The Time Is Ripe: Olive Drupe Maturation Can Be Simply Evidenced by a Miniaturized, Portable and Easy-to-Use MicroNIR Green Sensor
Abstract
:1. Introduction
- − The pre-harvest drop causes significant losses on future oil production; the product obtained in any case from cascaded olives is of poor quality. In the cultivars subject to this phenomenon, it is good to anticipate the harvest.
- − Anticipating the harvest avoids both damage from atmospheric events and parasitic attacks.
- − The olives harvested early, with maturation in any case already completed, have both a more pleasant flavor, with lower acidity, and a better oil yield.
- − The prolonged stay of already-ripe olives on the plant leads the new buds not to differentiate, thus favoring the alternation of production.
1.1. In-Lab Analysis
1.2. In-Field Analysis
1.3. Aim of the Study
2. Materials and Methods
2.1. NIR Sample Analysis
2.2. The MicroNIR/Chemometric Platform
2.3. Parallel Chromatographic Analysis
3. Results and Discussion
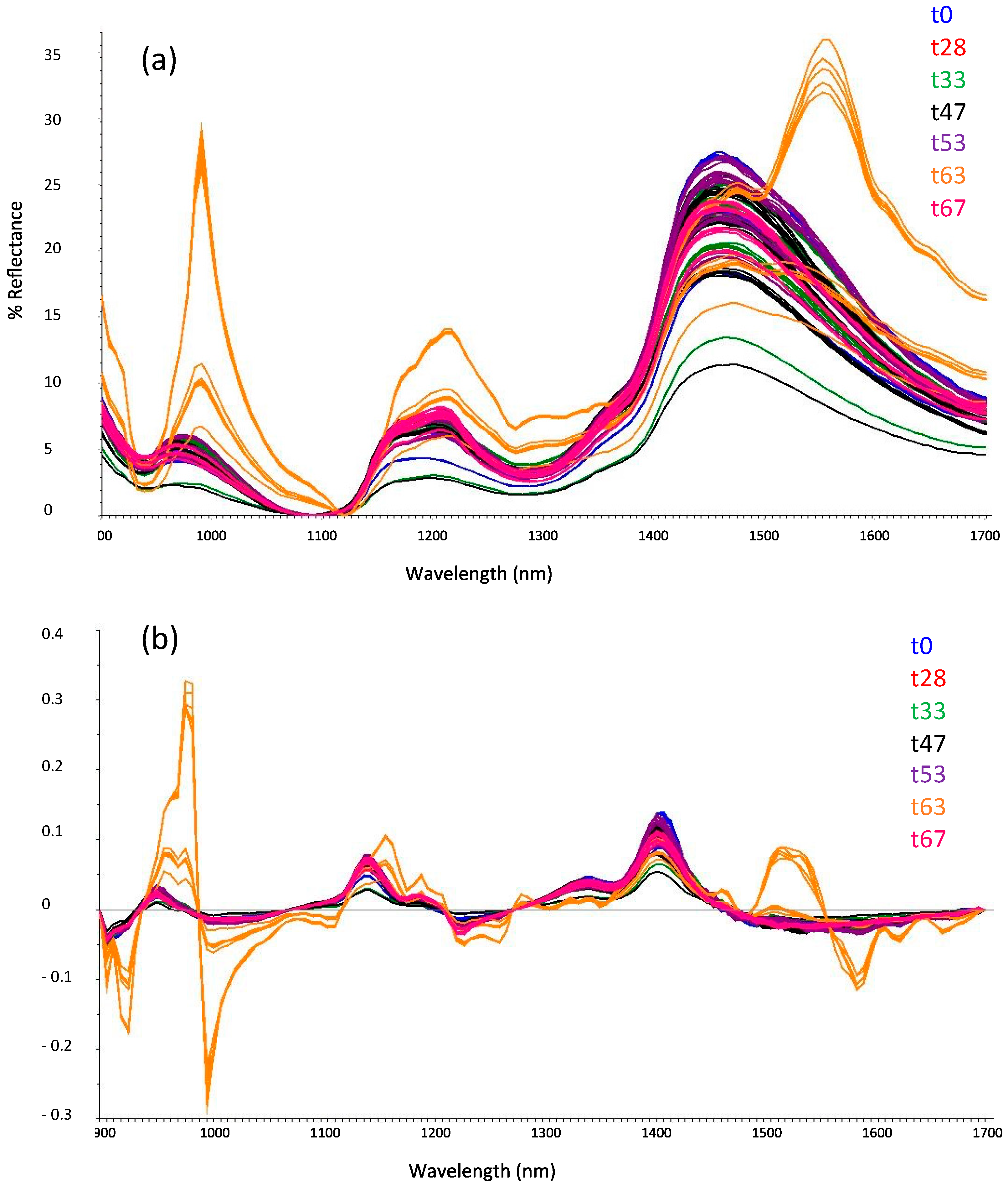
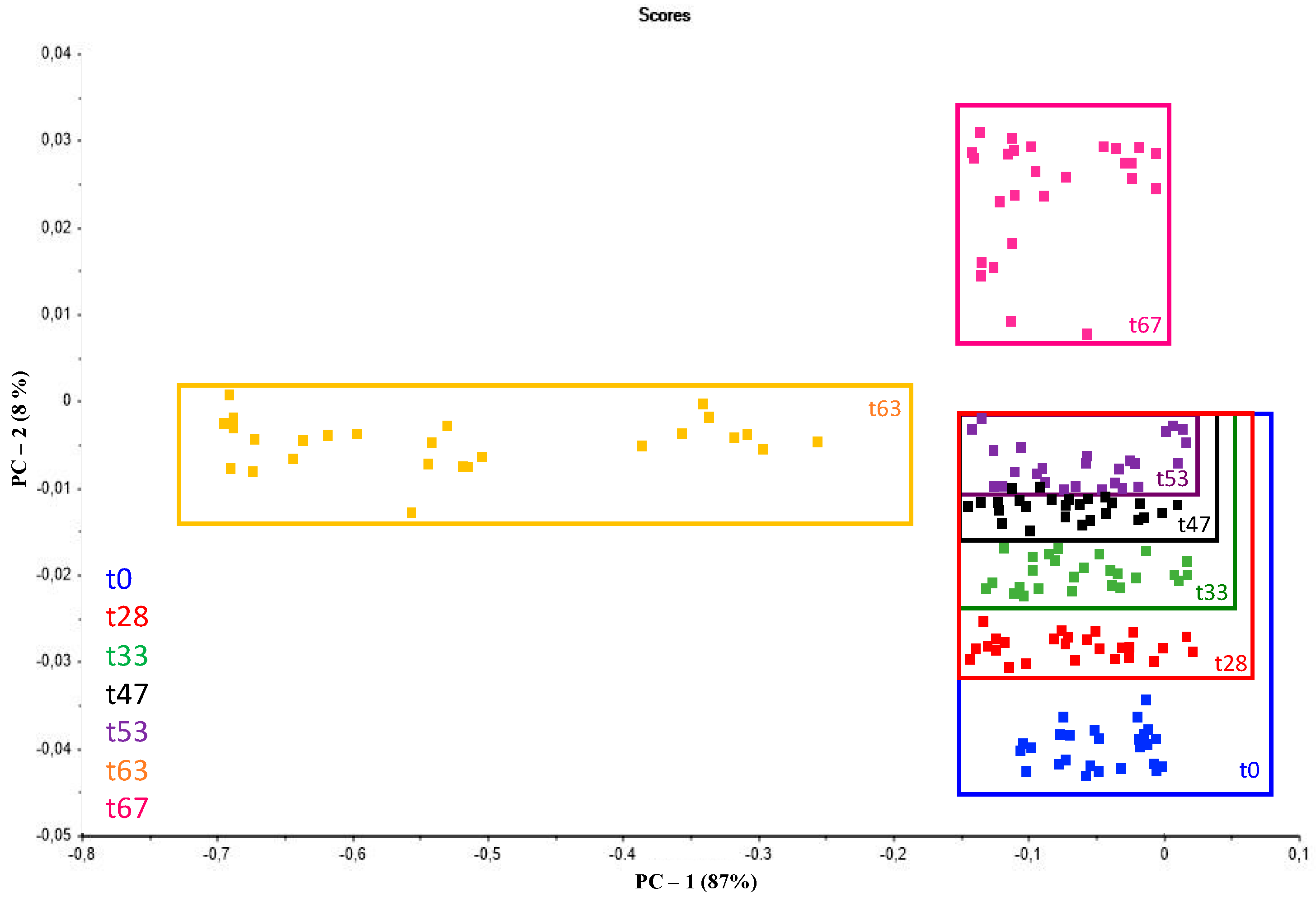
3.1. Portable Miniaturized Near-Infrared Spectroscopy Followed by Chemometrics
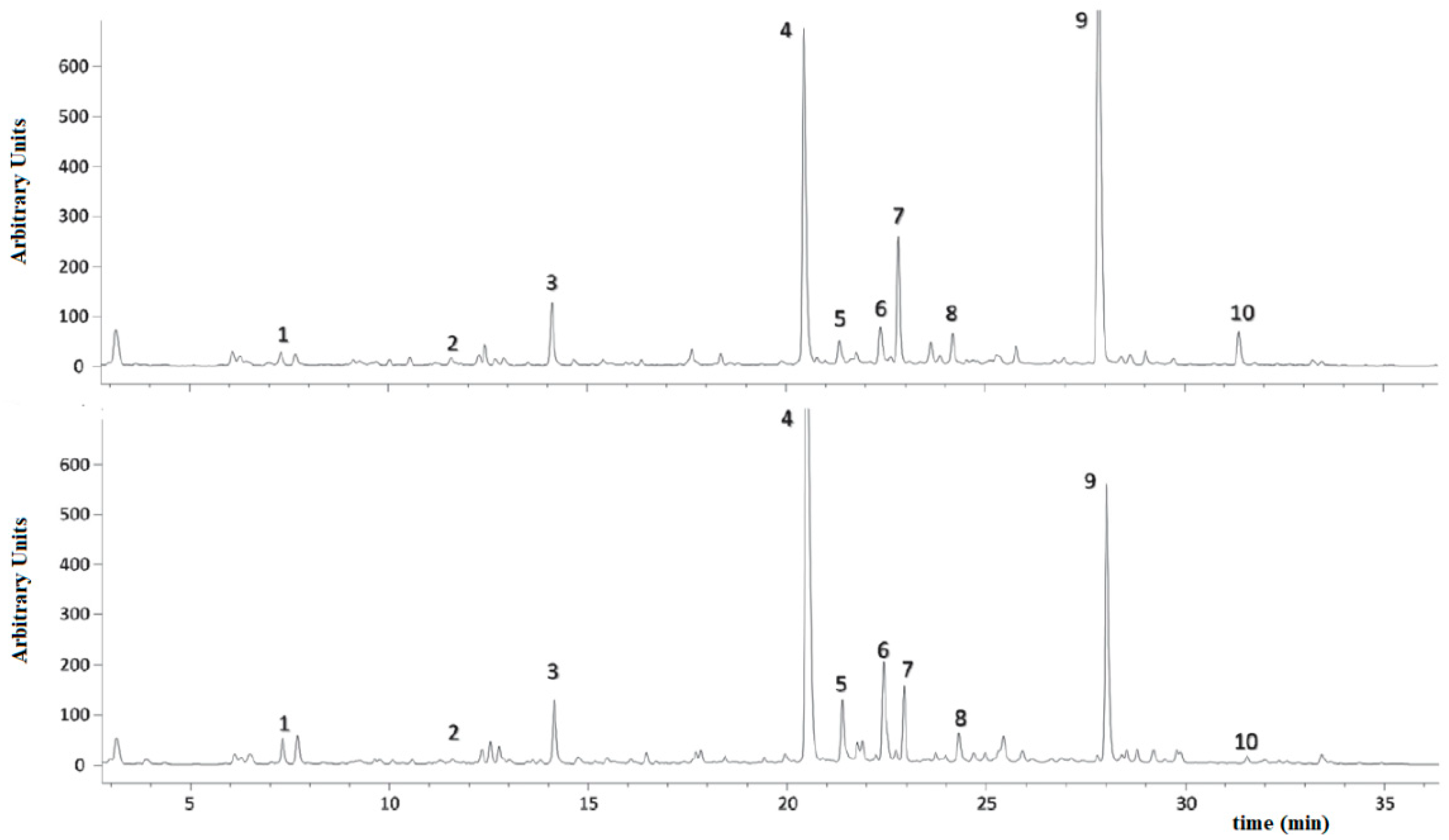
3.2. HPLC-UV and GC-MS Analysis
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- del Pilar Fernández-Poyatos, M.; Llorent-Martínez, E.J.; Ruiz-Medina, A. Effect of Ripening on the Phenolic Composition and Mineral Content of Three Varieties of Olive Fruits. Foods 2021, 10, 380. [Google Scholar] [CrossRef] [PubMed]
- Laribi, R.; Rovellini, P.; Defuoui, L.; Aidu, A.; Mettouchi, S.; Arrar, L.; Tamendjari, A. Evolution of biophenolic compounds in virgin olive oil during olive ripening of Algerian cultivars. Riv. Ital. Sostanze Grasse 2009, 86, 151–161. [Google Scholar]
- Ortega-Garcia, F.; Peragon, J. HPLC analysis of oleuropein, hydroxytyrosol, and tyrosol in stems and roots of Olea europaea L. cv. Picual during ripening. J. Sci. Food Agric. 2010, 90, 2295–2300. [Google Scholar] [CrossRef]
- Martakos, I.; Katsianou, P.; Koulis, G.; Efstratiou, E.; Nastou, E.; Nikas, S.; Dasenaki, M.; Pentogennis, M.; Thomaidis, N. Development of Analytical Strategies for the Determination of Olive Fruit Bioactive Compounds Using UPLC-HRMS and HPLC-DAD. Chemical Characterization of Kolovi Lesvos Variety as a Case Study. Molecules 2021, 26, 7182. [Google Scholar] [CrossRef] [PubMed]
- Del Coco, L.; Girelli, C.R.; Angile, F.; Mascio, I.; Montemurro, C.; Distaso, E.; Tamburrano, P.; Chiurlia, S.; Clodoveo, M.L.; Corbo, F.; et al. NMR-based metabolomic study of Apulian Coratina extra virgin olive oil extracted with a combined ultrasound and thermal conditioning process in an industrial setting. Food Chem. 2021, 345, 128778. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, R.; Patumi, M.; Fontanazza, G.; Barone, P.; Fiordiponti, P.; Mannina, L.; Rossi, E.; Segre, A.L. A high-field1H nuclear magnetic resonance study of the minor components in virgin olive oils. J. Am. Oil Chem. Soc. 1996, 73, 747–758. [Google Scholar] [CrossRef]
- Alonso-Salces, R.M.; Gallo, B.; Collado, M.I.; Sasía-Arriba, A.; Viacava, G.E.; García-González, D.L.; Gallina Toschi, T.; Servili, M.; Berrueta, L.A. 1H–NMR fingerprinting and supervised pattern recognition to evaluate the stability of virgin olive oil during storage. Food Control 2021, 123, 107831. [Google Scholar] [CrossRef]
- D’imperio, M.; Gobbino, M.; Picanza, A.; Costanzo, S.; Della Corte, A.; Mannina, L. Influence of Harvest Method and Period on Olive Oil Composition: An NMR and Statistical Study. J. Agric. Food Chem. 2010, 58, 11043–11051. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Tarapoulouzi, M.; Bedine Boat, M.A.; Rébufa, C.; Dupuy, N.; Theocharis, C.R.; Varzakas, T.; Roussos, S.; Artaud, J. Authentication and Chemometric Discrimination of Six Greek PDO Table Olive Varieties through Morphological Characteristics of Their Stones. Foods 2021, 10, 1829. [Google Scholar] [CrossRef]
- Bianco, L.; Alagna, F.; Baldoni, L.; Finnie, C.; Svensson, B.; Perrotta, G. Proteome regulation during Olea europaea fruit development. PLoS ONE 2013, 8, e53563. [Google Scholar] [CrossRef]
- Bruno, L.; Picardi, E.; Pacenza, M.; Chiappetta, A.; Muto, A.; Gagliardi, O.; Muzzalupo, I.; Pesole, G.; Bitonti, M.B. Changes in gene expression and metabolic profile of drupes of Olea europaea L. cv Carolea in relation to maturation stage and cultivation area. BMC Plant Biol. 2019, 19, 428. [Google Scholar] [CrossRef] [PubMed]
- Skodra, C.; Styliani Titeli, V.; Michailidis, M.; Bazakos, C.; Ganopoulos, I.; Molassiotis, A.; Tanou, G. Olive Fruit Development and Ripening: Break on through to the "-Omics" Side. Int. J. Mol. Sci. 2021, 22, 5806. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, V.J.; Baqueta, M.R.; Março, P.H.; Valderrama, P.; Visentainer, J.V. Proof-of-concept on the effect of human milk storage time: Lipid degradation and spectroscopic characterization using portable near-infrared spectrometer and chemometrics. Food Chem. 2022, 368, 30675. [Google Scholar] [CrossRef] [PubMed]
- Losso, K.; Bec, K.B.; Mayr, S.; Grabska, J.; Stuppner, S.; Jones, M.; Jakschitz, T.; Rainer, M.; Bonn, G.; Huck, G.C.W. Rapid discrimination of Curcuma longa and Curcuma xanthorrhiza using Direct Analysis in Real Time Mass Spectrometry and Near Infrared Spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 265, 120347. [Google Scholar] [CrossRef] [PubMed]
- Escuredo, O.; Meno, L.; Rodríguez-Flores, M.S.; Seijo, M.C. Rapid estimation of potato quality parameters by a portable near-infrared spectroscopy device. Sensors 2021, 21, 8222. [Google Scholar] [CrossRef]
- Pissard, A.; Marques, E.J.N.; Dardenne, P.; Lateur, M.; Pasquini, C.; Pimentel, M.F.; Fernández Pierna, J.A.; Baeten, V. Evaluation of a handheld ultra-compact NIR spectrometer for rapid and non-destructive determination of apple fruit quality. Postharvest Biol. Technol. 2021, 172, 111375. [Google Scholar] [CrossRef]
- Basri, K.N.; Hussain, M.N.; Bakar, J.; Khir, M.F.A.; Zoolfakar, A.S. Classification and quantification of palm oil adulteration via portable NIR spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 335–342. [Google Scholar] [CrossRef]
- Kappacher, C.; Trübenbacher, B.; Losso, K.; Rainer, M.; Bonn, G.; Huck, C.W. Portable vs. Benchtop NIR-Sensor Technology for Classification and Quality Evaluation of Black Truffle. Molecules 2022, 27, 589. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, J. Advanced chemometrics toward robust spectral analysis for fruit quality evaluation. Trends Food Sci. Technol. 2024, 150, 104612. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, L.; He, H.J.; Zhang, J.; Chew, K.W.; Ou, X. NIR sensors combined with chemometric algorithms in intelligent quality evaluation of sweetpotato roots from ‘Farm’to ‘Table’: Progresses, challenges, trends, and prospects. Food Chem. X 2024, 22, 101449. [Google Scholar] [CrossRef]
- Fonseca, A.C.S.; Pereira, J.F.Q.; Ricardo, S.; Bro, R.; Pimentel, M.F. Hierarchical classification models and handheld NIR spectrometer to human blood stains identification on different floor tiles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 267, 120533. [Google Scholar] [CrossRef] [PubMed]
- da Silva, N.C.; Cavalcanti, C.J.; Honorato, F.A.; Amigo, J.M.; Pimentel, M.F. Standardization from a benchtop to a handheld NIR spectrometer using mathematically mixed NIR spectra to determine fuel quality parameters. Anal. Chim. Acta 2017, 954, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Risoluti, R.; Materazzi, S. MicroNIR/Chemometrics assessment of occupational exposure to hydroxyurea. Front. Chem. 2018, 6, 228. [Google Scholar] [CrossRef] [PubMed]
- Risoluti, R.; Gullifa, G.; Battistini, A.; Materazzi, S. Monitoring of cannabinoids in hemp flours by MicroNIR/Chemometrics. Talanta 2020, 211, 120672. [Google Scholar] [CrossRef]
- Tao, F.; Liu, L.; Kucha, C.; Ngadi, M. Rapid and non-destructive detection of cassava flour adulterants in wheat flour using a handheld MicroNIR spectrometer. Biosyst. Eng. 2021, 203, 34–43. [Google Scholar] [CrossRef]
- Escuredo, O.; Rodríguez-Flores, M.S.; Meno, L.; Seijo, M.C. Prediction of physicochemical properties in honeys with portable near-infrared (Micronir) spectroscopy combined with multivariate data processing. Foods 2021, 10, 317. [Google Scholar] [CrossRef]
- Gooding, E.; Yu, P.; Solutions, V. Improving antibiotic yield with a MicroNIR spectrometer. In Applied Industrial Spectroscopy; Optica Publishing Group: Washington, DC, USA, 2021; Volume AW6E.3, p. 174071. [Google Scholar]
- Risoluti, R.; Gullifa, G.; Buiarelli, F.; Materazzi, S. Real time detection of amphetamine in oral fluids by MicroNIR/Chemometrics. Talanta 2020, 208, 120456. [Google Scholar] [CrossRef]
- Risoluti, R.; Gullifa, G.; Battistini, A.; Materazzi, S. MicroNIR/Chemometrics: A new analytical platform for fast and accurate detection of Δ9-Tetrahydrocannabinol (THC) in oral fluids. Drug Alcohol Depend. 2019, 205, 107578. [Google Scholar] [CrossRef]
- Modrono, S.; Soldado, A.; Martínez-Fernández, A.; de la Roza Delgado, B. Handheld NIRS sensors for routine compound feed quality control: Real time analysis and field monitoring. Talanta 2017, 162, 597–603. [Google Scholar] [CrossRef]
- Risoluti, R.; Gullifa, G.; Battistini, A.; Materazzi, S. “Lab-on-Click” Detection of Illicit Drugs in Oral Fluids by MicroNIR-Chemometrics. Anal. Chem. 2019, 91, 6435–6439. [Google Scholar] [CrossRef]
- Paiva, E.M.; Rohwedder, J.J.R.; Pasquini, C.; Pimentel, M.F.; Pereira, C.F. Quantification of biodiesel and adulteration with vegetable oils in diesel/biodiesel blends using portable near-infrared spectrometer. Fuel 2015, 160, 57–63. [Google Scholar] [CrossRef]
- Risoluti, R.; Materazzi, S.; Tau, F.; Russo, A.; Romolo, F.S. Towards innovation in paper dating: A MicroNIR analytical platform and chemometrics. Analyst 2018, 143, 4394–4399. [Google Scholar] [CrossRef] [PubMed]
- Risoluti, R.; Pichini, S.; Pacifici, R.; Materazzi, S. Miniaturized analytical platform for cocaine detection in oral fluids by MicroNIR/Chemometrics. Talanta 2019, 202, 546–553. [Google Scholar] [CrossRef] [PubMed]
- González-Cabrera, M.; Domínguez-Vidal, A.; Ayora-Cañada, M.J. Hyperspectral FTIR imaging of olive fruit for understanding ripening processes. Postharvest Biol. Technol. 2018, 145, 74–82. [Google Scholar] [CrossRef]
- Guzmán, E.; Baeten, V.; Fernández Pierna, J.A.; García-Mesa, J.A. A portable Raman sensor for the rapid discrimination of olives according to fruit quality. Talanta 2012, 93, 94–98. [Google Scholar] [CrossRef]
- León, L. Determination of Oil and Moisture Content in Intact Olive Fruits by Portable near Infrared Spectroscopy. NIR News 2012, 23, 4–8. [Google Scholar] [CrossRef]
- Furferi, R.; Governi, L.; Volpe, Y. ANN-based method for olive Ripening Index automatic prediction. J. Food Eng. 2010, 101, 318–328. [Google Scholar] [CrossRef]
- López-Sánchez, M.; Ayora-Cañada, M.J.; Molina-Díaz, A. Olive Fruit Growth and Ripening as Seen by Vibrational Spectroscopy. J. Agric. Food Chem. 2009, 58, 82–87. [Google Scholar] [CrossRef]
- Grassi, S.; Jolayemi, O.S.; Giovenzana, V.; Tugnolo, A.; Squeo, G.; Conte, P.; De Bruno, A.; Flamminii, F.; Casiraghi, E.; Alamprese, C. Near infrared spectroscopy as a green technology for the quality prediction of intact olives. Foods 2021, 10, 1042. [Google Scholar] [CrossRef]
- Cappelli, A.; Cividino, S.; Redaelli, V.; Tripodi, G.; Aiello, G.; Velotto, S.; Zaninelli, M. Applying spectroscopies, imaging analyses, and other non-destructive techniques to olives and extra virgin olive oil: A systematic review of current knowledge and future applications. Agriculture 2024, 14, 1160. [Google Scholar] [CrossRef]
- Barnes, R.J.; Dhanoa, M.S.; Lister, S.J. Standard Normal Variate Transformation and Detrending of Near-Infrared Diffuse Reflectance Spectra. Appl. Spectrosc. 1989, 43, 772–777. [Google Scholar] [CrossRef]
- Geladi, P.; MacDougall, D.; Martens, H. Linearization and scatter-correction for Near-Infrared reflectance spectra of meat. Appl. Spectrosc. 1985, 39, 491–500. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M. Chemometrics: Theory and Applications; Kowalski, B.R., Ed.; ACS Symposium Series 52; American Chemical Society: Washington, DC, USA, 1977; Volume 52, pp. 243–282. [Google Scholar]
- Savitzky, M.J.; Golay, E. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Rinnan, A.; van den Berg, F.; Engelsen, S.B. Review of the most common pre-processing techniques for near-infrared spectra. Trends Anal. Chem. 2009, 28, 10. [Google Scholar] [CrossRef]
- Risoluti, R.; Gullifa, G.; Battistini, A.; Materazzi, S. Development of a "single-click" analytical platform for the detection of cannabinoids in hemp seed oil. RSC Advances 2020, 10, 43394–43399. [Google Scholar] [CrossRef]
- Servili, M.; Sordini, B.; Esposto, S.; Taticchi, A.; Urbani, S.; Sebastiani, L. Metabolomics of olive fruit: A focus on the secondary metabolites. In The Olive Tree Genome; Springer: Cham, Switzerland, 2016; pp. 123–139. [Google Scholar]
- Gómez-González, S.; Ruiz-Jiménez, J.; Luque De Castro, M.D. Oil content and fatty acid profile of spanish cultivars during olive fruit ripening. J. Am. Oil Chem. Soc. 2011, 88, 1737–1745. [Google Scholar] [CrossRef]
- Cecchi, L.; Migliorini, M.; Cherubini, C.; Giusti, M.; Zanoni, B.; Innocenti, M.; Mulinacci, N. Phenolic profiles, oil amount and sugar content during olive ripening of three typical Tuscan cultivars to detect the best harvesting time for oil production. Food Res. Int. 2013, 54, 1876–1884. [Google Scholar] [CrossRef]
- Peragón, J. Time course of pentacyclic triterpeneoids from fruits and leaves of olive tree (Olea europaea L.) cv. Picual and cv. Cornezuelo during ripening. J. Agric. Food Chem. 2013, 61, 6671–6678. [Google Scholar] [CrossRef]
- Beltrán, G.; Bejaoui, M.A.; Jimenez, A.; Sanchez-Ortiz, A. Ethanol in Olive Fruit. Changes during Ripening. J. Agric. Food Chem. 2015, 63, 5309–5312. [Google Scholar] [CrossRef]
- Vicario, G.; Cantini, C.; Francini, A.; Raffaelli, A.; Cifelli, M.; Domenici, V.; Sebastiani, L. Olive Fruit Ripening Degree and Water Content Relationships with Phenolic Acids and Alcohols, Secoiridoids, Flavonoids and Pigments in Fruit and Oil. Molecules 2023, 28, 6943. [Google Scholar] [CrossRef]
- Xiaoxia, L.; Jianguo, Z.; Ying, L.; Guodong, R. Metabolome and Transcriptome Analyses Reveal Tissue-Specific Variations in Gene Expression and Metabolites of Olive. J. Plant Biol. 2020, 63, 73–82. [Google Scholar] [CrossRef]
- Aiello, D.; Siciliano, C.; Mazzotti, F.; Donna, L.D.; Risoluti, R.; Napoli, A. Protein extraction, enrichment and MALDI MS and MS/MS analysis from bitter orange leaves (citrus aurantium). Molecules 2020, 25, 1485. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, B.R.; Bender, C.F. An orthogonal feature selection method. Pattern Recognit. 1976, 8, 1–4. [Google Scholar] [CrossRef]



| Cultivar | Plant Number | Ripening | Location–Region | Notes |
|---|---|---|---|---|
| Leccino | 1 | Early | Central Italy–Lazio | Age 8 years |
| Rosciola | 1 | Early | Central Italy–Lazio | Age 10 years |
| Moraiolo | 1 | Early | Central Italy–Lazio | Age 8 years |
| Leccino | 2 | Early | Central Italy–Lazio | Age 10 years |
| Rosciola | 2 | Early | Central Italy–Lazio | Age > 20 years |
| Moraiolo | 2 | Early | Central Italy–Lazio | Age > 20 years |
| Leccino | 3 | Early | Central Italy–Lazio | Age 8 years |
| Rosciola | 3 | Early | Central Italy–Lazio | Age 10 years |
| Moraiolo | 3 | Early | Central Italy–Lazio | Age > 20 years |
| Cardoncella | 1 | Medium-early | Central Italy–Lazio | Age 11 years |
| Frantoio | 1 | Late | Central Italy–Lazio | Age 7 years |
| Leccino | 4 | Early | Central Italy–Umbria | Age > 20 years |
| Rosciola | 4 | Early | Central Italy–Umbria | Age > 20 years |
| Moraiolo | 4 | Early | Central Italy–Umbria | Age > 20 years |
| Cardoncella | 2 | Medium-early | Central Italy–Umbria | Age > 20 years |
| Cardoncella | 3 | Medium-early | Central Italy–Umbria | Age > 20 years |
| Frantoio | 2 | Late | Central Italy–Umbria | Age 7 years |
| Leccino | 5 | Early | Central Italy–Tuscany | Age 8 years |
| Leccino | 6 | Early | Central Italy–Tuscany | Age 11 years |
| Frantoio | 3 | Late | Central Italy–Tuscany | Age > 20 years |
| Frantoio | 4 | Late | Central Italy–Tuscany | Age > 20 years |
| Moraiolo | 5 | Early | Central Italy–Tuscany | Age > 20 years |
| Leccino | 7 | Early | South Italy–Puglia | Age > 20 years |
| Leccino | 8 | Early | South Italy–Puglia | Age > 20 years |
| Leccino | 9 | Early | South Italy–Puglia | Age > 20 years |
| Leccino | 10 | Early | South Italy–Puglia | Age > 20 years |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gullifa, G.; Albertini, C.; Ruocco, M.; Risoluti, R.; Materazzi, S. The Time Is Ripe: Olive Drupe Maturation Can Be Simply Evidenced by a Miniaturized, Portable and Easy-to-Use MicroNIR Green Sensor. Chemosensors 2024, 12, 182. https://doi.org/10.3390/chemosensors12090182
Gullifa G, Albertini C, Ruocco M, Risoluti R, Materazzi S. The Time Is Ripe: Olive Drupe Maturation Can Be Simply Evidenced by a Miniaturized, Portable and Easy-to-Use MicroNIR Green Sensor. Chemosensors. 2024; 12(9):182. https://doi.org/10.3390/chemosensors12090182
Chicago/Turabian StyleGullifa, Giuseppina, Chiara Albertini, Marialuisa Ruocco, Roberta Risoluti, and Stefano Materazzi. 2024. "The Time Is Ripe: Olive Drupe Maturation Can Be Simply Evidenced by a Miniaturized, Portable and Easy-to-Use MicroNIR Green Sensor" Chemosensors 12, no. 9: 182. https://doi.org/10.3390/chemosensors12090182
APA StyleGullifa, G., Albertini, C., Ruocco, M., Risoluti, R., & Materazzi, S. (2024). The Time Is Ripe: Olive Drupe Maturation Can Be Simply Evidenced by a Miniaturized, Portable and Easy-to-Use MicroNIR Green Sensor. Chemosensors, 12(9), 182. https://doi.org/10.3390/chemosensors12090182







