRef]
- Vicentini, F.C.; Silva, L.R.G.; Stefano, J.S.; Lima, A.R.F.; Prakash, J.; Bonacin, J.A.; Janegitz, B.C. Starch-Based Electrochemical Sensors and Biosensors: A Review. Biomed. Mater. Devices 2022, 1, 319–338. [Google Scholar] [CrossRef]
- Alves, G.M.; da Silva, J.L.; Stradiotto, N.R. A Novel Citrus Pectin-Modified Carbon Paste Electrochemical Sensor Used for Copper Determination in Biofuel. Measurement 2021, 169, 108356. [Google Scholar] [CrossRef]
- Arulraj, A.D.; Devasenathipathy, R.; Chen, S.-M.; Vasantha, V.S.; Wang, S.-F. Femtomolar Detection of Mercuric Ions Using Polypyrrole, Pectin and Graphene Nanocomposites Modified Electrode. J. Colloid Interface Sci. 2016, 483, 268–274. [Google Scholar] [CrossRef]
- El Hamdouni, Y.; El Hajjaji, S.; Szabo, T.; Trif, L.; Felhősi, I.; Abbi, K.; Labjar, N.; Harmouche, L.; Shaban, A. Biomass Valorization of Walnut Shell into Biochar as a Resource for Electrochemical Simultaneous Detection of Heavy Metal Ions in Water and Soil Samples: Preparation, Characterization, and Applications. Arab. J. Chem. 2022, 15, 104252. [Google Scholar] [CrossRef]
- de Oliveira Farias, E.A.; dos Santos, M.C.; de Araujo Dionísio, N.; Quelemes, P.V.; de Leite, J.R.S.A.; Eaton, P.; da Silva, D.A.; Eiras, C. Layer-by-Layer Films Based on Biopolymers Extracted from Red Seaweeds and Polyaniline for Applications in Electrochemical Sensors of Chromium VI. Mater. Sci. Eng. B 2015, 200, 9–21. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Fox, D.; Stanberry, J.; Anagnostopoulos, V.; Zhai, L.; Lee, W.H. Direct Mercury Detection in Landfill Leachate Using a Novel AuNP-Biopolymer Carbon Screen-Printed Electrode Sensor. Micromachines 2021, 12, 649. [Google Scholar] [CrossRef] [PubMed]
- Mondal, B.; Banerjee, S.; Samanta, S.K.; Senapati, S.; Tripathy, T. Highly Selective and Sensitive Electrochemical Sensing of Trace Zn2+ Ions, by Grafted Tricholoma Mushroom Polysaccharide/Ag Composite Nanoparticles in Aqueous Medium. Appl. Organomet. Chem. 2021, 35, e6171. [Google Scholar] [CrossRef]
- Silva, I.B.; de Araújo, D.M.; Vocciante, M.; Ferro, S.; Martínez-Huitle, C.A.; Dos Santos, E.V. Electrochemical Determination of Lead Using A Composite Sensor Obtained from Low-Cost Green Materials:Graphite/Cork. Appl. Sci. 2021, 11, 2355. [Google Scholar] [CrossRef]
- Palisoc, S.T.; Vitto, R.I.M.; Noel, M.G.; Palisoc, K.T.; Natividad, M.T. Highly Sensitive Determination of Heavy Metals in Water Prior to and after Remediation Using Citrofortunella Microcarpa. Sci. Rep. 2021, 11, 1394. [Google Scholar] [CrossRef]
- Qin, X.; Tang, D.; Zhang, Y.; Cheng, Y.; He, F.; Su, Z.; Jiang, H. An Electrochemical Sensor for Simultaneous Stripping Determination of Cd(II) and Pb(II) Based on Gold Nanoparticles Functionalized β-Cyclodextrin-Graphene Hybrids. Int. J. Electrochem. Sci. 2020, 15, 1517–1528. [Google Scholar] [CrossRef]
- Elamin, M.B.; Chrouda, A.; Ali, S.M.A.; Alhaidari, L.M.; Jabli, M.; Alrouqi, R.M.; Renault, N.J. Electrochemical Sensor Based on Gum Arabic Nanoparticles for Rapid and In-Situ Detection of Different Heavy Metals in Real Samples. Heliyon 2024, 10, 4. [Google Scholar] [CrossRef]
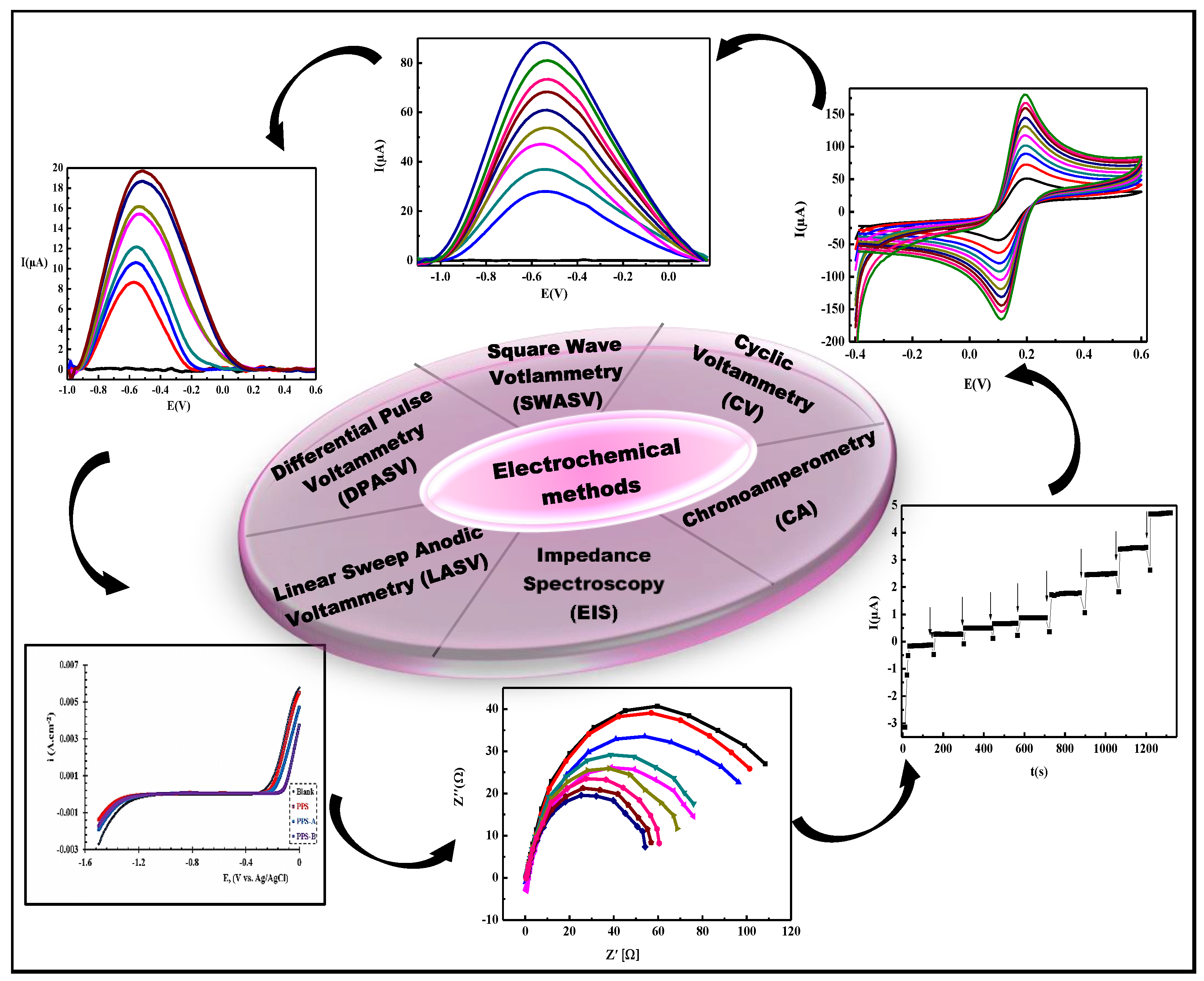
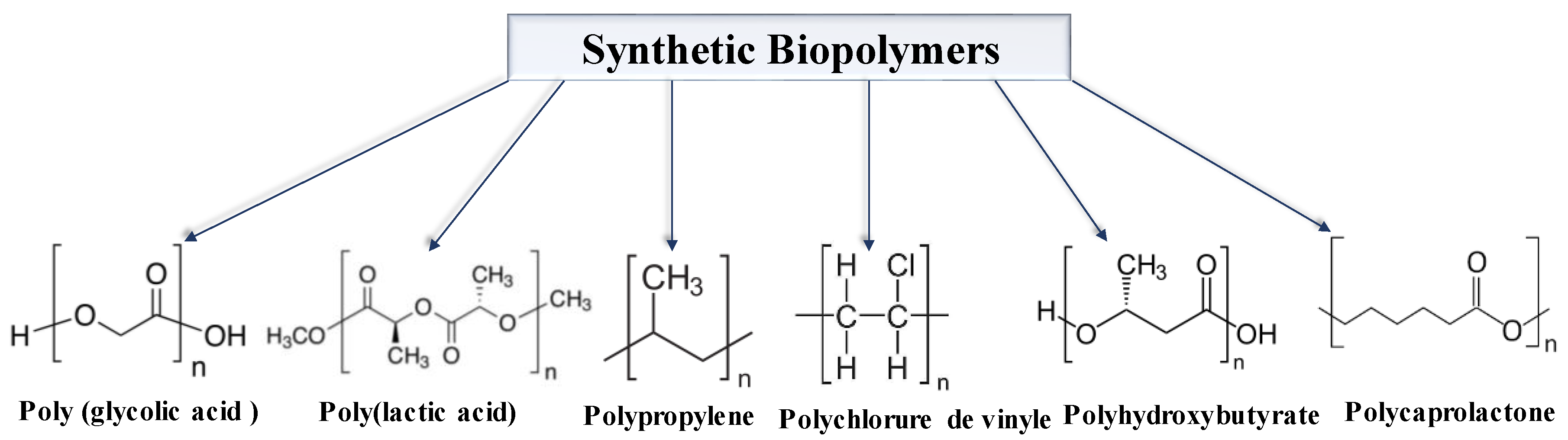
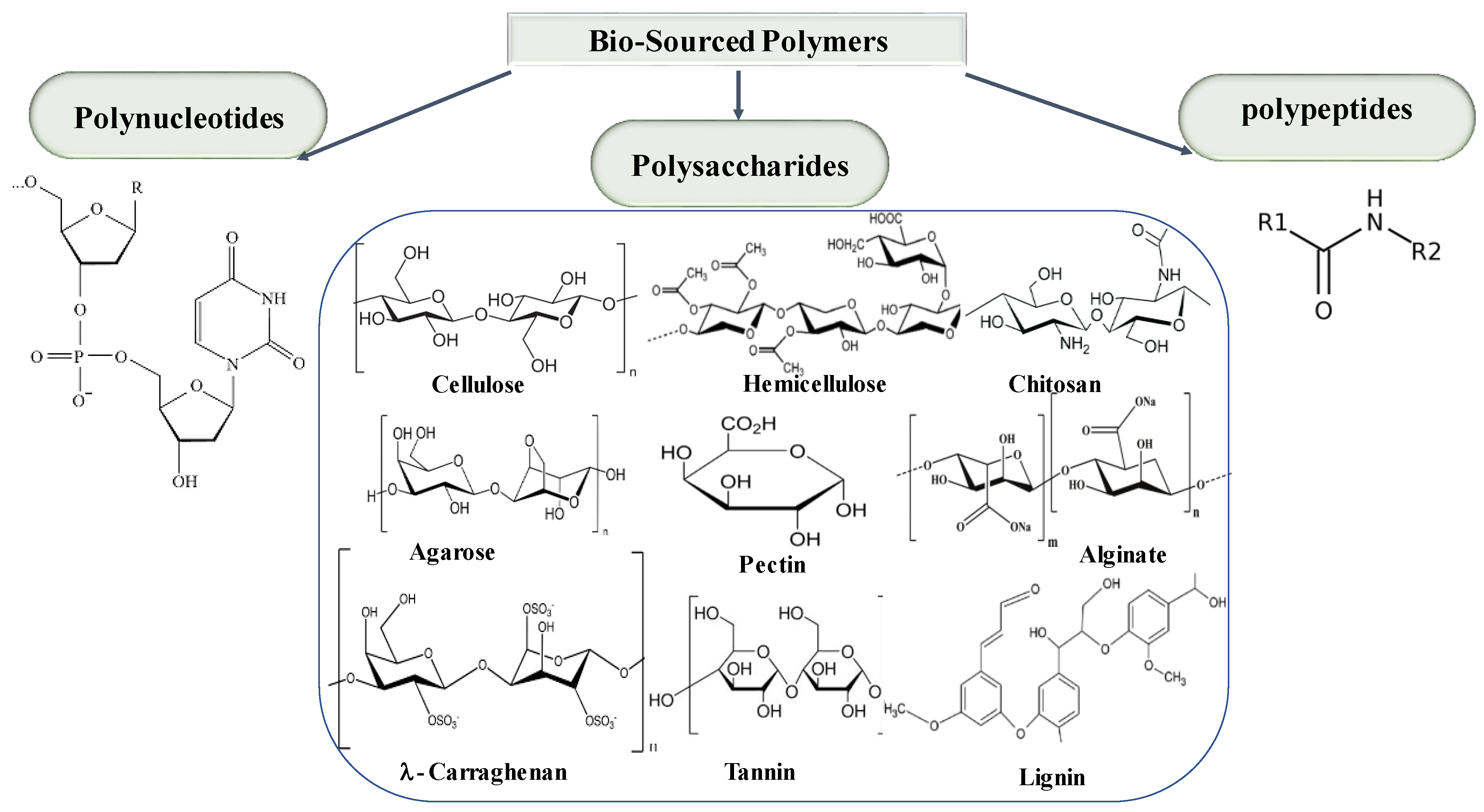
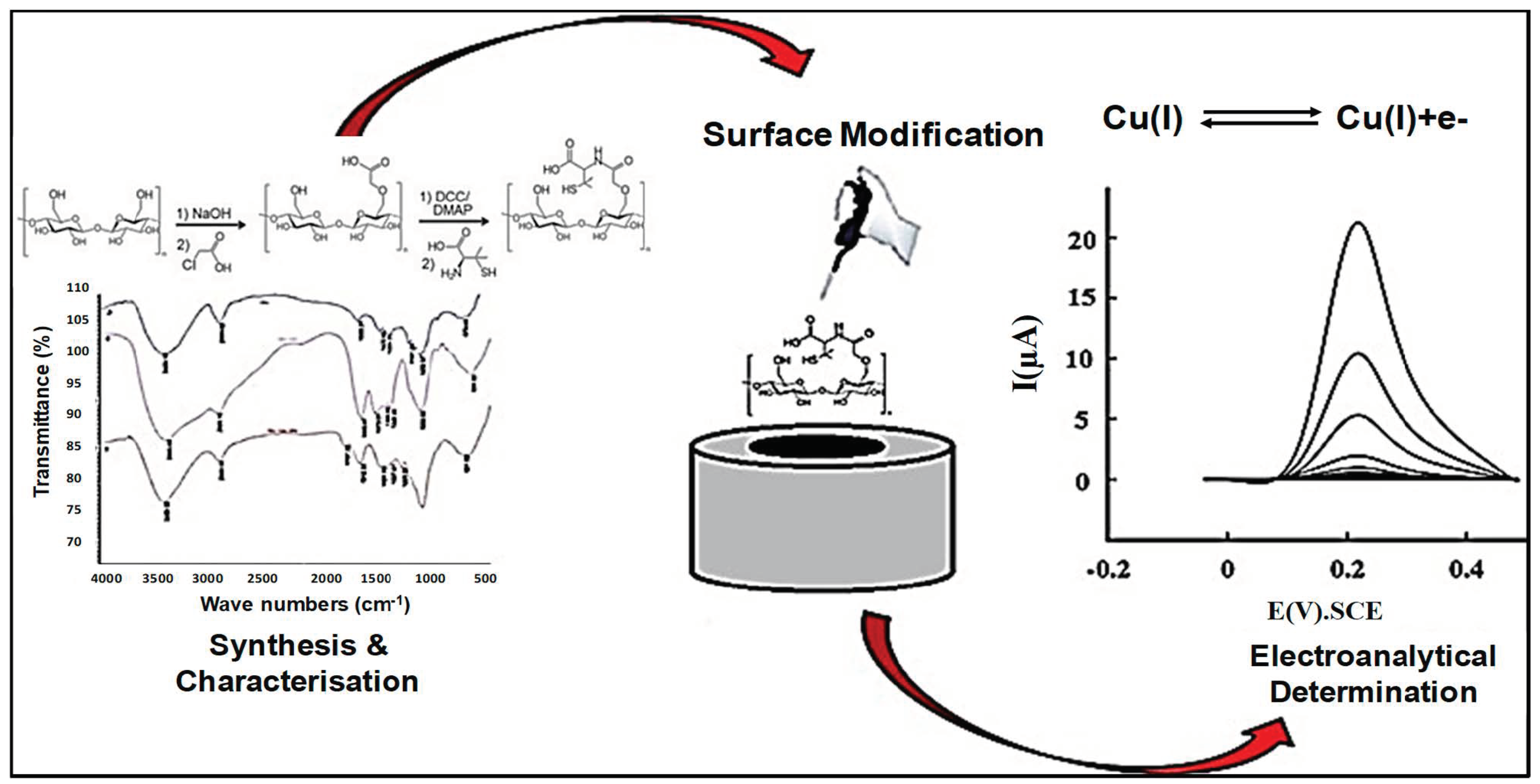

| Method | Advantages | Disadvantages |
|---|---|---|
| Atomic Absorption Spectroscopy (AAS) | High sensitivity and specificity, simple sample preparation, wide range of applications. | Requires individual measurements for each element, susceptible to interference from other elements. |
| Inductively Coupled Plasma Optical Emission | Simultaneous multi-element analysis, high sensitivity and precision, wide linear dynamic range. | Complex instrumentation, expensive, requires skilled operators. |
| Spectroscopy (ICP-OES) Inductively Coupled Plasma Mass Spectrometry (ICP-MS) | Extremely high sensitivity, excellent detection limits, isotopic analysis capability. | |
| Colorimetric Methods | Simple, inexpensive, and rapid. | Low sensitivity, susceptible to interference, limited dynamic range. |
| Biopolymers Used for Removal | Metal Ions | Adsorption Capacity (mg g−1) | Ref. |
|---|---|---|---|
| Nano-sized TiO2/carboxymethyl chitosan hemicellulose composites | Ni(II) | 370.4 | [49] |
| Cd(II) | 555.6 | ||
| Cu(II) | 526.3 | ||
| Hg(II) | 18.6 | ||
| Mn(VII) | 29.9 | ||
| Cr(VI) | 32.1 | ||
| Phosphorylated cellulose microsphere | Pb(II) | 139.38 | [50] |
| Porous carboxymethyl chitosan (PCMC) | Co(II) | 46.25 | [51] |
| Cellulose/N-isopropylacrylamide-glycidyl methacrylate Cell-g-NIPAM-co-GMA | Ni(II) | 74.68 | [52] |
| Cu(II) | 82.92 | ||
| Pd(II) | 119.76 | ||
| Carboxymethylated cellulose fiber (CMF) | Cu(II) | 23.48 | [53] |
| Glucan/chitosan (GL/CS) hydrogels | Cu(II) | 342 | [54] |
| Co(II) | 232 | ||
| Ni(II) | 184 | ||
| Pb(II) | 395 | ||
| Cd(II) | 269 | ||
| Chitosan/calcium alginate/bentonite composite hydrogel | Pb(II) | 434.89 | [55] |
| Cu(II) | 115.30 | ||
| Cd(II) | 102.38 | ||
| Carboxylated chitosan/carboxylated nanocellulose hydrogel beads | Pb(II) | 334.9 | [56] |
| Cellulose nanofiber and sodium alginate | Pb(II) | 318.47 | [57] |
| Picea smithiana sawdust | Pb(II) | 6.35 | [58] |
| Cr(VI) | 3.37 | ||
| Cd(II) | 2.87 | ||
| Sodium alginate@ polyethyleneimine-carbon dots | Pb(II) | 380.39 | [59] |
| Hemicellulose-based hydrogel | Pb(II) | 5.88 | [60] |
| Microwave-functionalized cellulose | Pb(II) | 5.43 | [61] |
| Cd(II) | 3.14 | ||
| Ni(II) | 2.77 | ||
| Thiol-functionalized cellulose nanofiber | Cu(II) | 49.0 | [62] |
| Cd(II) | 45.9 | ||
| Pb(II) | 22.0 | ||
| Lignin-based hybrid magnetic nanoparticles | Pb(II) | 150.33 | [63] |
| Cu(II) | 70.69 | ||
| Three-dimensional porous graphene/lignin/sodium alginate nanocomposite (denoted as 3D PG/L/SA) | Cd(II) | 79.88 | [64] |
| Pb(II) | 226.24 | ||
| Chitosan/nanoclay composite | Cu(II) | 176 | [65] |
| Ni(II) | 144 | ||
| Chitosan/Two-Dimensional Metal-Organic Frameworks (Ni3(HITP)2) and MXene (Ni3(HITP)2/MXene/CS) | Pb(II) | 448.93 | [66] |
| Chitosan/4-hydroxy-3-methoxybenzaldehyde (VAN)-Epichlorohydrin (Fe3O4@CTS-VAN) | Cr(VI) | 188.68 | [67] |
| Xylan hemicellulose modified with sulfonic acid 30% content HA3 Xylan hemicellulosemodified with sulfonate 50% content of Xylan | Pb(II) | 193 | [68] |
| Cd(II) | 182 | ||
| Cu(II) | 66 | ||
| Pb(II) | 273 | ||
| Cd(II) | 143 | ||
| Cu(II) | 45 |
| Electrode | Method | Analyte | LOD | Linear Range | Applications | Ref |
|---|---|---|---|---|---|---|
| Au/Agarose-Hemicellulose | SWASV | Pb(II) | 1.3 fM | 1 µM–1 fM | Tap, spring, and sea water | [27] |
| GCE/Cellulose nanofiber | DPASV | Cd(II) | 5 nM | 0.1 nM–10 µM | Sea water | [75] |
| Cu(II) | 0.5 nM | |||||
| Pb(II) | 0.5 nM | |||||
| Hg(II) | 5 nM | |||||
| Penicillamine functionalized nano-cellulose modified pencil graphite electrode | SWASV | Cu(II) | 0.048 pM | 0.2–50 pM | Tap and river water | [76] |
| PA6/Cellulose nanowhiskers /rGO | DPASV | Hg(II) | 0.52 µM | 2.5–75 μM | River and tap water | [78] |
| AuNPs/Cellulose nanofiber/GCE | SWASV | Cd(II) | 0.1 μM | 0.1–1.0 μM | - | [81] |
| Pb(II) | ||||||
| Cu(II) | ||||||
| γ-AlOOH-carbonated bacterial cellulose | DPASV | Cd(II) | 1.5 nM | 4.4–2200 nM | Drinking water | [74] |
| Pb(II) | 0.5 nM | 2–1200 nM | ||||
| Carbon nanodots Dot (CNDS) | DPASV | Hg(II) Pb(II) | 124 nM 551 nM | - | Drinking water | [82] |
| Hydroxyethylcellulose-CA | EIS | Pb(II) | 1.8 nM | - | Leachates from detonation chamber dust (DCD) and galvanic sludge from the plating industry (GS) | [83] |
| Electrode | Method | Metal | LOD | Linear Range | Application | Ref. |
|---|---|---|---|---|---|---|
| Thymine-Hg2+-Thymine/AuNPs/Chitosane (Aptamer/(AuNPs/CS)2/GCE) | DPASV | Hg(II) | 0.005 nM | 0.01–500 nM | Tap water | [100] |
| GC/Chitisane–(Bi–CX) | SWASV | Pb(II) | 0.07 nM | 0.2–2 nM | Drilled well water | [91] |
| Cd(II) | 5.06 µM | 11.2–124 µM | ||||
| AuNPs/CS-Aptamer/GCE | DPASV | Cd(II) | 0.05 pM | 0.001–100 nM | Tap water | [101] |
| Amino-functionalized graphene/chitosan (NH2–G/CS) | DPASV | Cu(II) | 0.064 µM−1 | 0.4–40 µM | Tap water | [102] |
| CS/AuNPs/GR/GCE | DPASV | Cd(II) | 16.2 nM | 0.1–0.9 μM | River water, tap water, and pure milk | [103] |
| Biochar-nanodiamond-chitosan electrode ND-BC-CS | SWASV | Cd(II) | 0.11 µM | 1.0–75 μM | - | [104] |
| Pb(II) | 0.056 µM | 0.25–6 μM | ||||
| Chitosan–graphene oxide composites (CS/GO-IIP) | DPASV | Cu(II) | 0.15 µM | 0.5–100 µM | Tap and river water | [96] |
| rGO/MoS2/CS (GCE) | SWASV | Pb(II) | 1.6 nM | 0.005–2.0 μM | Tobacco leaves | [105] |
| NiO-CS/CPE | EIS | Pb(II) | 0.3 µM | 1 µM–0.1 mM | Waste water | [106] |
| Electrode | Method | Analyte | LOD | Linear Range | Application | Ref. |
|---|---|---|---|---|---|---|
| Tea polyphenols mediated zero-valent iron/reduced graphene oxide nanocomposites (rGO-ZVI-P) | SWASV | Hg(II) | 1.2 nM | - | River | [112] |
| Tannic acid capped gold nanoparticle (AuNPs@TA) complexes | SWASV | Hg(II) | 100.0 fM | 100 fM–100 nM | Tap | [109] |
| Crosslinked Sodium alginate (SA) and chitosan (CS)SA-CS/GCE | DPASV | Cu(II) | 0.95 μM | 1–100 μM | Tap/river | [85] |
| Sodium alginate-decorated single-walled carbon nanotube | DPASV | Pb(II) | 0.1 nM | - | Tap | [86] |
| Cd(II) | 31 nM | |||||
| Cu(II) | 1 nM | |||||
| AuNPs-biopolymer-coated carbon SPE sensor | SWASV | Hg(II) | 1.7 nM | 10–100 nM | Landfill leachate. | [118] |
| Grafted Tricholoma mushroom polysaccharide–silver composite nanoparticles (TMPSGP-Ag NPs) | CA | Zn(II) | 0.5 nM | 1 nM | River | [119] |
| Cork–graphite electrodes | DPASV | Pb(II) | 0.3 µM | 1–25 µM | Tap Ground “produced water” (a brackish water) | [120] |
| Bi/AgNPs/Nafion-SPGE with Pectin of Citrofortunella Microcarpa | ASV | Pb(II) | 267 nM | - | River | [121] |
| β-cyclodextrin (β-CD)-graphene hybrids (AuNPs-CD-GS) | DPASV | Cd(II) | 0.21 µM | 0.035–10 µM | River | [122] |
| Pb(II) | 0.05 µM | 0.2–6 µM | ||||
| Green nanoparticles based on gum Arabic | DPASV | Zn(II) | 1.9 nM | 2–150 nM | Lake | [123] |
| Hg(II) | 0.9 nM | 1–100 nM | ||||
| Pb(II) | 4.2 nM | 5–300 nM | ||||
| Cu(II) | 9.6 nM | 10–300 nM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helim, R.; Zazoua, A.; Korri-Youssoufi, H. Sustainable Biopolymer-Based Electrochemical Sensors for Trace Heavy Metal Determination in Water: A Comprehensive Review. Chemosensors 2024, 12, 267. https://doi.org/10.3390/chemosensors12120267
Helim R, Zazoua A, Korri-Youssoufi H. Sustainable Biopolymer-Based Electrochemical Sensors for Trace Heavy Metal Determination in Water: A Comprehensive Review. Chemosensors. 2024; 12(12):267. https://doi.org/10.3390/chemosensors12120267
Chicago/Turabian StyleHelim, Rabiaa, Ali Zazoua, and Hafsa Korri-Youssoufi. 2024. "Sustainable Biopolymer-Based Electrochemical Sensors for Trace Heavy Metal Determination in Water: A Comprehensive Review" Chemosensors 12, no. 12: 267. https://doi.org/10.3390/chemosensors12120267
APA StyleHelim, R., Zazoua, A., & Korri-Youssoufi, H. (2024). Sustainable Biopolymer-Based Electrochemical Sensors for Trace Heavy Metal Determination in Water: A Comprehensive Review. Chemosensors, 12(12), 267. https://doi.org/10.3390/chemosensors12120267






