Selective Colorimetric Detection of Pb(II) Ions by Using Green Synthesized Gold Nanoparticles with Orange Peel Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Orange Peel Extract
2.3. Synthesis of AuNPs @OPE
2.4. Characterization of AuNPs@OPE
2.5. AuNPs@OPE as Colorimetric Sensors for Heavy Metal Ions
3. Results
3.1. Synthesis of AuNPs@OPE
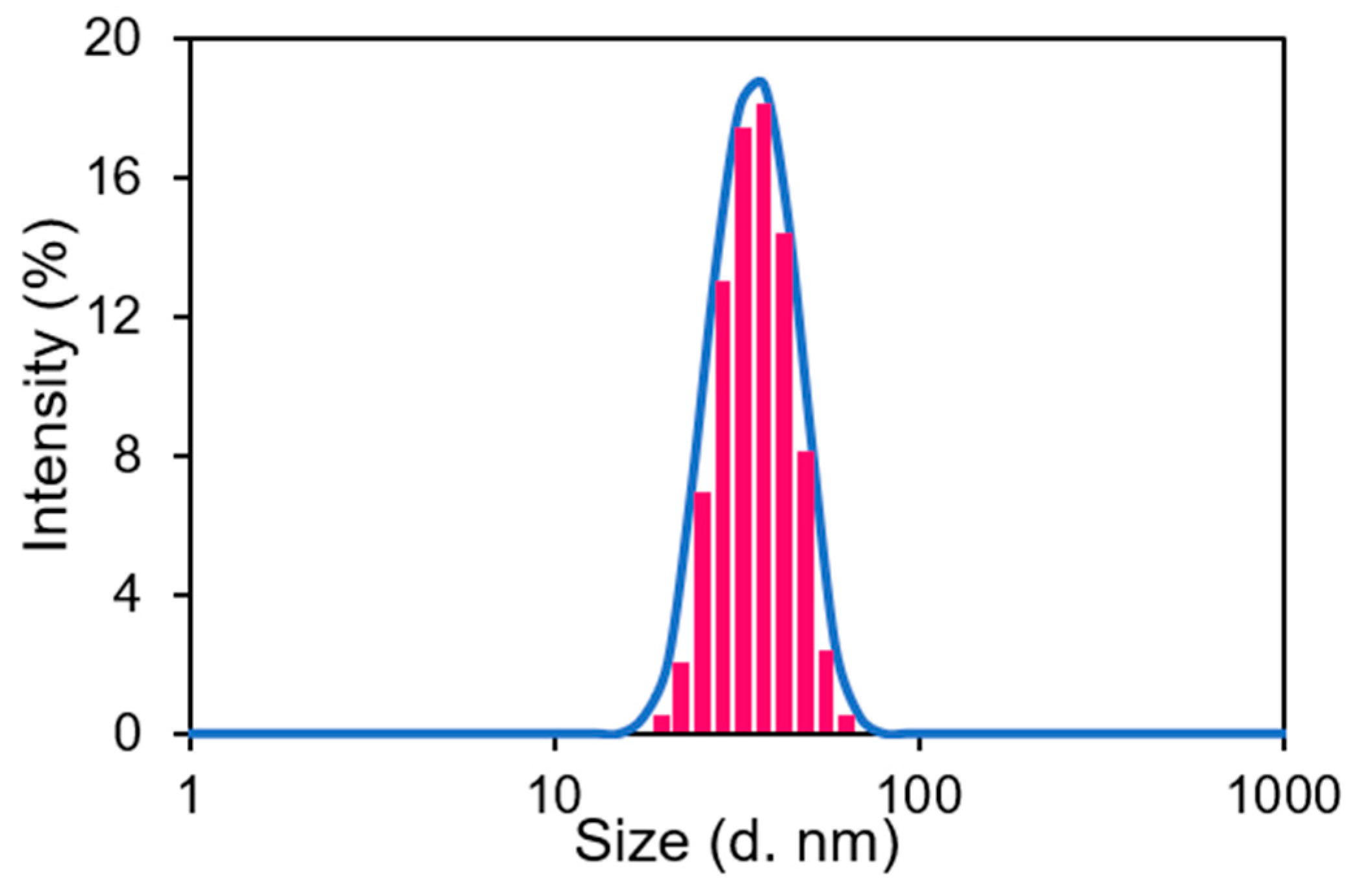
3.2. Characterization of AuNPs @OPE
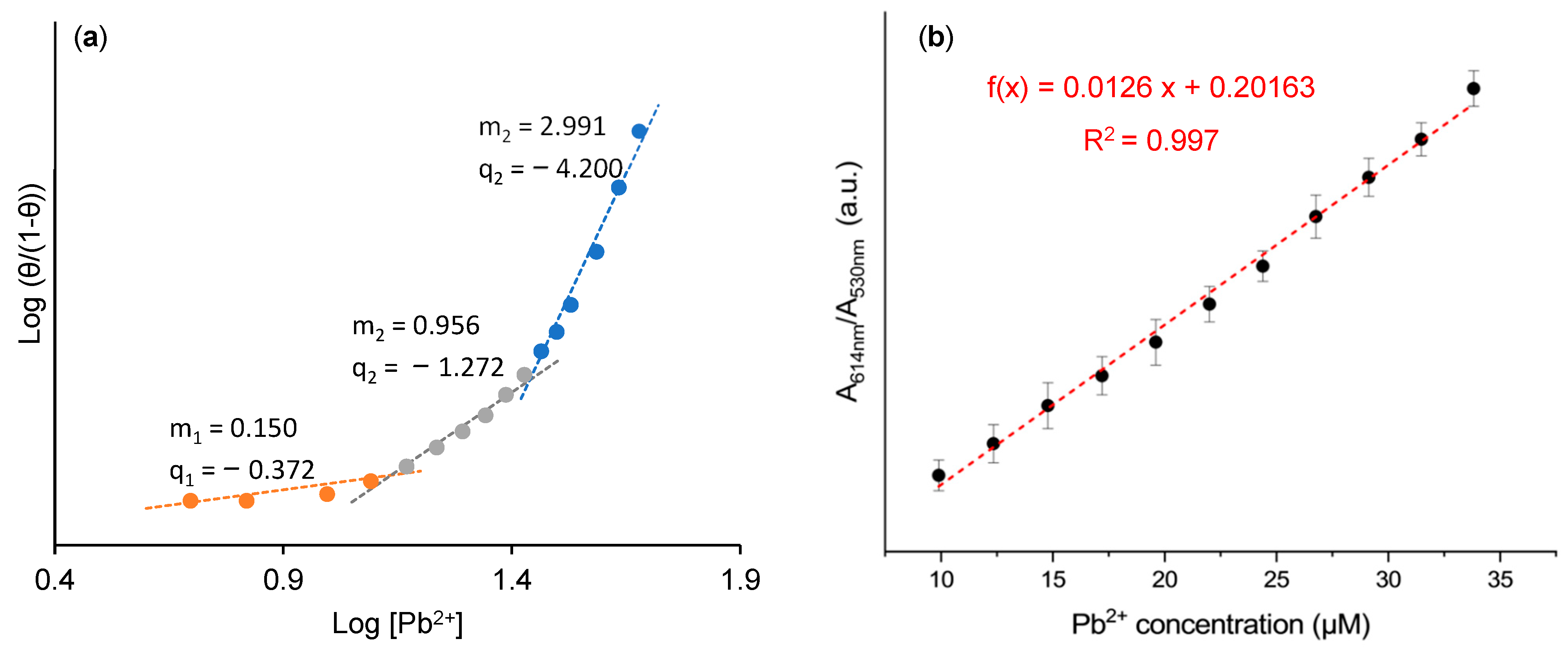
3.3. Sensing Mechanism and Selectivity of the Colorimetric Assay
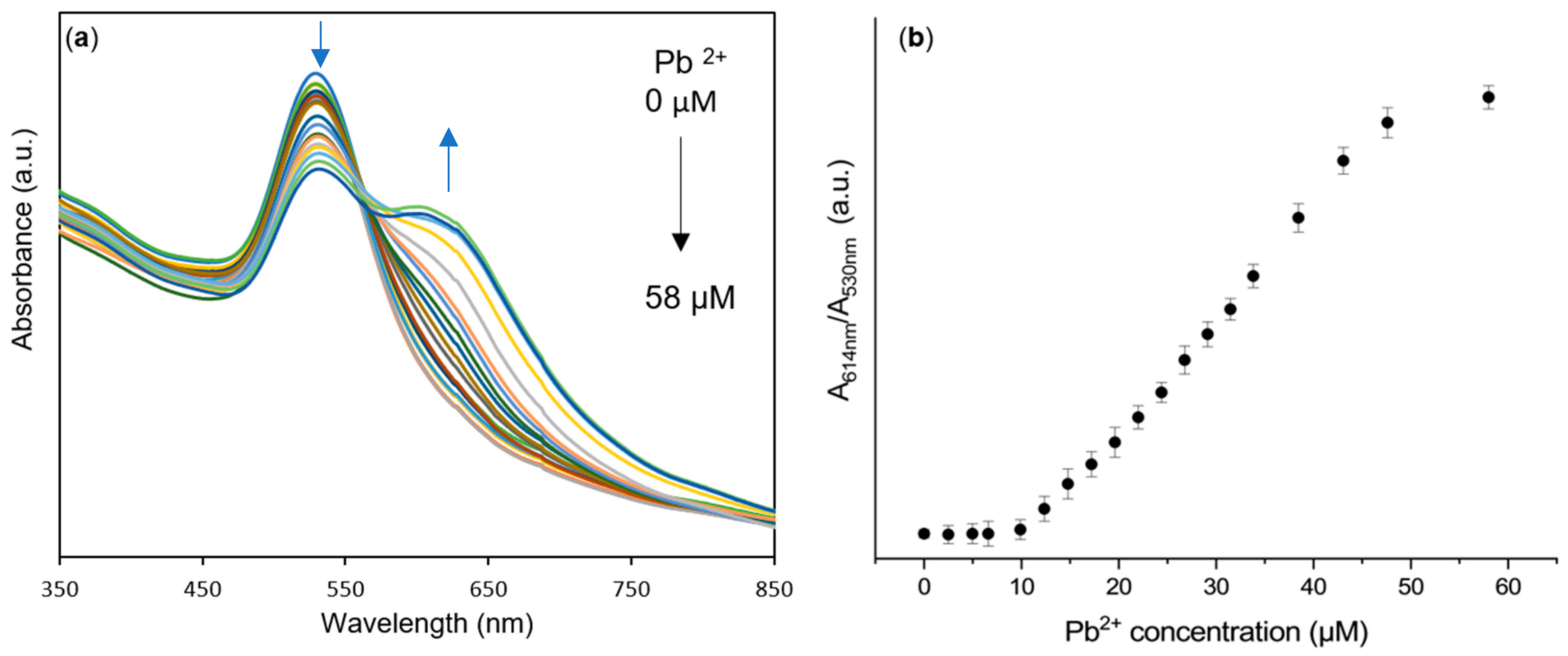
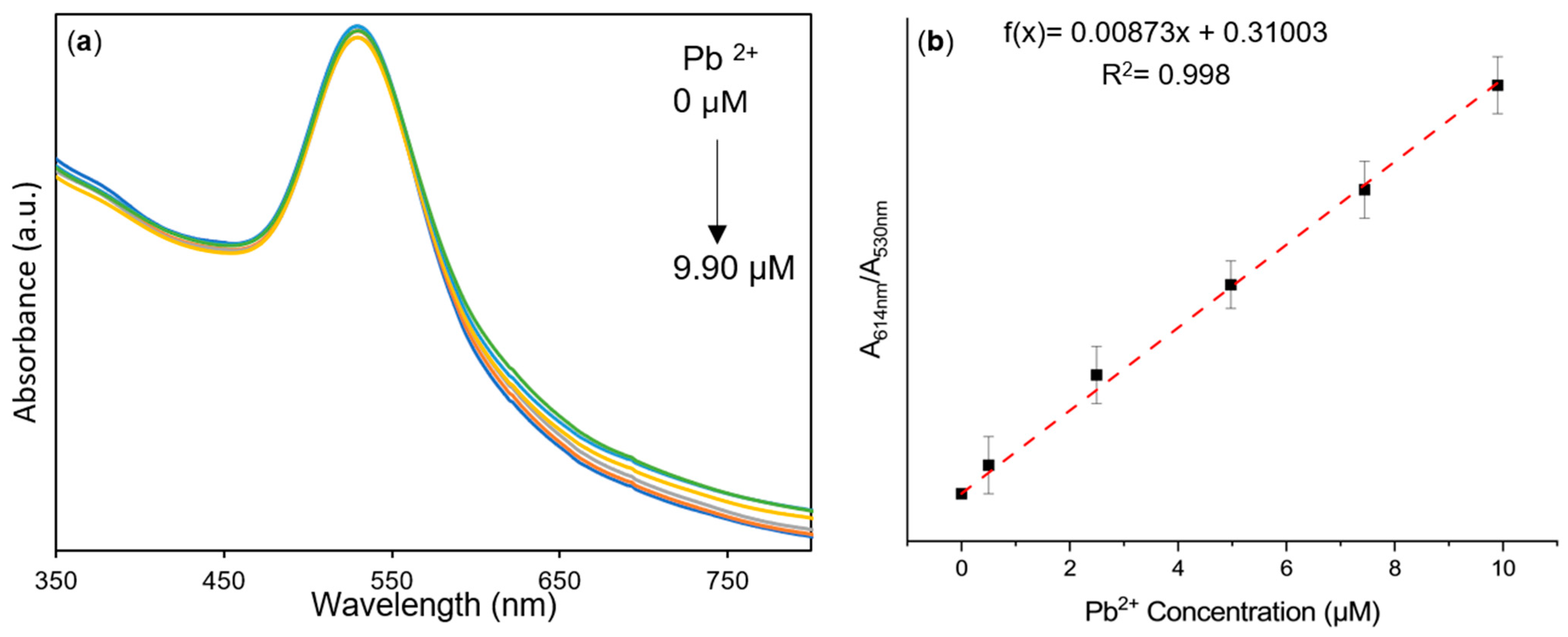
3.4. AuNPs@OPE as Sensors for Pb(II) in Water
3.5. Analysis of Pb(II) in Water Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Z.; Zhang, Z.Y.; Qi, J.; You, J.M.; Ma, J.P.; Chen, L.X. Colorimetric detection of heavy metal ions with various chromogenic materials: Strategies and applications. J. Hazard. Mater. 2023, 441, 129889. [Google Scholar] [CrossRef] [PubMed]
- Zamora–Ledezma, C.; Negrete–Bolagay, D.; Figueroa, F.; Zamora–Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V.H. Heavy metal water pollution: A fresh look about hazards, novel and conventional remediation methods. Environ. Technol. Innov. 2021, 22, 101504. [Google Scholar] [CrossRef]
- Raj, K.; Das, A.P. Lead pollution: Impact on environment and human health and approach for a sustainable solution. Environ. Chem. Ecotoxicol. 2023, 5, 79–85. [Google Scholar] [CrossRef]
- World Health Organization. Lead Poisoning. Available online: https://www.who.int/news–room/fact–sheets/detail/lead–poisoning–and–health (accessed on 20 January 2024).
- Singh, H.; Bamrah, A.; Bhardwaj, S.K.; Deep, A.; Khatri, M.; Kim, K.H.; Bhardwaj, N. Nanomaterial–based fluorescent sensors for the detection of lead ions. J. Hazard. Mater. 2021, 407, 124379. [Google Scholar] [CrossRef] [PubMed]
- Willner, M.R.; Vikesland, P.J. Nanomaterial enabled sensors for environmental contaminants. J. Nanobiotechnol. 2018, 16, 95. [Google Scholar] [CrossRef] [PubMed]
- Arduini, F.; Cinti, S.; Scognamiglio, V.; Moscone, D. 13—Nanomaterial–based sensors. In Handbook of Nanomaterials in Analytical Chemistry; Mustansar Hussain, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 329–359. [Google Scholar]
- Zannotti, M.; Vicomandi, V.; Rossi, A.; Minicucci, M.; Ferraro, S.; Petetta, L.; Giovannetti, R. Tuning of hydrogen peroxide etching during the synthesis of silver nanoparticles. An application of triangular nanoplates as plasmon sensors for Hg2+ in aqueous solution. J. Mol. Liq. 2020, 309, 113238. [Google Scholar] [CrossRef]
- Zannotti, M.; Rossi, A.; Giovannetti, R. SERS Activity of Silver Nanosphere, Triangular Nanoplates, Hexagonal Nanoplates and Quasi–Spherical Nanoparticles: Effect of Shape and Morphology. Coatings 2020, 10, 288. [Google Scholar] [CrossRef]
- Rossi, A.; Cuccioloni, M.; Magnaghi, L.R.; Biesuz, R.; Zannotti, M.; Petetta, L.; Angeletti, M.; Giovannetti, R. Optimizing the Heavy Metal Ion Sensing Properties of Functionalized Silver Nanoparticles: The Role of Surface Coating Density. Chemosensors 2022, 10, 483. [Google Scholar] [CrossRef]
- Gehrke, I.; Geiser, A.; Somborn–Schulz, A. Innovations in nanotechnology for water treatment. Nanotechnol. Sci. Appl. 2015, 8, 1–17. [Google Scholar] [CrossRef]
- Guerra, F.D.; Attia, M.F.; Whitehead, D.C.; Alexis, F. Nanotechnology for Environmental Remediation: Materials and Applications. Molecules 2018, 23, 1760. [Google Scholar] [CrossRef]
- D’Amato, C.A.; Giovannetti, R.; Zannotti, M.; Rommozzi, E.; Ferraro, S.; Seghetti, C.; Minicucci, M.; Gunnella, R.; Di Cicco, A. Enhancement of visible–light photoactivity by polypropylene coated plasmonic Au/TiO for dye degradation in water solution. Appl. Surf. Sci. 2018, 441, 575–587. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Biswas, R.; Alam, M.; Sarkar, A.; Haque, M.I.; Hasan, M.M.; Hoque, M. Application of nanotechnology in food: Processing, preservation, packaging and safety assessment. Heliyon 2022, 8, e11795. [Google Scholar] [CrossRef]
- Kusuma, S.A.F.; Harmonis, J.A.; Pratiwi, R.; Hasanah, A.N. Gold Nanoparticle–Based Colorimetric Sensors: Properties and Application in Detection of Heavy Metals and Biological Molecules. Sensors 2023, 23, 8172. [Google Scholar] [CrossRef]
- Jain, P.K.; Huang, X.H.; El–Sayed, I.H.; El–Sayed, M.A. Noble Metals on the Nanoscale: Optical and Photothermal Properties and Some Applications in Imaging, Sensing, Biology, and Medicine. Acc. Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef]
- Gao, L.; Mei, S.H.; Ma, H.L.; Chen, X.M. Ultrasound–assisted green synthesis of gold nanoparticles using citrus peel extract and their enhanced anti–inflammatory activity. Ultrason. Sonochem. 2022, 83, 105940. [Google Scholar] [CrossRef] [PubMed]
- Akintelu, S.A.; Olugbeko, S.C.; Folorunso, A.S. A review on synthesis, optimization, characterization and antibacterial application of gold nanoparticles synthesized from plants. Internat. Nano Lett. 2020, 10, 237–248. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. Citrus Fruit Statistical Compendium; FAO: Roma, Italy, 2020. [Google Scholar]
- Sivakavinesan, M.; Vanaja, M.; Lateef, R.; Alhadlaq, H.A.; Mohan, R.; Annadurai, G.; Ahamed, M. Risso peel mediated green synthesis of gold nanoparticles and its antioxidant and catalytic activity. J. King Saud. Univ. Sci. 2022, 34, 102235. [Google Scholar] [CrossRef]
- Ringwal, S.; Bartwal, A.S.; Sati, S.C. Photo–catalytic degradation of different toxic dyes using silver nanoparticles as photo–catalyst, mediated via peels extract. J. Indian Chem. Soc. 2021, 98, 100221. [Google Scholar] [CrossRef]
- Dutta, T.; Ghosh, N.N.; Das, M.; Adhikary, R.; Mandal, V.; Chattopadhyay, A.P. Green synthesis of antibacterial and antifungal silver nanoparticles using peel extract: Experimental and theoretical studies. J. Environm. Chem. Eng. 2020, 8, 104019. [Google Scholar] [CrossRef]
- Pagar, K.; Chavan, K.; Kasav, S.; Basnet, P.; Rahdar, A.; Kataria, N.; Oza, R.; Abhale, Y.; Ravindran, B.; Pardeshi, O.; et al. Bio–inspired synthesis of CdO nanoparticles using Citrus limetta peel extract and their diverse biomedical applications. J. Drug. Deliv. Sci. Technol. 2023, 82, 104373. [Google Scholar] [CrossRef]
- Fall, A.; Sackey, J.; Mayedwa, N.; Ngom, B.D. Investigation of structural and optical properties of CdO nanoparticles via peel of Citrus x sinensis. Mater. Today Proc. 2021, 36, 298–302. [Google Scholar] [CrossRef]
- Villegas–Fuentes, A.; Garrafa–Gálvez, H.E.; Quevedo–Robles, R.V.; Luque–Morales, M.; Vilchis–Nestor, A.R.; Luque, P.A. Synthesis of semiconductor ZnO nanoparticles using extract and the influence of concentration on their optical properties. J. Mol. Struct. 2023, 1281, 135067. [Google Scholar] [CrossRef]
- Gao, Y.L.; Xu, D.; Ren, D.; Zeng, K.F.; Wu, X.Y. Green synthesis of zinc oxide nanoparticles using peel extract and application to strawberry preservation: A comparison study. Food Sci. Techol. 2020, 126, 109297. [Google Scholar] [CrossRef]
- Wei, Y.F.; Fang, Z.Q.; Zheng, L.C.; Tan, L.; Tsang, E.P. Green synthesis of Fe nanoparticles using peels aqueous extracts. Mater. Lett. 2016, 185, 384–386. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Galeas, S.; Sharma, V.; Guerrero, V.H.; Debut, A.; Cumbal, L. Characterization and application of biosynthesized iron oxide nanoparticles using peel: A sustainable approach. Inorg. Chem. Commun. 2020, 119, 108116. [Google Scholar] [CrossRef]
- Baglari, S.; Wary, R.R.; Kalita, P.; Baruah, M.B. Green synthesis of CuO nanoparticles using citrus maxima peel aqueous extract. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Singh, S.; Maurya, I.C.; Tiwari, A.; Srivastava, P.; Bahadur, L. Green synthesis of TiO nanoparticles using juice extract as a bio–capping agent for enhanced performance of dye–sensitized solar cells. Surf. Interfaces 2022, 28, 101652. [Google Scholar] [CrossRef]
- Priyadarshini, E.; Pradhan, N. Gold nanoparticles as efficient sensors in colorimetric detection of toxic metal ions: A review. Sens. Actuators B Chem. 2017, 238, 888–902. [Google Scholar] [CrossRef]
- Kesharwani, P.; Ma, R.Y.; Sang, L.; Fatima, M.; Sheikh, A.; Abourehab, M.A.S.; Gupta, N.; Chen, Z.S.; Zhou, Y. Gold nanoparticles and gold nanorods in the landscape of cancer therapy. Mol. Cancer 2023, 22, 98. [Google Scholar] [CrossRef]
- Rossi, A.; Zannotti, M.; Cuccioloni, M.; Minicucci, M.; Petetta, L.; Angeletti, M.; Giovannetti, R. Silver Nanoparticle–Based Sensor for the Selective Detection of Nickel Ions. Nanomaterials 2021, 11, 1733. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Bamrah, A.; Bhardwaj, S.K.; Deep, A.; Khatri, M.; Brown, R.J.C.; Bhardwaj, N.; Kim, K.H. Recent advances in the application of noble metal nanoparticles in colorimetric sensors for lead ions. Environ. Sci. Nano 2021, 8, 863–889. [Google Scholar] [CrossRef]
- Tripathi, R.M.; Park, S.H.; Kim, G.; Kim, D.H.; Ahn, D.; Kim, Y.M.; Kwon, S.J.; Yoon, S.Y.; Kang, H.J.; Chung, S.J. Metal–induced redshift of optical spectra of gold nanoparticles: An instant, sensitive, and selective visual detection of lead ions. Int. Biodeterior. Biodegrad. 2019, 144, 104740. [Google Scholar] [CrossRef]
- Ding, N.; Cao, Q.A.; Zhao, H.; Yang, Y.M.; Zeng, L.X.; He, Y.J.; Xiang, K.X.; Wang, G.W. Colorimetric Assay for Determination of Lead (II) Based on Its Incorporation into Gold Nanoparticles during Their Synthesis. Sensors 2010, 10, 11144–11155. [Google Scholar] [CrossRef] [PubMed]
- Sener, G.; Uzun, L.; Denizli, A. Colorimetric sensor array based on gold nanoparticles and amino acids for identification of toxic metal ions in water. ACS Appl. Mater. Interfaces 2014, 6, 18395–18400. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.Y.; Li, Z.H.; Miao, L.J.; Wang, H.S.; Zhang, Y.N.; Wu, S.S.; Zhang, Y.J.; Li, X.; Wu, A.G. Colorimetric detection of Ba, Cd and Pb based on a multifunctionalized Au NP sensor. Analyst 2019, 144, 5081–5089. [Google Scholar] [CrossRef]
- Lei, L.L.; Song, H.M.; Zhao, J.H.; Yang, Q.X.; Chen, Z.J. Preparation of gold nanoparticles using pyridine–formaldehyde as a reducing agent and its application in high sensitivity colorimetric detection of Pb. Anal. Methods 2019, 11, 4362–4369. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, C.J.; Liu, C.; Li, Y.L.; Liu, H.B.; Li, Y.J.; Zhu, D.B. A dual sensor of fluorescent and colorimetric for the rapid detection of lead. Analyst 2012, 137, 1446–1450. [Google Scholar] [CrossRef]
- Chai, F.; Wang, C.A.; Wang, T.T.; Li, L.; Su, Z.M. Colorimetric Detection of Pb Using Glutathione Functionalized Gold Nanoparticles. Acs Appl. Mater. Inter. 2010, 2, 1466–1470. [Google Scholar] [CrossRef]
- Priyadarshini, E.; Pradhan, N. Metal–induced aggregation of valine capped gold nanoparticles: An efficient and rapid approach for colorimetric detection of Pb ions. Sci. Rep. 2017, 7, 9278. [Google Scholar] [CrossRef]
- Fakhri, N.; Hosseini, M.; Tavakoli, O. Aptamer–based colorimetric determination of Pb using a paper–based microfluidic platform. Anal. Methods 2018, 10, 4438–4444. [Google Scholar] [CrossRef]
- Nguyen, T.H.A.; Le, T.T.V.; Huynh, B.A.; Nguyen, N.V.; Le, V.T.; Doan, V.; Tran, V.A.; Nguyen, A.T.; Cao, X.T.; Vasseghian, Y. Novel biogenic gold nanoparticles stabilized on poly(styrene–co–maleic anhydride) as an effective material for reduction of nitrophenols and colorimetric detection of Pb(II). Environ. Res. 2022, 212, 113281. [Google Scholar] [CrossRef]
- Rizvi, A.H.; Aziz, M.A.; Naqvi, A.H.; Ahmad, A. Towards sustainable and selective detection of lead Ions: Assisted green synthesis of gold nanoparticles with tunable properties. J. Environ. Chem. Eng. 2023, 11, 110757. [Google Scholar] [CrossRef]
- Do Dat, T.; Cong, C.Q.; Nhi, T.L.; Khang, P.T.; Nam, N.T.H.; Tinh, N.T.; Hue, D.; Hieu, N.H. Green synthesis of gold nanoparticles using leave extract for lead ion detection, degradation of dyes, and bioactivities. Biochem. Eng. J. 2023, 200, 109103. [Google Scholar] [CrossRef]
- Krysa, M.; Szymanska–Chargot, M.; Zdunek, A. FT–IR and FT–Raman fingerprints of flavonoids—A review. Food Chem. 2022, 393, 133430. [Google Scholar] [CrossRef] [PubMed]
- Terenteva, E.A.; Apyari, V.V.; Dmitrienko, S.G.; Zolotov, Y.A. Formation of plasmonic silver nanoparticles by flavonoid reduction: A comparative study and application for determination of these substances. Spectrochim. Acta A 2015, 151, 89–95. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Vikulina, A.; Loughlin, M.; Volodkin, D. How similar is the antibacterial activity of silver nanoparticles coated with different capping agents? RSC Adv. 2023, 13, 10542–10555. [Google Scholar] [CrossRef]
- Zhong, G.W.; Liu, J.X.; Liu, X.Y. A Fast Colourimetric Assay for Lead Detection Using Label–Free Gold Nanoparticles (AuNPs). Micromachines 2015, 6, 462–472. [Google Scholar] [CrossRef]
- Mao, X.; Li, Z.P.; Tang, Z.Y. One pot synthesis of monodispersed L–glutathione stabilized gold nanoparticles for the detection of Pb2+ions. Front. Mater. Sci. 2011, 5, 322–328. [Google Scholar] [CrossRef]
- Beqa, L.; Singh, A.K.; Khan, S.A.; Senapati, D.; Arumugam, S.R.; Ray, P.C. Gold Nanoparticle–Based Simple Colorimetric and Ultrasensitive Dynamic Light Scattering Assay for the Selective Detection of Pb(II) from Paints, Plastics, and Water Samples. ACS Appl. Mater. Interfaces 2011, 3, 668–673. [Google Scholar] [CrossRef]
- Sengan, M.; Kamlekar, R.K.; Veerappan, A. Highly selective rapid colorimetric sensing of Pb2+ ion in water samples and paint based on metal induced aggregation of N–decanoyltromethamine capped gold nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 239, 118485. [Google Scholar] [CrossRef] [PubMed]




| Capping/Functionalization Agent | Linear Range | LOD | Ref. |
|---|---|---|---|
| Sodium citrate | 10–100 µM | 18 µM | [36] |
| Gallic acid | 50 nM–1 µM | 25 nM | [37] |
| 11–Mercaptoundecanoic acid | 2–50 µM | 2 µM | [38] |
| 3–Mercaptonicotinic acid 4–aminobenzo–18crown–6 | -- | 50 nM | [39] |
| Pyridine–formaldehyde | 1–6 µM | 1 µM | [40] |
| Tetra–n–octylammonium bromide | -- | 0.5 µM | [41] |
| GSH | 0.1–10 µM | 100 nM | [42] |
| Valine | -- | 96.5 µM | [43] |
| T– and G–rich aptamers | -- | 0.57 µM | [44] |
| N–decanoyl–tromethamine | -- | 350 nM | [54] |
| Poly(styrene–co–maleic anhydride) | -- | 30 nM | [45] |
| Selaginella bryopteris plant extract | 10–60 µM | 0.24 µM | [46] |
| Andrographis paniculata leave extract | 0–100 | 12.66 µM | [47] |
| OPE extract | 9.9–33.8 µM (15 min) 0.8–9.9 µM (90 min) | 13.31 µM 0.05 µM | This work |
| Samples | Pb2+ Added (µM) | A614nm/A530nm | Recovery (µM) | Recovery % |
|---|---|---|---|---|
| Water 1 (15 min) | 15 | 0.385 | 14.566 | 97.11 |
| Water 2 (15 min) | 20 | 0.457 | 20.257 | 101.29 |
| Water 1 (15 min) | 28 | 0.546 | 27.338 | 97.64 |
| Water 2 (15 min) | 33 | 0.608 | 32.198 | 97.75 |
| Water 1 (90 min) | 9 | 0.389 | 9.160 | 101.78 |
| Water 2 (90 min) | 3 | 0.341 | 3.433 | 114.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zannotti, M.; Piras, S.; Remia, L.; Appignanesi, D.; Giovannetti, R. Selective Colorimetric Detection of Pb(II) Ions by Using Green Synthesized Gold Nanoparticles with Orange Peel Extract. Chemosensors 2024, 12, 33. https://doi.org/10.3390/chemosensors12030033
Zannotti M, Piras S, Remia L, Appignanesi D, Giovannetti R. Selective Colorimetric Detection of Pb(II) Ions by Using Green Synthesized Gold Nanoparticles with Orange Peel Extract. Chemosensors. 2024; 12(3):33. https://doi.org/10.3390/chemosensors12030033
Chicago/Turabian StyleZannotti, Marco, Sara Piras, Lorenzo Remia, Diego Appignanesi, and Rita Giovannetti. 2024. "Selective Colorimetric Detection of Pb(II) Ions by Using Green Synthesized Gold Nanoparticles with Orange Peel Extract" Chemosensors 12, no. 3: 33. https://doi.org/10.3390/chemosensors12030033





