O2-Sensitive Inks for Measuring Total (Aerobic) Viable Count Using Micro-Respirometry
Abstract
:1. Introduction
| Company | Product | Dye | Cost ($) ** | τo */μs | τ(air) */μs | Ksv/%O2−1 | Ref. |
|---|---|---|---|---|---|---|---|
| Oculer | TVC Assay | PtBP | 6.5 | 44.1 | 22.5 | 0.046 | [23] |
| PyroScience | OXSP5 | PtBP | 33 | 52.5 | 20.8 | 0.073 | [24] |
| PreSens | PSt3 | PtTFPP | 33 | 59.0 | 17.5 | 0.115 | [24] |
| Ocean Insight | FOSPOR | PtTFPP | 36 | 22.4 | 9.1 | 0.070 | [24] |
| OxySense | O2xyDot | [Ru(dpp)3](ClO4)2 | 4.3 | 6.5 | 0.9 | 0.30 | [25] |
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of O2-Sensitive Inks
2.2.2. Preparation of E. coli Stock and Calibration Dispersion of Bacteria
2.2.3. μR-TVC Using O2-Sensitive Inks
2.2.4. Other Methods
3. Results and Discussion
3.1. Modelling the Effect of O2 Sensor Sensitivity on μR-TVC
3.2. Characterisation of the PtBP/PVB O2 Sensor in Growth Medium
3.3. μR-TVC Using a PtBP/PVB Ink and the Oculer Rapid 930
3.4. Micro-Respirometry Using a PtTFPP/PVB Ink
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elisseeva, S.; Santovito, E.; Linehan, E.; Kerry, J.P.; Papkovsky, D.B. Performance assessment of the two oxygen sensor based respirometric platforms with complex media and in selective bacterial assays. Sens. Actuators B 2023, 383, 133582. [Google Scholar] [CrossRef]
- ISO 18593:2018; Microbiology of the Food Chain—Horizontal Methods for Surface Sampling. International Organization for Standardization: Geneva, Switzerland, 2018.
- ISO 4833-1:2013; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony count at 30 °C by the Pour Plate Technique. International Organization for Standardization: Geneva, Switzerland, 2013.
- Tanaka, Y.; Takahashi, H.; Imai, A.; Asao, T.; Kozaki, S.; Igimi, S.; Kimura, B. Reconsideration of flexibility in verifying rapid alternative food microbiological methods. Food Control 2010, 21, 1075–1079. [Google Scholar] [CrossRef]
- Jasson, V.; Jacxsens, L.; Luning, P.; Rajkovic, A.; Uyttendaele, M. Alternative microbial methods: An overview and selection criteria. Food Microbiol. 2010, 27, 710–730. [Google Scholar] [CrossRef] [PubMed]
- Santovito, E.; Elisseeva, S.; Smyth, C.; Cruz-Romero, M.; Kerry, J.P.; Duffy, G.; Papkovsky, D.B. A sensor-based system for rapid on-site testing of microbial contamination in meat samples and carcasses. J. Appl. Microbiol. 2022, 132, 1210–1220. [Google Scholar] [CrossRef]
- Hempel, A.; Borchert, N.; Walsh, H.; Choudhury, K.R.; Kerry, J.P.; Papkovsky, D.B. Analysis of Total Aerobic Viable Counts in Raw Fish by High-Throughput Optical Oxygen Respirometry. J. Food Prot. 2011, 74, 776–782. [Google Scholar] [CrossRef]
- Fernandes, R.; Carey, C.; Hynes, J.; Papkovsky, D. GreenLight™ model 960. J. AOAC Int. 2013, 96, 369–385. [Google Scholar] [CrossRef]
- The Oculer 930 Series. Available online: https://oculer.com/technology_tech_Soft.html (accessed on 24 June 2024).
- Papkovsky, D.B.; Kerry, J.P. Oxygen Sensor-Based Respirometry and the Landscape of Microbial Testing Methods as Applicable to Food and Beverage Matrices. Sensors 2023, 23, 4519. [Google Scholar] [CrossRef] [PubMed]
- Hałasa, R.; Turecka, K.; Smoktunowicz, M.; Mizerska, U.; Orlewska, C. Application of tris-(4, 7-Diphenyl-1, 10 phenanthroline) ruthenium (II) Dichloride to Detection of Microorganisms in Pharmaceutical Products. Pharmaceuticals 2023, 16, 856. [Google Scholar] [CrossRef] [PubMed]
- Santovito, E.; Elisseeva, S.; Kerry, J.P.; Papkovsky, D.B. Rapid detection of bacterial load in food samples using disposable respirometric sensor sachets. Sens. Actuators B 2023, 390, 134016. [Google Scholar] [CrossRef]
- Keb-Fonseca, G.; Guerrero-Barajas, C.; Ordaz, A. Characterization of the biological sulfide oxidation process: In-situ pulse respirometry and ex-situ pulse microrespirometry approach. Rev. Mex. Ing. Quim. 2022, 21, IA2575. [Google Scholar] [CrossRef]
- Müller, B.; Sulzer, P.; Walch, M.; Zirath, H.; Buryška, T.; Rothbauer, M.; Ertl, P.; Mayr, T. Measurement of respiration and acidification rates of mammalian cells in thermoplastic microfluidic devices. Sens. Actuators B 2021, 334, 129664. [Google Scholar] [CrossRef]
- Gwak, J.-H.; Awala, S.I.; Nguyen, N.-L.; Yu, W.-J.; Yang, H.-Y.; von Bergen, M.; Jehmlich, N.; Kits, K.D.; Loy, A.; Dunfield, P.F. Sulfur and methane oxidation by a single microorganism. Proc. Natl. Acad. Sci. USA 2022, 119, e2114799119. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Camacho, I.; Chavarría-Hernández, N.; del Rocío López-Cuellar, M.; Guerrero-Barajas, C.; Ordaz, A. Microrespirometric Validation of a Two-stage Process for Polyhydroxyalkanoates Production from Peanut Oil and Propionate with Cupriavidus necator. Open Chem. Eng. J. 2024, 18, e18741231301420. [Google Scholar] [CrossRef]
- Li, L.; Zhdanov, A.V.; Papkovsky, D.B. Advanced multimodal solid-state optochemical pH and dual pH/O2 sensors for cell analysis. Sens. Actuators B 2022, 371, 132486. [Google Scholar] [CrossRef]
- Zanetti, C.; Li, L.; Gaspar, R.D.L.; Santovito, E.; Elisseeva, S.; Collins, S.G.; Maguire, A.R.; Papkovsky, D.B. Susceptibility of the Different Oxygen-Sensing Probes to Interferences in Respirometric Bacterial Assays with Complex Media. Sensors 2024, 24, 267. [Google Scholar] [CrossRef]
- Flamholz, A.I.; Saccomano, S.; Cash, K.; Newman, D.K. Optical O2 sensors also respond to redox active molecules commonly secreted by bacteria. mBio 2022, 13, e02076-22. [Google Scholar] [CrossRef]
- Kelly, C.; Yusufu, D.; Okkelman, I.; Banerjee, S.; Kerry, J.P.; Mills, A.; Papkovsky, D.B. Extruded phosphorescence based oxygen sensors for large-scale packaging applications. Sens. Actuators B 2020, 304, 127357. [Google Scholar] [CrossRef]
- Quaranta, M.; Borisov, S.M.; Klimant, I. Indicators for optical oxygen sensors. Bioanal. Rev. 2012, 4, 115–157. [Google Scholar]
- Santovito, E.; Elisseeva, S.; Bukulin, A.; Kerry, J.P.; Papkovsky, D.B. Facile biosensor-based system for on-site quantification of total viable counts in food and environmental swabs. Biosens. Bioelectron. 2021, 176, 112938. [Google Scholar] [CrossRef]
- Automated TVC Enumeration. Available online: https://www.oculer.com/assays/total-viable-count (accessed on 24 June 2024).
- Yusufu, D.; Han, R.; Mills, A. 3D printed O2 indicators. Analyst 2020, 145, 4124–4129. [Google Scholar] [CrossRef]
- Banerjee, S.; Kelly, C.; Kerry, J.P.; Papkovsky, D.B. High throughput non-destructive assessment of quality and safety of packaged food products using phosphorescent oxygen sensors. Trends Food Sci. Technol. 2016, 50, 85–102. [Google Scholar] [CrossRef]
- Mills, A. Effect of plasticizer viscosity on the sensitivity of an [Ru(bpy)32+(Ph4B−)2]-based optical oxygen sensor. Analyst 1998, 123, 1135–1140. [Google Scholar] [CrossRef]
- Pt(II) meso-Tetraphenyl Tetrabenzoporphine. Available online: https://www.fishersci.com/shop/products/pd-ii-meso-tetraphenyl-te50mg/501374487 (accessed on 24 June 2024).
- Pt(II) meso-Tetra(pentafluorophenyl)porphine. Available online: https://frontierspecialtychemicals.com/product/ptii-meso-tetrapentafluorophenylporphine/ (accessed on 24 June 2024).


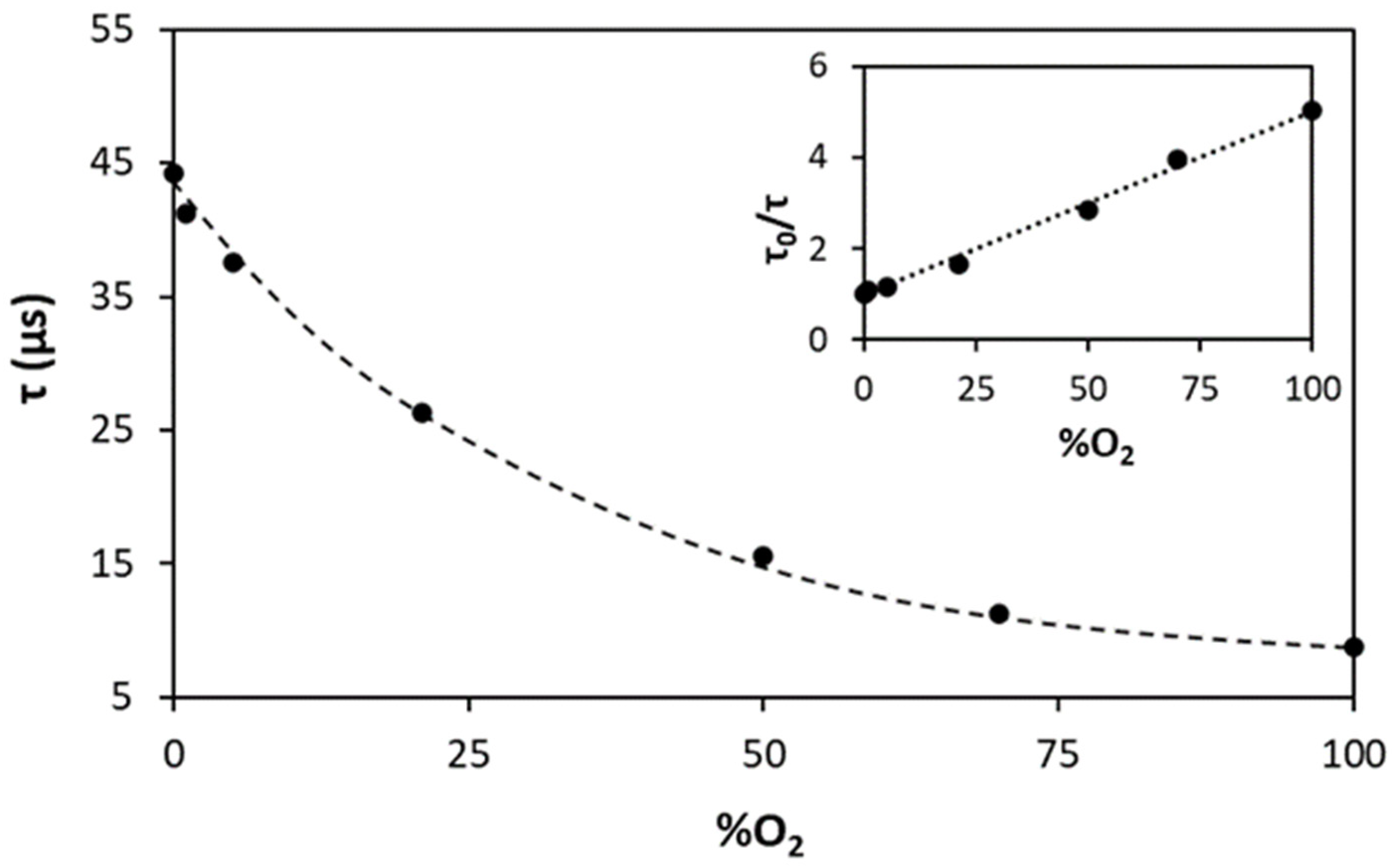
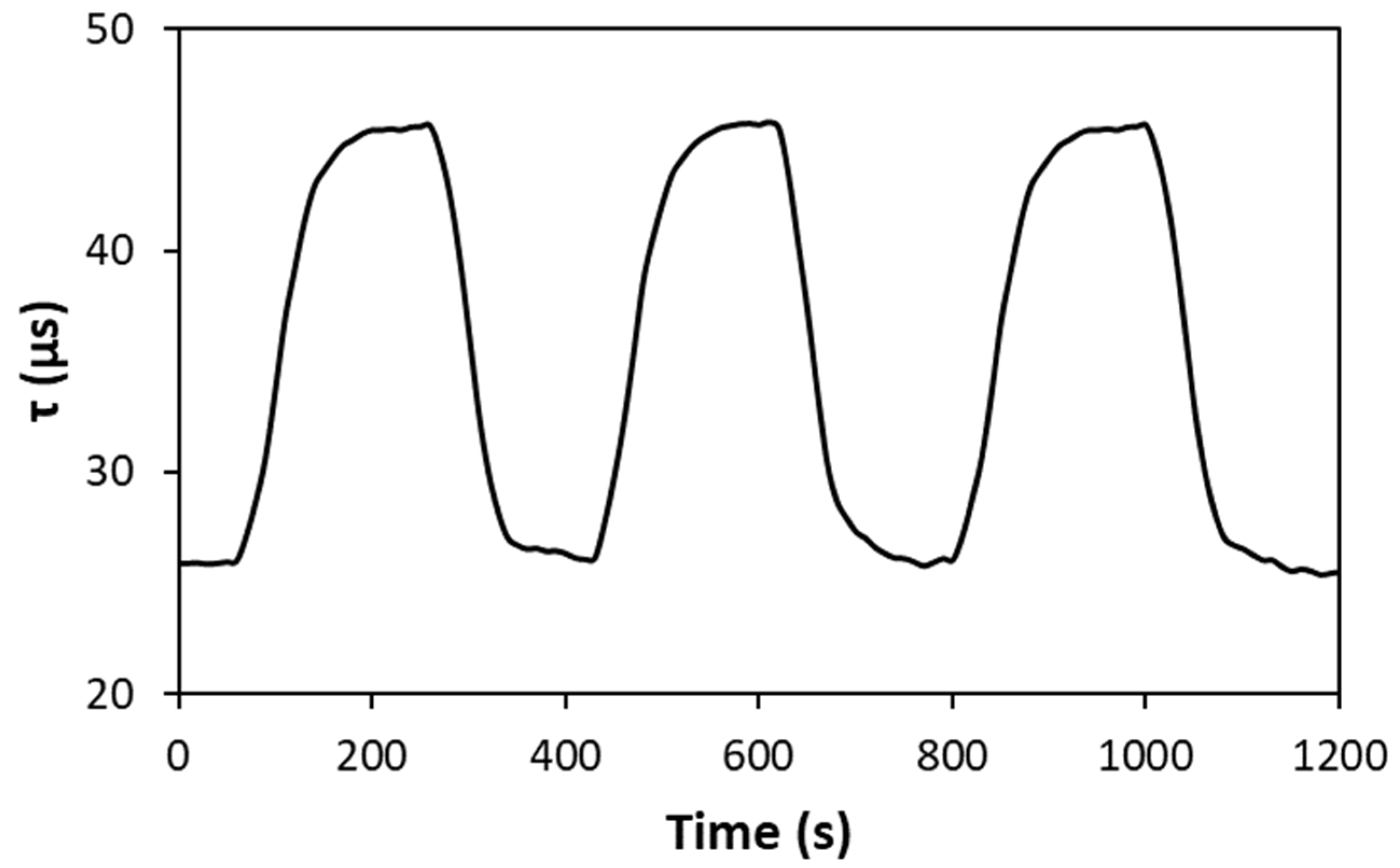


| Ink (Dye/Polymer) | Preparation |
|---|---|
| Dye */PVB | A 0.1% w/v dye solution was prepared by adding 3 mg dye to 3 mL dichloromethane (DCM). The ink was then prepared by adding 0.1 g of the 0.1% w/v dye solution to 1 g of a 10% w/v PVB/ethanol (EtOH) solution. |
| Dye */PS | As above, but using a 5% w/v PS/ethyl acetate solution. |
| Dye */PVC | As above, but using a 2% w/v PVC/DCM solution. |
| Dye | Polymer | τo/μs | τ(air)/μs | KSV/%O2−1 | TT */h |
|---|---|---|---|---|---|
| PtBP | PVB | 41.6 | 20.4 | 0.050 | 3.80 |
| PS | 46.2 | 11.0 | 0.152 | 3.80 | |
| PVC | 45.7 | 30.2 | 0.024 | 3.85 | |
| PdBP | PVB | 290.0 | 72.0 | 0.144 | 3.60 |
| [Ru(dpp)32+(Ph4B2)2] | PVB | 6.0 | 5.6 | 0.003 | 3.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cross, S.; Yusufu, D.; O’Rourke, C.; Mills, A. O2-Sensitive Inks for Measuring Total (Aerobic) Viable Count Using Micro-Respirometry. Chemosensors 2024, 12, 190. https://doi.org/10.3390/chemosensors12090190
Cross S, Yusufu D, O’Rourke C, Mills A. O2-Sensitive Inks for Measuring Total (Aerobic) Viable Count Using Micro-Respirometry. Chemosensors. 2024; 12(9):190. https://doi.org/10.3390/chemosensors12090190
Chicago/Turabian StyleCross, Sean, Dilidaer Yusufu, Christopher O’Rourke, and Andrew Mills. 2024. "O2-Sensitive Inks for Measuring Total (Aerobic) Viable Count Using Micro-Respirometry" Chemosensors 12, no. 9: 190. https://doi.org/10.3390/chemosensors12090190






