Low-Drift NO2 Sensor Based on Polyaniline/Black Phosphorus Composites at Room Temperature
Abstract
:1. Introduction
2. Materials and Methods
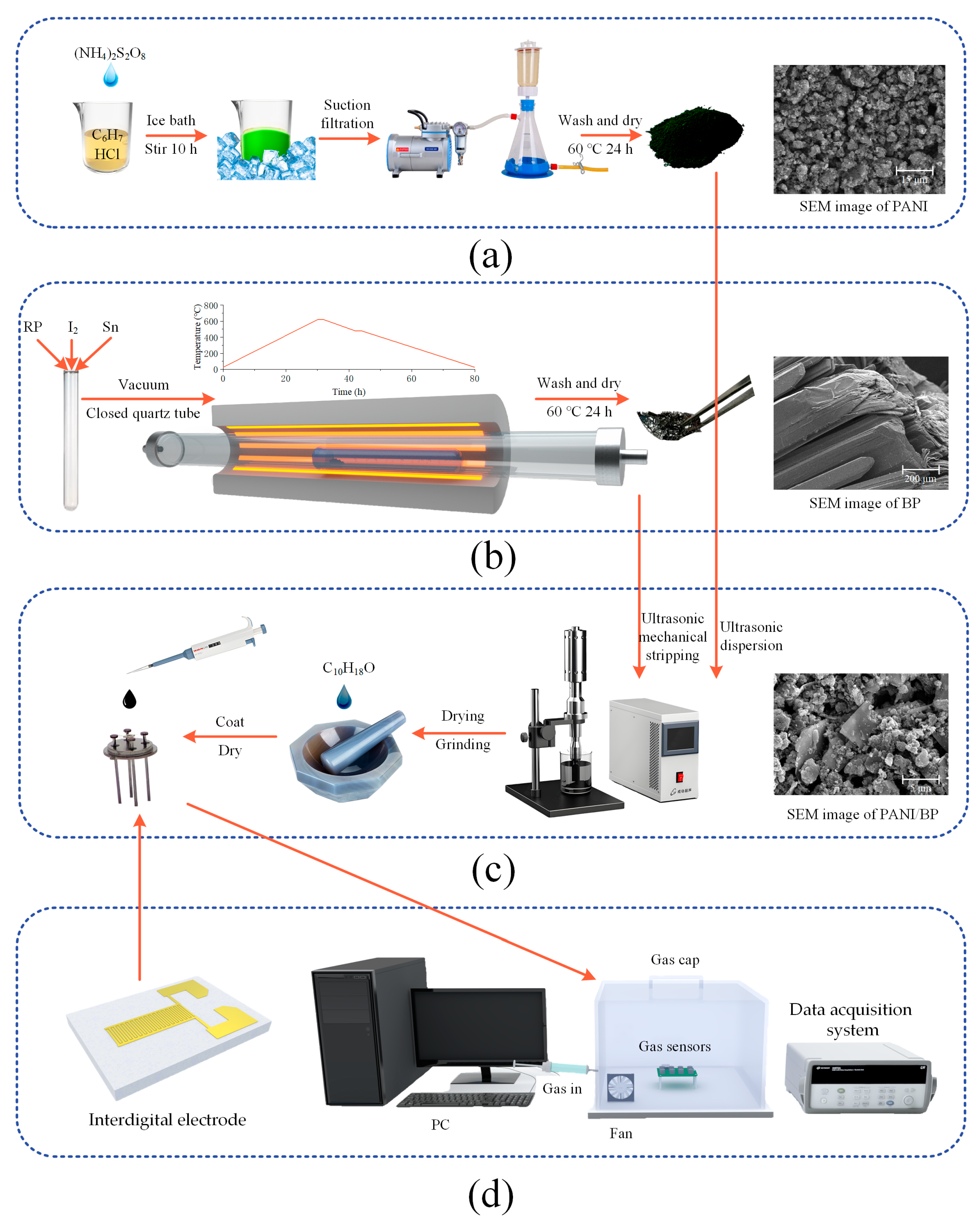
2.1. Synthesis of PANI
2.2. Synthesis of BP
2.3. Preparation of Composite Materials and Sensors
2.4. Characterization
2.5. Test Environment
3. Results and Discussion
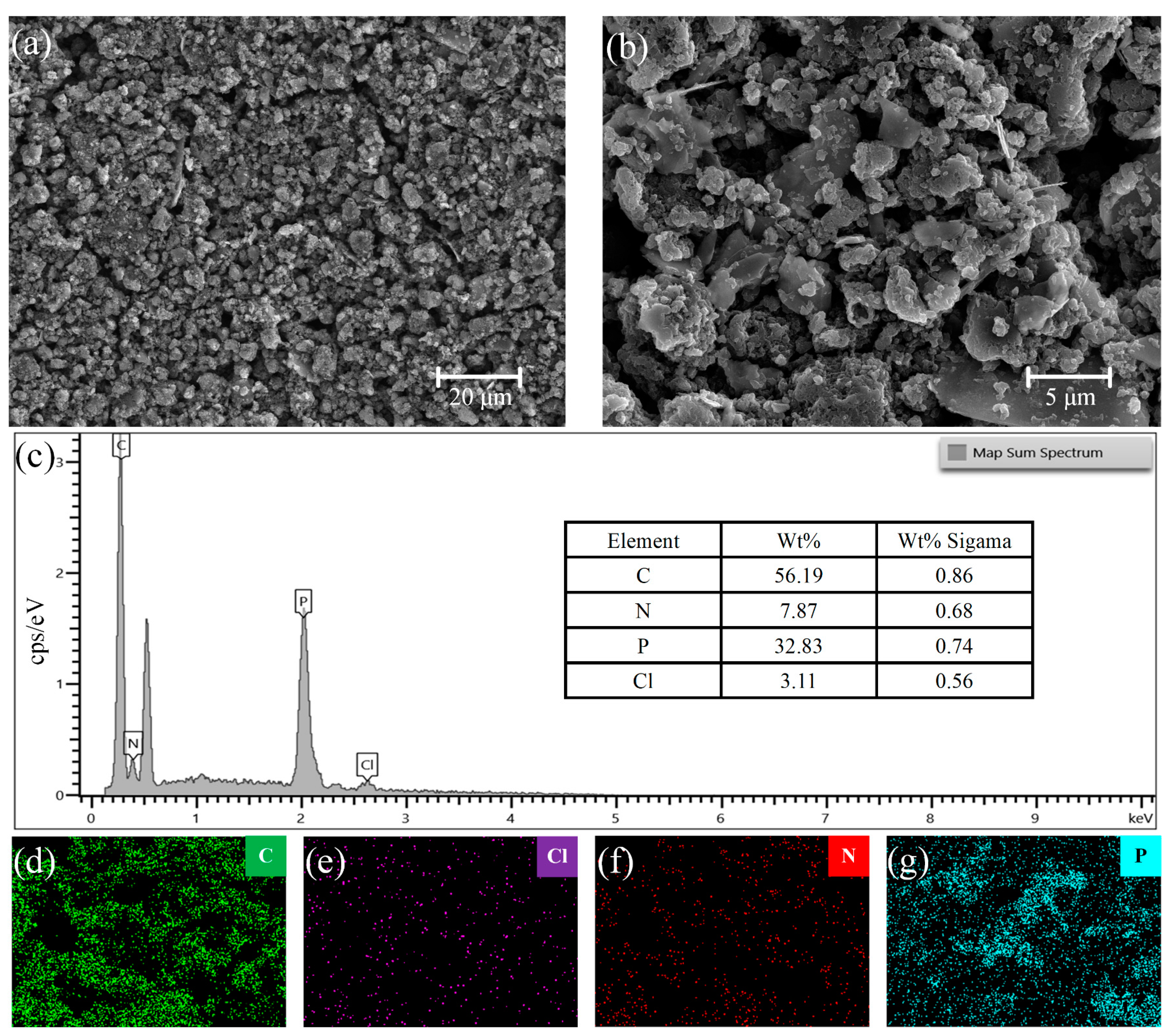
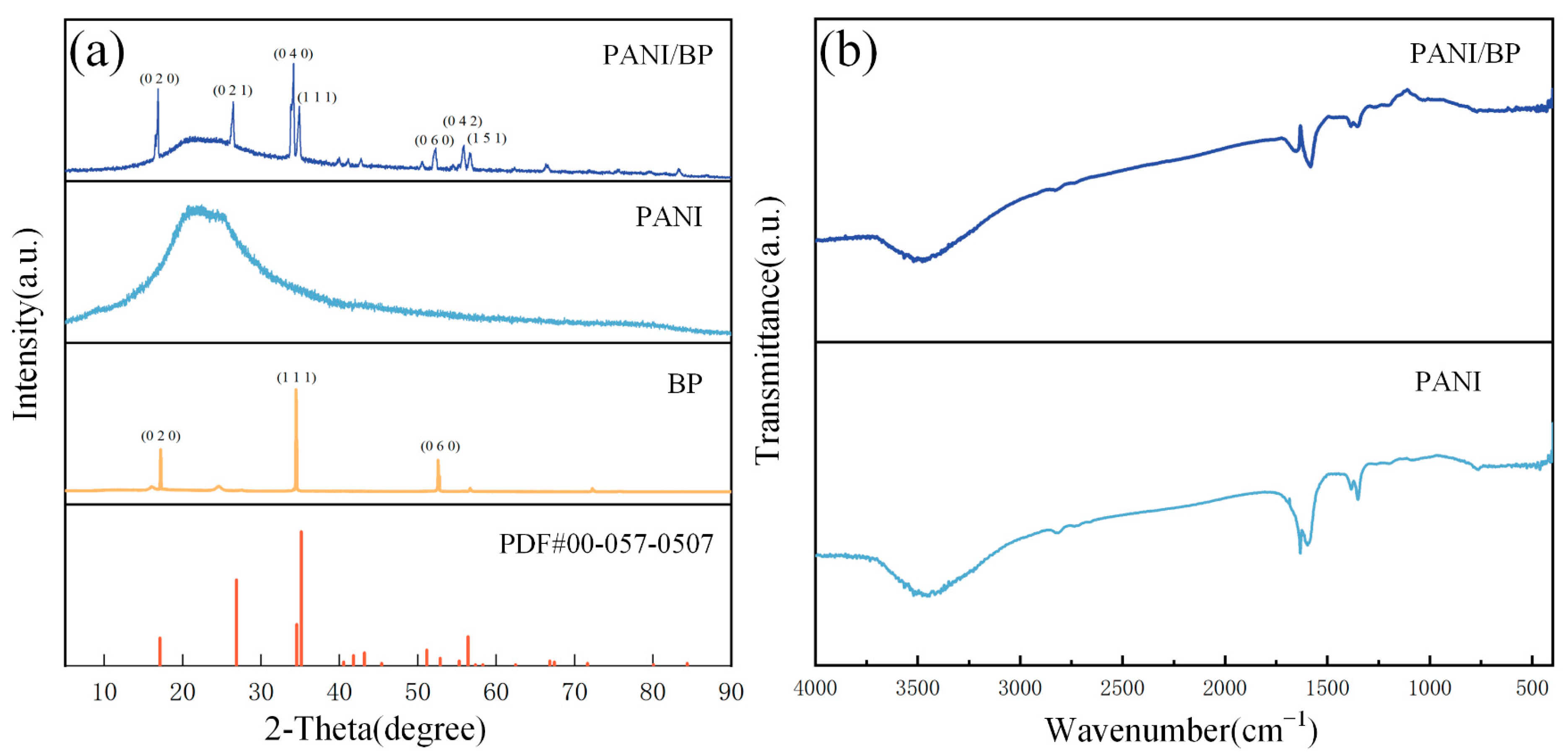
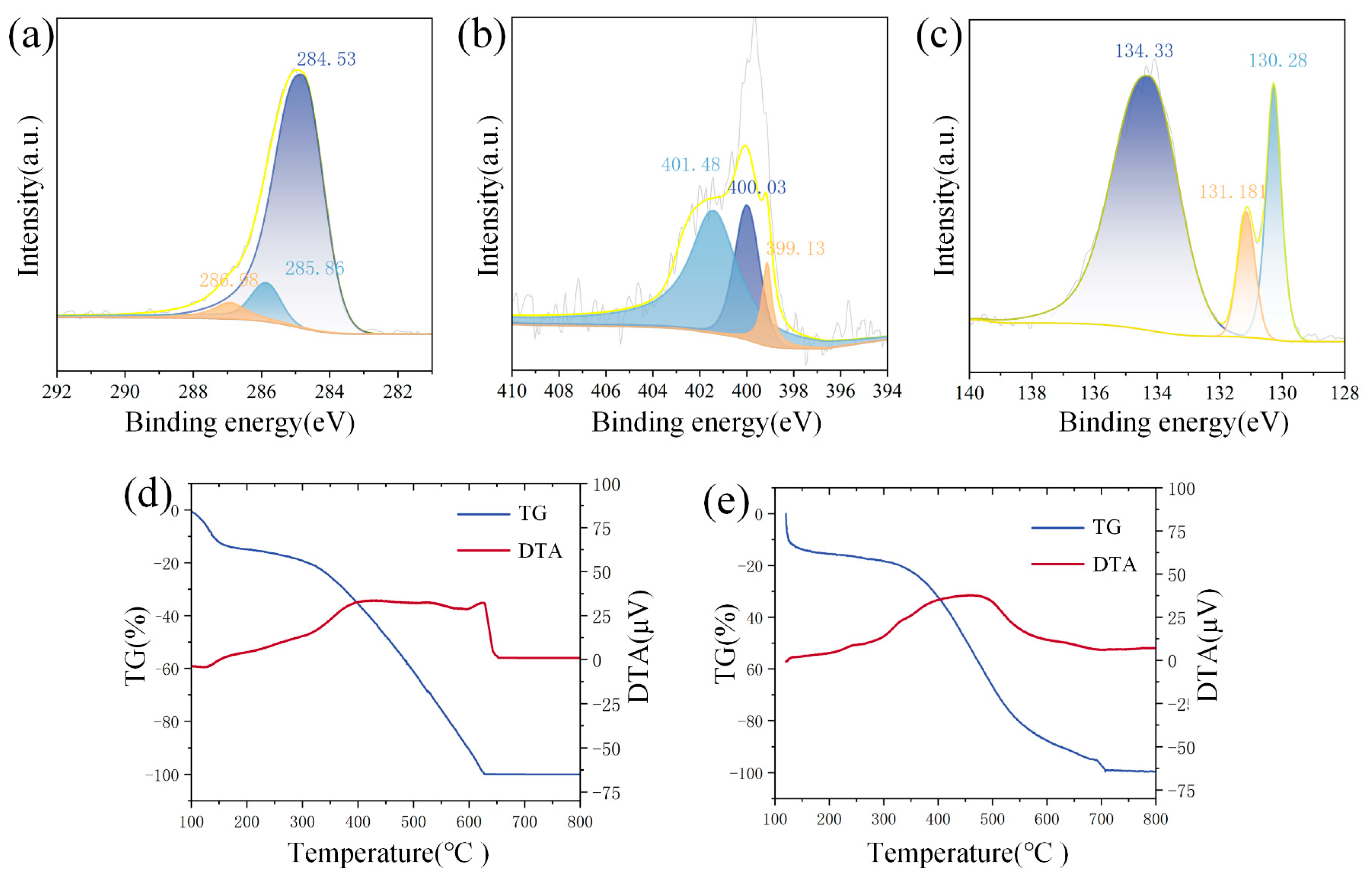
3.1. Characterization of Material
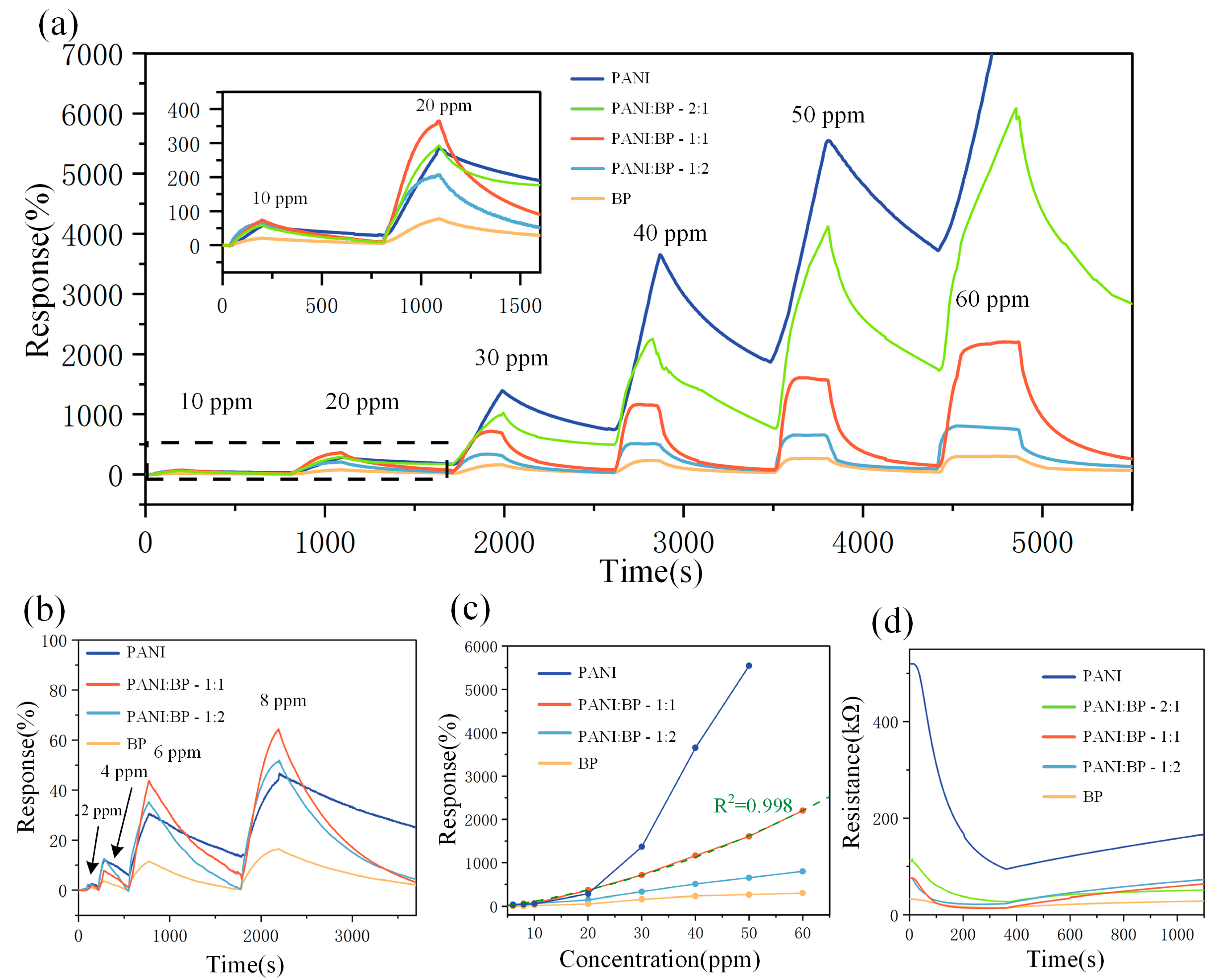
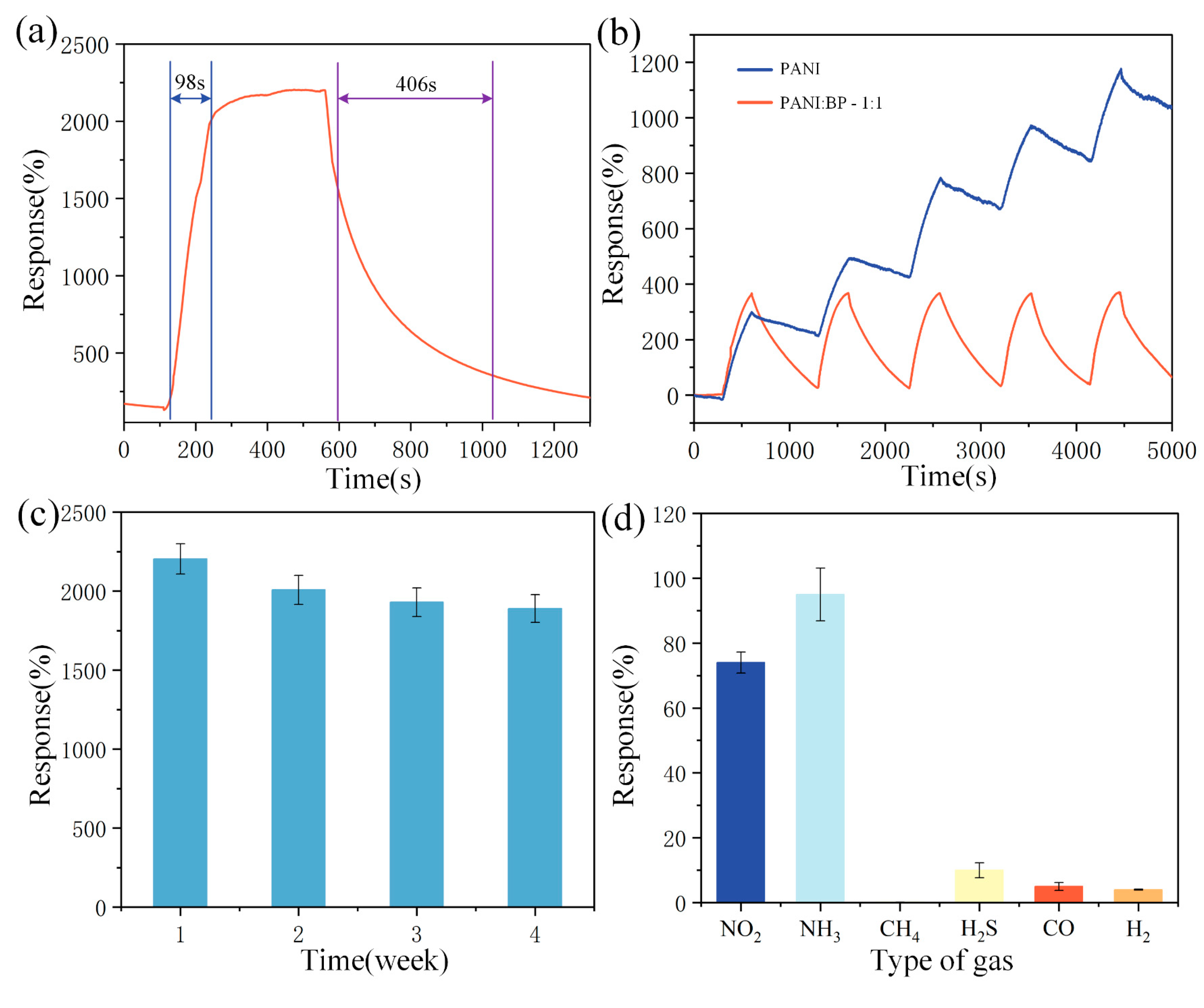
3.2. Gas-Sensitive Performance Testing
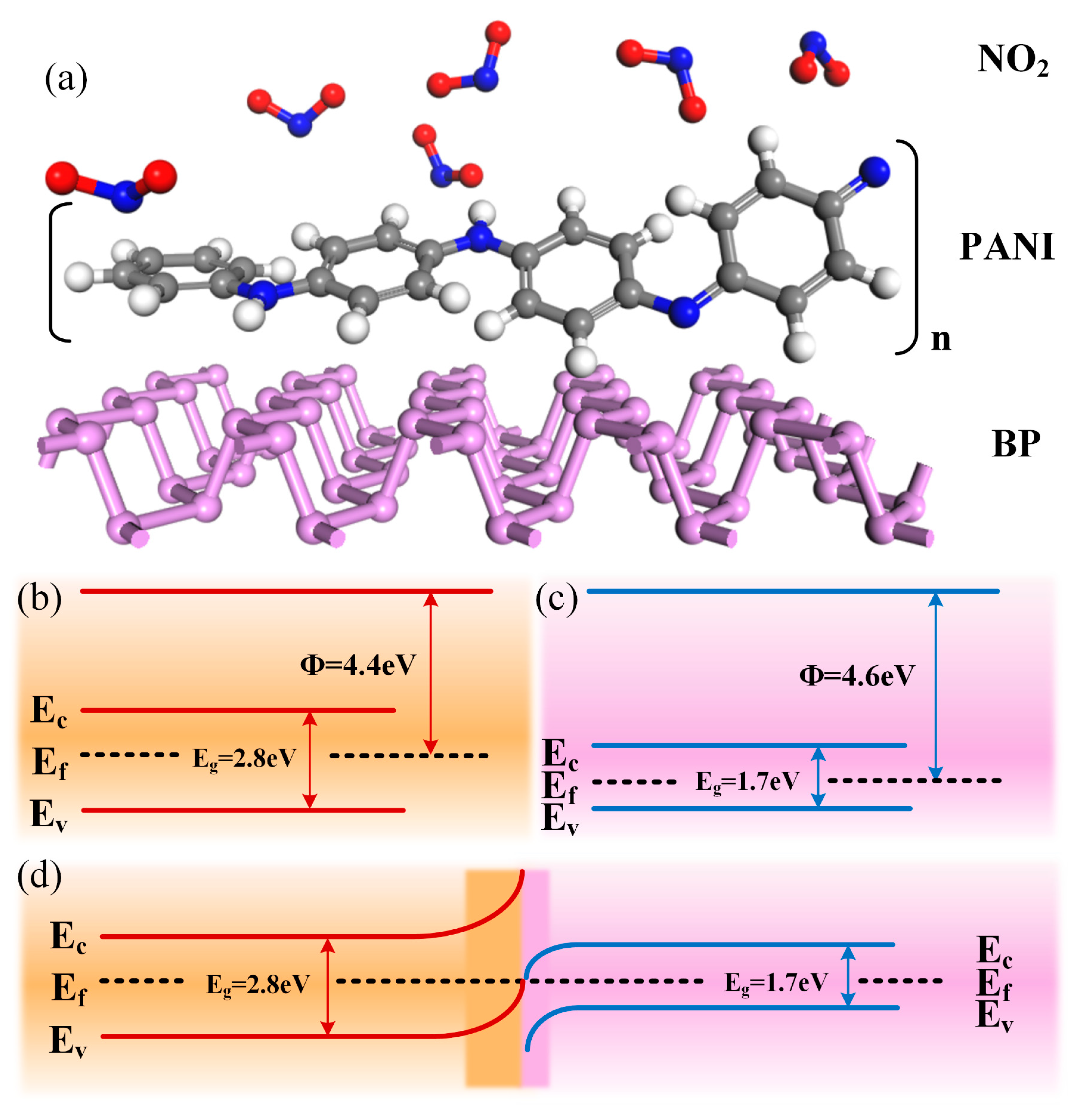
3.3. Gas-Sensitive Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gamon, L.F.; Wille, U. Oxidative Damage of Biomolecules by the Environmental Pollutants NO2 and NO3. Acc. Chem. Res. 2016, 49, 2136–2145. [Google Scholar] [CrossRef]
- Chauhan, A.J.; Inskip, H.M.; Linaker, C.H.; Smith, S.; Schreiber, J.; Johnston, S.L.; Holgate, S.T. Personal exposure to nitrogen dioxide (NO2) and the severity of virus-induced asthma in children. Lancet 2003, 361, 1939–1944. [Google Scholar] [CrossRef]
- Chapman, J.; Truong, V.K.; Elbourne, A.; Gangadoo, S.; Cheeseman, S.; Rajapaksha, P.; Latham, K.; Crawford, R.J.; Cozzolino, D. Combining Chemometrics and Sensors: Toward New Applications in Monitoring and Environmental Analysis. Chem. Rev. 2020, 120, 6048–6069. [Google Scholar] [CrossRef]
- Zhu, L.Y.; Ou, L.X.; Mao, L.W.; Wu, X.Y.; Liu, Y.P.; Lu, H.L. Advances in Noble Metal-Decorated Metal Oxide Nanomaterials for Chemiresistive Gas Sensors: Overview. Nanomicro Lett. 2023, 15, 89. [Google Scholar] [CrossRef] [PubMed]
- Burgues, J.; Marco, S. Low Power Operation of Temperature-Modulated Metal Oxide Semiconductor Gas Sensors. Sensors 2018, 18, 339. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Shi, Y.; Li, J.; Tang, J.; Feng, Q. Design, Simulation, and Fabrication of Multilayer Al2O3 Ceramic Micro-Hotplates for High Temperature Gas Sensors. Sensors 2022, 22, 6778. [Google Scholar] [CrossRef] [PubMed]
- Henriquez, D.; Kang, M.; Cho, I.; Choi, J.; Park, J.; Gul, O.; Ahn, J.; Lee, D.S.; Park, I. Low-Power, Multi-Transduction Nanosensor Array for Accurate Sensing of Flammable and Toxic Gases. Small Methods 2023, 7, e2201352. [Google Scholar] [CrossRef]
- Wu, H.; Bu, X.; Deng, M.; Chen, G.; Zhang, G.; Li, X.; Wang, X.; Liu, W. A Gas Sensing Channel Composited with Pristine and Oxygen Plasma-Treated Graphene. Sensors 2019, 19, 625. [Google Scholar] [CrossRef]
- Han, J.K.; Kang, M.; Jeong, J.; Cho, I.; Yu, J.M.; Yoon, K.J.; Park, I.; Choi, Y.K. Artificial Olfactory Neuron for an In-Sensor Neuromorphic Nose. Adv. Sci. 2022, 9, e2106017. [Google Scholar] [CrossRef]
- Farea, M.A.; Mohammed, H.Y.; Shirsat, S.M.; Sayyad, P.W.; Ingle, N.N.; Al-Gahouari, T.; Mahadik, M.M.; Bodkhe, G.A.; Shirsat, M.D. Hazardous gases sensors based on conducting polymer composites: Review. Chem. Phys. Lett. 2021, 776, 138703. [Google Scholar] [CrossRef]
- Wen, J.Y.; Wang, S.; Feng, J.Y.; Ma, J.X.; Zhang, H.; Wu, P.; Li, G.; Wu, Z.H.; Meng, F.Z.; Li, L.Q.; et al. Recent progress in polyaniline-based chemiresistive flexible gas sensors: Design, nanostructures, and composite materials. J. Mater. Chem. A 2024, 12, 6190–6210. [Google Scholar] [CrossRef]
- Sharma, S.; Hussain, S.; Singh, S.; Islam, S.S. MWCNT-conducting polymer composite based ammonia gas sensors: A new approach for complete recovery process. Sens. Actuators B Chem. 2014, 194, 213–219. [Google Scholar] [CrossRef]
- Sonker, R.K.; Yadav, B.C. Development of Fe2O3-PANI nanocomposite thin film based sensor for NO2 detection. J. Taiwan Inst. Chem. Eng. 2017, 77, 276–281. [Google Scholar] [CrossRef]
- Khalifa, M.; Anandhan, S. Highly sensitive and wearable NO2 gas sensor based on PVDF nanofabric containing embedded polyaniline/g-C3N4 nanosheet composites. Nanotechnology 2021, 32, 485504. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, P.F.; Xiao, K.T.; Meng, X.N.; Han, L.; Yu, C.C. Sensor Drift Compensation Based on the Improved LSTM and SVM Multi-Class Ensemble Learning Models. Sensors 2019, 19, 3844. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chu, R.Z.; Tang, Z.A. Metal Oxide Gas Sensor Drift Compensation Using a Two-Dimensional Classifier Ensemble. Sensors 2015, 15, 10180–10193. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, D.Q.; Chen, J.J.; Chen, Y.B.; Yang, T.; Cao, J.H. Gas-Sensor Drift Counteraction with Adaptive Active Learning for an Electronic Nose. Sensors 2018, 18, 4028. [Google Scholar] [CrossRef]
- Kim, H.; Uddin, S.Z.; Lien, D.H.; Yeh, M.; Azar, N.S.; Balendhran, S.; Kim, T.; Gupta, N.; Rho, Y.; Grigoropoulos, C.P.; et al. Actively variable-spectrum optoelectronics with black phosphorus. Nature 2021, 596, 232. [Google Scholar] [CrossRef]
- Dhanabalan, S.C.; Ponraj, J.S.; Guo, Z.N.; Li, S.J.; Bao, Q.L.; Zhang, H. Emerging Trends in Phosphorene Fabrication towards Next Generation Devices. Adv. Sci. 2017, 4, 1600305. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, M.X.; Guo, Z.N.; Miao, L.L.; Han, S.T.; Wang, Z.Y.; Zhang, X.W.; Zhang, H.; Peng, Z.C. Recent advances in black phosphorus-based photonics, electronics, sensors and energy devices. Mater. Horiz. 2017, 4, 997–1019. [Google Scholar] [CrossRef]
- Hu, Z.H.; Niu, T.C.; Guo, R.; Zhang, J.L.; Lai, M.; He, J.; Wang, L.; Chen, W. Two-dimensional black phosphorus: Its fabrication, functionalization and applications. Nanoscale 2018, 10, 21575–21603. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.Z.; Frauenheim, T.; Chen, C.F. Phosphorene as a Superior Gas Sensor: Selective Adsorption and Distinct I-V Response. J. Phys. Chem. Lett. 2014, 5, 2675–2681. [Google Scholar] [CrossRef]
- Abbas, A.N.; Liu, B.L.; Chen, L.; Ma, Y.Q.; Cong, S.; Aroonyadet, N.; Köpf, M.; Nilges, T.; Zhou, C.W. Black Phosphorus Gas Sensors. Acs Nano 2015, 9, 5618–5624. [Google Scholar] [CrossRef] [PubMed]
- Valt, M.; Caporali, M.; Fabbri, B.; Gaiardo, A.; Krik, S.; Iacob, E.; Vanzetti, L.; Malagù, C.; Banchelli, M.; D’Andrea, C.; et al. Air Stable Nickel-Decorated Black Phosphorus and Its Room-Temperature Chemiresistive Gas Sensor Capabilities. Acs Appl. Mater. Interfaces 2021, 13, 44711–44722. [Google Scholar] [CrossRef]
- Sajedi-Moghaddam, A.; Mayorga-Martinez, C.C.; Sofer, Z.; Bousa, D.; Saievar-Iranizad, E.; Pumera, M. Black Phosphorus Nanoflakes/Polyaniline Hybrid Material for High-Performance Pseudocapacitors. J. Phys. Chem. C 2017, 121, 20532–20538. [Google Scholar] [CrossRef]
- Jain, S.; Karmakar, N.; Shah, A.; Shimpi, N.G. Development of Ni doped ZnO/polyaniline nanocomposites as high response room temperature NO2 sensor. Mater. Sci. Eng. B-Adv. Funct. Solid-State Mater. 2019, 247, 114381. [Google Scholar] [CrossRef]
- Xu, H.Y.; Ju, D.X.; Li, W.R.; Gong, H.B.; Zhang, J.; Wang, J.Q.; Cao, B.Q. Low-working-temperature, fast-response-speed NO2 sensor with nanoporous-SnO2/polyaniline double-layered film. Sens. Actuators B Chem. 2016, 224, 654–660. [Google Scholar] [CrossRef]
- Reddy, N.R.; Anandhan, S. Polyaniline/poly(styrene-co-acrylonitrile) blend nanofibers exhibit enhanced ammonia and nitrogen dioxide sensing characteristics. J. Mater. Sci. Mater. Electron. 2016, 27, 13329–13337. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Cao, S.; Wu, Z.F.; Zhang, M.; Cao, Y.L.; Guo, J.X.; Zhong, F.R.; Duan, H.M.; Jia, D.Z. High-Performance Gas Sensor of Polyaniline/Carbon Nanotube Composites Promoted by Interface Engineering. Sensors 2020, 20, 149. [Google Scholar] [CrossRef]
- Kailasa, S.; Reddy, M.S.B.; Rani, B.G.; Maseed, H.; Rao, K.V. Twisted Polyaniline Nanobelts @ rGO for Room Temperature NO2 Sensing. Mater. Lett. 2019, 257, 126687. [Google Scholar] [CrossRef]
- Cui, S.M.; Pu, H.H.; Wells, S.A.; Wen, Z.H.; Mao, S.; Chang, J.B.; Hersam, M.C.; Chen, J.H. Ultrahigh sensitivity and layer-dependent sensing performance of phosphorene-based gas sensors. Nat. Commun. 2015, 6, 8632. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.Y.; Zhang, T.T.; Liu, P.; Rodriguez, J.A.; Liu, G.; Liu, M.H. Bandgap- and Local Field-Dependent Photoactivity of Ag/Black Phosphorus Nanohybrids. Acs Catal. 2016, 6, 8009–8020. [Google Scholar] [CrossRef]
| Materials | Sensing Range | Response | Res/Rec Time | Temperature | Reference |
|---|---|---|---|---|---|
| PANI/Ni@ZnO | 5–100 ppm | 75% (100 ppm) | 82/399 s | RT | [26] |
| SnO2/PANI | 5–50 ppm | 810% (37 ppm) | 17/25 s | 140 °C | [27] |
| PANI/g-C3N4 | 8–108 ppm | 92% (108 ppm) | RT | [14] | |
| PANI/SAN | 5–100 ppm | 72% (80 ppm) | 1/1900 s | 30 °C | [28] |
| PANI/CNT | 10–50 ppm | 65.9% (50 ppm) | 5.2/3.2 s | RT | [29] |
| PANI/rGO | 15–35 ppm | 21.56% (30 ppm) | 12/14 s | RT | [30] |
| PANI/BP | 2–60 ppm | 2204% (60 ppm) | 98/406 s | RT | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, B.; Shi, Y.; Liu, J.; Zheng, C.; Zhao, K.; Zhang, J.; Feng, Q. Low-Drift NO2 Sensor Based on Polyaniline/Black Phosphorus Composites at Room Temperature. Chemosensors 2024, 12, 181. https://doi.org/10.3390/chemosensors12090181
Tang B, Shi Y, Liu J, Zheng C, Zhao K, Zhang J, Feng Q. Low-Drift NO2 Sensor Based on Polyaniline/Black Phosphorus Composites at Room Temperature. Chemosensors. 2024; 12(9):181. https://doi.org/10.3390/chemosensors12090181
Chicago/Turabian StyleTang, Bolun, Yunbo Shi, Jijiang Liu, Canda Zheng, Kuo Zhao, Jianhua Zhang, and Qiaohua Feng. 2024. "Low-Drift NO2 Sensor Based on Polyaniline/Black Phosphorus Composites at Room Temperature" Chemosensors 12, no. 9: 181. https://doi.org/10.3390/chemosensors12090181
APA StyleTang, B., Shi, Y., Liu, J., Zheng, C., Zhao, K., Zhang, J., & Feng, Q. (2024). Low-Drift NO2 Sensor Based on Polyaniline/Black Phosphorus Composites at Room Temperature. Chemosensors, 12(9), 181. https://doi.org/10.3390/chemosensors12090181





