Hydrogen Sensing Properties of FET-Type Sensors with Pt-In2O3 at Room Temperature
Abstract
1. Introduction
2. Materials and Methods
2.1. FET Platforms
2.2. Sensing Materials
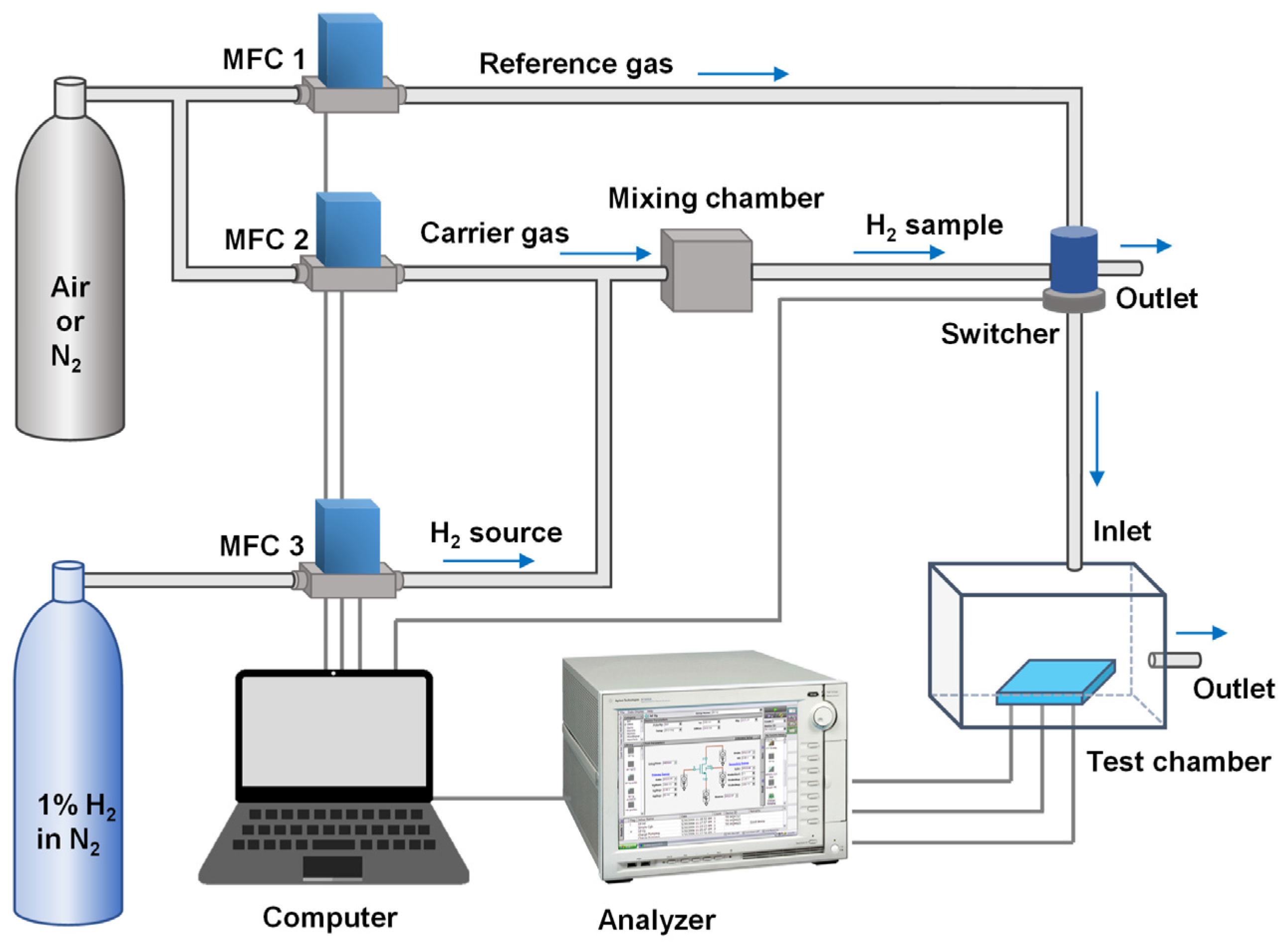
2.3. Measurement Setups
3. Results and Discussion
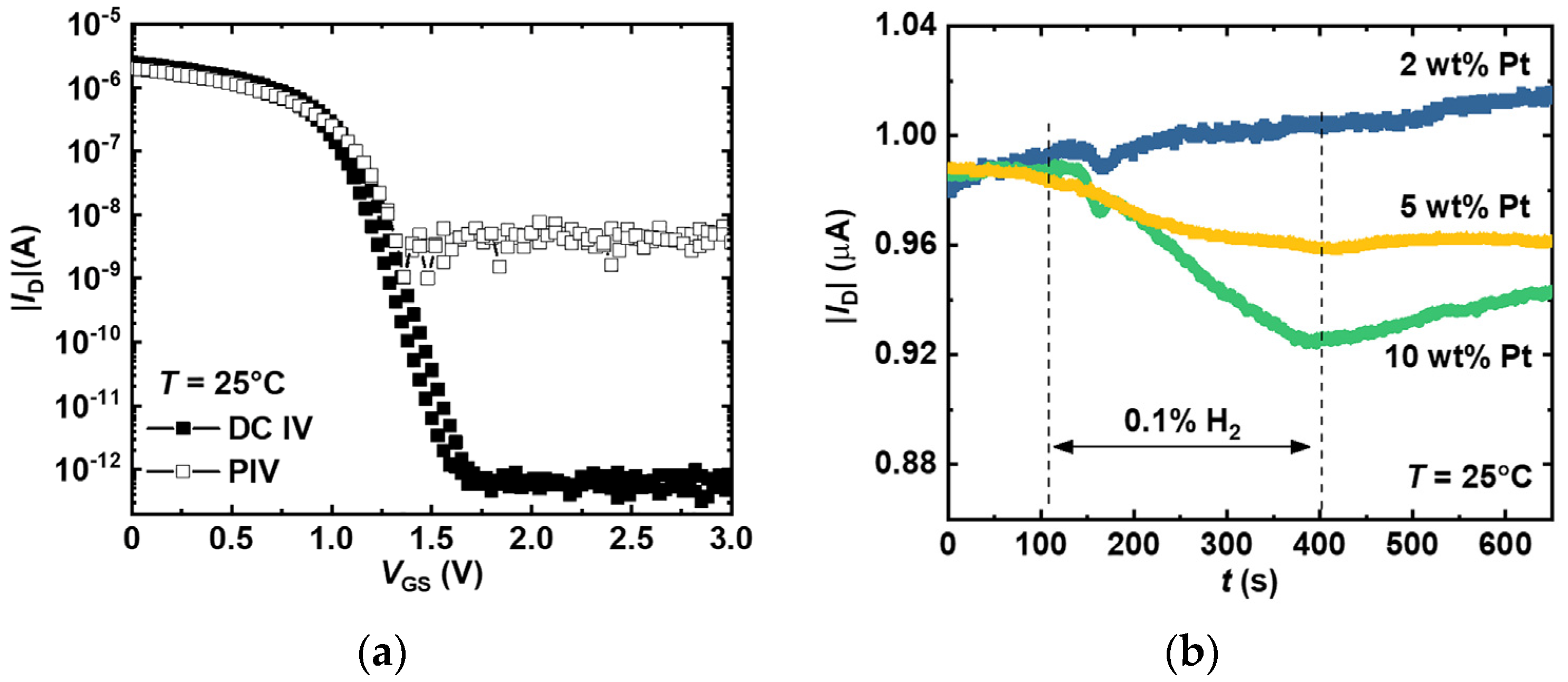
3.1. Pt Concentration Impact
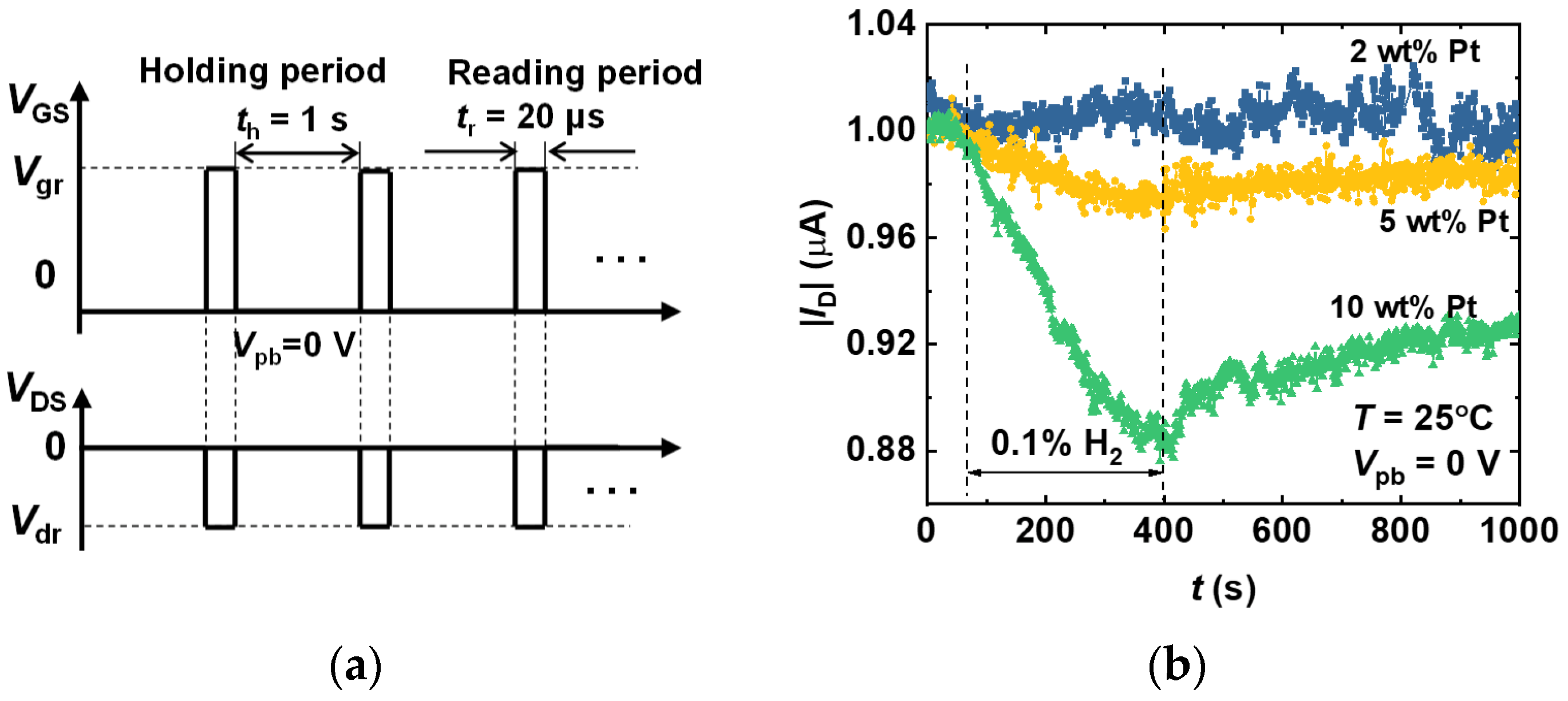
3.2. Pulse Measurement
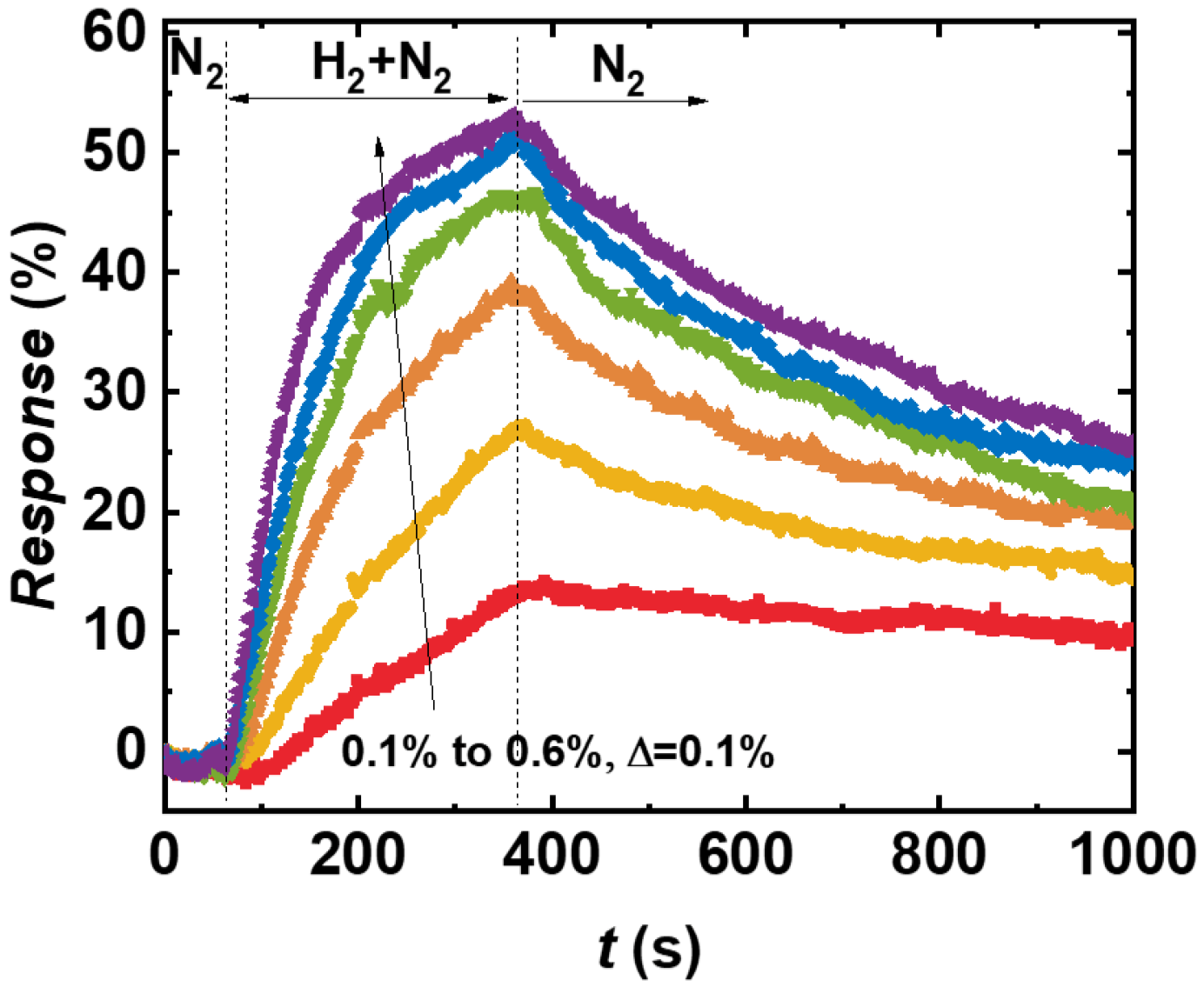
3.3. H2 Concentration Sensing
3.4. Impact of O2 on H2 Sensing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuczyński, S.; Łaciak, M.; Olijnyk, A.; Szurlej, A.; Włodek, T. Thermodynamic and Technical Issues of Hydrogen and Methane-Hydrogen Mixtures Pipeline Transmission. Energies 2019, 12, 569. [Google Scholar] [CrossRef]
- Singh, G.; Ramadass, K.; DasiReddy, V.D.B.C.; Yuan, X.; Ok, Y.S.; Bolan, N.; Xiao, X.; Ma, T.; Karakoti, A.; Yi, J.; et al. Material-based generation, storage, and utilisation of hydrogen. Prog. Mater. Sci. 2023, 135, 101104. [Google Scholar] [CrossRef]
- Mirzaei, A.; Yousefi, H.R.; Falsafi, F.; Bonyani, M.; Lee, J.-H.; Kim, J.-H.; Kim, H.W.; Kim, S.S. An overview on how Pd on resistive-based nanomaterial gas sensors can enhance response toward hydrogen gas. Int. J. Hydrogen Energy 2019, 44, 20552–20571. [Google Scholar] [CrossRef]
- Handwerker, M.; Wellnitz, J.; Marzbani, H. Comparison of Hydrogen Powertrains with the Battery Powered Electric Vehicle and Investigation of Small-Scale Local Hydrogen Production Using Renewable Energy. Hydrogen 2021, 2, 76–100. [Google Scholar] [CrossRef]
- Yusaf, T.; Mahamude, A.S.F.; Kadirgama, K.; Ramasamy, D.; Farhana, K.; Dhahad, H.A.; Talib, A.R.A.D. Sustainable hydrogen energy in aviation—A narrative review. Int. J. Hydrogen Energy 2024, 52, 1026–1045. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Wu, Z.; Wang, M.; Luo, J.; Torun, H.; Hu, P.-A.; Yang, C.; Grundmann, M.; Liu, X.; et al. Advances in designs and mechanisms of semiconducting metal oxide nanostructures for high-precision gas sensors operated at room temperature. Mater. Horiz. 2019, 6, 470–506. [Google Scholar] [CrossRef]
- Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Improving the hydrogen sensing properties of SnO2 nanowire-based conductometric sensors by Pd-decoration. Sens. Actuators B Chem. 2019, 285, 358–367. [Google Scholar] [CrossRef]
- Zhao, J.; Song, J.; Lu, X.; Wu, M.; Yan, Z.; Chen, F.; Chen, W. Room-Temperature Hydrogen-Sensitive Pt-SnO2 Composite Nanoceramics: Contrasting Roles of Pt Nano-Catalysts Loaded via Two Different Methods. Inorganics 2023, 11, 366. [Google Scholar] [CrossRef]
- Wadhwa, R.; Kumar, A.; Sarkar, R.; Mohanty, P.P.; Kumar, D.; Deswal, S.; Kumar, P.; Ahuja, R.; Chakraborty, S.; Kumar, M.; et al. Pt Nanoparticles on Vertically Aligned Large-Area MoS2 Flakes for Selective H2 Sensing at Room Temperature. ACS Appl. Nano Mater. 2023, 6, 2527–2537. [Google Scholar] [CrossRef]
- Dasari, S.G.; Nagaraju, P.; Yelsani, V.; Tirumala, S.; Reddy, M.V.R. Nanostructured Indium Oxide Thin Films as a Room Temperature Toluene Sensor. ACS Omega 2021, 6, 17442–17454. [Google Scholar] [CrossRef]
- Pawar, K.K.; Mali, S.S.; Navale, Y.H.; Patil, V.B.; Sharma, K.K.; Hong, C.K.; Patil, P.S. Fabrication of enhanced sensitive and selective porous indium oxide nanocube sensor for NO2 detection. Ceram. Int. 2021, 47, 2430–2440. [Google Scholar] [CrossRef]
- Tian, J.; Chen, X.; Wang, T.; Pei, W.; Li, F.; Li, D.; Yang, Y.; Dong, X. Modification of indium oxide nanofibers by polyoxometalate electron acceptor doping for enhancement of gas sensing at room temperature. Sens. Actuators B Chem. 2021, 344, 130227. [Google Scholar] [CrossRef]
- Inyawilert, K.; Wisitsoraat, A.; Liewhiran, C.; Tuantranont, A.; Phanichphant, S. H2 gas sensor based on PdOx-doped In2O3 nanoparticles synthesized by flame spray pyrolysis. Appl. Surf. Sci. 2019, 475, 191–203. [Google Scholar] [CrossRef]
- Luo, Y.; An, B.; Bai, J.; Wang, Y.; Cheng, X.; Wang, Q.; Li, J.; Yang, Y.; Wu, Z.; Xie, E. Ultrahigh-response hydrogen sensor based on PdO/NiO co-doped In2O3 nanotubes. J. Colloid Interface Sci. 2021, 599, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Wu, M.; Hong, Y.; Jeong, Y.; Jung, G.; Shin, W.; Park, J.; Kim, D.; Jang, D.; Lee, J.-H. FET-type gas sensors: A review. Sens. Actuators B Chem. 2021, 330, 129240. [Google Scholar] [CrossRef]
- Zhang, P.; Xiao, Y.; Zhang, J.; Liu, B.; Ma, X.; Wang, Y. Highly sensitive gas sensing platforms based on field effect Transistor—A review. Anal. Chim. Acta 2021, 1172, 338575. [Google Scholar] [CrossRef]
- Mukherjee, A.; Rosenwaks, Y. Recent Advances in Silicon FET Devices for Gas and Volatile Organic Compound Sensing. Chemosensors 2021, 9, 260. [Google Scholar] [CrossRef]
- Kim, E.-B.; Imran, M.; Lee, E.-H.; Akhtar, M.S.; Ameen, S. Multiple ions detection by field-effect transistor sensors based on ZnO@GO and ZnO@rGO nanomaterials: Application to trace detection of Cr (III) and Cu (II). Chemosphere 2022, 286, 131695. [Google Scholar] [CrossRef]
- Morati, N.; Contaret, T.; Gomri, S.; Fiorido, T.; Seguin, J.-L.; Bendahan, M. Noise spectroscopy data analysis-based gas identification with a single MOX sensor. Sens. Actuators B Chem. 2021, 334, 129654. [Google Scholar] [CrossRef]
- Shin, W.; Jung, G.; Hong, S.; Jeong, Y.; Park, J.; Jang, D.; Park, B.-G.; Lee, J.-H. Low frequency noise characteristics of resistor- and Si MOSFET-type gas sensors fabricated on the same Si wafer with In2O3 sensing layer. Sens. Actuators B Chem. 2020, 318, 128087. [Google Scholar] [CrossRef]
- Hassanalieragh, M.; Ignjatovic, Z.; Newman, J.D.; Fourspring, K. Design and Characterization of a 10 × 10 Pixel Array THz Camera in 350 nm CMOS Technology. IEEE Sens. J. 2020, 20, 9834–9848. [Google Scholar] [CrossRef]
- Rezende, N.P.; Cadore, A.R.; Gadelha, A.C.; Pereira, C.L.; Ornelas, V.; Watanabe, K.; Taniguchi, T.; Ferlauto, A.S.; Malachias, A.; Campos, L.C.; et al. Probing the Electronic Properties of Monolayer MoS2 via Interaction with Molecular Hydrogen. Adv. Electron. Mater. 2019, 5, 1800591. [Google Scholar] [CrossRef]
- Sokolovskij, R.; Zhang, J.; Zheng, H.; Li, W.; Jiang, Y.; Yang, G.; Yu, H.; Sarro, P.M.; Zhang, G. The Impact of Gate Recess on the H Detection Properties of Pt-AlGaN/GaN HEMT Sensors. IEEE Sens. J. 2020, 20, 8947–8955. [Google Scholar] [CrossRef]
- Ghosh, S.; Rajan, L. Zinc Oxide Thin-Film Transistor with Catalytic Electrodes for Hydrogen Sensing at Room Temperature. IEEE Trans. Nanotechnol. 2021, 20, 303–310. [Google Scholar] [CrossRef]
- Ahn, J.; Kim, D.; Park, K.H.; Yoo, G.; Heo, J. Pt-Decorated Graphene Gate AlGaN/GaN MIS-HEMT for Ultrahigh Sensitive Hydrogen Gas Detection. IEEE Trans. Electron Devices 2021, 68, 1255–1261. [Google Scholar] [CrossRef]
- Chen, Y.-Z.; Lee, S.-H.; Su, T.-Y.; Wu, S.-C.; Chen, P.-J.; Chueh, Y.-L. Phase-modulated 3D-hierarchical 1T/2H WSe2 nanoscrews by a plasma-assisted selenization process as high performance NO gas sensors with a ppb-level detection limit. J. Mater. Chem. A 2019, 7, 22314–22322. [Google Scholar] [CrossRef]
- Wang, C.; Lei, S.; Li, X.; Guo, S.; Cui, P.; Wei, X.; Liu, W.; Liu, H. A Reduced GO-Graphene Hybrid Gas Sensor for Ultra-Low Concentration Ammonia Detection. Sensors 2018, 18, 3147. [Google Scholar] [CrossRef] [PubMed]
- Hellsing, B.; Kasemo, B.; Zhdanov, V.P. Kinetics of the hydrogen-oxygen reaction on platinum. J. Catal. 1991, 132, 210–228. [Google Scholar] [CrossRef]
- Mansour, H.; Iglesia, E. Theoretical and Experimental Assessments of Elementary Steps and Bound Intermediates in Catalytic H2–O2 Reactions on Dispersed Pt Nanoparticles. J. Phys. Chem. C 2023, 127, 4553–4569. [Google Scholar] [CrossRef]
- Wang, X.; Liu, W.; Wang, C.; Zhang, S.; Ding, M.; Xu, X. Enhanced formaldehyde gas sensing performance of ternary CuB2O4 oxides through oxygen vacancy manipulation and surface platinum decoration. Sens. Actuators B Chem. 2021, 344, 130190. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.; Hu, S.; Wu, Z.; Wang, Z.; Li, M.; Liu, X.; Jin, X.; Lee, J.-H. Hydrogen Sensing Properties of FET-Type Sensors with Pt-In2O3 at Room Temperature. Chemosensors 2024, 12, 32. https://doi.org/10.3390/chemosensors12030032
Wu M, Hu S, Wu Z, Wang Z, Li M, Liu X, Jin X, Lee J-H. Hydrogen Sensing Properties of FET-Type Sensors with Pt-In2O3 at Room Temperature. Chemosensors. 2024; 12(3):32. https://doi.org/10.3390/chemosensors12030032
Chicago/Turabian StyleWu, Meile, Shixin Hu, Zhanyu Wu, Zebin Wang, Meng Li, Xi Liu, Xiaoshi Jin, and Jong-Ho Lee. 2024. "Hydrogen Sensing Properties of FET-Type Sensors with Pt-In2O3 at Room Temperature" Chemosensors 12, no. 3: 32. https://doi.org/10.3390/chemosensors12030032
APA StyleWu, M., Hu, S., Wu, Z., Wang, Z., Li, M., Liu, X., Jin, X., & Lee, J.-H. (2024). Hydrogen Sensing Properties of FET-Type Sensors with Pt-In2O3 at Room Temperature. Chemosensors, 12(3), 32. https://doi.org/10.3390/chemosensors12030032




