A Mini-Review on Metal Oxide Semiconductor Gas Sensors for Carbon Monoxide Detection at Room Temperature
Abstract
1. Introduction
2. General Definition and Sensing Model
3. MOS Sensing Materials
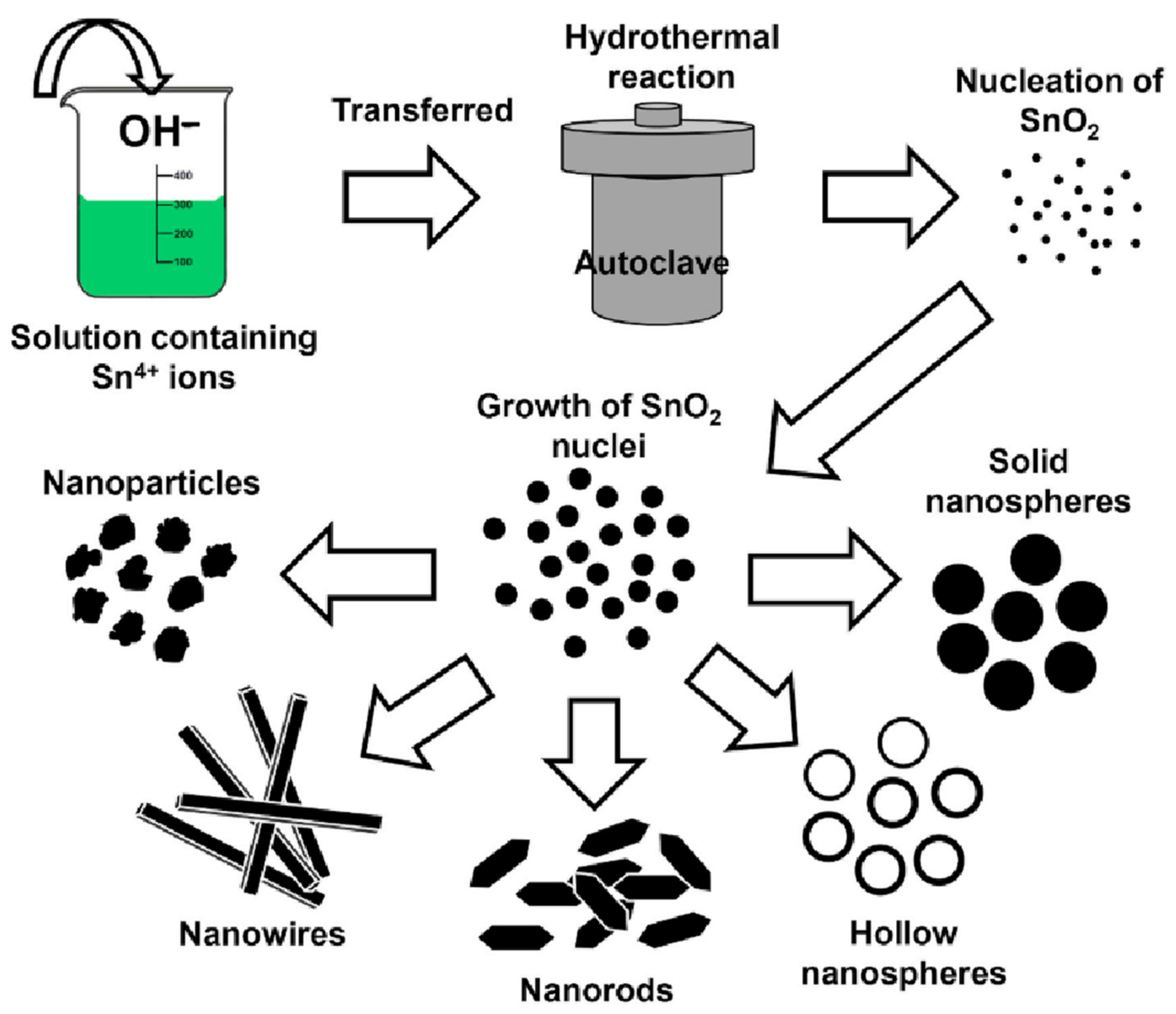
3.1. SnO2
| Sensing Material | CO Concentration (ppm) | Response | Response Time (s) | Recovery Time (s) | Reference |
|---|---|---|---|---|---|
| Au-SnO2 | 500 | ~50 | 20 (50 °C) | NA | [42] |
| Pt- SnO2 nanoparticle | 5000 | 3.57 | ~720 | NA | [45] |
| Pt-SnO2 porous nanosolid | 100 | 64.5 | 144 | 882 | [44] |
| Pd-SnO2 nanoparticle | 50 | ~5 | 20 | 40 | [47] |
| Polyaniline-Pd-SnO2 | 300 | 4 | 88 | 62 | [48] |
| CH3NH3SnI3/SnO2/Pd/Au | 50 | 68 | 25 | 32 | [46] |
| CNT-Co3O4− SnO2 | 1000 | 1.46 (Va/Vg) | 120 | 150 | [2] |
3.2. ZnO
3.3. Other Metal Oxides
3.4. Metal Oxide–2D Material Composites
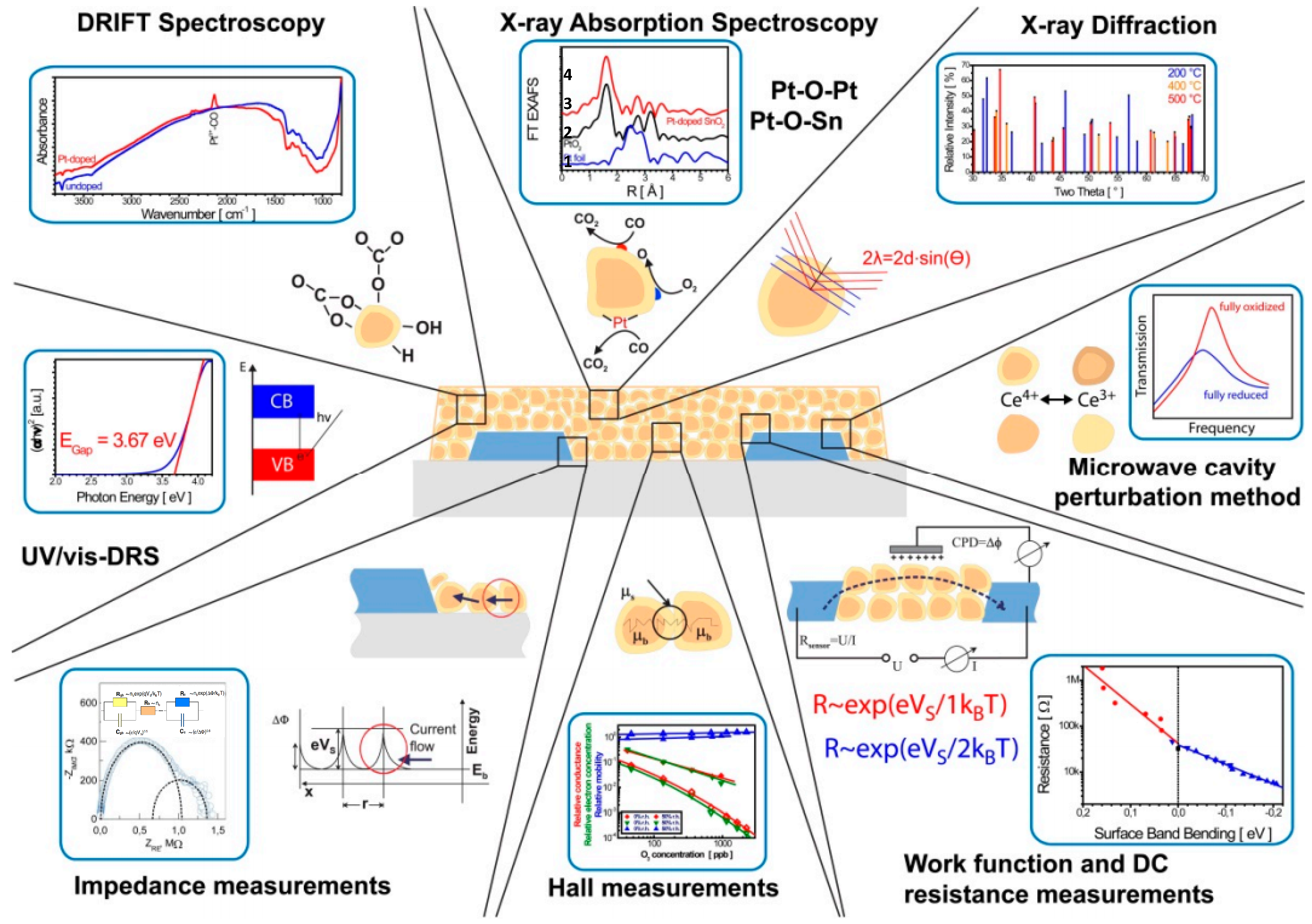
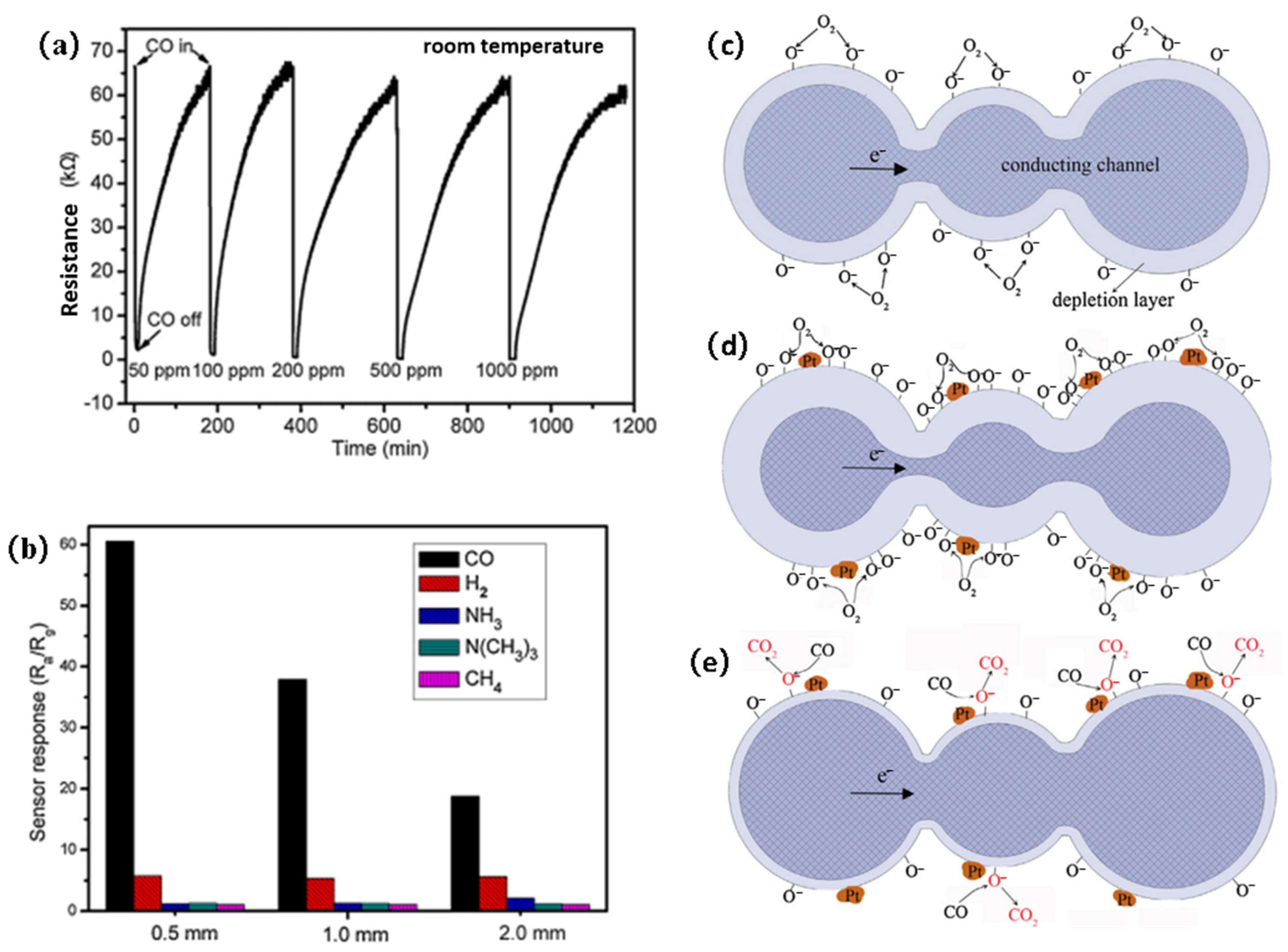
4. Discussion of Sensing Mechanism
- (1)
- Structures of MOS nanostructures
- (2)
- Surface modification of MOSs
- (3)
- Annealing effect
- (4)
- UV activation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, S.W.; Kim, J.; Lee, J.H.; Byun, Y.T. Remarkable Improvement of CO-Sensing Performances in Single-Walled Carbon Nanotubes Due to Modification of the Conducting Channel by Functionalization of Au Nanoparticles. Sens. Actuators B Chem. 2016, 232, 625–632. [Google Scholar] [CrossRef]
- Wu, R.J.; Wu, J.G.; Yu, M.R.; Tsai, T.K.; Yeh, C.T. Promotive Effect of CNT on Co3O4-SnO2 in a Semiconductor-Type CO Sensor Working at Room Temperature. Sens. Actuators B Chem. 2008, 131, 306–312. [Google Scholar] [CrossRef]
- Roy, A.; Ray, A.; Sadhukhan, P.; Naskar, K.; Lal, G.; Bhar, R.; Sinha, C.; Das, S. Polyaniline-Multiwalled Carbon Nanotube (PANI-MWCNT): Room Temperature Resistive Carbon Monoxide (CO) Sensor. Synth. Met. 2018, 245, 182–189. [Google Scholar] [CrossRef]
- Wang, H. Research Progress of Low-Power Carbon Monoxide Sensor. Ind. Mine Autom. 2021, 47, 16–23. [Google Scholar] [CrossRef]
- Dong, K.N.; Yang, J.F. Development of Mine-Used Infrared Carbon Monoxide Sensor Based on Mini Pump Suctio. Ind. Mine Autom. 2021, 47, 128–132. [Google Scholar]
- Shrivastava, S.; Mahana, D.; Nehra, S.; Gangwar, S.; Singh, S.; Yadav, C.S.; Muthusamy, S.K.; Dogra, A. CO Gas Sensing Characteristics of BaSnO3 Epitaxial Films Prepared by PLD: The Effect of Film Thickness. Sens. Actuators B Chem. 2024, 400, 134882. [Google Scholar] [CrossRef]
- Ani, A.; Poornesh, P.; Antony, A.; Chattopadhyay, S. Synergism of Spray-Pyrolyzed Aliovalent Ga (Iii) Ions and ZnO Nanostructures for Selective Sensing of Hazardous CO Gas towards Low Ppm Levels. Sens. Actuators B Chem. 2024, 399, 134827. [Google Scholar] [CrossRef]
- Zegebreal, L.T.; Tegegne, N.A.; Hone, F.G. Recent Progress in Hybrid Conducting Polymers and Metal Oxide Nanocomposite for Room-Temperature Gas Sensor Applications: A Review. Sens. Actuators A Phys. 2023, 359, 114478. [Google Scholar] [CrossRef]
- Desimone, P.M.; Zonta, G.; Giulietti, G.; Ortega, P.P.; Aldao, C.M.; Simões, A.Z.; Moura, F.; Ponce, M.A.; Foschini, C.R. Towards Carbon Monoxide Detection Based on ZnO Nanostructures. Mater. Sci. Eng. B 2024, 299, 117003. [Google Scholar] [CrossRef]
- Sadredini, A.R.; Nikfarjam, A.; Naeimi Pour, M.; Nazeri, N. Intensity Modulation of UV Light in Gas Sensor Array to Discriminate Several Analytes at Room Temperature. IET Sci. Meas. Technol. 2023, 18, 65–73. [Google Scholar] [CrossRef]
- Tang, M.; Zhang, D.; Sun, Y.; Chen, Q.; Chen, Y.; Xi, G.; Wang, Z.; Shao, X. CVD-Fabricated Co3O4-Co3S4 Heterojunction for Ultra-Sensitive Detection of CO in SF6 Discharge Decomposition Products. Sens. Actuators B Chem. 2024, 401, 134968. [Google Scholar] [CrossRef]
- Chen, M.; Li, X.; Li, Y.; Li, Y.; Qin, Z.; Wang, Q. MOF-Derived Co3O4 Nanoparticles over Direct Grown ZnO Nanoflower on Ceramic for CO Sensor with High Selectivity. Sens. Actuators B Chem. 2024, 401, 134951. [Google Scholar] [CrossRef]
- de Araújo, E.P.; Paiva, M.P.; Moisés, L.A.; Santo, G.S.D.E.; Blanco, K.C.; Chiquito, A.J.; Amorim, C.A. Improving Hazardous Gas Detection Behavior with Palladium Decorated SnO2 Nanobelts Networks. Sensors 2023, 23, 4783. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Cao, W.; Wang, P.H.; Wang, J.; Yu, L.; Qiao, Z.; Ding, Z.J. Fast and Sensitive Detection of CO by Bi-MOF-Derived Porous In2O3/Fe2O3 Core-Shell Nanotubes. ACS Sens. 2023, 8, 4577–4586. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.M.; Murshed, M.N.; El Sayed, M.E.; Samir, A.; Alsharabi, R.M.; Farea, M.O. A New Fabrication Strategy of Ag2O Doped PANI as a Highly Stable and Room Temperature Operable Carbon Monoxide Gas Sensor. Opt. Mater. 2023, 144, 114324. [Google Scholar] [CrossRef]
- Al-Maamori, M.H.; Al-Jamal, A.N.; Habeeb, S.A.; Hassan, A.S.; Majdi, H.S. Al3+-Modified ZnO Thin Film Sensor Fabricated by the Sputtering Method: Characterization and a Carbon Monoxide Gas Detection Study. J. Chem. Res. 2024, 48, 17475198231221453. [Google Scholar] [CrossRef]
- Meng, F.J.; Guo, X.M. Co/Au Bimetal Synergistically Modified SnO2-In2O3 Nanocomposite for Efficient CO Sensing. Ceram. Int. 2023, 49, 15979–15989. [Google Scholar] [CrossRef]
- You, S.; Li, G.; Fan, Z.; Li, X.; Fu, L.; Wu, W. Nanotechnology-Assisted Sensors for the Detection of Carbon Monoxide: A Review. Int. J. Electrochem. Sci. 2023, 18, 100314. [Google Scholar] [CrossRef]
- Qin, C.; Wei, Z.; Wang, B.; Wang, Y. Sn and Mn Co-Doping synergistically promotes the sensing properties of Co3O4 sensor for high-sensitive CO detection. Sens. Actuators B Chem. 2023, 390, 133930. [Google Scholar] [CrossRef]
- Pandit, N.A.; Ahmad, T. ZrO2/CeO2-Heterostructured Nanocomposites for Enhanced Carbon Monoxide Gas Sensing. ACS Appl. Nano Mater. 2023, 6, 7299–7309. [Google Scholar] [CrossRef]
- Baier, D.; Priamushko, T.; Weinberger, C.; Kleitz, F.; Tiemann, M. Selective Discrimination between CO and H2 with Copper-Ceria-Resistive Gas Sensors. ACS Sens. 2023, 8, 1616–1623. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, J.; Shukla, M.; Singh, V. Investigation of the Effect of ZnO Film Thickness over the Gas Sensor Developed for Sensing Carbon Monoxide. Phys. Status Solidi (A) Appl. Mater. Sci. 2023, 220, 2300047. [Google Scholar] [CrossRef]
- Zhao, W.; Fam, D.W.H.; Yin, Z.; Sun, T.; Tan, H.T.; Liu, W.; Tok, A.I.Y.; Boey, Y.C.F.; Zhang, H.; Hng, H.H.; et al. A Carbon Monoxide Gas Sensor Using Oxygen Plasma Modified Carbon Nanotubes. Nanotechnology 2012, 23, 425502. [Google Scholar] [CrossRef] [PubMed]
- Afrin, R.; Shah, N.A. Room Temperature Gas Sensors Based on Carboxyl and Thiol Functionalized Carbon Nanotubes Buckypapers. Diam. Relat. Mater. 2015, 60, 42–49. [Google Scholar] [CrossRef]
- Panda, D.; Nandi, A.; Datta, S.K.; Saha, H.; Majumdar, S. Selective Detection of Carbon Monoxide (CO) Gas by Reduced Graphene Oxide (RGO) at Room Temperature. RSC Adv. 2016, 6, 47337–47348. [Google Scholar] [CrossRef]
- Simões, A.N.; Lustosa, G.M.M.M.; de Souza Morita, E.; de Souza, A.N.; Torres, F.; Bizzo, W.A.; Mazon, T. Room-Temperature SnO2-Based Sensor with Pd-Nanoparticles for Real-Time Detection of CO Dissolved Gas in Transformer Oil. Mater. Chem. Phys. 2024, 311, 128576. [Google Scholar] [CrossRef]
- Fort, A.; Landi, E.; Mugnaini, M.; Parri, L.; Pozzebon, A.; Vignoli, V. A LoRaWAN Carbon Monoxide Measurement System with Low-Power Sensor Triggering for the Monitoring of Domestic and Industrial Boilers. IEEE Trans. Instrum. Meas. 2021, 70, 5500609. [Google Scholar] [CrossRef]
- Mahajan, S.; Jagtap, S. Metal-Oxide Semiconductors for Carbon Monoxide (CO) Gas Sensing: A Review. Appl. Mater. Today 2020, 18, 100483. [Google Scholar] [CrossRef]
- Lawaniya, S.D.; Kumar, S.; Yu, Y.; Rubahn, H.G.; Mishra, Y.K.; Awasthi, K. Functional Nanomaterials in Flexible Gas Sensors: Recent Progress and Future Prospects. Mater. Today Chem. 2023, 29, 101428. [Google Scholar] [CrossRef]
- Jiao, M.Z.; Chen, X.Y.; Hu, K.X.; Qian, D.Y.; Zhao, X.H.; Ding, E.J. Recent Developments of Nanomaterials-Based Conductive Type Methane Sensors. Rare Met. 2021, 40, 1515–1527. [Google Scholar] [CrossRef]
- Yamazoe, N.; Shimanoe, K. New Perspectives of Gas Sensor Technology. Sens. Actuators B Chem. 2009, 138, 100–107. [Google Scholar] [CrossRef]
- Blackman, C. Do We Need “Ionosorbed” Oxygen Species? (Or, “a Surface Conductivity Model of Gas Sensitivity in Metal Oxides Based on Variable Surface Oxygen Vacancy Concentration”). ACS Sens. 2021, 6, 3509–3516. [Google Scholar] [CrossRef] [PubMed]
- Degler, D. Trends and Advances in the Characterization of Gas Sensing Materials Based on Semiconducting Oxides. Sensors 2018, 18, 3544. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Meng, Q.; Zhang, L.; Han, C.; Gao, H.; Zhang, Y.; Yan, H. SnO2-Based Electron Transporting Layer Materials for Perovskite Solar: A Review of Recent Progress. J. Energy Chem. 2019, 35, 144–167. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, S.; Chang, J.; Ocola, L.E.; Chen, J. Highly Sensitive Room Temperature Carbon Monoxide Detection Using SnO2 Nanoparticle-Decorated Semiconducting Single-Walled Carbon Nanotubes. Nanotechnology 2013, 24, 025503. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Chen, M.; Li, X.; Wang, Q. One-Step CVD Growth of ZnO Nanorod/SnO2 Film Heterojunction for NO2 Gas Sensor. Sens. Actuators B Chem. 2022, 373, 132738. [Google Scholar] [CrossRef]
- Yang, A.; Tao, X.; Wang, R.; Lee, S.; Surya, C. Room Temperature Gas Sensing Properties of Sn O2 /Multiwall-Carbon-Nanotube Composite Nanofibers. Appl. Phys. Lett. 2007, 91, 133110. [Google Scholar] [CrossRef]
- Shojaee, M.; Nasresfahani, S.; Sheikhi, M.H. Hydrothermally Synthesized Pd-Loaded SnO2/Partially Reduced Graphene Oxide Nanocomposite for Effective Detection of Carbon Monoxide at Room Temperature. Sens. Actuators B Chem. 2018, 254, 457–467. [Google Scholar] [CrossRef]
- Nguyen, V.D.; Duong, T.T.; Dang, T.T.; Chu, M.H.; Matteo, T.; Hugo, N.; Nguyen, D.H. Enhancement of NH3 Gas Sensing with Ag-Pt Co-Catalyst on SnO2 Nanofilm towards Medical Diagnosis. Thin Solid Films 2023, 767, 139682. [Google Scholar] [CrossRef]
- Nguyen, V.D.; Nguyen, X.T.; Trinh, M.N.; Dang, T.T.; Chu, M.H.; Hugo, N.; Matteo, T.; Nguyen, V.H.; Nguyen, D.H. Design and Fabrication of Effective Gradient Temperature Sensor Array Based on Bilayer SnO2/Pt Gas Classification. Sens. Actuators B Chem. 2022, 351, 130979. [Google Scholar] [CrossRef]
- Emanuel, P.N.; Hellen, C.T.F.; Gelmires, A.N.; Romualdo, R.M. A Review of Recent Developments in Tin Dioxide Nanostructured Materials for Gas Sensors. Ceram. Int. 2022, 48, 7405–7440. [Google Scholar] [CrossRef]
- Manjula, P.; Arunkumar, S.; Manorama, S.V. Au/SnO2 an Excellent Material for Room Temperature Carbon Monoxide Sensing. Sens. Actuators B Chem. 2011, 152, 168–175. [Google Scholar] [CrossRef]
- Matsushima, S.; Teraoka, Y.; Norio, M.; Yamazoe, N. Electronic Interaction between Metal Additives and Tin Dioxide in Tin Dioxide-Based Gas Sensors. Jpn. J. Appl. Phys. 1988, 27, 1798–1802. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, T.; Lian, G.; Yu, Q.; Luan, C.; Wang, Q.; Cui, D. Room Temperature CO Sensor Fabricated from Pt-Loaded SnO2 Porous Nanosolid. Sens. Actuators B Chem. 2013, 184, 33–39. [Google Scholar] [CrossRef]
- Kang, K.-S.; Lee, S.P. CO Gas Sensors Operating at Room Temperature. J. Mater. Sci. 2003, 38, 4319–4323. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Y.; Dong, Z.; Wang, X.; Xu, J. The Preparation of CH3NH3SnI3/SnO2/Pd/Au Gas Sensor Material for Detecting CO and the Function of Each Component. J. Mater. Sci. Mater. Electron. 2022, 33, 7463–7476. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, Y.; Wu, G.; Fei, L.; Zhang, S.; Hu, Y.; Yan, Z.; Wang, Y.; Gu, H.; Chen, W. Mechanism Study on Extraordinary Room-Temperature CO Sensing Capabilities of Pd-SnO2 Composite Nanoceramics. Sens. Actuators B Chem. 2019, 285, 49–55. [Google Scholar] [CrossRef]
- Kishnani, V.; Verma, G.; Pippara, R.K.; Yadav, A.; Chauhan, P.S.; Gupta, A. Highly Sensitive, Ambient Temperature CO Sensor Using Tin Oxide Based Composites. Sens. Actuators A Phys. 2021, 332, 113111. [Google Scholar] [CrossRef]
- Marikutsa, A.; Rumyantseva, M.; Gaskov, A. Specific Interaction of PdOx- and RuOy-Modified Tin Dioxide with CO and NH3 Gases: Kelvin Probe and DRIFT Studies. J. Phys. Chem. C 2015, 119, 24342–24350. [Google Scholar] [CrossRef]
- Li, L.; Wang, C.; Ying, Z.; Wu, W.; Hu, Y.; Yang, W.; Xuan, W.; Li, Y.; Wen, F. SnO2/Graphene Nanocomposite for Effective Detection of CO at Room Temperature. Chem. Phys. Lett. 2023, 830, 140803. [Google Scholar] [CrossRef]
- Pineda-Reyes, A.M.; Herrera-Rivera, M.R.; Rojas-Chávez, H.; Cruz-Martínez, H.; Medina, D.I. Recent Advances in ZnO-Based Carbon Monoxide Sensors: Role of Doping. Sensors 2021, 21, 4425. [Google Scholar] [CrossRef]
- Heo, Y.W.; Norton, D.P.; Tien, L.C.; Kwon, Y.; Kang, B.S.; Ren, F.; Pearton, S.J.; Laroche, J.R. ZnO Nanowire Growth and Devices. Mater. Sci. Eng. R Rep. 2004, 47, 1–47. [Google Scholar] [CrossRef]
- Schmidt-Mende, L.; Macmanus-Driscoll, J.L. ZnO-Nanostructures, Defects, and Devices. Mater. Today 2007, 10, 40–48. [Google Scholar] [CrossRef]
- Zhu, L.; Zeng, W. Room-Temperature Gas Sensing of ZnO-Based Gas Sensor: A Review. Sens. Actuators A Phys. 2017, 267, 242–261. [Google Scholar] [CrossRef]
- Huang, M.H.; Wu, Y.; Feick, H.; Tran, N.; Weber, E.; Yang, P. Catalytic Growth of Zinc Oxide Nanowires by Vapor Transport. Adv. Mater. 2001, 13, 113–116. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Z.L. One-Dimensional ZnO Nanostructures: Solution Growth and Functional Properties. Nano Res. 2011, 4, 1013–1098. [Google Scholar] [CrossRef]
- Wang, L.; Kang, Y.; Liu, X.; Zhang, S.; Huang, W.; Wang, S. ZnO Nanorod Gas Sensor for Ethanol Detection. Sens. Actuators B Chem. 2012, 162, 237–243. [Google Scholar] [CrossRef]
- Wang, J.X.; Sun, X.W.; Yang, Y.; Huang, H.; Lee, Y.C.; Tan, O.K.; Vayssieres, L. Hydrothermally Grown Oriented ZnO Nanorod Arrays for Gas Sensing Applications. Nanotechnology 2006, 17, 4995–4998. [Google Scholar] [CrossRef]
- Yuan, Z.; Jiaqiang, X.; Qun, X.; Hui, L.; Qingyi, P.; Pengcheng, X. Brush-like Hierarchical Zno Nanostructures: Synthesis, Photoluminescence and Gas Sensor Properties. J. Phys. Chem. C 2009, 113, 3430–3435. [Google Scholar] [CrossRef]
- Alenezi, M.R.; Henley, S.J.; Emerson, N.G.; Silva, S.R.P. From 1D and 2D ZnO Nanostructures to 3D Hierarchical Structures with Enhanced Gas Sensing Properties. Nanoscale 2014, 6, 235–247. [Google Scholar] [CrossRef]
- Pan, X.; Zhao, X. Ultra-High Sensitivity Zinc Oxide Nanocombs for on-Chip Room Temperature Carbon Monoxide Sensing. Sensors 2015, 15, 8919–8930. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi Bandari, A.; Nasirian, S. Carbon Monoxide Gas Sensing Features of Zinc Oxide Nanoneedles: Practical Selectivity and Long-Term Stability. J. Mater. Sci. Mater. Electron. 2019, 30, 10073–10081. [Google Scholar] [CrossRef]
- Joshi, R.K.; Hu, Q.; Alvi, F.; Joshi, N.; Kumar, A. Au Decorated Zinc Oxide Nanowires for CO Sensing. J. Phys. Chem. C 2009, 113, 16199–16202. [Google Scholar] [CrossRef]
- Arunkumar, S.; Hou, T.; Kim, Y.B.; Choi, B.; Park, S.H.; Jung, S.; Lee, D.W. Au Decorated ZnO Hierarchical Architectures: Facile Synthesis, Tunable Morphology and Enhanced CO Detection at Room Temperature. Sens. Actuators B Chem. 2017, 243, 990–1001. [Google Scholar] [CrossRef]
- Schipani, F.; Villegas, E.A.; Ramajo, L.A.; Parra, R. Zinc Oxide Thin Films for a Room Temperature Dual Carbon Dioxide and Carbon Monoxide Sensor. J. Mater. Sci. Mater. Electron. 2023, 34, 1092. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, D.; Pan, Q.; Wang, T.; Chen, F. Gas Sensing Performance of Carbon Monoxide Sensor Based on Rod-Shaped Tin Diselenide/MOFs Derived Zinc Oxide Polyhedron at Room Temperature. Sens. Actuators B Chem. 2022, 371, 132481. [Google Scholar] [CrossRef]
- Yu, M.R.; Wu, R.J.; Chavali, M. Effect of “Pt” Loading in ZnO-CuO Hetero-Junction Material Sensing Carbon Monoxide at Room Temperature. Sens. Actuators B Chem. 2011, 153, 321–328. [Google Scholar] [CrossRef]
- Cozzoli, P.D.; Kornowski, A.; Weller, H. Low-Temperature Synthesis of Soluble and Processable Organic-Capped Anatase TiO2 Nanorods. J. Am. Chem. Soc. 2003, 125, 14539–14548. [Google Scholar] [CrossRef]
- Galstyan, V.; Comini, E.; Faglia, G.; Sberveglieri, G. TiO2 Nanotubes: Recent Advances in Synthesis and Gas Sensing Properties. Sensors 2013, 13, 14813–14838. [Google Scholar] [CrossRef]
- Galstyan, V. Porous TiO2-Based Gas Sensors for Cyber Chemical Systems to Provide Security and Medical Diagnosis. Sensors 2017, 17, 2947. [Google Scholar] [CrossRef]
- Kumarage, G.W.C.; Hakkoum, H.; Comini, E. Recent Advancements in TiO2 Nanostructures: Sustainable Synthesis and Gas Sensing. Nanomaterials 2023, 13, 1424. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.J.; Chang, W.C.; Tsai, K.M.; Wu, J.G. The Novel CO Sensing Material CoOOH-WO3 with Au and SWCNT Performance Enhancement. Sens. Actuators B Chem. 2009, 138, 35–41. [Google Scholar] [CrossRef]
- Bittencourt, C.; Felten, A.; Espinosa, E.H.; Ionescu, R.; Llobet, E.; Correig, X.; Pireaux, J.J. WO3 Films Modified with Functionalised Multi-Wall Carbon Nanotubes: Morphological, Compositional and Gas Response Studies. Sens. Actuators B Chem. 2006, 115, 33–41. [Google Scholar] [CrossRef]
- Al-Kuhaili, M.F.; Durrani, S.M.A.; Bakhtiari, I.A. Carbon Monoxide Gas-Sensing Properties of CeO2-WO3 Thin Films. Mater. Sci. Technol. 2010, 26, 726–731. [Google Scholar] [CrossRef]
- Estananto; Septiani, N.L.W.; Iqbal, M.; Suyatman; Nuruddin, A.; Yuliarto, B. Nanocomposite of Graphene and WO3 Nanowires for Carbon Monoxide Sensors. Nanocomposites 2021, 7, 225–236. [Google Scholar] [CrossRef]
- Chen, K.W.; Liu, J.P.; Hsu, Y.S.; Liu, C.H.; Pai, Y.H.; Chen, C.H. Controlled Synthesis of Pt and Co3O4 Dual-Functionalized In2O3 Nanoassemblies for Room Temperature Detection of Carbon Monoxide. New J. Chem. 2018, 42, 16478–16482. [Google Scholar] [CrossRef]
- Geng, B.; Zhan, F.; Jiang, H.; Xing, Z.; Fang, C. Facile Production of Self-Assembly Hierarchical Dumbbell-like CoOOH Nanostructures and Their Room-Temperature CO-Gas-Sensing Properties. Cryst. Growth Des. 2008, 8, 3497–3500. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, D.; Gupta, A.K. Fe-Doped TiO2 Thin Films for CO Gas Sensing. J. Electron. Mater. 2015, 44, 152–157. [Google Scholar] [CrossRef]
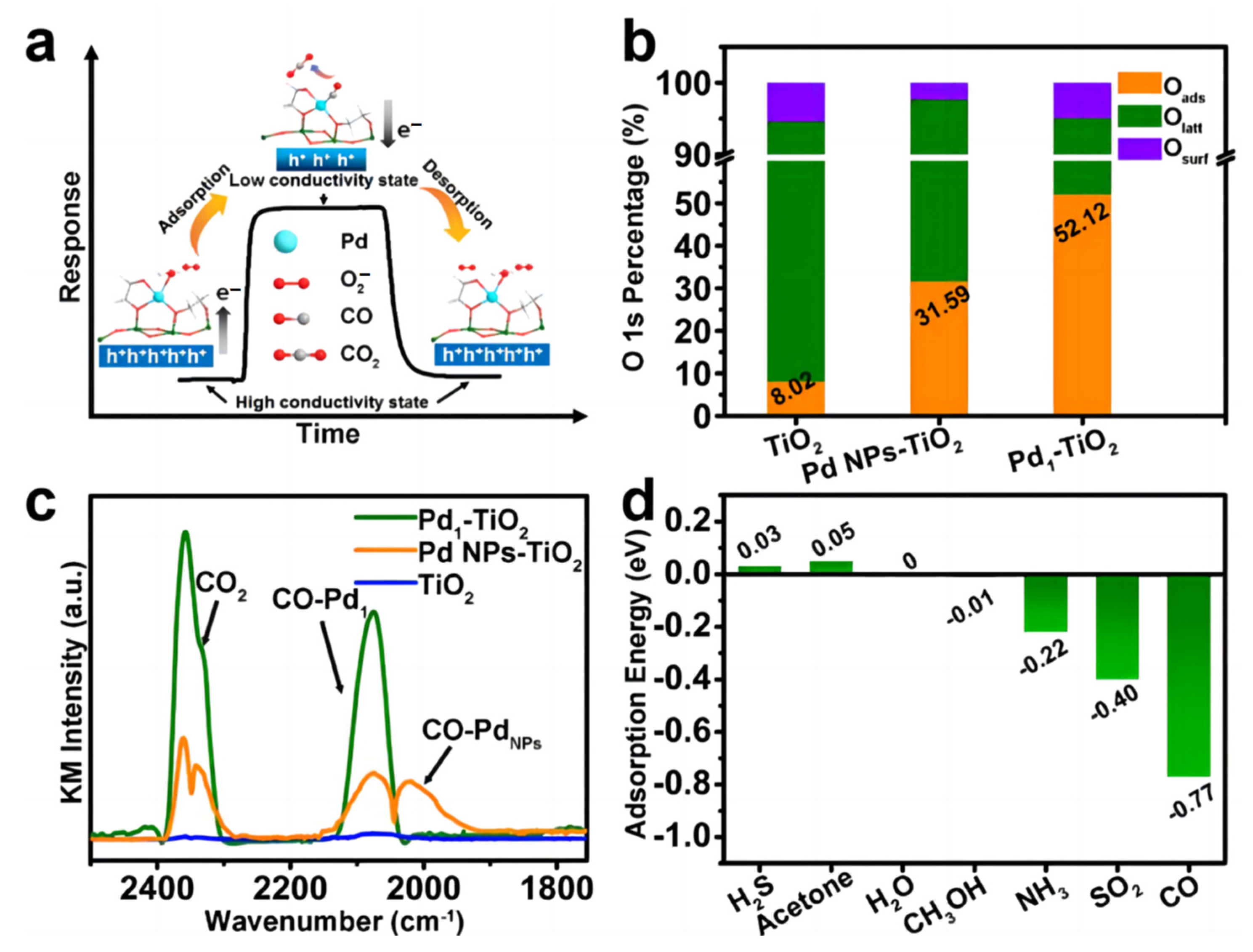
- Ye, X.L.; Lin, S.J.; Zhang, J.W.; Jiang, H.J.; Cao, L.A.; Wen, Y.Y.; Yao, M.S.; Li, W.H.; Wang, G.E.; Xu, G. Boosting Room Temperature Sensing Performances by Atomically Dispersed Pd Stabilized via Surface Coordination. ACS Sens. 2021, 6, 1103–1110. [Google Scholar] [CrossRef]
- Su, J.; Zou, X.X.; Zou, Y.C.; Li, G.D.; Wang, P.P.; Chen, J.S. Porous Titania with Heavily Self-Doped Ti3+ for Specific Sensing of CO at Room Temperature. Inorg. Chem. 2013, 52, 5924–5930. [Google Scholar] [CrossRef]
- Kumar, M.; Gupta, A.K.; Kumar, D. Mg-Doped TiO2 Thin Films Deposited by Low Cost Technique for CO Gas Monitoring. Ceram. Int. 2016, 42, 405–410. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, H.; Tao, T.; Xia, X.; Bao, Y.; Lourenço, M.; Homewood, K.; Huang, Z.; Gao, Y. A CuO/TiO2 Heterojunction Based CO Sensor with High Response and Selectivity. Energy Environ. Mater. 2023, 6, e12570. [Google Scholar] [CrossRef]
- Wu, Y.; Li, J.; Lv, M.; Zhang, X.; Gao, R.; Guo, C.; Cheng, X.; Zhou, X.; Xu, Y.; Gao, S.; et al. Selective Detection of Trace Carbon Monoxide at Room Temperature Based on CuO Nanosheets Exposed to (111) Crystal Facets. J. Hazard. Mater. 2023, 442, 130041. [Google Scholar] [CrossRef] [PubMed]
- Adeyemo, A.; Hunter, G.; Dutta, P.K. Interaction of CO with Hydrous Ruthenium Oxide and Development of a Chemoresistive Ambient CO Sensor. Sens. Actuators B Chem. 2011, 152, 307–315. [Google Scholar] [CrossRef]
- Li, H.; Wu, J.; Yin, Z.; Zhang, H. Preparation and Applications of Mechanically Exfoliated Single-Layer and Multilayer MoS2 and WSe2 Nanosheets. Acc. Chem. Res. 2014, 47, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Perkins, F.K.; Friedman, A.L.; Cobas, E.; Campbell, P.M.; Jernigan, G.G.; Jonker, B.T. Chemical Vapor Sensing with Monolayer MoS2. Nano Lett. 2013, 13, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Sadaf, S.; Zhang, H.; Akhtar, A. MoS 2–NiO Nanocomposite for H 2 S Sensing at Room Temperature. RSC Adv. 2023, 13, 28564–28575. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sanger, A.; Kang, S.B.; Chandra, R. Interface Engineering-Driven Room-Temperature Ultralow Gas Sensors with Elucidating Sensing Performance of Heterostructure Transition Metal Dichalcogenide Thin Films. ACS Sens. 2023, 8, 3824–3835. [Google Scholar] [CrossRef] [PubMed]
- Piosik, E.; Szary, M.J. Development of MoS2 Doping Strategy for Enhanced SO2 Detection at Room Temperature. Appl. Surf. Sci. 2023, 638, 158013. [Google Scholar] [CrossRef]
- Choi, W.; Choudhary, N.; Han, G.H.; Park, J.; Akinwande, D.; Lee, Y.H. Recent Development of Two-Dimensional Transition Metal Dichalcogenides and Their Applications. Mater. Today 2017, 20, 116–130. [Google Scholar] [CrossRef]
- Li, H.; Yin, Z.; He, Q.; Li, H.; Huang, X.; Lu, G.; Fam, D.W.H.; Tok, A.I.Y.; Zhang, Q.; Zhang, H. Fabrication of Single- and Multilayer MoS2 Film-Based Field-Effect Transistors for Sensing NO at Room Temperature. Small 2012, 8, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Sun, Y.; Jiang, C.; Yao, Y.; Dongyue, W.; Zhang, Y. Room-Temperature Highly Sensitive CO Gas Sensor Based on Ag-Loaded Zinc Oxide/Molybdenum Disulfide Ternary Nanocomposite and Its Sensing Properties. Sens. Actuators B Chem. 2017, 253, 1120–1128. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, J.; Cao, Y. Cobalt-Doped Indium Oxide/Molybdenum Disulfide Ternary Nanocomposite toward Carbon Monoxide Gas Sensing. J. Alloys Compd. 2019, 777, 443–453. [Google Scholar] [CrossRef]
- Li, Y.; Song, X.; Li, L.; Wu, W.; Tao, K.; Ying, Z.; Hu, Y.; Zhou, Y.; Zhang, R.; Wang, G.; et al. Low Concentration CO Gas Sensor Constructed from MoS2 Nanosheets Dispersed SnO2 Nanoparticles at Room Temperature under UV Light. Ceram. Int. 2023, 49, 10249–10254. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, D.; Wang, D. Carbon Monoxide Gas Sensing Properties of Metal-Organic Frameworks-Derived Tin Dioxide Nanoparticles/Molybdenum Diselenide Nanoflowers. Sens. Actuators B Chem. 2020, 304, 127369. [Google Scholar] [CrossRef]
- Staerz, A.; Weimar, U.; Barsan, N. Current State of Knowledge on the Metal Oxide Based Gas Sensing Mechanism. Sens. Actuators B Chem. 2022, 358, 131531. [Google Scholar] [CrossRef]
- Degler, D.; Weimar, U.; Barsan, N. Current Understanding of the Fundamental Mechanisms of Doped and Loaded Semiconducting Metal-Oxide-Based Gas Sensing Materials. ACS Sens. 2019, 4, 2228–2249. [Google Scholar] [CrossRef] [PubMed]
- Boehme, I.; Weimar, U.; Barsan, N. Unraveling the Surface Chemistry of CO Sensing with In2O3 Based Gas Sensors. Sens. Actuators B Chem. 2021, 326, 129004. [Google Scholar] [CrossRef]
- Fu, H.; Hou, C.; Gu, F.; Han, D.; Wang, Z. Facile Preparation of Rod-like Au/In2O3 Nanocomposites Exhibiting High Response to CO at Room Temperature. Sens. Actuators B Chem. 2017, 243, 516–524. [Google Scholar] [CrossRef]
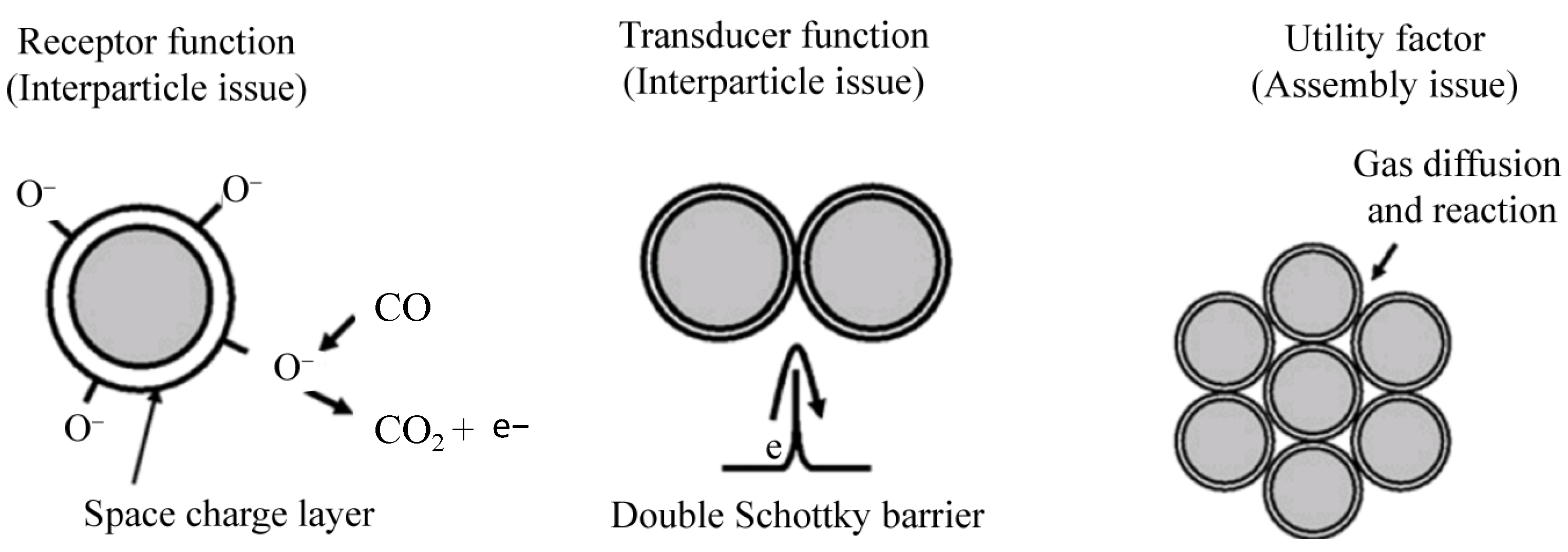
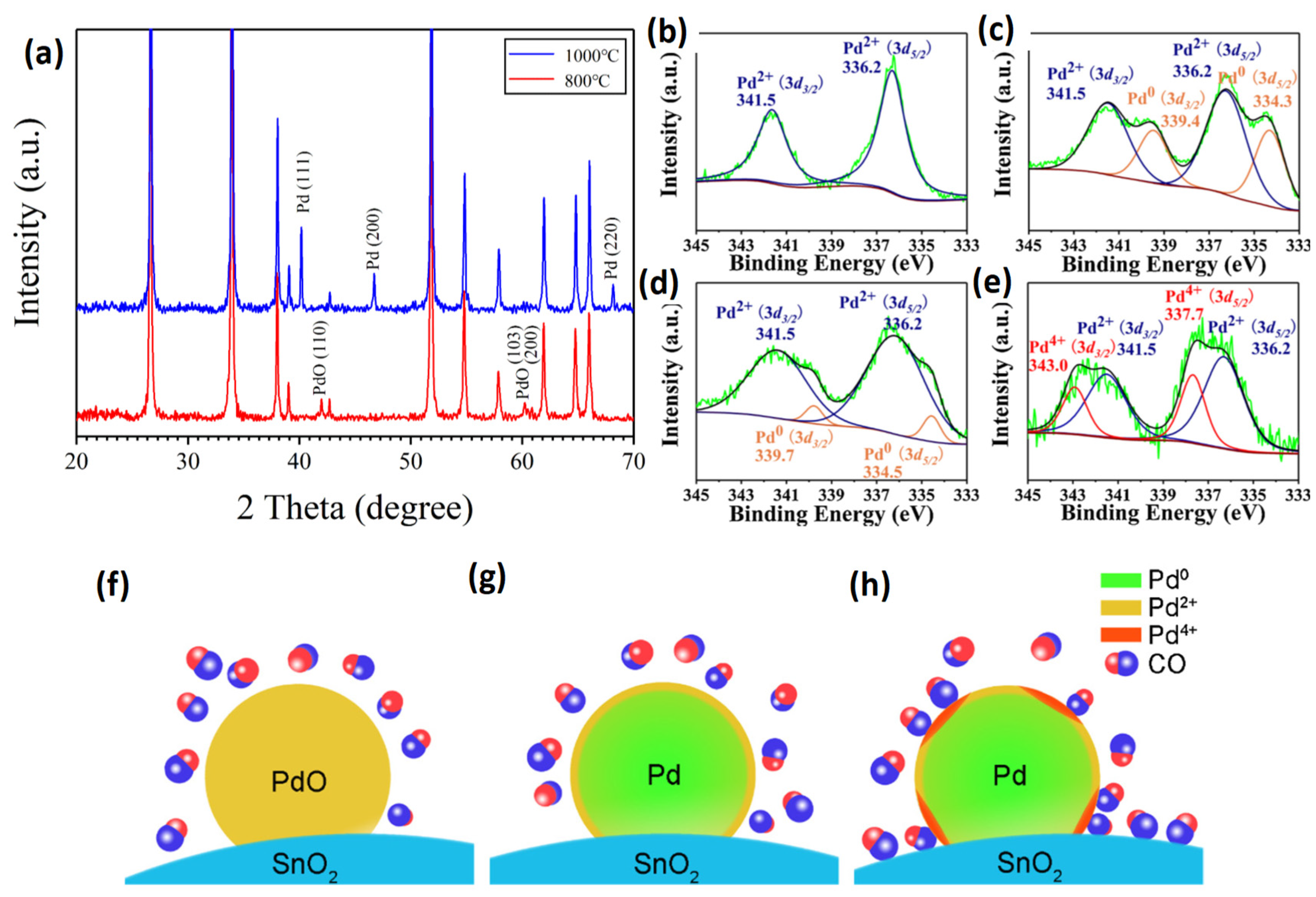
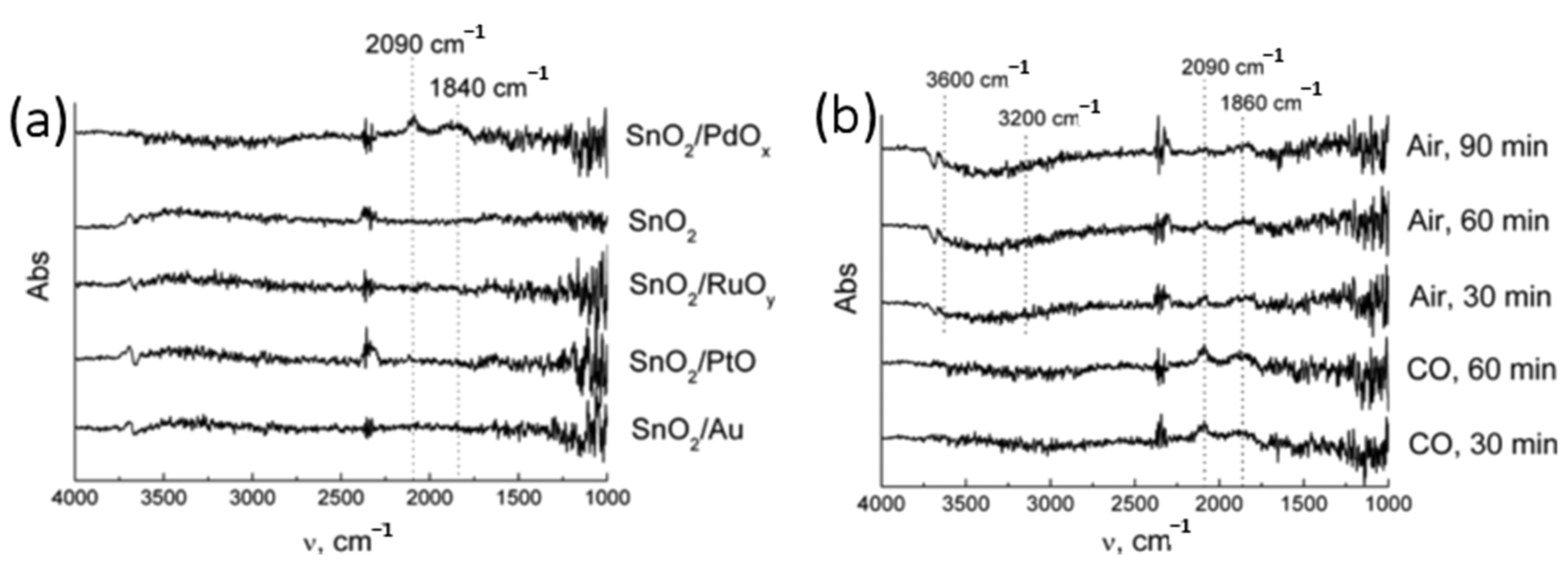

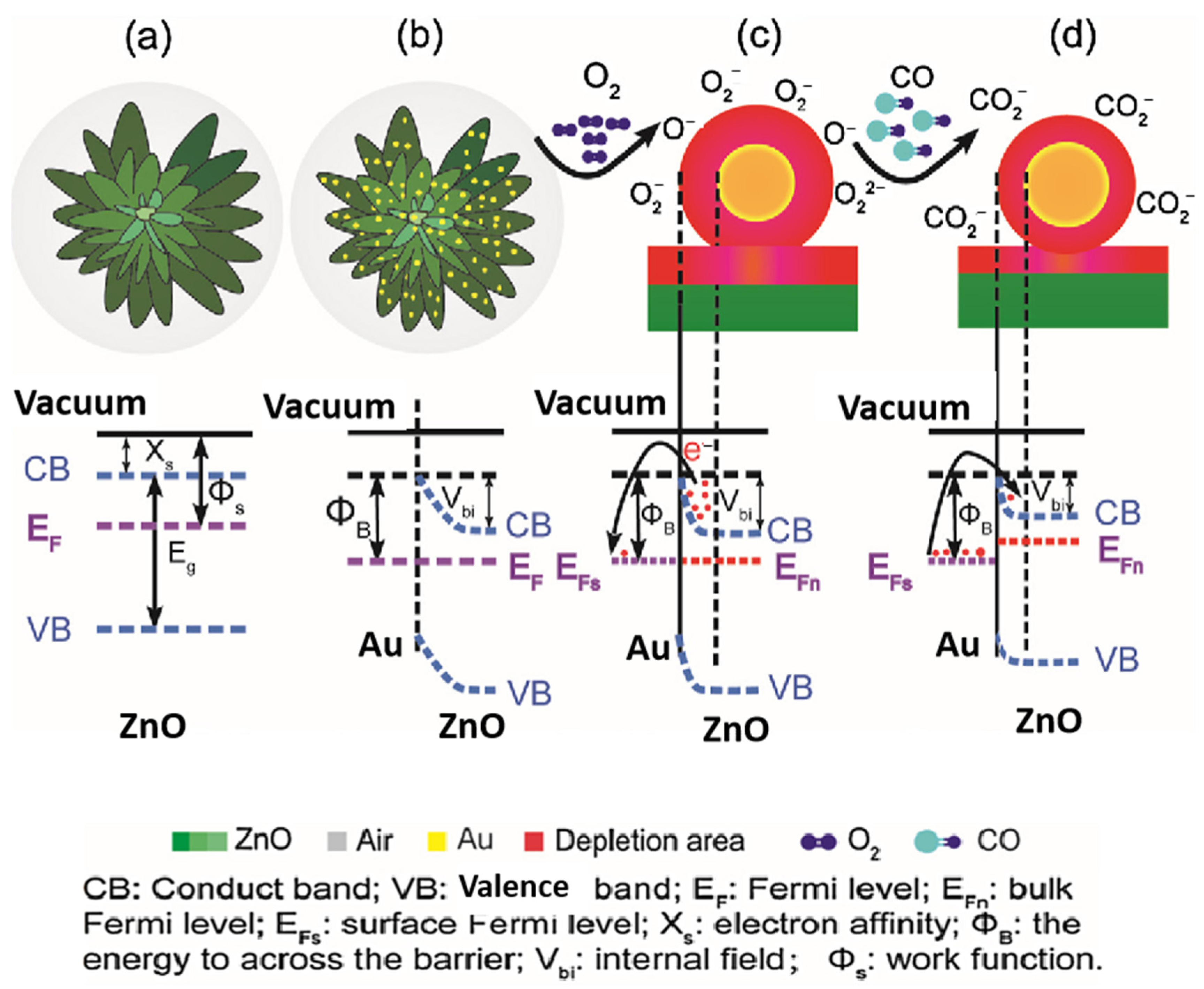
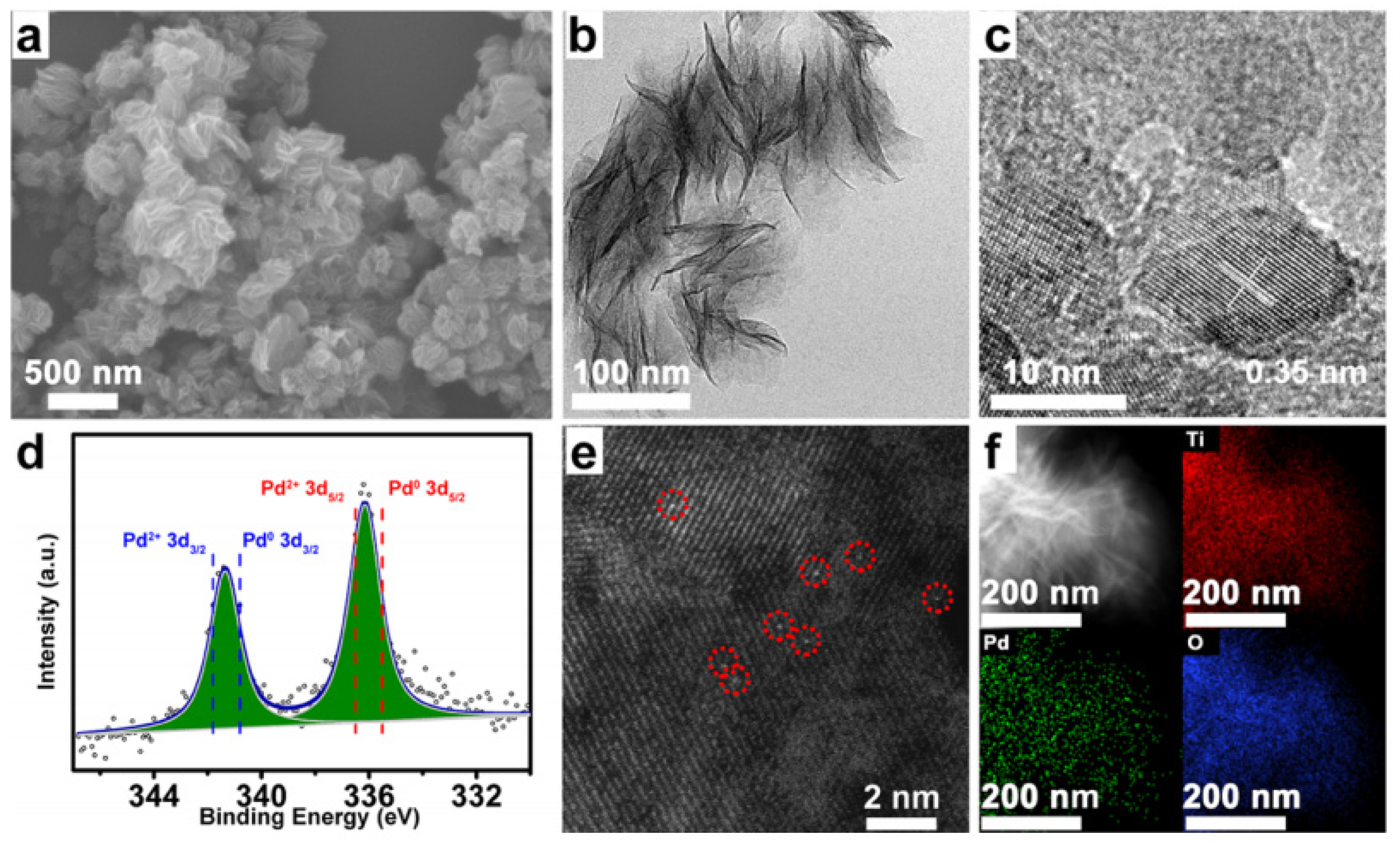

| Possible Reaction | Temperature Range | |
|---|---|---|
| 2CO + O2− (ads) → 2CO2 + e− | T < 150 °C | (1) |
| CO + O− (ads) → CO2 + e− | 150 °C ≤ T ≤ 300 °C | (2) |
| CO + O2− (ads) → CO2 + 2e− | T > 300 °C | (3) |
| Sensing Material | CO Concentration (ppm) | Response | Response Time (s) | Recovery Time (s) | Reference |
|---|---|---|---|---|---|
| ZnO nanocomb | 250 | 7.22 | 200 | 50 | [61] |
| ZnO thin films | 50 | 1.10 | ~180 | - | [65] |
| ZnO nanoneedles | 375 | 1.51 | 186 | 38 | [62] |
| Au-ZnO nanowires | 100 | ~5 | - | - | [63] |
| Au-ZnO nanostars | 500 | 55.3 | 41 | 40 | [64] |
| SnSe2-ZnO polyhedron | 200 | 1.17 | 19 | 13 | [66] |
| Pt-ZnO-CuO | 1000 | 2.64 | 81 | 81 | [67] |
| Sensing Material | CO Concentration (ppm) | Response | Response Time (s) | Recovery Time (s) | Reference |
|---|---|---|---|---|---|
| Pt-Co3O4-In2O3 | 5 | 4 | NA | NA | [76] |
| Dumbbell CoOOH nanostructures | 50 | NA | ~20 | ~18 | [77] |
| Fe-TiO2 | 100 | ~4.8 | 43 | 25 | [78] |
| Atomically dispersed Pd-TiO2 | 100 | 125.49 | 28 | 70 | [79] |
| Self-doped Ti3+-porous TiO2 | 5000 | ~2 | ~10 | ~30 | [80] |
| Mg-TiO2 thin films | 120 | 8.40(CO+Ar) | 62 | 30 | [81] |
| CuO-TiO2 heterojunction | 1 | ~2.2 | NA | NA | [82] |
| CuO (111) nanosheets | 100 | 39.6 | 100 | 72.4 | [83] |
| RuOx (OH)y | 250 | ~2 | NA | NA | [84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Jiao, M. A Mini-Review on Metal Oxide Semiconductor Gas Sensors for Carbon Monoxide Detection at Room Temperature. Chemosensors 2024, 12, 55. https://doi.org/10.3390/chemosensors12040055
He Y, Jiao M. A Mini-Review on Metal Oxide Semiconductor Gas Sensors for Carbon Monoxide Detection at Room Temperature. Chemosensors. 2024; 12(4):55. https://doi.org/10.3390/chemosensors12040055
Chicago/Turabian StyleHe, Yaoyi, and Mingzhi Jiao. 2024. "A Mini-Review on Metal Oxide Semiconductor Gas Sensors for Carbon Monoxide Detection at Room Temperature" Chemosensors 12, no. 4: 55. https://doi.org/10.3390/chemosensors12040055
APA StyleHe, Y., & Jiao, M. (2024). A Mini-Review on Metal Oxide Semiconductor Gas Sensors for Carbon Monoxide Detection at Room Temperature. Chemosensors, 12(4), 55. https://doi.org/10.3390/chemosensors12040055







