SERS Substrate Based on Ag Nanoparticles@Layered Double Hydroxide@graphene Oxide and Au@Ag Core–Shell Nanoparticles for Detection of Two Taste and Odor Compounds
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Apparatus and Equipment
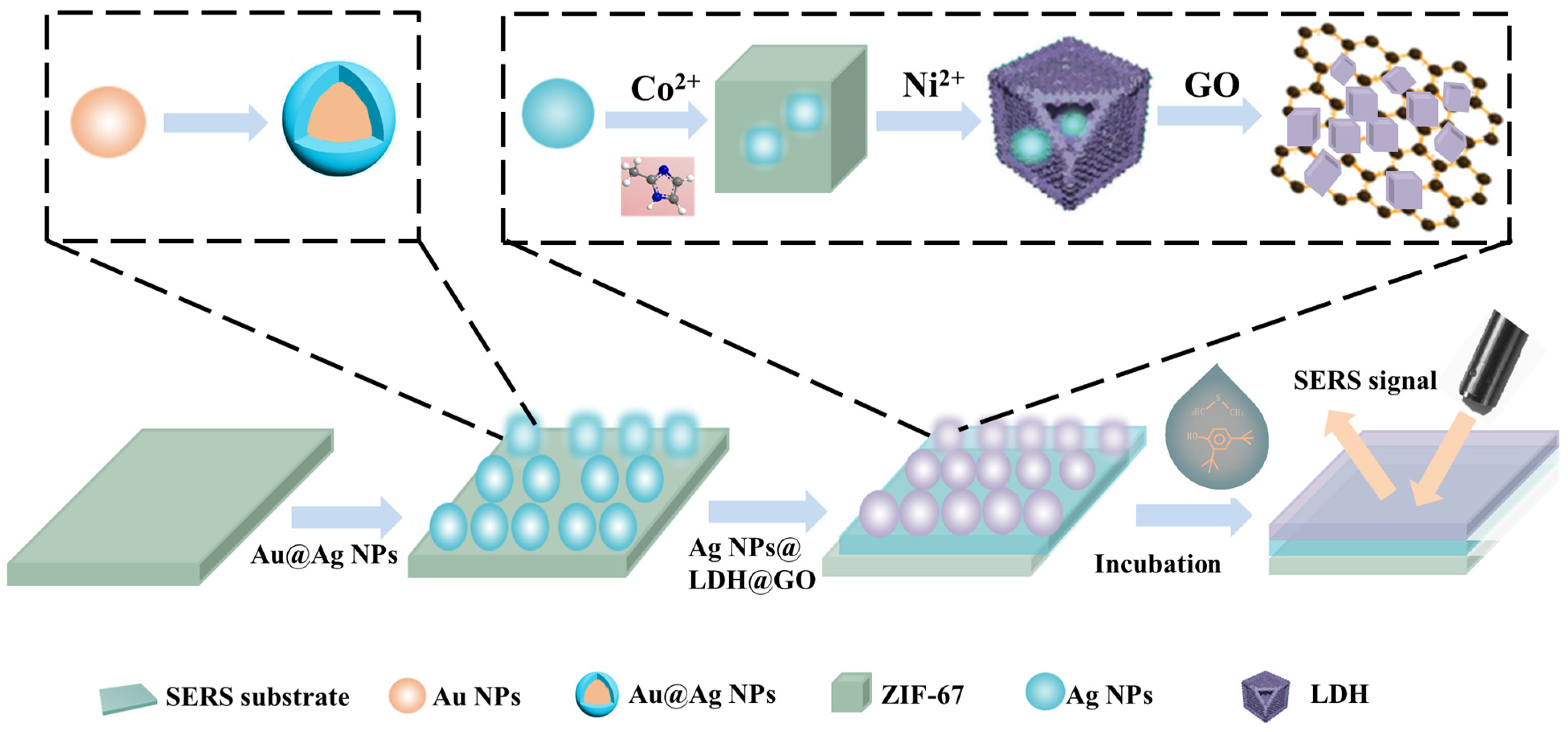
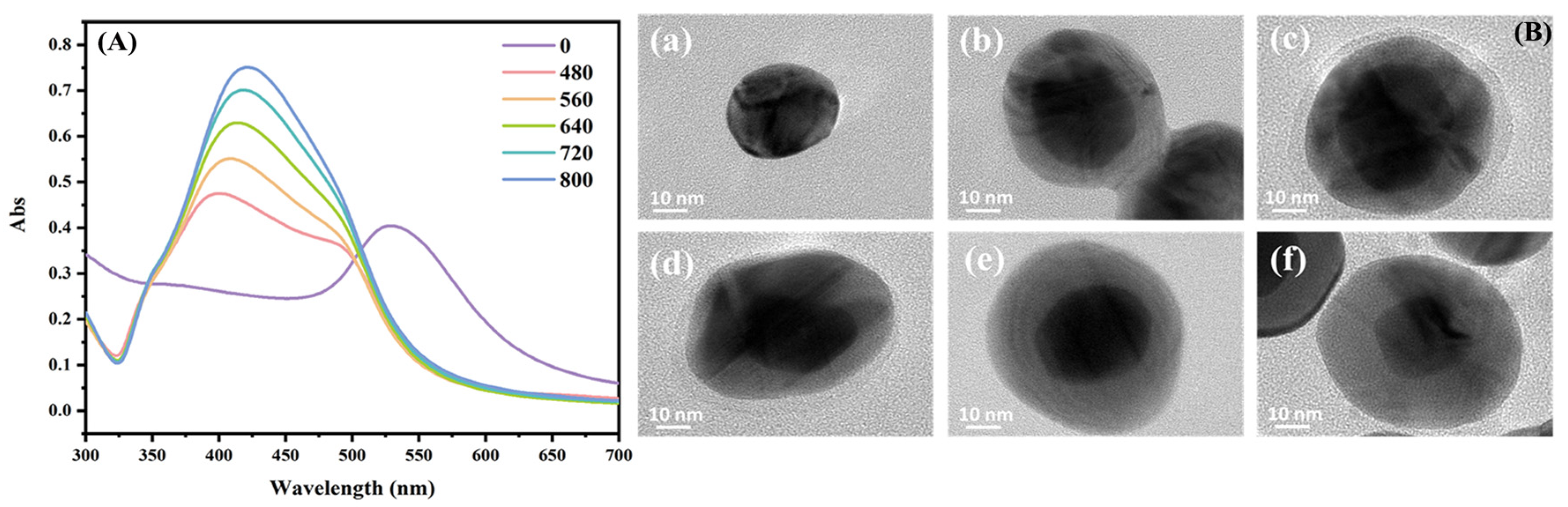
2.3. Preparation of Core–Shell Au@Ag NPs
2.4. Synthesis of Ag NPs
2.5. Fabrication of Ag NPs@LDH@GO
2.6. Preparation of SERS Substrate and the Process of SERS Detection
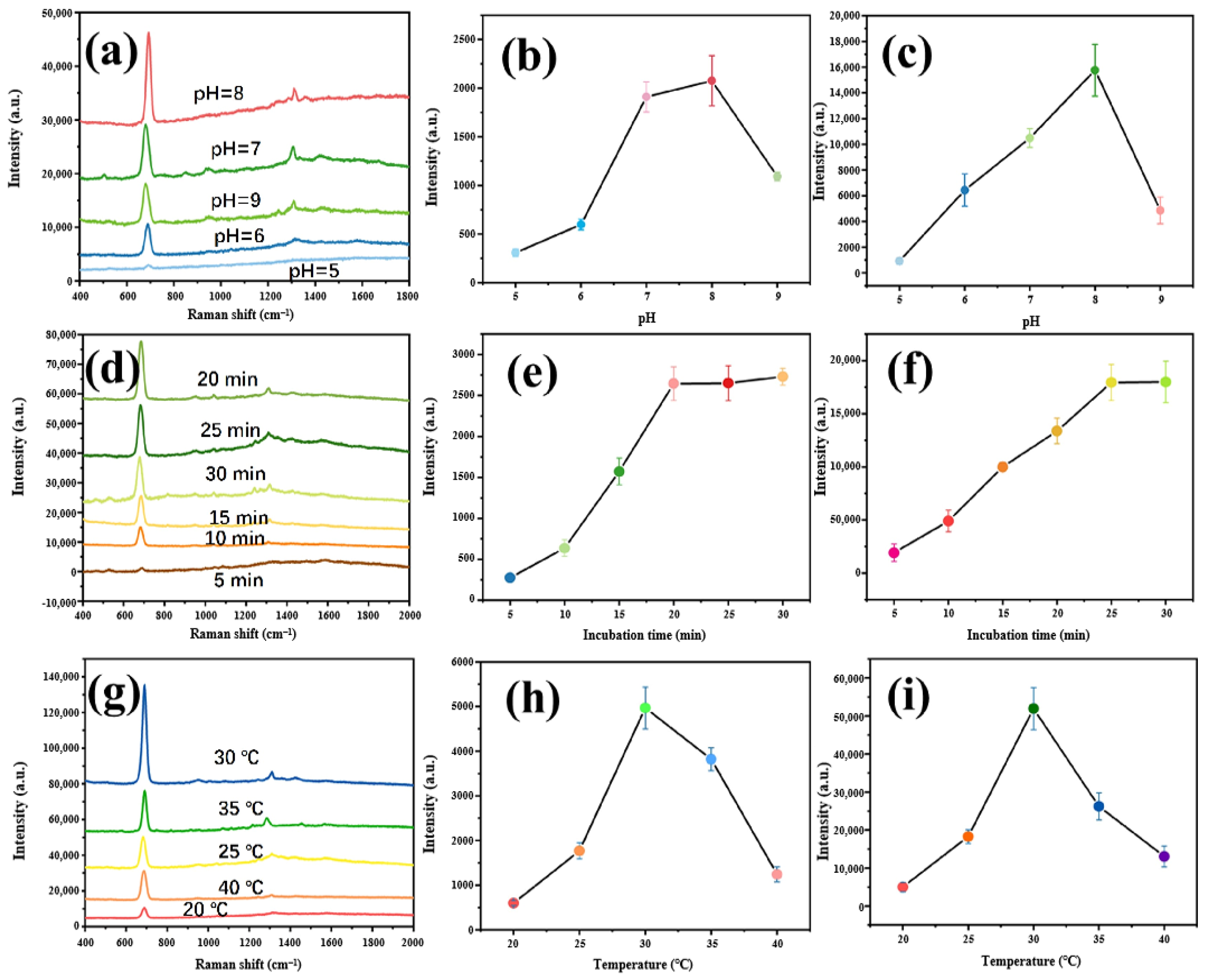
2.7. Optimization of the SERS Detection Conditions
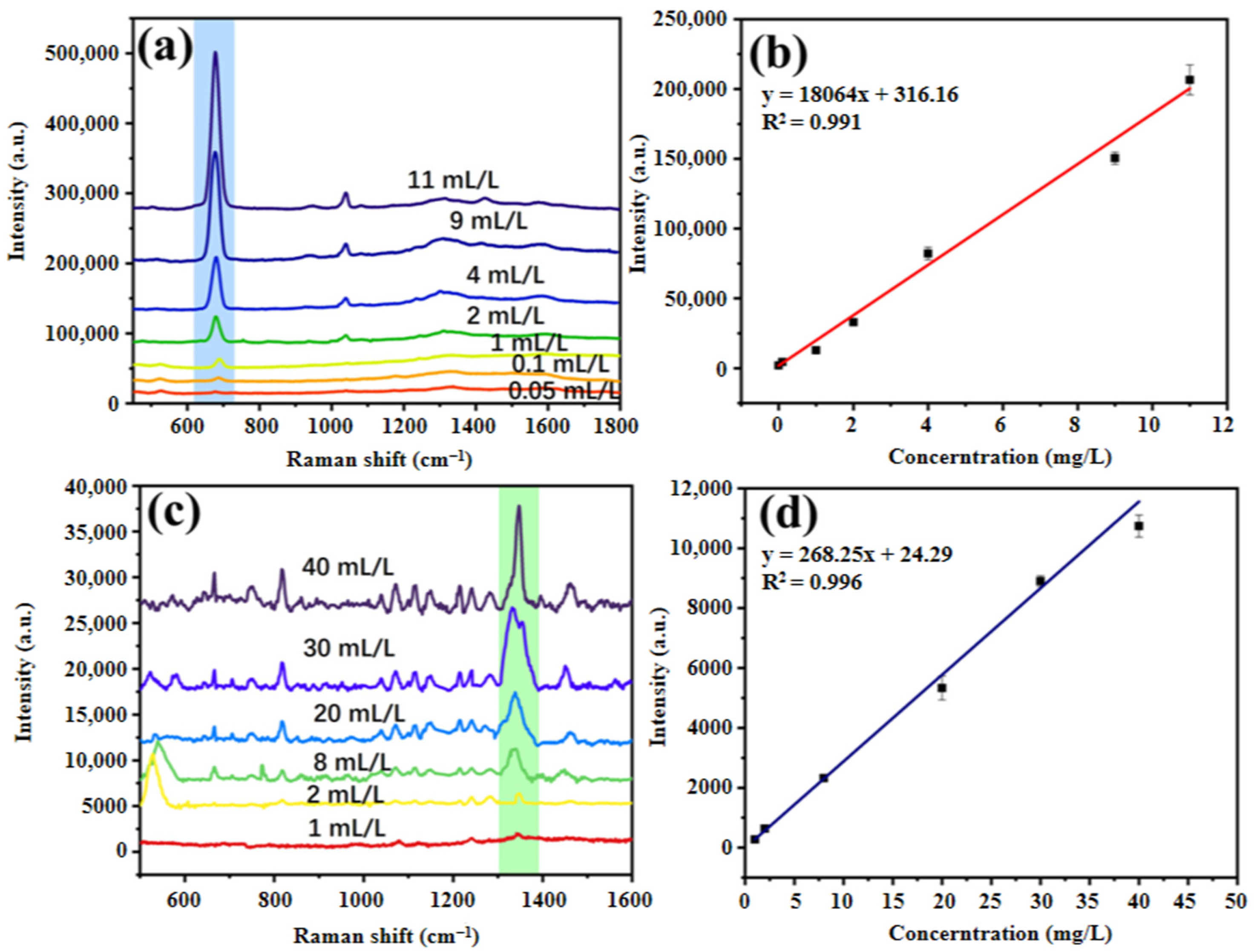
2.8. Determination of the Standard Solutions of MS and DTBP
2.9. Determination of MS and DTBP in Actual Water Samples
3. Results and Discussion
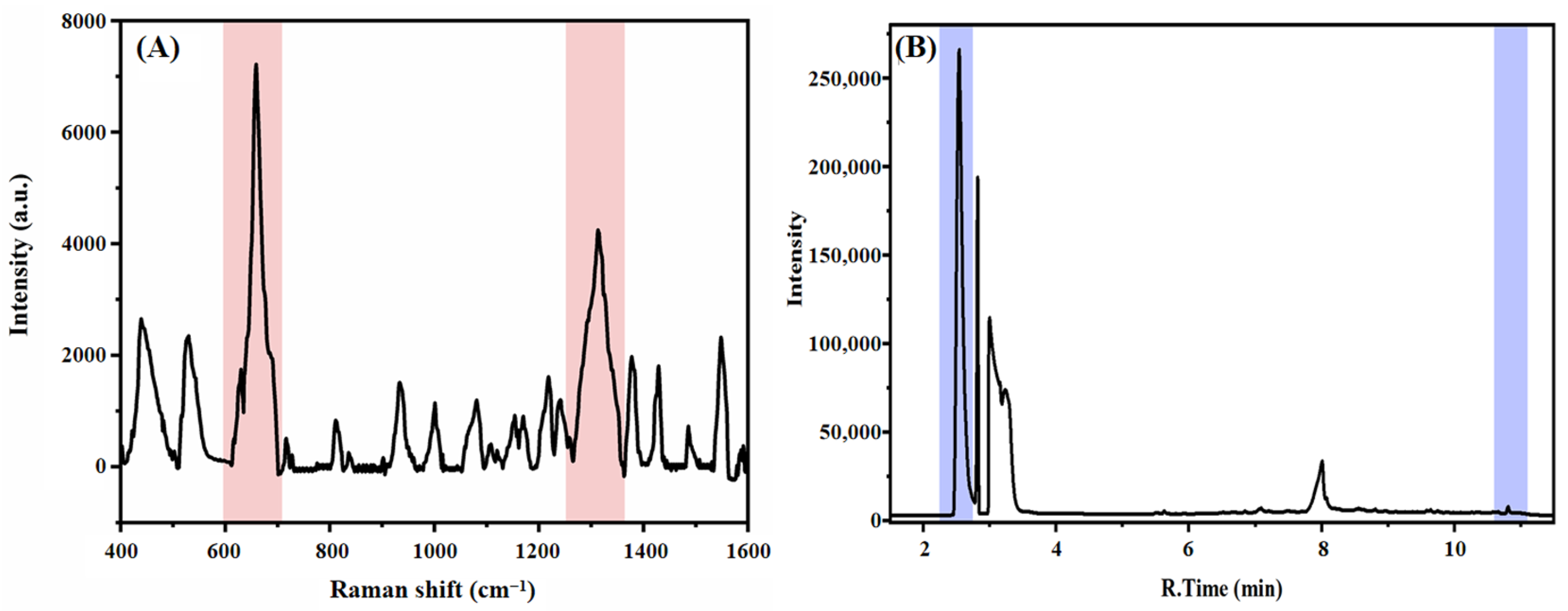
3.1. Characterization of Materials
3.2. Comparison of SERS Performance of Au@Ag NPs with Different Sizes
3.3. Influence of Incubation Conditions on SERS Effect of the Substrate
3.4. Drawing of Standard Curve for Analytes
3.5. Simultaneous Detection of Two Analytes in Actual Water Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jahnichen, S.; Jaschke, K.; Wieland, F.; Packroff, G.; Benndorf, J. Spatio-temporal distribution of cell-bound and dissolved geosmin in Wahnbach Reservoir: Causes and potential odour nuisances in raw water. Water Res. 2011, 45, 4973–4982. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Yu, J.; Zhang, J.; Chen, H.; An, W.; Vogt, R.D.; Andersen, T.; Jia, D.; Wang, J.; Yang, M. MIB-producing cyanobacteria (Planktothrix sp.) in a drinking water reservoir: Distribution and odor producing potential. Water Res. 2015, 68, 444–453. [Google Scholar]
- Kim, T.; Kim, T.K.; Zoh, K.D. Degradation kinetics and pathways of β-cyclocitral and β-ionone during UV photolysis and UV/chlorination reactions. J. Environ. Manag. 2019, 239, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Li, X.; Yu, J.W.; Zhang, H.; Zhang, Y.; Yang, M.; Lu, N.; Zhang, D. Comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry for the screening of potent swampy/septic odor-causing compounds in two drinking water sources in China. Anal. Methods 2015, 7, 2458–2468. [Google Scholar] [CrossRef]
- Yu, C.; Shi, C.; Ji, M.; Xu, X.; Zhang, Z.; Ma, J.; Wang, G. Taste and odor compounds associated with aquatic plants in Taihu Lake: Distribution and producing potential. Environ. Sci. Pollut. Res. 2019, 26, 34510–34520. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Xu, X.; Shi, C.; Liu, X.; Wang, G. Response of Taste and Odor Compounds to Elevated Cyanobacteria Biomass and Temperature. Bull. Environ. Contam. Toxicol. 2018, 101, 272–278. [Google Scholar]
- Li, S.; Xie, S.; Wei, Q.; Zeng, T.; Rong, L. Phenolic Wastewater Treatment via Catalytic Gasification. Pol. J. Environ. Stud. 2015, 24, 1147–1151. [Google Scholar] [CrossRef]
- Quintana, J.; Hernandez, A.; Ventura, F.; Devesa, R.; Boleda, M.R. Identification of 3-(trifluoromethyl)phenol as the malodorous compound in a pollution incident in the water supply in Catalonia (NE Spain). Environ. Sci. Pollut. Res. 2019, 26, 16076–16084. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, P.; Shu, J.; Yang, B.; Huang, J. Rapid detection of taste and odor compounds in water using the newly invented chemi-ionization technique coupled with time-of-flight mass spectrometry. Anal. Chim. Acta 2018, 1035, 119–128. [Google Scholar] [CrossRef]
- Ioime, P.; Piva, E.; Pozzebon, M.; Pascali, J.P. Automated sample preparation and analysis by gas chromatography tandem mass spectrometry (GC-MS/MS) for the determination of 3-and 2-monochloropropanediol (MCPD) esters and glycidol esters in edible oils. J. Chromatogr. A 2021, 1650, 462253. [Google Scholar] [CrossRef]
- Xu, D.; Muhammad, M.; Chu, L.; Sun, Q.; Shen, C.; Huang, Q. SERS Approach to Probe the Adsorption Process of Trace Volatile Benzaldehyde on Layered Double Hydroxide Material. Anal. Chem. 2021, 93, 8228–8237. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Yang, T.X.; He, L.L. Review of surface enhanced Raman spectroscopic (SERS) detection of synthetic chemical pesticides. Trends Anal. Chem. 2016, 85, 73–82. [Google Scholar] [CrossRef]
- Qu, L.L.; Liu, Y.Y.; Liu, M.K.; Yang, G.H.; Li, D.W.; Li, H.T. Highly Reproducible Ag NPS/CNT-Intercalated GO Membranes for Enrichment and SERS Detection of Antibiotics. ACS Appl. Mater. Interfaces 2016, 8, 28180–28186. [Google Scholar] [CrossRef] [PubMed]
- Gjergjizi, B.; Cogun, F.; Yildirim, E.; Eryilmaz, M.; Selbes, Y.; Saglam, N.; Tamer, U. SERS-based ultrafast and sensitive detection of luteinizing hormone in human serum using a passive microchip. Sens. Actuators B Chem. 2018, 269, 314–321. [Google Scholar] [CrossRef]
- Muhammad, M.; Shao, C.-S.; Bashir, M.A.; Yu, X.; Wu, Y.; Zhan, J.; Zhang, L.; Huang, Q. Application of Aptamer-SERS Nanotags for Unveiling the PD-L1 Immunomarker Progression Correlated to the Cell Metabolic Bioprocess. Anal. Chem. 2024, 6, 6236–6244. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Sun, D.; Pu, H.; Wei, Q. Shell thickness-dependent Au@Ag nanoparticals aggregates for high-performance SERS applications. Talanta 2019, 195, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wan, J.; Pang, G. Hollow Metal-Organic-Framework Micro/ Nanostructures and their Derivatives: Emerging Multifunctional Materials. Adv. Mater. 2019, 31, 1803291. [Google Scholar] [CrossRef]
- He, P.; Yu, X.; Luo, X. Carbon-Incorporated Nickel-Cobalt Mixed Metal Phosphide Nanoboxes with Enhanced Electrocatalytic Activity for Oxygen Evolution. Angew. Chem. Int. Ed. 2017, 56, 3897–3900. [Google Scholar] [CrossRef]
- Sharifi-Bonab, M.; Aber, S.; Salari, D.; Khodam, A. Synthesis of CoZnAl-layered double hydroxide/graphene oxide nanocomposite for the removal of methylene blue: Kinetic, thermodynamic, and isotherm studies. Environ. Prog. Sustain. 2019, 39, e13316. [Google Scholar] [CrossRef]
- Chen, Z.; Cheng, F.; Nan, D.; Yang, Y.; Zhong, L.; Ma, Z.; Hao, L.; Wang, Q. Plasmonic nanorod arrays of a two-segment dimer and a coaxial cable with 1 nm gap for large field confinement and enhancement. Nanoscale 2015, 7, 2862–2868. [Google Scholar]
- Thomsen, V.; Schatzlein, D.; Mercuro, D. Limits of detection in spectroscopy. Spectroscopy 2003, 18, 112–118. [Google Scholar]
- Jena, T.; Choudhary, G.; Hossain, M.T.; Nath, U.; Sarma, M.; Giri, P.K. Salt-Catalyzed Directed Growth of Bilayer Palladium Diselenide (PdSe2) Dendrites and Pd Nanoparticle-Decorated PdSe2-Pd2Se3 Junction Exhibiting Very High Surface Enhanced Raman Scattering Sensitivity. Chem. Mater. 2024, 36, 5922–5934. [Google Scholar] [CrossRef]
- Kumar, K.M.A.; Kumar, E.A.; Wang, T.-J.; Kokulnathan, T.; Chang, Y.-H. Ultrasensitive and Reusable SERS Substrates Based on Ag-Photodecorated Mn2O3 Microspheres for Nitrofurazone Detection. ACS Sustain. Chem. Eng. 2023, 11, 15808–15817. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lao, Z.; Zhong, M.; Liang, Y.; Tan, J.; Liang, X.; Xie, W.; Liang, Y.; Wang, J. SERS Substrate Based on Ag Nanoparticles@Layered Double Hydroxide@graphene Oxide and Au@Ag Core–Shell Nanoparticles for Detection of Two Taste and Odor Compounds. Chemosensors 2024, 12, 137. https://doi.org/10.3390/chemosensors12070137
Lao Z, Zhong M, Liang Y, Tan J, Liang X, Xie W, Liang Y, Wang J. SERS Substrate Based on Ag Nanoparticles@Layered Double Hydroxide@graphene Oxide and Au@Ag Core–Shell Nanoparticles for Detection of Two Taste and Odor Compounds. Chemosensors. 2024; 12(7):137. https://doi.org/10.3390/chemosensors12070137
Chicago/Turabian StyleLao, Zhixiong, Mingmin Zhong, Yin Liang, Jianrong Tan, Xiaoyan Liang, Wucheng Xie, Yong Liang, and Jun Wang. 2024. "SERS Substrate Based on Ag Nanoparticles@Layered Double Hydroxide@graphene Oxide and Au@Ag Core–Shell Nanoparticles for Detection of Two Taste and Odor Compounds" Chemosensors 12, no. 7: 137. https://doi.org/10.3390/chemosensors12070137
APA StyleLao, Z., Zhong, M., Liang, Y., Tan, J., Liang, X., Xie, W., Liang, Y., & Wang, J. (2024). SERS Substrate Based on Ag Nanoparticles@Layered Double Hydroxide@graphene Oxide and Au@Ag Core–Shell Nanoparticles for Detection of Two Taste and Odor Compounds. Chemosensors, 12(7), 137. https://doi.org/10.3390/chemosensors12070137




