Plasmonic Optical Fiber Sensors and Molecularly Imprinted Polymers for Glyphosate Detection at an Ultra-Wide Range
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. MIP and NIP Preparation
2.3. Measuring Protocol
3. POF-Based GLY Sensor Chips
3.1. SPR–POF Probe Covered by MIP Layer
3.2. Experimental Setup
4. Results and Discussion
4.1. MIP Layer Characterization
4.2. Experimental Results of the SPR–POF–MIP Sensor
4.3. Selectivity Analysis
4.3.1. Test Based on SPR–POF–MIP Sensor
4.3.2. Test Based on SPR–POF–NIP Sensor
4.4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woodburn, A.T. Glyphosate: Production, pricing and use worldwide. Pest Manag. Sci. Former. Pestic. Sci. 2000, 56, 309–312. [Google Scholar] [CrossRef]
- Soares, D.; Silva, L.; Duarte, S.; Pena, A.; Pereira, A. Glyphosate use, toxicity and occurrence in food. Foods 2021, 10, 2785. [Google Scholar] [CrossRef] [PubMed]
- Bøhn, T.; Cuhra, M.; Traavik, T.; Sanden, M.; Fagan, J.; Primicerio, R. Compositional differences in soybeans on the market: Glyphosate accumulates in Roundup Ready GM soybeans. Food Chem. 2014, 153, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Kolakowski, B.M.; Miller, L.; Murray, A.; Leclair, A.; Bietlot, H.; van de Riet, J.M. Analysis of glyphosate residues in foods from the Canadian retail markets between 2015 and 2017. J. Agric. Food Chem. 2020, 68, 5201–5211. [Google Scholar] [CrossRef] [PubMed]
- Zoller, O.; Rhyn, P.; Rupp, H.; Zarn, J.A.; Geiser, C. Glyphosate residues in Swiss market foods: Monitoring and risk evaluation. Food Addit. Contam. Part B 2018, 11, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Ledoux, M.L.; Hettiarachchy, N.; Yu, X.; Howard, L.; Lee, S.O. Penetration of glyphosate into the food supply and the incidental impact on the honey supply and bees. Food Control 2020, 109, 106859. [Google Scholar] [CrossRef]
- Cook, K.; Fund, C.E. Glyphosate in Beer and Wine; California Public Interest Research Group (CALPIRG) Education Fund: Sacramento, CA, USA, 2019. [Google Scholar]
- Wang, Y.; Gao, W.; Li, Y.; Xiao, Y.; Song, W.; Yao, T.; Hou, R. Establishment of a HPLC–MS/MS detection method for glyphosate, glufosinate-ammonium, and aminomethyl phosphoric acid in tea and its use for risk exposure assessment. J. Agric. Food Chem. 2021, 69, 7969–7978. [Google Scholar] [CrossRef] [PubMed]
- Bandana, B.; Sharma, N.; Joshi, R.; Gulati, A.; Sondhia, S. Dissipation kinetics of glyphosate in tea and tea-field under northwestern mid-hill conditions of India. J. Pestic. Sci. 2015, 40, 82–86. [Google Scholar] [CrossRef]
- Duke, S.O. Glyphosate: Environmental fate and impact. Weed Sci. 2020, 68, 201–207. [Google Scholar] [CrossRef]
- Annett, R.; Habibi, H.R.; Hontela, A. Impact of glyphosate and glyphosate-based herbicides on the freshwater environment. J. Appl. Toxicol. 2014, 34, 458–479. [Google Scholar] [CrossRef]
- Sprankle, P.; Meggitt, W.F.; Penner, D. Absorption, action, and translocation of glyphosate. Weed Sci. 1975, 23, 235–240. [Google Scholar] [CrossRef]
- Samsel, A.; Seneff, S. Glyphosate’s suppression of cytochrome P450 enzymes and amino acid biosynthesis by the gut microbiome: Pathways to modern diseases. Entropy 2013, 15, 1416–1463. [Google Scholar] [CrossRef]
- Benachour, N.; Séralini, G.E. Glyphosate formulations induce apoptosis and necrosis in human umbilical, embryonic, and placental cells. Chem. Res. Toxicol. 2009, 22, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Thongprakaisang, S.; Thiantanawat, A.; Rangkadilok, N.; Suriyo, T.; Satayavivad, J. Glyphosate induces human breast cancer cells growth via estrogen receptors. Food Chem. Toxicol. 2013, 59, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Guyton, K.Z.; Loomis, D.; Grosse, Y.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Straif, K. Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. Lancet Oncol. 2015, 16, 490–491. [Google Scholar] [CrossRef] [PubMed]
- Tarone, R.E. On the International Agency for Research on Cancer classification of glyphosate as a probable human carcinogen. Eur. J. Cancer Prev. 2018, 27, 82–87. [Google Scholar] [CrossRef]
- Rana, I.; Nguyen, P.K.; Rigutto, G.; Louie, A.; Lee, J.; Smith, M.T.; Zhang, L. Mapping the key characteristics of carcinogens for glyphosate and its formulations: A systematic review. Chemosphere 2023, 339, 139572. [Google Scholar] [CrossRef] [PubMed]
- Alcántara-de la Cruz, R.; Cruz-Hipolito, H.E.; Domínguez-Valenzuela, J.A.; De Prado, R. Glyphosate ban in Mexico: Potential impacts on agriculture and weed management. Pest Manag. Sci. 2021, 77, 3820–3831. [Google Scholar] [CrossRef]
- European Commission Brussels. Proposal for a Directive of the European Parliament and of the Council amending Directive 2000/60/EC Establishing a Framework for Community Action in the Field of Water Policy, Directive 2006/118/EC on the Protection of Groundwater against Pollution and Deterioration and Directive 2008/105/EC on Environmental Quality Standards in the Field of Water Policy 26.10.2022, COM(2022) 540 Final 2022/0344 (COD); European Commission: Brussels, Belgium, 2022; pp. 1–81. [Google Scholar]
- Valle, A.L.; Mello, F.C.C.; Alves-Balvedi, R.P.; Rodrigues, L.P.; Goulart, L.R. Glyphosate detection: Methods, needs and challenges. Environ. Chem. Lett. 2019, 17, 291–317. [Google Scholar] [CrossRef]
- Royer, A.; Beguin, S.; Tabet, J.C.; Hulot, S.; Reding, M.A.; Communal, P.Y. Determination of glyphosate and aminomethylphosphonic acid residues in water by gas chromatography with tandem mass spectrometry after exchange ion resin purification and derivatization. Application on vegetable matrixes. Anal. Chem. 2000, 72, 3826–3832. [Google Scholar] [CrossRef]
- Rawat, K.A.; Majithiya, R.P.; Rohit, J.V.; Basu, H.; Singhal, R.K.; Kailasa, S.K. Mg2+ ion as a tuner for colorimetric sensing of glyphosate with improved sensitivity via the aggregation of 2-mercapto-5-nitrobenzimidazole capped silver nanoparticles. RSC Adv. 2016, 6, 47741–47752. [Google Scholar] [CrossRef]
- Waiman, C.V.; Avena, M.J.; Garrido, M.; Band, B.F.; Zanini, G.P. A simple and rapid spectrophotometric method to quantify the herbicide glyphosate in aqueous media. Application to adsorption isotherms on soils and goethite. Geoderma 2012, 170, 154–158. [Google Scholar] [CrossRef]
- Cennamo, N.; Pesavento, M.; Zeni, L. A review on simple and highly sensitive plastic optical fiber probes for bio-chemical sensing. Sens. Actuators B Chem. 2021, 331, 129393. [Google Scholar] [CrossRef]
- Gupta, B.D.; Shrivastav, A.M.; Usha, S.P. Surface plasmon resonance-based fiber optic sensors utilizing molecular imprinting. Sensors 2016, 16, 1381. [Google Scholar] [CrossRef] [PubMed]
- Cennamo, N.; D’Agostino, G.; Porto, G.; Biasiolo, A.; Perri, C.; Arcadio, F.; Zeni, L. A molecularly imprinted polymer on a plasmonic plastic optical fiber to detect perfluorinated compounds in water. Sensors 2018, 18, 1836. [Google Scholar] [CrossRef] [PubMed]
- Haupt, K.; Mosbach, K. Molecularly imprinted polymers and their use in biomimetic sensors. Chem. Rev. 2000, 100, 2495–2504. [Google Scholar] [CrossRef] [PubMed]
- Kupai, J.; Razali, M.; Buyuktiryaki, S.; Kecili, R.; Szekely, G. Long-term stability and reusability of molecularly imprinted polymers. Polym. Chem. 2017, 8, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Do, M.H.; Florea, A.; Farre, C.; Bonhomme, A.; Bessueille, F.; Vocanson, F.; Tran-Thi, N.T.; Jaffrezic-Renault, N. Molecularly imprinted polymer-based electrochemical sensor for the sensitive detection of glyphosate herbicide. Int. J. Environ. Anal. Chem. 2015, 95, 1489–1501. [Google Scholar] [CrossRef]
- Sawetwong, P.; Chairam, S.; Jarujamrus, P.; Amatatongchai, M. Enhanced selectivity and sensitivity for colorimetric determination of glyphosate using Mn–ZnS quantum dot embedded molecularly imprinted polymers combined with a 3D-microfluidic paper-based analytical device. Talanta 2021, 225, 122077. [Google Scholar] [CrossRef] [PubMed]
- Balciunas, D.; Plausinaitis, D.; Ratautaite, V.; Ramanaviciene, A.; Ramanavicius, A. Towards electrochemical surface plasmon resonance sensor based on the molecularly imprinted polypyrrole for glyphosate sensing. Talanta 2022, 241, 123252. [Google Scholar] [CrossRef]
- Alberti, G.; Spina, S.; Arcadio, F.; Pesavento, M.; De Maria, L.; Cennamo, N.; Zeni, L.; Merli, D. MIP-Assisted 3-Hole POF Chip Faced with SPR-POF Sensor for Glyphosate Detection. Chemosensors 2023, 11, 414. [Google Scholar] [CrossRef]
- Pesavento, M.; Marchetti, S.; De Maria, L.; Zeni, L.; Cennamo, N. Sensing by molecularly imprinted polymer: Evaluation of the binding properties with different techniques. Sensors 2019, 19, 1344. [Google Scholar] [CrossRef] [PubMed]
- da Mata, K.; Corazza, M.Z.; de Oliveira, F.M.; de Toffoli, A.L.; Tarley, C.R.T.; Moreira, A.B. Synthesis and characterization of cross-linked molecularly imprinted polyacrylamide for the extraction/preconcentration of glyphosate and aminomethylphosphonic acid from water samples. React. Funct. Polym. 2014, 83, 76–83. [Google Scholar] [CrossRef]
- Cennamo, N.; Massarotti, D.; Conte, L.; Zeni, L. Low cost sensors based on SPR in a plastic optical fiber for biosensor implementation. Sensors 2011, 11, 11752–11760. [Google Scholar] [CrossRef]
- Pesavento, M.; Cennamo, N.; Zeni, L.; De Maria, L. A Molecularly Imprinted Polymer Based SPR Sensor for 2-Furaldehyde Determination in Oil Matrices. Appl. Sci. 2021, 11, 10390. [Google Scholar] [CrossRef]
- Kushwaha, A.S.; Kumar, A.; Kumar, R.; Srivastava, S.K. A study of surface plasmon resonance (SPR) based biosensor with improved sensitivity. Photonics Nanostruct.-Fundam. Appl. 2018, 31, 99–106. [Google Scholar] [CrossRef]
- Cennamo, N.; Arcadio, F.; Zeni, L.; Alberti, G.; Pesavento, M. Optical-Chemical Sensors based on plasmonic phenomena modulated via micro-holes in Plastic Optical Fibers filled by Molecularly Imprinted Polymers. Sens. Actuators B Chem. 2022, 372, 132672. [Google Scholar] [CrossRef]
- Miller, J.N.; Miller, J.C. Calibration methods in instrumental analysis: Regression and correlation. In Statistics and Chemometrics for Analytical Chemistry, 6th ed.; Pearson Education Limited: Harlow Essex, UK, 2010; pp. 124–126. [Google Scholar]
- Butmee, P.; Samphao, A.; Tumcharern, G. Reduced graphene oxide on silver nanoparticle layers-decorated titanium dioxide nanotube arrays as SERS-based sensor for glyphosate direct detection in environmental water and soil. J. Hazard. Mater. 2022, 437, 129344. [Google Scholar] [CrossRef]
- Li, Z.; Liang, S.; Zhou, L.; Luo, F.; Lou, Z.; Chen, Z.; Yang, M. A Turn-On Fluorescence Sensor Based on Nitrogen-Doped Carbon Dots and Cu2+ for Sensitively and Selectively Sensing Glyphosate. Foods 2023, 12, 2487. [Google Scholar] [CrossRef]
- Qiu, C.; Zhang, L.; Miao, F.; Tao, B.; Li, H.; Qiu, Z.; Zang, Y. Development of a sensitive ZnO/CuO/Au electrochemical sensor for measuring glyphosate. Vacuum 2023, 214, 112138. [Google Scholar] [CrossRef]
- Costa, I.A.; Gross, M.A.; Alves, E.D.; Fonseca, F.J.; Paterno, L.G. An impedimetric e-tongue based on CeO2-graphene oxide chemical sensors for detection of glyphosate and its potential interferents. J. Electroanal. Chem. 2022, 922, 116719. [Google Scholar] [CrossRef]
- Chang, Y.C.; Lin, Y.S.; Xiao, G.T.; Chiu, T.C.; Hu, C.C. A highly selective and sensitive nanosensor for the detection of glyphosate. Talanta 2016, 161, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Bera, M.K.; Mohapatra, S. Ultrasensitive detection of glyphosate through effective photoelectron transfer between CdTe and chitosan derived carbon dot. Colloids Surf. A Physicochem. Eng. Asp. 2020, 596, 124710. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, H.; Qu, J.; Zhu, Q.; Chen, X. Visual detection of glyphosate in environmental water samples using cysteamine-stabilized gold nanoparticles as colorimetric probe. Anal. Methods 2013, 5, 917–924. [Google Scholar] [CrossRef]
- Pesavento, M.; Zeni, L.; De Maria, L.; Alberti, G.; Cennamo, N. SPR-Optical Fiber-Molecularly Imprinted Polymer Sensor for the Detection of Furfural in Wine. Biosensors 2021, 11, 72. [Google Scholar] [CrossRef]




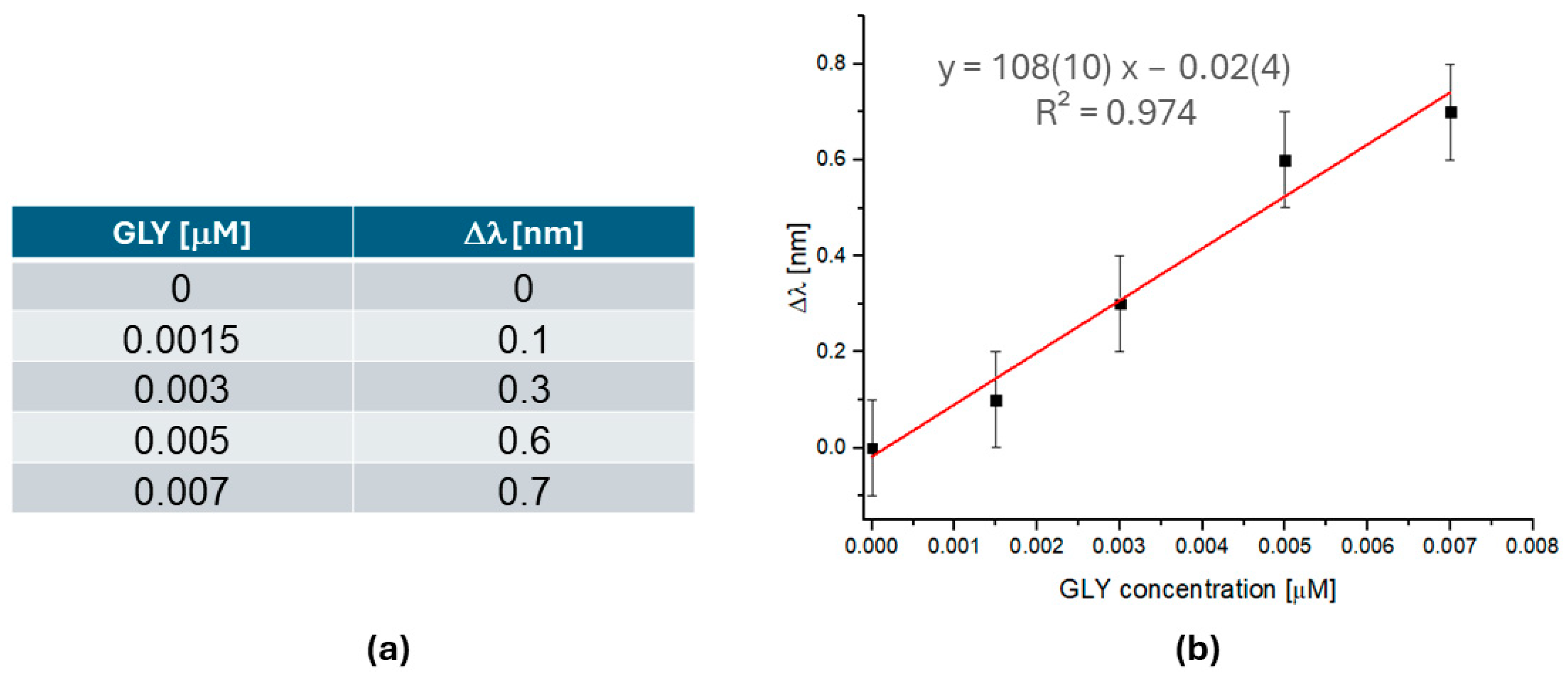


| Sensor | Sensitivity [nm µM−1] | R2 | LOD [µM] | LOQ [µM] | Linear Range [µM] | Dynamic Range [µM] |
|---|---|---|---|---|---|---|
| SPR-POF-MIP | 108(10) | 0.974 | 0.0017 | 0.0052 | 0.0015–0.007 | 0.0015–2 |
| Sensing Method | LOD [μM] | Kaff [μM]−1 | Detection Range (μM) | Reference |
|---|---|---|---|---|
| Surface-enhanced Raman scattering | 0.017 | n.r. | 29–296 | [41] |
| Fluorescence | 0.124 | n.r. | 0.59–590 | [42] |
| Electrochemical | 0.35 | n.r. | 6.9–230 | [43] |
| Electrochemical | 0.03 | n.r. | 0.11–0.29 | [44] |
| Fluorescence | 0.004 | n.r. | 0.01–0.06 | [45] |
| Photoluminescence | 0.05 | n.r. | 0–0.1 | [46] |
| Electrochemical/indirect method | 5×10−9 | n.r. | 6 10−9–6 10−3 | [30] |
| Colorimetric | 0.06 | n.r. | 0.5–7 | [47] |
| MIP-Assisted 3-Hole POF Chip Faced with SPR–POF Sensor | 0.0002 | 280 | 0.0003–0.05 | [33] |
| SPR–POF–MIP | 0.0015 | 325 (site 1) * 1.4 (site 2) * | 0.0015–2 | [This work] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renzullo, L.P.; Tavoletta, I.; Alberti, G.; Zeni, L.; Pesavento, M.; Cennamo, N. Plasmonic Optical Fiber Sensors and Molecularly Imprinted Polymers for Glyphosate Detection at an Ultra-Wide Range. Chemosensors 2024, 12, 142. https://doi.org/10.3390/chemosensors12070142
Renzullo LP, Tavoletta I, Alberti G, Zeni L, Pesavento M, Cennamo N. Plasmonic Optical Fiber Sensors and Molecularly Imprinted Polymers for Glyphosate Detection at an Ultra-Wide Range. Chemosensors. 2024; 12(7):142. https://doi.org/10.3390/chemosensors12070142
Chicago/Turabian StyleRenzullo, Luca Pasquale, Ines Tavoletta, Giancarla Alberti, Luigi Zeni, Maria Pesavento, and Nunzio Cennamo. 2024. "Plasmonic Optical Fiber Sensors and Molecularly Imprinted Polymers for Glyphosate Detection at an Ultra-Wide Range" Chemosensors 12, no. 7: 142. https://doi.org/10.3390/chemosensors12070142






