Effects of Au Addition to Porous CuO2-Added SnO2 Gas Sensors on Their VOC-Sensing Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of pr-wAu-SnO2 Powders
2.2. Sensor Fabrication and Gas-Sensing Measurements
2.3. Characterization
2.4. Catalytic Combustion Activity
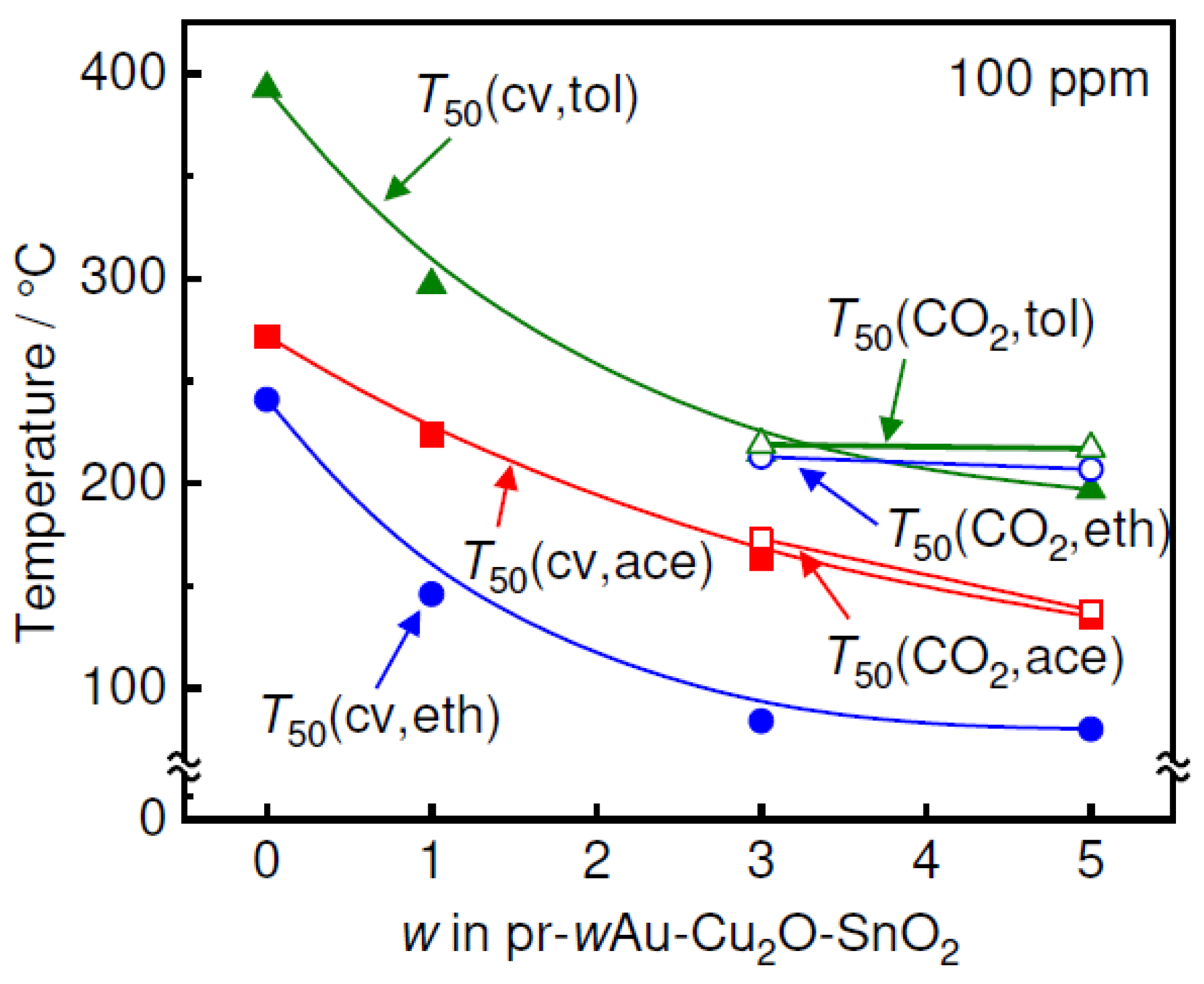
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koo, W.-T.; Choi, S.-J.; Kim, N.-H.; Jang, J.-S.; Kim, I.-D. Catalyst-decorated hollow WO3 nanotubes using layer-by-layer self-assembly on polymeric nanofiber templates and their application in exhaled breath sensor. Sens. Actuators B Chem. 2016, 223, 301–310. [Google Scholar] [CrossRef]
- Li, W.; Dai, W.; Liu, M.; Long, Y.; Wang, C.; Xie, S.; Liu, Y.; Zhang, Y.; Shi, Q.; Peng, X.; et al. VOC biomarkers identification and predictive model construction for lung cancer based on exhaled breath analysis: Research protocol for an exploratory study. BMJ Open 2019, 9, e028448. [Google Scholar] [CrossRef] [PubMed]
- Kalidoss, R.; Umapathy, R. An overview on the exponential growth of non-invasive diagnosis of diabetes mellitus from exhaled breath by nanostructured metal oxide Chemi-resistive gas sensors and μ-preconcentrator. Biomed. Microdevices 2020, 22, 2. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Choi, S.-J.; Lee, I.; Youn, D.-Y.; Park, C.O.; Lee, J.-H.; Tuller, H.L.; Kim, I.-D. Thin-wall assembled SnO2 fibers functionalized by catalytic Pt nanoparticles and their superior exhaled-breath-sensing properties for the diagnosis of diabetes. Adv. Funct. Mater. 2013, 23, 2357–2367. [Google Scholar] [CrossRef]
- Kalapos, M.P. On the mammalian acetone metabolism: From chemistry to clinical implications. Biochim. Biophys. Acta 2003, 1621, 122–139. [Google Scholar] [CrossRef] [PubMed]
- Miekisch, W.; Schubert, J.K.; Noeldge-Schomburg, G.F. Diagnostic potential of breath analysis focus on volatile organic compounds. Clin. Chim. Acta 2004, 347, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Duan, Y. Breath Analysis: Potential for clinical diagnosis and exposure assessment. Clin. Chem. 2006, 52, 800–811. [Google Scholar] [CrossRef]
- Righettoni, M.; Tricoli, A.; Pratsinis, S.E. Si:WO3 Sensors for highly selective detection of acetone for easy diagnosis of diabetes by breath analysis. Anal. Chem. 2010, 82, 3581–3587. [Google Scholar] [CrossRef]
- Righettoni, M.; Tricoli, A.; Gass, S.; Schmid, A.; Amann, A.; Pratsinis, S.E. Breath acetone monitoring by portable Si:WO3 gas sensors. Anal. Chim. Acta 2012, 738, 69–75. [Google Scholar] [CrossRef]
- Güntner, A.T.; Sievi, N.A.; Theodore, S.J.; Gulich, T.; Kohler, M.; Pratsinis, S.E. Noninvasive body fat burn monitoring from exhaled acetone with Si-doped WO3-sensing nanoparticles. Anal. Chem. 2017, 89, 10578–10584. [Google Scholar] [CrossRef]
- Weber, I.C.; Braun, H.P.; Krumeich, F.; Güntner, A.T.; Pratsinis, S.E. Superior acetone selectivity in gas mixtures by catalyst-filtered chemoresistive sensors. Adv. Sci. 2020, 7, 2001503. [Google Scholar] [CrossRef] [PubMed]
- Gschwend, P.M.; Schenk, F.M.; Gogos, A.; Pratsinis, S.E. Acetone sensing and catalytic conversion by Pd-loaded SnO2. Materials 2021, 14, 5921. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, P.; Li, X.; Wang, C.; Feng, C.; Lu, G. Revealing the relationship between the Au decoration method and the enhanced acetone sensing performance of a mesoporous In2O3-based gas sensor. J. Mater. Chem. C 2020, 8, 78–88. [Google Scholar] [CrossRef]
- Yang, M.; Lu, J.; Wang, X.; Zhang, H.; Chen, F.; Sun, J.; Yang, J.; Sun, Y.; Lu, G. Acetone sensors with high stability to humidity changes based on Ru-doped NiO flower-like microspheres. Sens. Actuators B Chem. 2020, 313, 127965. [Google Scholar] [CrossRef]
- Weber, I.C.; Oosthuizen, D.N.; Mohammad, R.W.; Mayhew, C.A.; Pratsinis, S.E.; Güntner, A.T. Dynamic breath limonene sensing at high selectivity. ACS Sens. 2023, 8, 2618–2626. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Jiang, Y.; Zhao, L.; Wang, T.; Liu, X.; Liu, F.; Yan, X.; Sun, P.; Lu, G. High sensitivity and low detection limit of acetone sensor based on Ru-doped Co3O4 flower-like hollow microspheres. Sens. Actuators B Chem. 2022, 363, 131839. [Google Scholar] [CrossRef]
- Yang, J.; Jiang, B.; Wang, X.; Wang, C.; Sun, Y.; Zhang, H.; Shimanoe, K.; Lu, G. MOF-derived porous NiO/NiFe2O4 nanocubes for improving the acetone detection. Sens. Actuators B Chem. 2022, 366, 131985. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, L.; Liu, Y.; Liu, D.; Liu, F.; Liu, F.; Yan, X.; Liang, X.; Gao, Y.; Lu, G. Gas sensor based on samarium oxide loaded mulberry-shaped tin oxide for highly selective and sub ppm-level acetone detection. J. Colloid Interface Sci. 2018, 531, 74–82. [Google Scholar] [CrossRef]
- Chen, F.; Yang, M.; Wang, X.; Song, Y.; Guo, L.; Xie, N.; Kou, X.; Xu, X.; Sun, Y.; Lu, G. Template-free synthesis of cubic-rhombohedral-In2O3 flower for ppb level acetone detection. Sens. Actuators B Chem. 2019, 290, 459–466. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, W.; Li, Y.; Yang, X.; Sun, P.; Liu, F.; Yan, X.; Gao, Y.; Liang, X.; Ma, J.; et al. Highly-sensitivity acetone sensors based on spinel-type oxide (NiFe2O4) through optimization of porous structure. Sens. Actuators B Chem. 2019, 291, 266–274. [Google Scholar] [CrossRef]
- Kou, X.; Meng, F.; Chen, K.; Wang, T.; Sun, P.; Liu, F.; Yan, X.; Sun, Y.; Liu, F.; Shimanoe, K.; et al. High-performance acetone gas sensor based on Ru-doped SnO2 nanofibers. Sens. Actuators B Chem. 2020, 320, 128292. [Google Scholar] [CrossRef]
- Wu, E.; Xie, Y.; Yuan, B.; Hao, D.; An, C.; Zhang, H.; Wu, S.; Hu, X.; Liu, J.; Zhang, D. Specific and highly sensitive detection of ketone compounds based on p-Type MoTe2 under Ultraviolet Illumination. ACS Appl. Mater. Interfaces 2018, 10, 35664–35669. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.J.; Koo, W.-T.; Jang, J.-S.; Kim, D.-H.; Kim, M.-H.; Kim, I.-D. Nanoscale PtO2 catalysts-loaded SnO2 multichannel nanofibers toward highly sensitive acetone sensor. ACS Appl. Mater. Interfaces 2018, 10, 2016–2025. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.-S.; Choi, S.-J.; Kim, S.-J.; Hakim, M.; Kim, I.-D. Rational design of highly porous SnO2 nanotubes functionalized with biomimetic nanocatalysts for direct observation of simulated diabetes. Adv. Funct. Mater. 2016, 26, 4740–4748. [Google Scholar] [CrossRef]
- Kim, S.-J.; Choi, S.-J.; Jang, J.-S.; Kim, N.-H.; Hakim, M.; Tuller, H.L.; Kim, I.-D. Mesoporous WO3 nanofibers with protein-templated nanoscale catalysts for detection of trace biomarkers in exhaled breath. ACS Nano 2016, 10, 5891–5899. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-I.; Hwang, S.-J.; Dai, Z.; Kang, Y.C.; Lee, J.-H. Rh-catalyzed WO3 with anomalous humidity dependence of gas sensing characteristics. RSC Adv. 2014, 4, 53130–53136. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, T.H.; Sohn, W.; Suh, J.M.; Shim, Y.; Kwon, K.C.; Hong, K.; Choi, S.; Byun, H.; Lee, J.; et al. Au decoration of vertical hematite nanotube arrays for further selective detection of acetone in exhaled breath. Sens. Actuators B Chem. 2018, 274, 587–594. [Google Scholar] [CrossRef]
- Kwak, C.-H.; Kim, T.-H.; Jeong, S.-Y.; Yoon, J.-W.; Kim, J.-S.; Lee, J.-H. Humidity-independent oxide semiconductor chemiresistors using terbium-doped SnO2 yolk–shell spheres for real-time breath analysis. ACS Appl. Mater. Interfaces 2018, 10, 18886–18894. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xu, H.; Yu, X.; Li, L.; Gao, Y.; Sun, P.; Lu, G. Detection of low concentration acetone utilizing semiconductor gas sensor. J. Mater. Sci. Mater. Electron. 2020, 31, 5478–5484. [Google Scholar] [CrossRef]
- Ahn, S.; Chun, K.W.; Park, C. Long-term stability test for femtosecond laser-irradiated SnO2-nanowire gas sensor for C7H8 gas sensing. Photonics 2024, 11, 550. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, B.; Wang, B.; Zhao, Z.; Zhang, W.; Zhang, W.; Suematsu, K.; Hu, J. Construction of flower-like PtOx@ZnO/In2O3 hollow microspheres for ultrasensitive and rapid trace detection of isopropanol. ACS Appl. Mater. Interfaces 2023, 15, 12041–12051. [Google Scholar] [CrossRef]
- Torai, S.; Ueda, T.; Kamada, K.; Hyodo, T.; Shimizu, Y. Effects of addition of CuxO to porous SnO2 microspheres prepared by ultrasonic spray pyrolysis on sensing properties to volatile organic compounds. Chemosensors 2023, 11, 59. [Google Scholar] [CrossRef]
- Hieda, K.; Hyodo, T.; Shimizu, Y.; Egashira, M. Preparation of porous tin dioxide powder by ultrasonic spray pyrolysis and their application to sensor materials. Sens. Actuators B Chem. 2008, 133, 144–150. [Google Scholar] [CrossRef]
- Hyodo, T.; Fujii, E.; Ishida, K.; Ueda, T.; Shimizu, Y. Microstructural control of porous In2O3 powders prepared by ultrasonic-spray pyrolysis employing self-synthesized polymethylmethacrylate microspheres as a template and their NO2-sensing properties. Sens. Actuators B Chem. 2017, 244, 992–1003. [Google Scholar] [CrossRef]
- Ueda, T.; Ishida, K.; Kamada, K.; Hyodo, T.; Shimizu, Y. Improvement in NO2 sensing properties of semiconductor-type gas sensors by loading of Au into porous In2O3 powders. Front. Mater. 2019, 6, 81. [Google Scholar] [CrossRef]
- Tammanoon, N.; Iwamoto, T.; Ueda, T.; Hyodo, T.; Wisitsoraat, A.; Liewhiran, C.; Shimizu, Y. Synergistic effects of PdOx-CuOx loadings on methylmercaptan sensing of porous WO3 microspheres prepared by ultrasonic spray pyrolysis. ACS Appl. Mater. Interfaces 2020, 12, 41728–41739. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Sun, L.; Han, N.; Chu, H.; Bai, S.; Shu, X.; Luo, R.; Chen, A. rGO decorated CdS/CdO composite for detection of low concentration NO2. Sens. Actuators B Chem. 2019, 299, 126832. [Google Scholar] [CrossRef]
- Mahdavifar, A.; Navaei, M.; Hesketh, P.J.; Findlay, M.; Stetter, J.R.; Hunter, W.G. Transient thermal response of micro-thermal conductivity detector (µTCD) for the identification of gas mixtures: An ultra-fast and low power method. Microsyst. Nanoeng. 2015, 1, 15025. [Google Scholar] [CrossRef]
- Struk, D.; Shirke, A.; Mahdavifar, A.; Hesketh, P.J.; Stetter, J.R. Investigating time-resolved response of micro thermal conductivity sensor under various modes of operation. Sens. Actuators B Chem. 2018, 254, 771–777. [Google Scholar] [CrossRef]
- Jung, G.; Shin, W.; Hong, S.; Jeong, Y.; Park, J.; Kim, D.; Bae, J.H.; Park, B.G.; Lee, J.H. Comparison of the characteristics of semiconductor gas sensors with different transducers fabricated on the same substrate. Sens. Actuators B Chem. 2021, 335, 129661. [Google Scholar] [CrossRef]
- Degler, D.; Wicker, S.; Weimar, U.; Barsan, N. Identifying the active oxygen species in SnO2 based gas sensing materials: An operando IR spectrsocopy study. J. Phys. Chem. C 2015, 119, 11792–11799. [Google Scholar] [CrossRef]
- Degler, D.; Rank, S.; Müller, S.; Carvalho, H.W.P.; Grunwaldt, J.-D.; Weimar, U.; Barsan, N. Gold-loaded tin dioxide gas sensing materials: Mechanistic insights and the role of gold dispersion. ACS Sens. 2016, 1, 1322–1329. [Google Scholar] [CrossRef]
- Ueda, T.; Boehme, I.; Hyodo, T.; Shimizu, Y.; Weimar, U.; Barsan, N. Effects of Gas adsorption properties of an Au-loaded porous In2O3 Sensor on NO2-Sensing Properties. ACS Sens. 2021, 6, 4019–4028. [Google Scholar] [CrossRef] [PubMed]

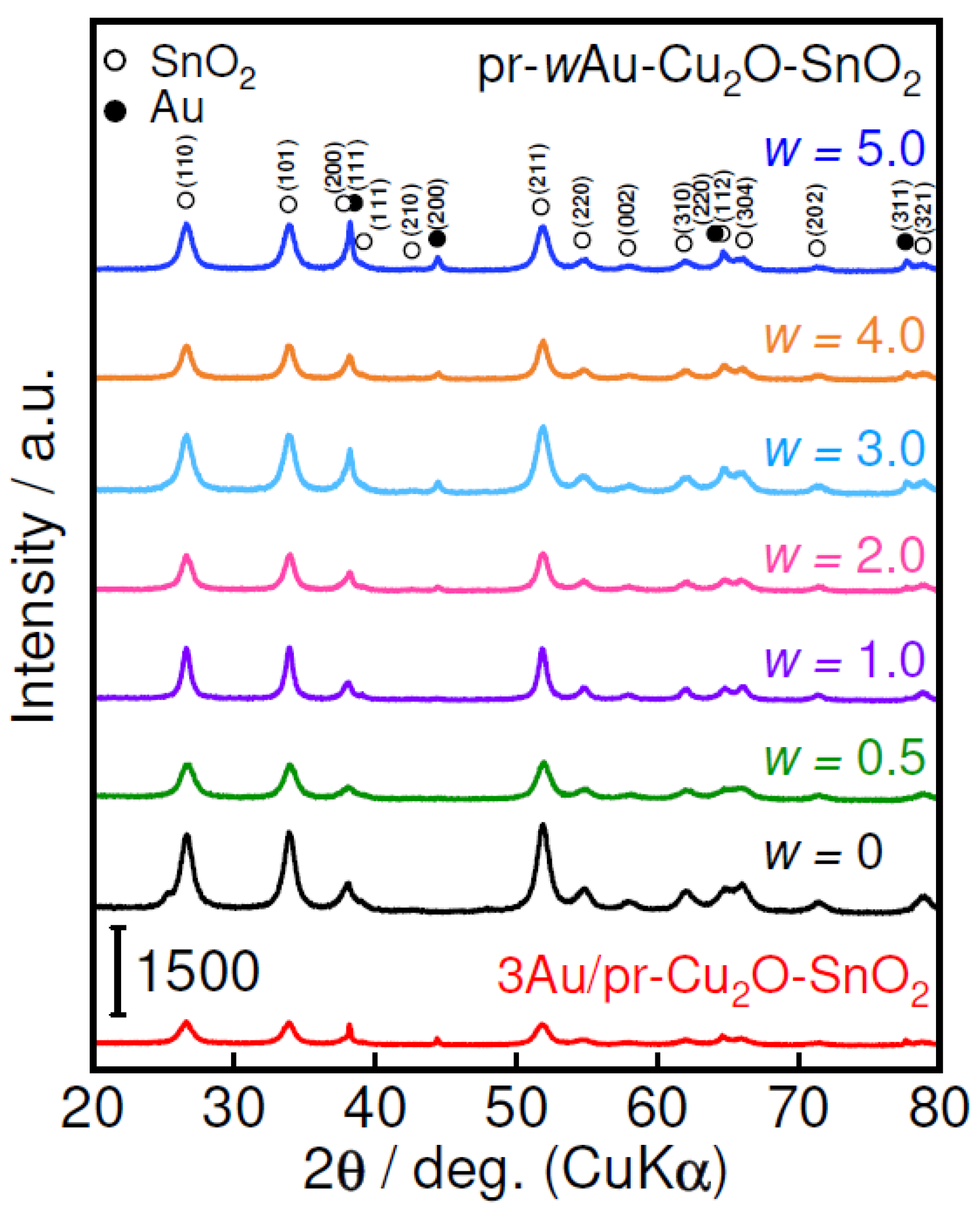
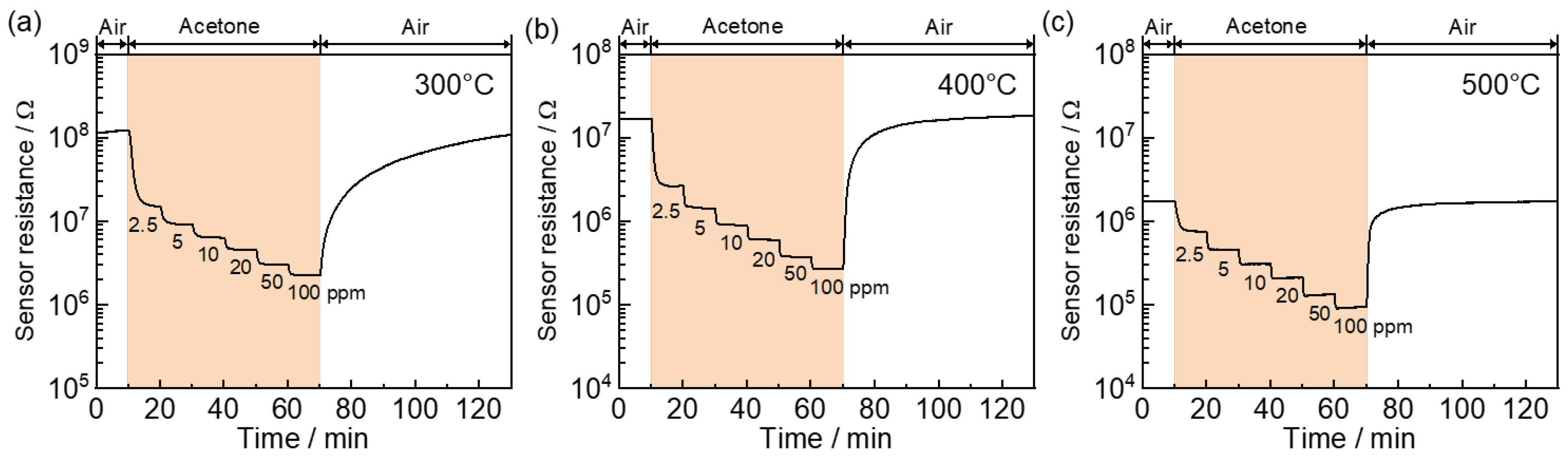

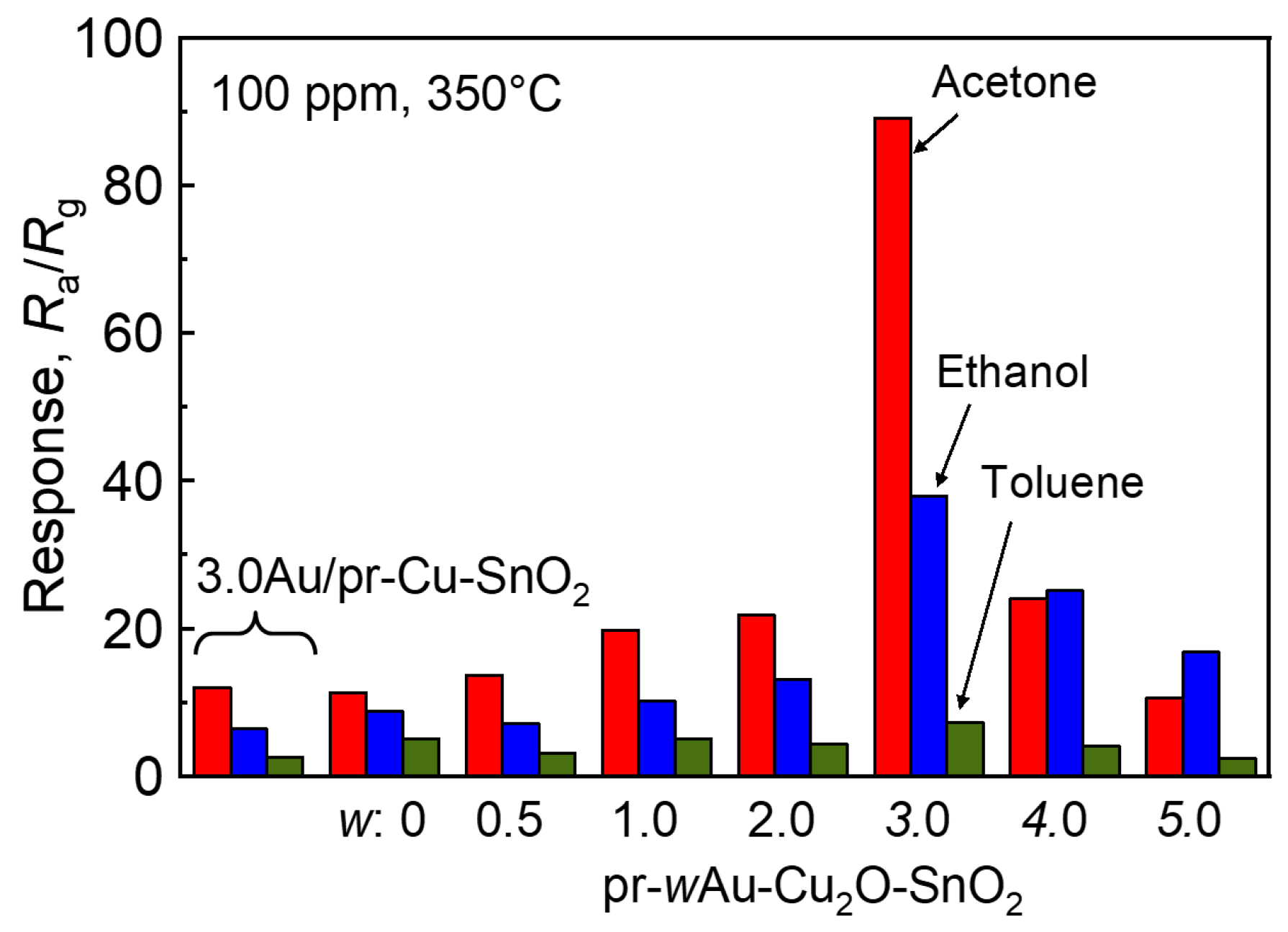

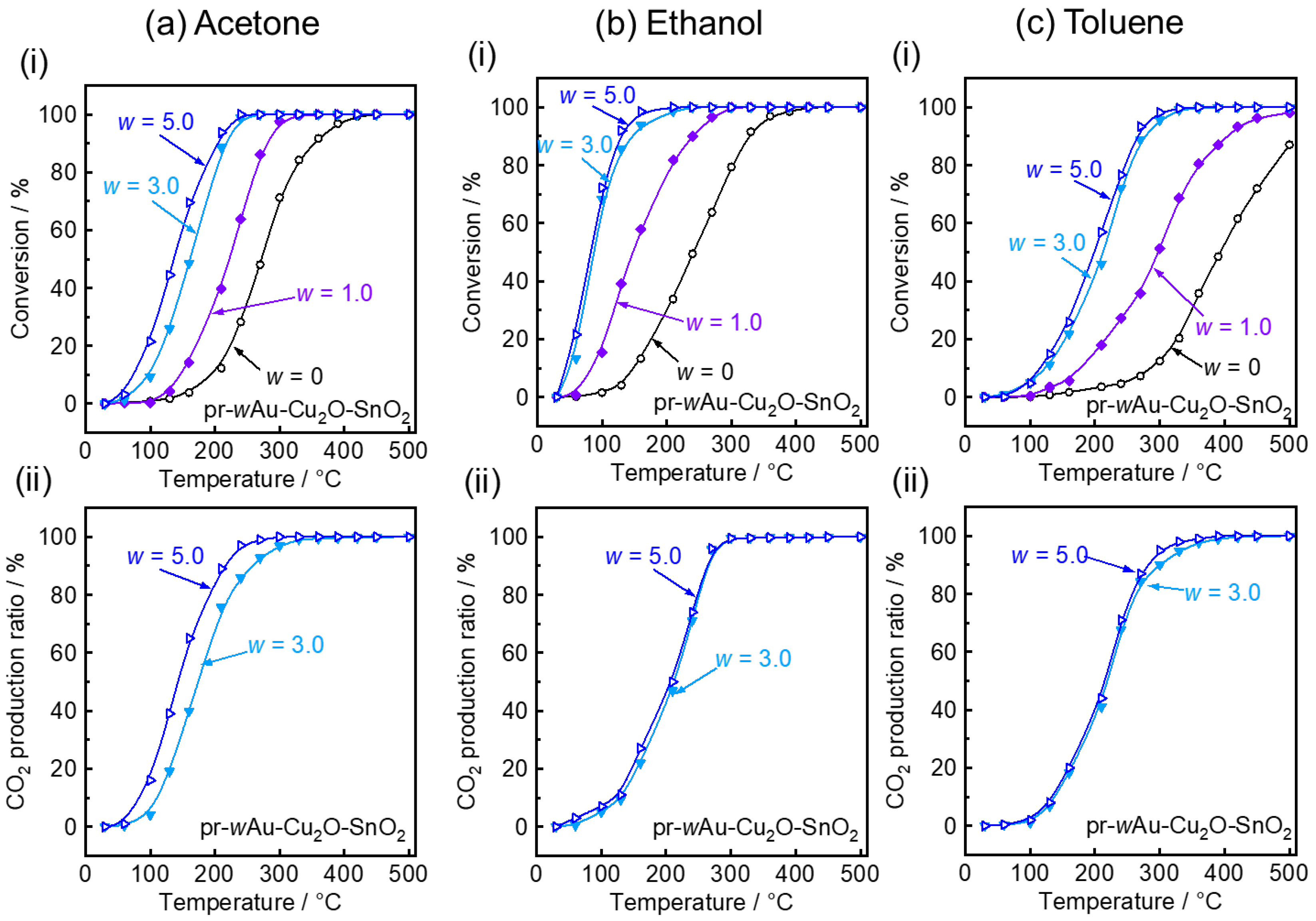
| Sample | w | CS * of SnO2/nm | SSA **/m2 g−1 |
|---|---|---|---|
| pr-wAu-Cu2O-SnO2 | 0 | 7.08 | 48.3 |
| 0.5 | 6.98 | 50.0 | |
| 1.0 | 7.87 | 38.1 | |
| 3.0 | 8.32 | 39.6 | |
| 5.0 | 8.41 | 38.7 | |
| 3.0Au/pr-Cu2O-SnO2 | — | 7.88 | 48.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueda, T.; Torai, S.; Fujita, K.; Shimizu, Y.; Hyodo, T. Effects of Au Addition to Porous CuO2-Added SnO2 Gas Sensors on Their VOC-Sensing Properties. Chemosensors 2024, 12, 153. https://doi.org/10.3390/chemosensors12080153
Ueda T, Torai S, Fujita K, Shimizu Y, Hyodo T. Effects of Au Addition to Porous CuO2-Added SnO2 Gas Sensors on Their VOC-Sensing Properties. Chemosensors. 2024; 12(8):153. https://doi.org/10.3390/chemosensors12080153
Chicago/Turabian StyleUeda, Taro, Soichiro Torai, Koki Fujita, Yasuhiro Shimizu, and Takeo Hyodo. 2024. "Effects of Au Addition to Porous CuO2-Added SnO2 Gas Sensors on Their VOC-Sensing Properties" Chemosensors 12, no. 8: 153. https://doi.org/10.3390/chemosensors12080153






