Oriented Immobilization of IgG for Immunosensor Development
Abstract
:1. Introduction
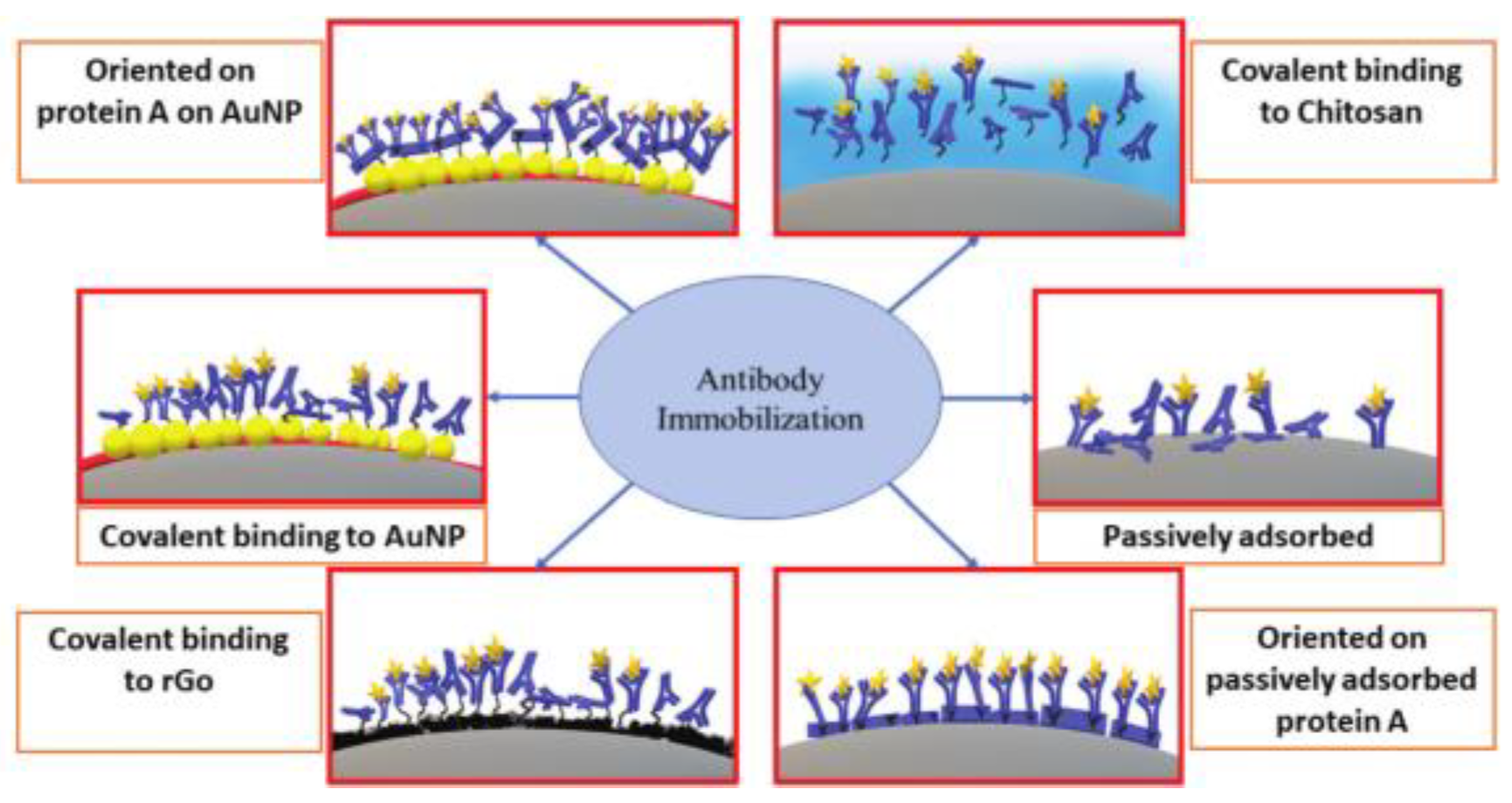
2. Strategies for Immobilization Design Approaches
2.1. Plasma Treatment Substrate
2.2. Three-Dimensional Substrate
2.3. Self-Assembled Monolayer Substrate
2.4. Layer-by-Layer Assembly Substrate
3. Covalent Binding Pathway
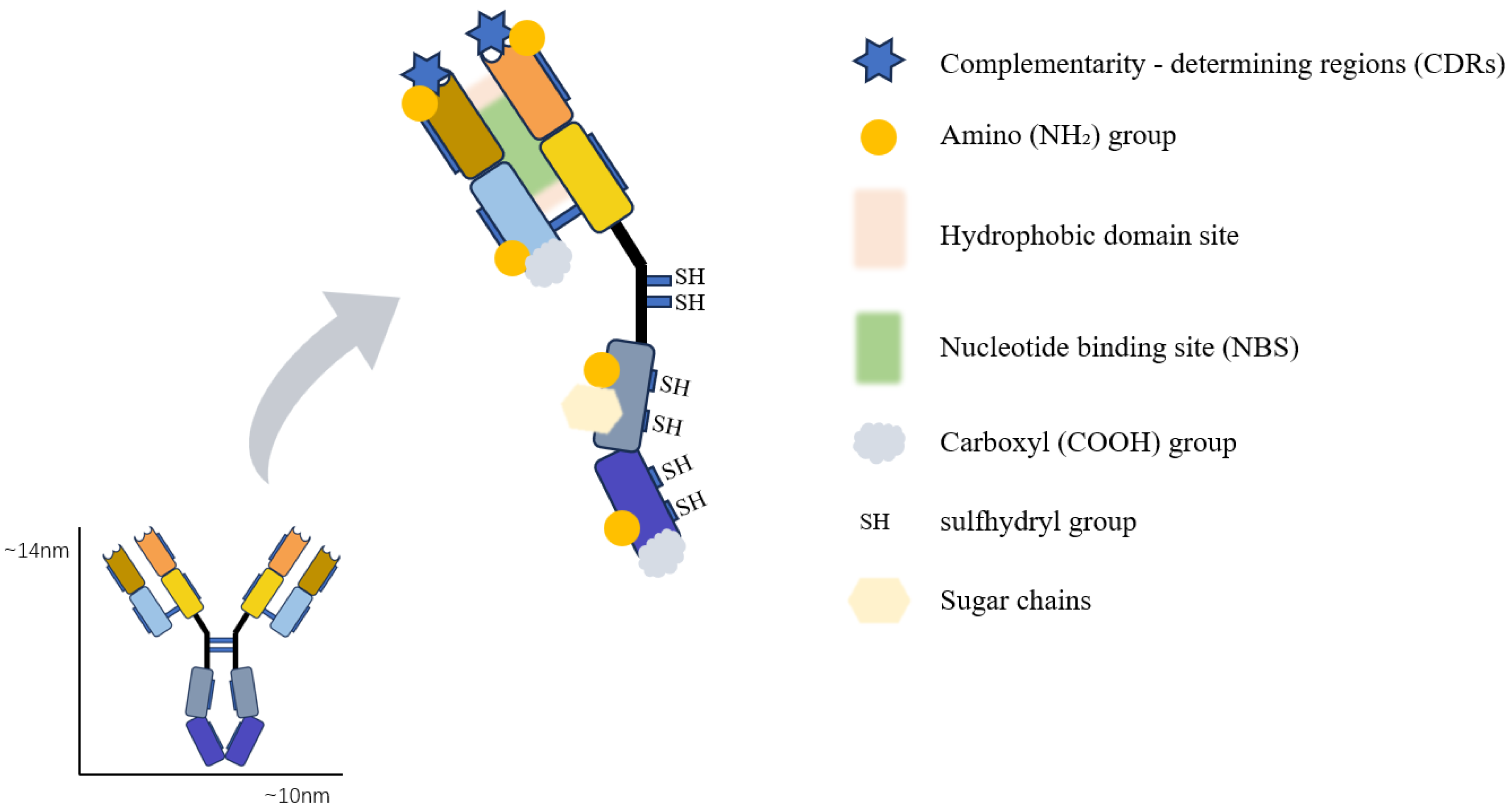
3.1. Nucleotide-Binding Site (NBS)
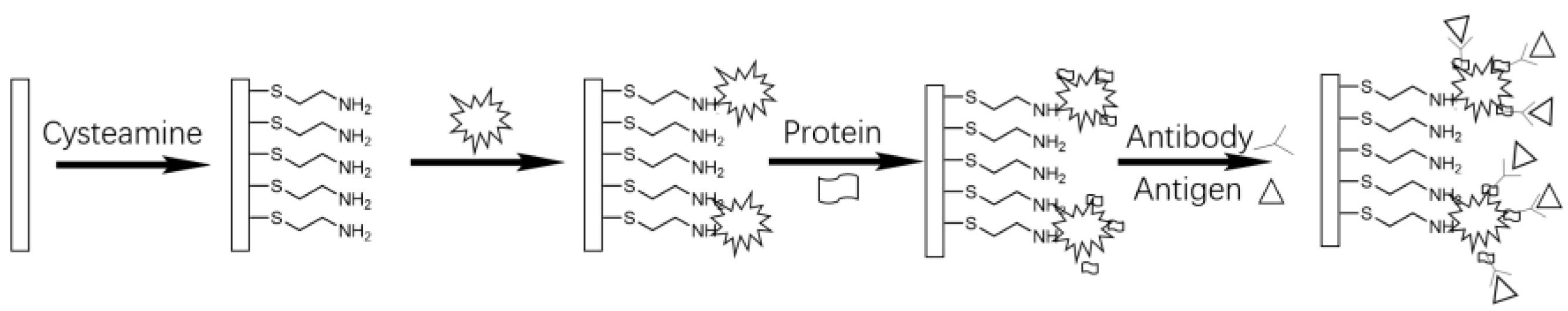
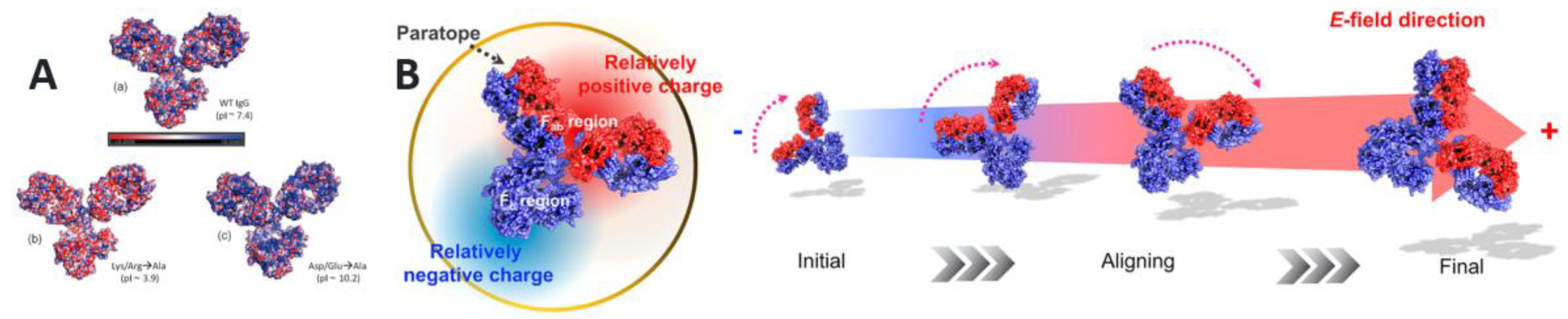
3.2. Amine, Carboxyl, and Hydroxyl Binding Sites
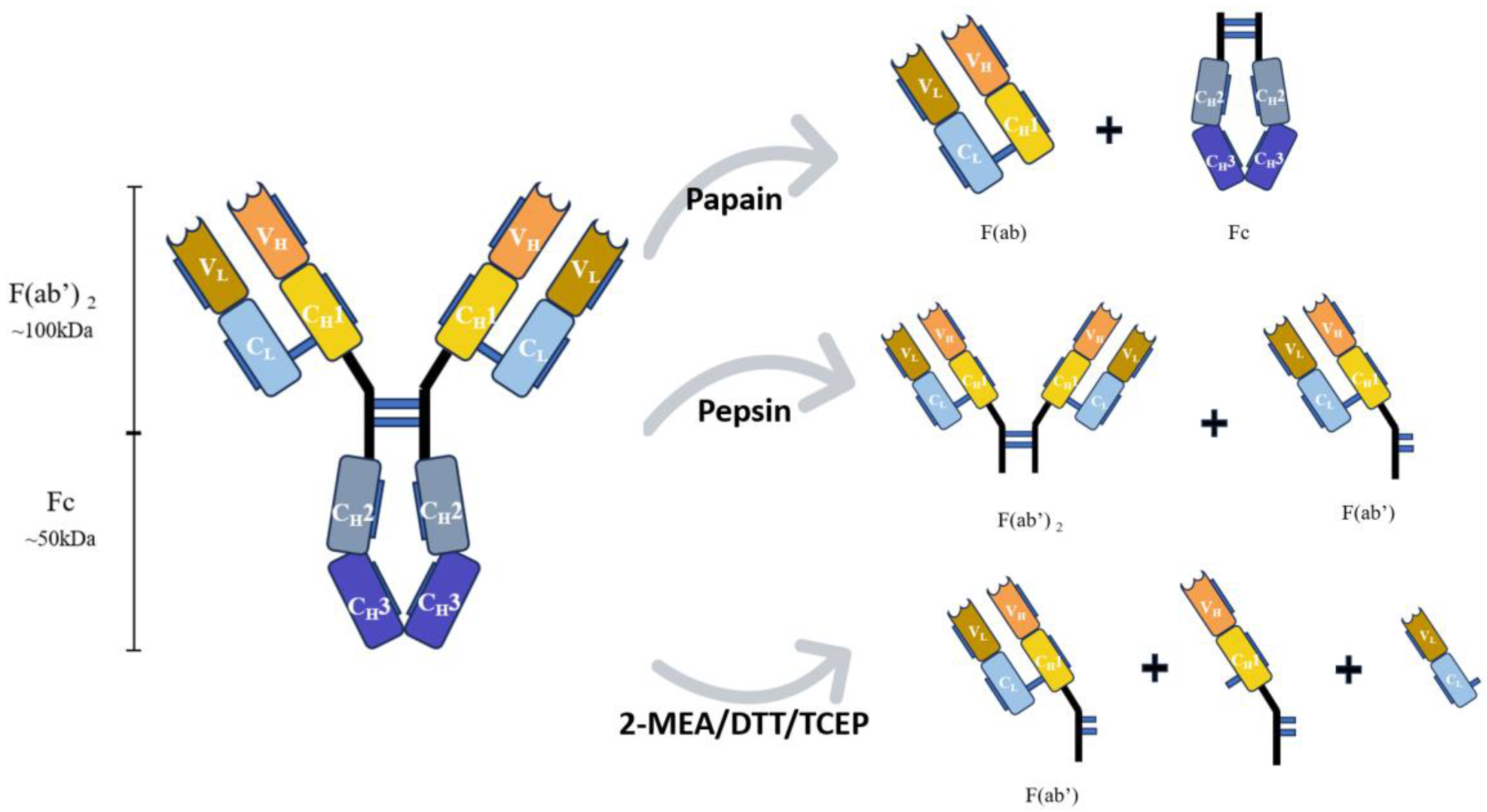
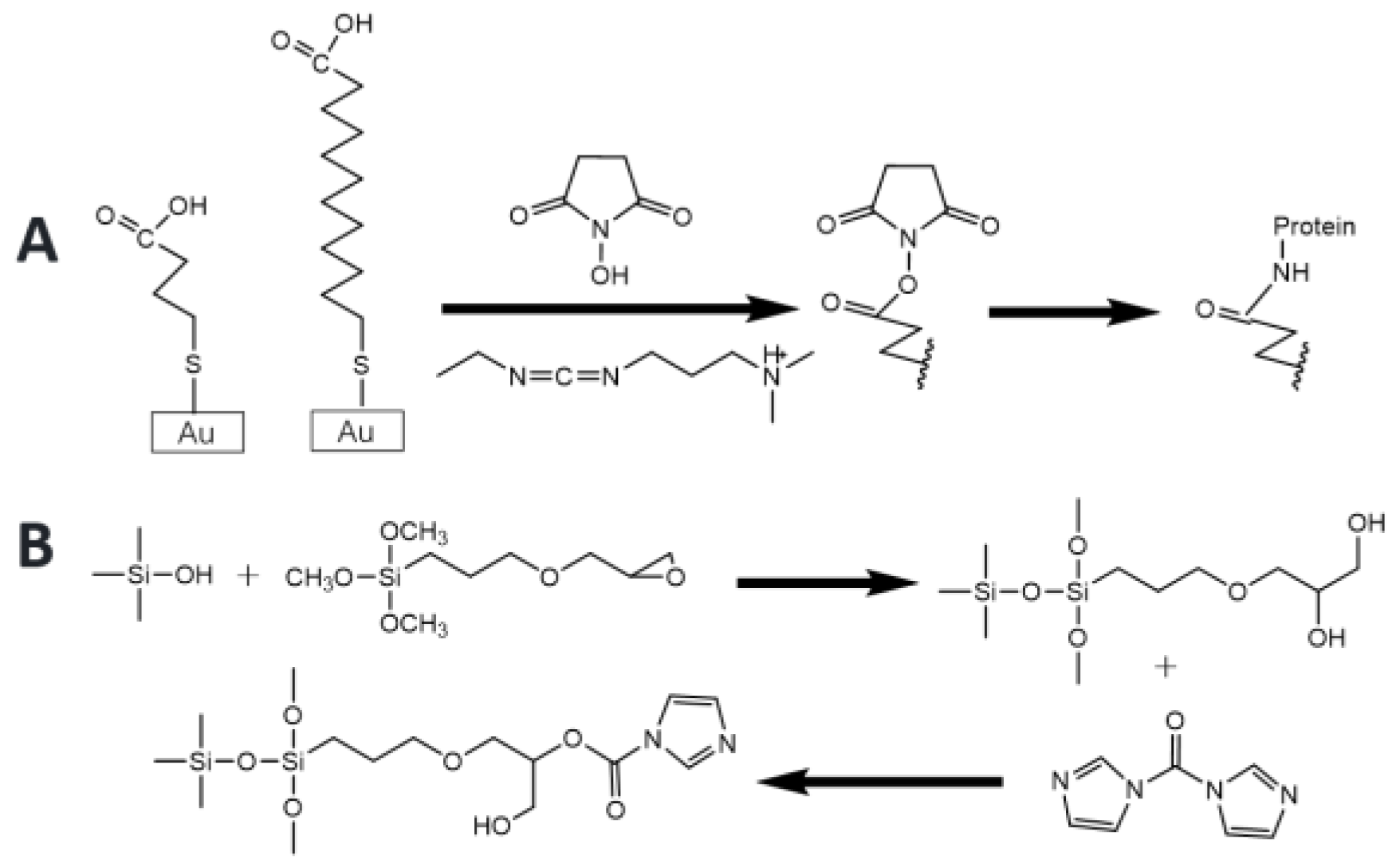
3.3. Thiol Binding Sites
3.4. Carbohydrate Binding Sites
4. Bioaffinity Immobilization Technology
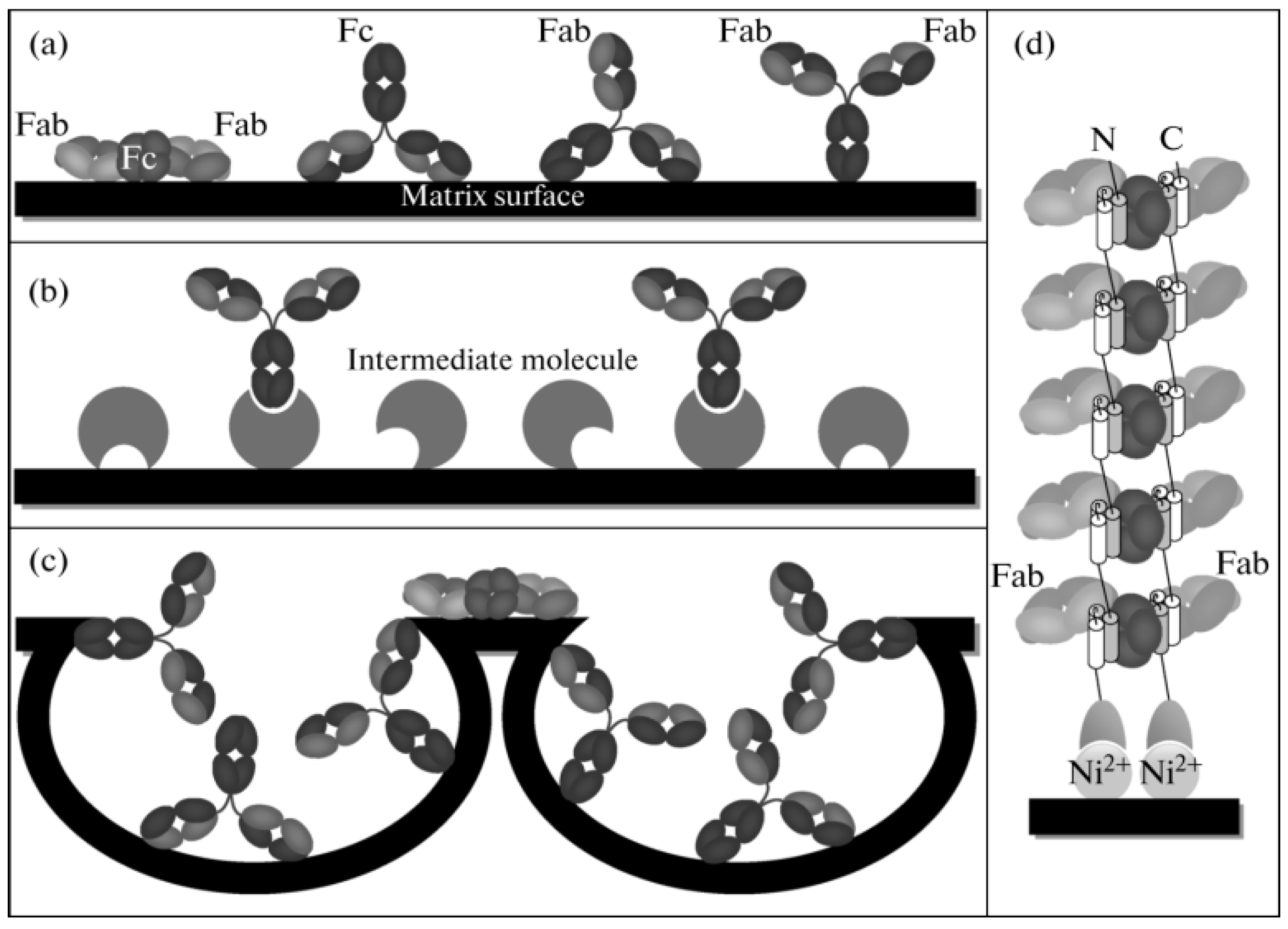
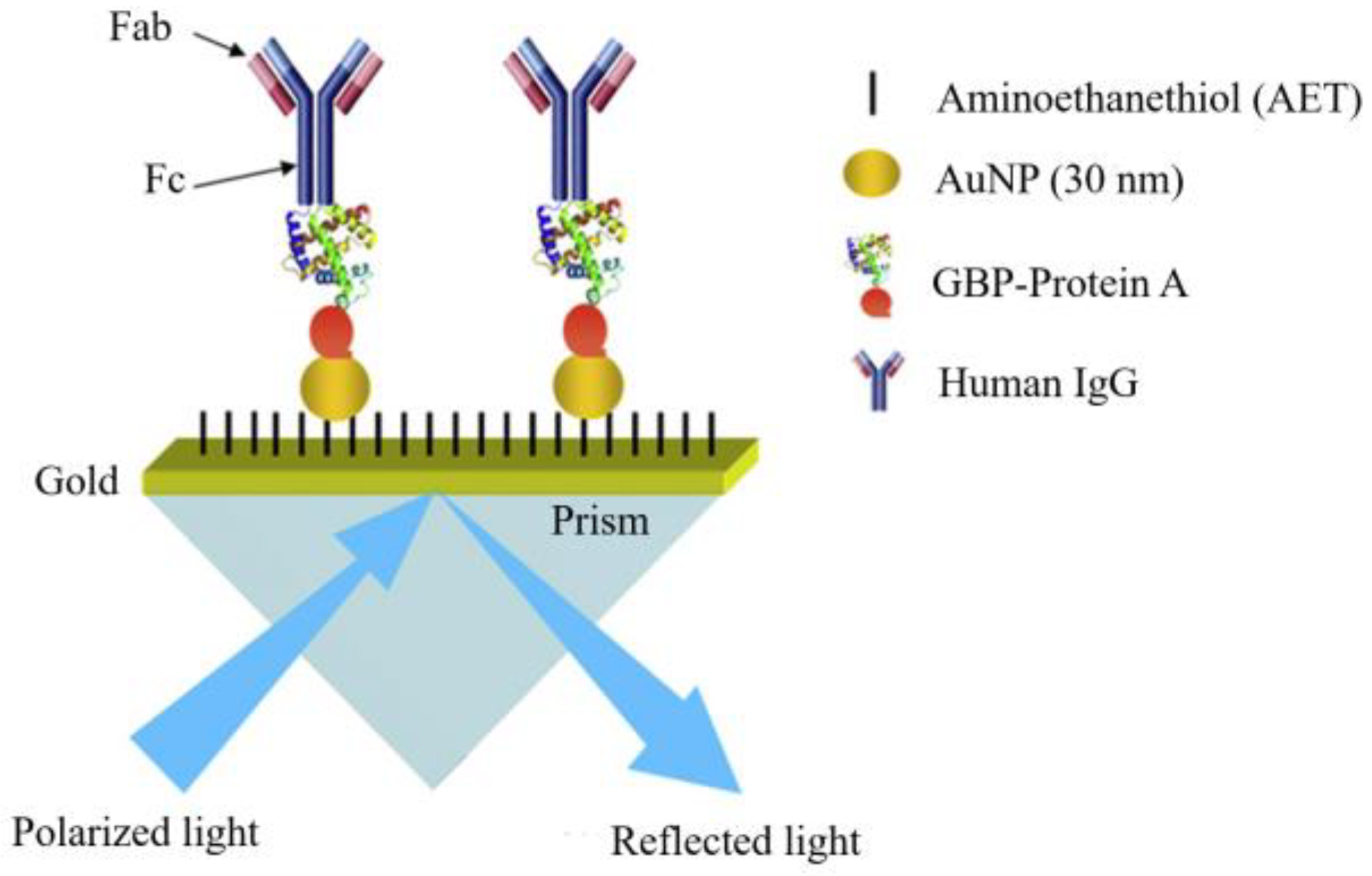
4.1. Specific Binding of Protein A and Protein G
4.2. Biotin Immobilization
4.3. DNA Bio-Immobilization Technology
4.4. Material-Binding Peptide Orientation Technology
5. Other Binding and Fixation Technologies
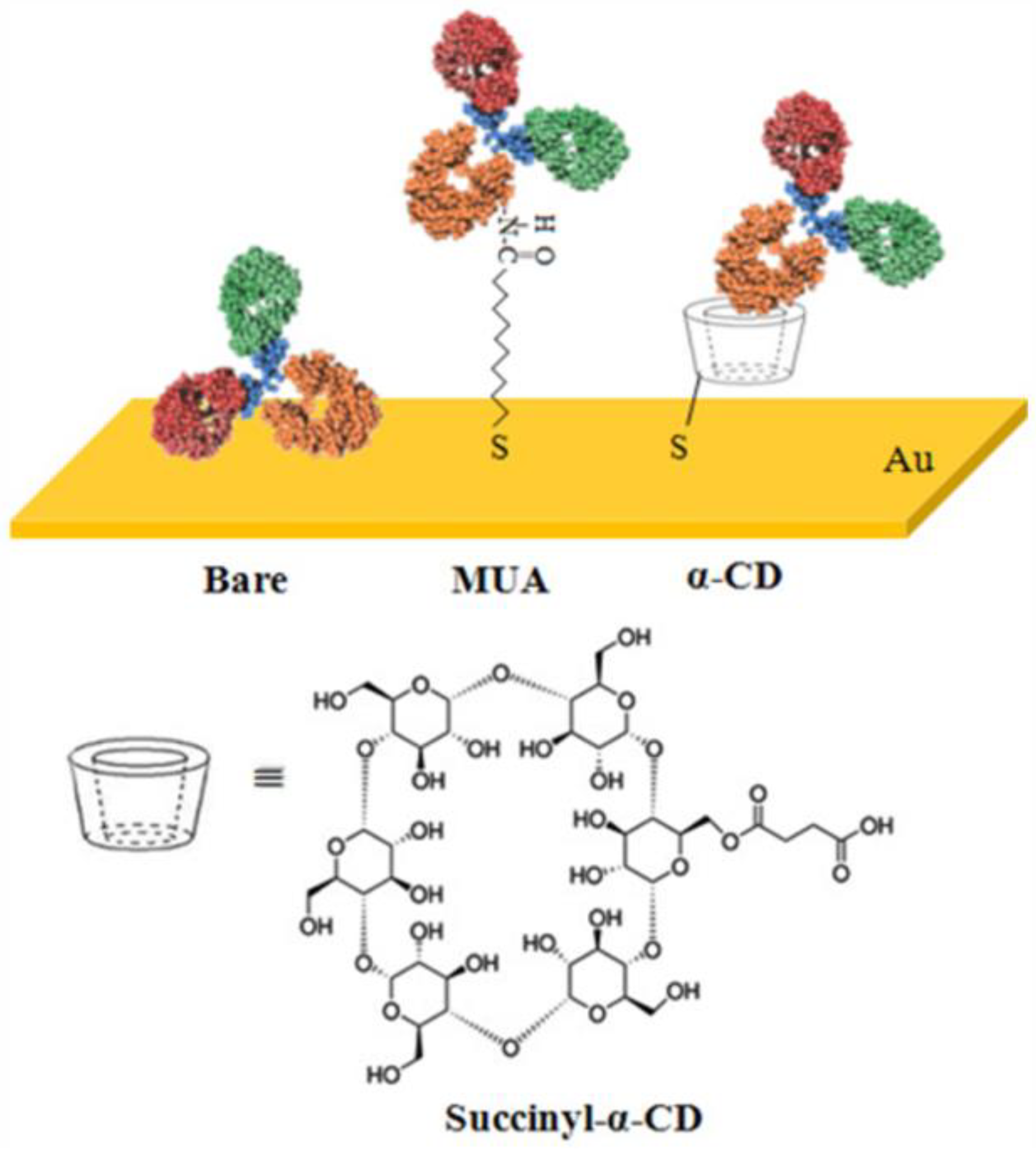
5.1. Hydrophobic Domain Binding Sites
5.2. Metal Bonding
6. Conclusions and Outlook
| Linking Method | Active Site Region | Mechanism | Path Advantage | Path Disadvantage |
|---|---|---|---|---|
| Nucleotide-binding site (NBS) | Within the Fab domain, between the light and heavy chains [33]. | Exposed to 254 nm ultraviolet light, NBS forms active free radicals and covalently photo-crosslinks with small aromatic-ring ligand molecules like IBA. | The directional immobilization of NBS proceeds under mild conditions, maximizing the retention of antibody 3D structure and antigen-binding affinity [33,34,35]. | Exposed to ultraviolet radiation of shorter wavelengths and higher energy, antibodies may denature [36,37]. |
| Amine, carboxyl and hydroxyl binding sites | In antibodies, active primary amine groups (exemplified by lysine and arginine), active carboxyl groups (represented by aspartic acid and glutamic acid), and a few hydroxyl groups (typified by serine and threonine) are present. | Employ chemical binding between active sites and bases or double-active interlayers [40]. | Active sites are ubiquitous, extensively studied with mature methods. | Owing to the high distribution of active sites, screening or external condition interference is necessary [2,5,25]. |
| Thiol binding sites | Disulfide bonds are present between light and heavy chains and also between two heavy chains [56]. | Disulfide bonds, cleavable by reducing agents, yield active thiol groups with strong interaction on gold surface [57,58]. | The fixed locations of disulfide bonds allow antibody upward adsorption; reductive cleavage doubles thiol groups, improving antibody binding efficiency [9,60,62]. | Disulfide bond cleavage may impact protein activity; reduction by agents may alter antibody 3D structure and biological activity [86]. |
| Carbohydrate binding sites | The heavy chain’s CH2 domain, within the Fc region, harbors a polysaccharide chain [65,66]. | 1. Transform multiple hydroxyls into active aldehydes [67]. 2. Employ boric acid derivatives for conjugation [75]. 3. Attach special structural functional groups to glycan residues [84]. | Glycan residues possess relatively fixed positions and have been intensively studied. Special-structured functional groups can be attached to enable click reactions. | As a long spacer, glycan may lead to antibody collapse and inactivation post-immobilization. Excessive antibody exposure to oxidants may reduce its activity [73]. Certain reactions, requiring metal ion catalysis, might cause protein denaturation and lower affinity efficiency. |
| Specific binding of protein A and G | The Fc portion of an antibody [87,88]. | Proteins A and G, capable of specifically binding to the Fc portion of antibodies, enable an orientation system. | 1. The antibody requires no chemical environment modification and optimization, preventing the impact on its biological activity. 2. Specifically link the Fc part [90]. | 1. Proteins A and G bind merely to specific IgG subclasses, restricting their application [91]. 2. The binding of Protein A to the Fc domain is reversible, necessitating metal ions or other aids like chemical bonding for irreversible binding [92]. |
| Biotin immobilization and binding | Streptavidin comprises four identical subunits, each possessing a biotin-binding site [151]. | CH/pi interaction [110]. | The biotin-streptavidin interaction exhibits an extremely high affinity, over a million times stronger than that of antigen-antibody interaction, being irreversible and highly tolerant to extreme pH and temperature [98]. | The coupling system commonly employs light for activation. However, certain aromatic amino acids in antibodies are prone to photo-oxidation, leading to antibody inactivation [33]. |
| DNA bio-immobilization technology | Amino acid sequences or domains with specific interaction to DNA [152]. | Enzymes are directionally immobilized on the carrier surface via DNA base complementary pairing to enhance multi-enzyme cascade activity [152]. | Efficient and controllable protein localization and immobilization. This technology averts protein denaturation common in traditional methods, with high operability and repeatability [152]. | The DDI coupling is essentially random. For a remarkable effect, antibodies usually bear multiple functional groups, causing non-specific binding and inactive conjugate formation. Moreover, it leads to elevated production costs and extended operation time [73]. |
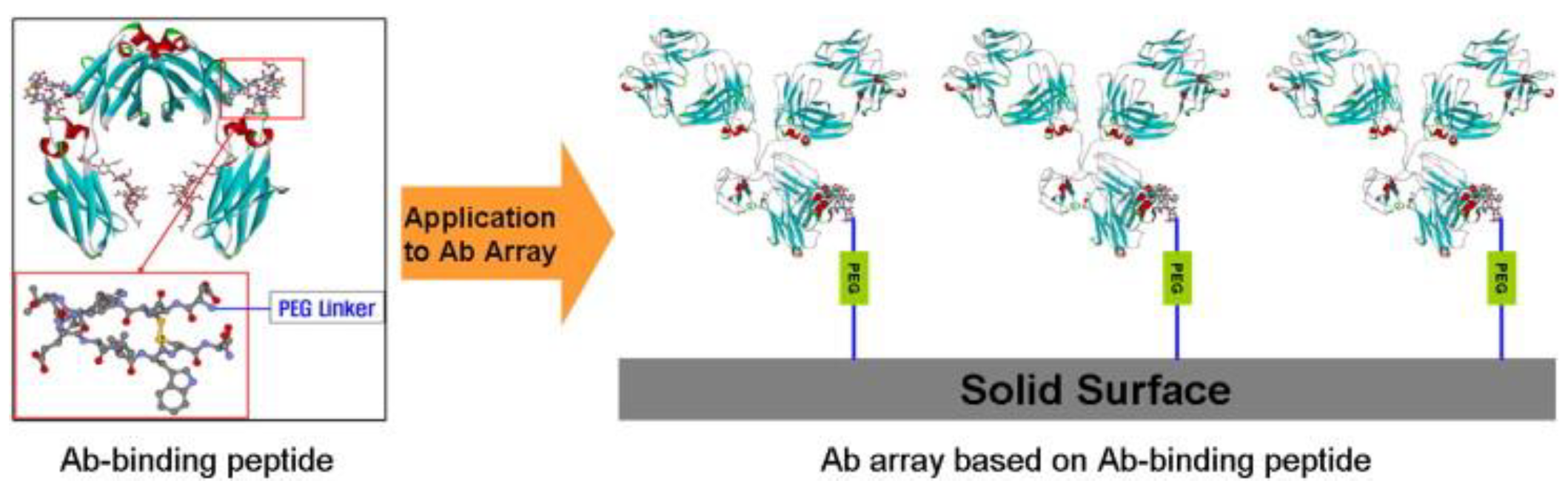
| Material-binding peptide orientation technology | The Fc portion of an antibody [153]. | The short peptide sequence shows specific binding affinity to the antibody Fc domain [154]. | Requiring no antibody engineering and binding rapidly under mild conditions, short synthetic peptides facilitate screening numerous antibodies in a single experiment. Their size allows for enhanced control of surface molecule grafting, averts steric hindrance, and renders them more apt for antibody immobilization than proteins such as SpA or SpG [155]. | The Fc binding site on the peptide is pH-dependent, with its affinity affected by pH fluctuations of the system [155]. |
| Hydrophobic domain binding site | Hydrophobic groups such as leucine and isoleucine exist within the antibody. Hydrophobic regions are also present between the two heavy chains and at the junctions of light and heavy chains. | In antibody immobilization, hydrophobic compounds (e.g., cyclodextrin) can capture hydrophobic side-chain amino acids via stable host-guest inclusion complex formation. | 1. Antibody requires no chemical environment modification and optimization. 2. Functional modification of hydrophobic compounds (e.g., cyclodcdextrin [135]) is feasible. | The antibody stabilization and immobilization via hydrophobic interactions is unstable and reversible. Irreversible binding necessitates metal ions or other aids like chemical bonding. |
| Metal bonding mode | Polypeptides binding metals frequently contain peptide segments with specific bioactivities like immunologically and neuroactive peptides, while also exposing antigen-binding sites [156]. | Resonance angles vary with ligand-analyte binding on metal surface. Monitoring such changes reveals biomolecular interaction details like affinity, association constant and binding kinetics [157]. | Flexible design and abundant diversity can be attained by modulating particle size or dispersion state, while peptide chain editing offers numerous possibilities [156]. | Covalent binding has a long reaction time, may inactivate some biomolecules and impede other molecules’ functionalization. Non-covalent interaction has a weak binding force and is sensitive to the environment (e.g., temperature, pH), so the bound molecules tend to detach from the particle surface [156]. |
Author Contributions
Funding
Conflicts of Interest
References
- Beaver, K.; Dantanarayana, A.; Minteer, S.D. Materials Approaches for Improving Electrochemical Sensor Performance. J. Phys. Chem. B 2021, 125, 11820–11834. [Google Scholar] [CrossRef]
- Boubeta, F.M.; Soler-Illia, G.; Tagliazucchi, M. Electrostatically Driven Protein Adsorption: Charge Patches versus Charge Regulation. Langmuir 2018, 34, 15727–15738. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; Niwa, R.; Satoh, M.; Akinaga, S.; Shitara, K.; Hanai, N. Engineered therapeutic antibodies with improved effector functions. Cancer Sci. 2009, 100, 1566–1572. [Google Scholar] [CrossRef] [PubMed]
- Brezski, R.J.; Georgiou, G. Immunoglobulin isotype knowledge and application to Fc engineering. Curr. Opin. Immunol. 2016, 40, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Boswell, C.A.; Tesar, D.B.; Mukhyala, K.; Theil, F.P.; Fielder, P.J.; Khawli, L.A. Effects of charge on antibody tissue distribution and pharmacokinetics. Bioconjugate Chem. 2010, 21, 2153–2163. [Google Scholar] [CrossRef]
- Trilling, A.K.; Beekwilder, J.; Zuilhof, H. Antibody orientation on biosensor surfaces: A minireview. Analyst 2013, 138, 1619–1627. [Google Scholar] [CrossRef]
- Collis, A.V.; Brouwer, A.P.; Martin, A.C. Analysis of the antigen combining site: Correlations between length and sequence composition of the hypervariable loops and the nature of the antigen. J. Mol. Biol. 2003, 325, 337–354. [Google Scholar] [CrossRef]
- Kausaite-Minkstimiene, A.; Ramanaviciene, A.; Kirlyte, J.; Ramanavicius, A. Comparative study of random and oriented antibody immobilization techniques on the binding capacity of immunosensor. Anal. Chem. 2010, 82, 6401–6408. [Google Scholar] [CrossRef]
- Brogan, K.L.; Wolfe, K.N.; Jones, P.A.; Schoenfisch, M.H. Direct oriented immobilization of F(ab′) antibody fragments on gold. Anal. Chim. Acta 2003, 496, 73–80. [Google Scholar] [CrossRef]
- Sharma, H.; Mutharasan, R. Half antibody fragments improve biosensor sensitivity without loss of selectivity. Anal. Chem. 2013, 85, 2472–2477. [Google Scholar] [CrossRef]
- Karyakin, A.A.; Presnova, G.V.; Rubtsova, M.Y.; Egorov, A.M. Oriented immobilization of antibodies onto the gold surfaces via their native thiol groups. Anal. Chem. 2000, 72, 3805–3811. [Google Scholar] [CrossRef]
- Trilling, A.K.; Hesselink, T.; van Houwelingen, A.; Cordewener, J.H.; Jongsma, M.A.; Schoffelen, S.; van Hest, J.C.; Zuilhof, H.; Beekwilder, J. Orientation of llama antibodies strongly increases sensitivity of biosensors. Biosens. Bioelectron. 2014, 60, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Coluccio, M.L.; Grillo, F.; Onesto, V.; Garo, V.; Scala, C.; Cuzzola, P.; Calfa, M.; Candeloro, P.; Gentile, F.; Piletsky, S.; et al. Enhancing Antibodies’ Binding Capacity through Oriented Functionalization of Plasmonic Surfaces. Nanomaterials 2021, 11, 2620. [Google Scholar] [CrossRef] [PubMed]
- Welch, N.G.; Scoble, J.A.; Muir, B.W.; Pigram, P.J. Orientation and characterization of immobilized antibodies for improved immunoassays (Review). Biointerphases 2017, 12, 02d301. [Google Scholar] [CrossRef]
- Kosobrodova, E.; Jones, R.T.; Kondyurin, A.; Chrzanowski, W.; Pigram, P.J.; McKenzie, D.R.; Bilek, M.M. Orientation and conformation of anti-CD34 antibody immobilised on untreated and plasma treated polycarbonate. Acta Biomater. 2015, 19, 128–137. [Google Scholar] [CrossRef]
- Wang, C.; Feng, B. Research progress on site-oriented and three-dimensional immobilization of protein. J. Mol. Biol. 2015, 49, 1–20. [Google Scholar] [CrossRef]
- Fahmy, H.M.; Abu Serea, E.S.; Salah-Eldin, R.E.; Al-Hafiry, S.A.; Ali, M.K.; Shalan, A.E.; Lanceros-Méndez, S. Recent Progress in Graphene- and Related Carbon-Nanomaterial-based Electrochemical Biosensors for Early Disease Detection. ACS Biomater. Sci. Eng. 2022, 8, 964–1000. [Google Scholar] [CrossRef]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 2005, 105, 1103–1169. [Google Scholar] [CrossRef]
- Chen, S.; Liu, L.; Zhou, J.; Jiang, S. Controlling Antibody Orientation on Charged Self-Assembled Monolayers. Langmuir 2003, 19, 2859–2864. [Google Scholar] [CrossRef]
- Sharafeldin, M.; McCaffrey, K.; Rusling, J.F. Influence of antibody immobilization strategy on carbon electrode immunoarrays. Analyst 2019, 144, 5108–5116. [Google Scholar] [CrossRef]
- Kumar, S.; Ch, R.; Rath, D.; Panda, S. Densities and orientations of antibodies on nano-textured silicon surfaces. Mater. Sci. Eng. C 2011, 31, 370–376. [Google Scholar] [CrossRef]
- Song, L.; Zhao, J.; Luan, S.; Ma, J.; Ming, W.; Yin, J. High-efficiency immunoassay platforms with controllable surface roughness and oriented antibody immobilization. J. Mat. Chem. B 2015, 3, 7499–7502. [Google Scholar] [CrossRef] [PubMed]
- Goyon, A.; Excoffier, M.; Janin-Bussat, M.C.; Bobaly, B.; Fekete, S.; Guillarme, D.; Beck, A. Determination of isoelectric points and relative charge variants of 23 therapeutic monoclonal antibodies. J. Chromatogr. B 2017, 1065–1066, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Zhou, Y.; Sun, Y. Influence of pH and ionic strength on the steric mass-action model parameters around the isoelectric point of protein. Biotechnol. Prog. 2005, 21, 516–523. [Google Scholar] [CrossRef]
- Lautenbach, V.; Hosseinpour, S.; Peukert, W. Isoelectric Point of Proteins at Hydrophobic Interfaces. Front. Chem. 2021, 9, 712978. [Google Scholar] [CrossRef]
- Zhou, J.; Tsao, H.K.; Sheng, Y.J.; Jiang, S. Monte Carlo simulations of antibody adsorption and orientation on charged surfaces. J. Chem. Phys. 2004, 121, 1050–1057. [Google Scholar] [CrossRef]
- Gao, S.; Rojas-Vega, F.; Rocha-Martin, J.; Guisán, J.M. Oriented immobilization of antibodies through different surface regions containing amino groups: Selective immobilization through the bottom of the Fc region. Int. J. Biol. Macromol. 2021, 177, 19–28. [Google Scholar] [CrossRef]
- Ruiz, G.; Tripathi, K.; Okyem, S.; Driskell, J.D. pH Impacts the Orientation of Antibody Adsorbed onto Gold Nanoparticles. Bioconjugate Chem. 2019, 30, 1182–1191. [Google Scholar] [CrossRef]
- Goddard, J.M.; Hotchkiss, J.H. Polymer surface modification for the attachment of bioactive compounds. Prog. Polym. Sci. 2007, 32, 698–725. [Google Scholar] [CrossRef]
- Wiseman, M.E.; Frank, C.W. Antibody adsorption and orientation on hydrophobic surfaces. Langmuir 2012, 28, 1765–1774. [Google Scholar] [CrossRef]
- Kanthe, A.; Ilott, A.; Krause, M.; Zheng, S.; Li, J.; Bu, W.; Bera, M.K.; Lin, B.; Maldarelli, C.; Tu, R.S. No ordinary proteins: Adsorption and molecular orientation of monoclonal antibodies. Sci. Adv. 2021, 7, eabg2873. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, A.; De Rossi, D.; Ristori, C.; Schirone, A.; Serra, G. A comparative study of protein immobilization techniques for optical immunosensors. Biosens. Bioelectron. 1992, 7, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.Y.; Tye, G.J.; Chew, A.L.; Ng, W.K.; Lai, N.S. Linker-mediated oriented antibody immobilisation strategies for a more efficient immunosensor and diagnostic applications: A review. Biosens. Bioelectron. 2023, 14, 100379. [Google Scholar] [CrossRef]
- Zhang, L.; Mazouzi, Y.; Salmain, M.; Liedberg, B.; Boujday, S. Antibody-Gold Nanoparticle Bioconjugates for Biosensors: Synthesis, Characterization and Selected Applications. Biosens. Bioelectron. 2020, 165, 112370. [Google Scholar] [CrossRef]
- Alves, N.J.; Mustafaoglu, N.; Bilgicer, B. Oriented antibody immobilization by site-specific UV photocrosslinking of biotin at the conserved nucleotide binding site for enhanced antigen detection. Biosens. Bioelectron. 2013, 49, 387–393. [Google Scholar] [CrossRef]
- Mustafaoglu, N.; Kiziltepe, T.; Bilgicer, B. Site-specific conjugation of an antibody on a gold nanoparticle surface for one-step diagnosis of prostate specific antigen with dynamic light scattering. Nanoscale 2017, 9, 8684–8694. [Google Scholar] [CrossRef]
- von Witting, E.; Hober, S.; Kanje, S. Affinity-Based Methods for Site-Specific Conjugation of Antibodies. Bioconjugate Chem. 2021, 32, 1515–1524. [Google Scholar] [CrossRef]
- Patel, N.; Davies, M.C.; Hartshorne, M.; Heaton, R.J.; Roberts, C.J.; Tendler, S.J.B.; Williams, P.M. Immobilization of Protein Molecules onto Homogeneous and Mixed Carboxylate-Terminated Self-Assembled Monolayers. Langmuir 1997, 13, 6485–6490. [Google Scholar] [CrossRef]
- Carrigan, S.D.; Scott, G.; Tabrizian, M. Real-time QCM-D immunoassay through oriented antibody immobilization using cross-linked hydrogel biointerfaces. Langmuir 2005, 21, 5966–5973. [Google Scholar] [CrossRef]
- Sun, Y.; Du, H.; Feng, C.; Lan, Y. Oriented immobilization of antibody through carbodiimide reaction and controlling electric field. J. Solid State Electrochem. 2015, 19, 3035–3043. [Google Scholar] [CrossRef]
- Subramanian, A.; Velander, W.H. Effect of antibody orientation on immunosorbent performance. J. Mol. Recognit. 1996, 9, 528–535. [Google Scholar] [CrossRef]
- Ferreira, N.S.; Sales, M.G. Disposable immunosensor using a simple method for oriented antibody immobilization for label-free real-time detection of an oxidative stress biomarker implicated in cancer diseases. Biosens. Bioelectron. 2014, 53, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K. Comparison of 1-Ethyl-3-(3-Dimethylaminopropyl) Carbodiimide Based Strategies to Crosslink Antibodies on Amine-Functionalized Platforms for Immunodiagnostic Applications. Diagnostics 2012, 2, 23–33. [Google Scholar] [CrossRef]
- Vashist, S.K.; Marion Schneider, E.; Lam, E.; Hrapovic, S.; Luong, J.H. One-step antibody immobilization-based rapid and highly-sensitive sandwich ELISA procedure for potential in vitro diagnostics. Sci. Rep. 2014, 4, 4407. [Google Scholar] [CrossRef]
- Potyrailo, R.A.; Conrad, R.C.; Ellington, A.D.; Hieftje, G.M. Adapting selected nucleic acid ligands (aptamers) to biosensors. Anal. Chem. 1998, 70, 3419–3425. [Google Scholar] [CrossRef]
- Jiang, H.; Xu, F.J. Biomolecule-functionalized polymer brushes. Chem. Soc. Rev. 2013, 42, 3394–3426. [Google Scholar] [CrossRef]
- Marco, M.-P.; Gee, S.; Hammock, B.D. Immunochemical techniques for environmental analysis II. Antibody production and immunoassay development. Trac-Trends Anal. Chem. 1995, 14, 415–425. [Google Scholar] [CrossRef]
- Gunda, N.S.K.; Singh, M.; Norman, L.; Kaur, K.; Mitra, S.K. Optimization and characterization of biomolecule immobilization on silicon substrates using (3-aminopropyl)triethoxysilane (APTES) and glutaraldehyde linker. Appl. Surf. Sci. 2014, 305, 522–530. [Google Scholar] [CrossRef]
- Yuan, Y.; Yin, M.; Qian, J.; Liu, C. Site-directed immobilization of antibodies onto blood contacting grafts for enhanced endothelial cell adhesion and proliferation. Soft Matter 2011, 7, 7207–7216. [Google Scholar] [CrossRef]
- Huy, T.Q.; Hanh, N.T.H.; Van Chung, P.; Anh, D.D.; Nga, P.T.; Tuan, M.A. Characterization of immobilization methods of antiviral antibodies in serum for electrochemical biosensors. Appl. Surf. Sci. 2011, 257, 7090–7095. [Google Scholar] [CrossRef]
- Walsh, M.K.; Wang, X.; Weimer, B.C. Optimizing the immobilization of single-stranded DNA onto glass beads. J. Biochem. Biophys. Methods 2001, 47, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Suter, J.D.; White, I.M.; Fan, X. Aptamer Based Microsphere Biosensor for Thrombin Detection. Sensors 2006, 6, 785–795. [Google Scholar] [CrossRef]
- Um, H.J.; Kim, M.; Lee, S.H.; Min, J.; Kim, H.; Choi, Y.W.; Kim, Y.H. Electrochemically oriented immobilization of antibody on poly-(2-cyano-ethylpyrrole)-coated gold electrode using a cyclic voltammetry. Talanta 2011, 84, 330–334. [Google Scholar] [CrossRef]
- Parolo, C.; de la Escosura-Muñiz, A.; Polo, E.; Grazú, V.; de la Fuente, J.M.; Merkoçi, A. Design, preparation, and evaluation of a fixed-orientation antibody/gold-nanoparticle conjugate as an immunosensing label. ACS Appl. Mater. Interfaces 2013, 5, 10753–10759. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, D.; Park, Y.; Kim, D.H.; Kim, J. Electric-Field-Mediated In-Sensor Alignment of Antibody’s Orientation to Enhance the Antibody-Antigen Binding for Ultrahigh Sensitivity Sensors. Nano Lett. 2022, 22, 6537–6544. [Google Scholar] [CrossRef]
- Shishido, M.; Kitagawa, D. Preparation of ordered mono-particulate film from colloidal solutions on the surface of water and continuous transcription of film to substrate. Colloids Surf. A-Physicochem. Eng. Asp. 2007, 311, 32–41. [Google Scholar] [CrossRef]
- Emaminejad, S.; Javanmard, M.; Gupta, C.; Chang, S.; Davis, R.W.; Howe, R.T. Tunable control of antibody immobilization using electric field. Proc. Natl. Acad. Sci. USA 2015, 112, 1995–1999. [Google Scholar] [CrossRef]
- Iarossi, M.; Schiattarella, C.; Rea, I.; De Stefano, L.; Fittipaldi, R.; Vecchione, A.; Velotta, R.; Ventura, B.D. Colorimetric Immunosensor by Aggregation of Photochemically Functionalized Gold Nanoparticles. ACS Omega 2018, 3, 3805–3812. [Google Scholar] [CrossRef]
- Baniukevic, J.; Kirlyte, J.; Ramanavicius, A.; Ramanaviciene, A. Application of oriented and random antibody immobilization methods in immunosensor design. Sens. Actuator B-Chem. 2013, 189, 217–223. [Google Scholar] [CrossRef]
- Wang, H.; Wu, J.; Li, J.; Ding, Y.; Shen, G.; Yu, R. Nanogold particle-enhanced oriented adsorption of antibody fragments for immunosensing platforms. Biosens. Bioelectron. 2005, 20, 2210–2217. [Google Scholar] [CrossRef]
- Bonroy, K.; Frederix, F.; Reekmans, G.; Dewolf, E.; De Palma, R.; Borghs, G.; Declerck, P.; Goddeeris, B. Comparison of random and oriented immobilisation of antibody fragments on mixed self-assembled monolayers. J. Immunol. Methods 2006, 312, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.R.; Mitchell, D.T. Peer reviewed: Nanomaterials in analytical chemistry. Anal. Chem. 1998, 70, 322a–327a. [Google Scholar] [CrossRef]
- Greene, M.K.; Richards, D.A.; Nogueira, J.C.F.; Campbell, K.; Smyth, P.; Fernández, M.; Scott, C.J.; Chudasama, V. Forming next-generation antibody-nanoparticle conjugates through the oriented installation of non-engineered antibody fragments. Chem. Sci. 2018, 9, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Giersig, M.; Mulvaney, P. Preparation of ordered colloid monolayers by electrophoretic deposition. Langmuir 1993, 9, 3408–3413. [Google Scholar] [CrossRef]
- Suzuki, N.; Khoo, K.H.; Chen, C.M.; Chen, H.C.; Lee, Y.C. N-glycan structures of pigeon IgG: A major serum glycoprotein containing Galalpha1-4 Gal termini. J. Biol. Chem. 2003, 278, 46293–46306. [Google Scholar] [CrossRef] [PubMed]
- Yoo, E.M.; Chintalacharuvu, K.R.; Penichet, M.L.; Morrison, S.L. Myeloma expression systems. J. Immunol. Methods 2002, 261, 1–20. [Google Scholar] [CrossRef]
- Byeon, J.Y.; Limpoco, F.T.; Bailey, R.C. Efficient bioconjugation of protein capture agents to biosensor surfaces using aniline-catalyzed hydrazone ligation. Langmuir 2010, 26, 15430–15435. [Google Scholar] [CrossRef]
- Hoffman, W.L.; O’Shannessy, D.J. Site-specific immobilization of antibodies by their oligosaccharide moieties to new hydrazide derivatized solid supports. J. Immunol. Methods 1988, 112, 113–120. [Google Scholar] [CrossRef]
- Lou, D.; Fan, L.; Cui, Y.; Zhu, Y.; Gu, N.; Zhang, Y. Fluorescent Nanoprobes with Oriented Modified Antibodies to Improve Lateral Flow Immunoassay of Cardiac Troponin I. Anal. Chem. 2018, 90, 6502–6508. [Google Scholar] [CrossRef]
- Han, H.J.; Kannan, R.M.; Wang, S.; Mao, G.; Kusanovic, J.P.; Romero, R. Multifunctional Dendrimer-templated Antibody Presentation on Biosensor Surfaces for Improved Biomarker Detection. Adv. Funct. Mater. 2010, 20, 409–421. [Google Scholar] [CrossRef]
- Gering, J.P.; Quaroni, L.; Chumanov, G. Immobilization of antibodies on glass surfaces through sugar residues. J. Colloid Interface Sci. 2002, 252, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Simón, B.; Saint, C.; Voelcker, N.H. Electrochemical biosensors featuring oriented antibody immobilization via electrografted and self-assembled hydrazide chemistry. Anal. Chem. 2014, 86, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, J. Oriented immobilization of proteins on solid supports for use in biosensors and biochips: A review. Microchim. Acta 2016, 183, 1–19. [Google Scholar] [CrossRef]
- Adak, A.K.; Li, B.Y.; Huang, L.D.; Lin, T.W.; Chang, T.C.; Hwang, K.C.; Lin, C.C. Fabrication of antibody microarrays by light-induced covalent and oriented immobilization. ACS Appl. Mater. Interfaces 2014, 6, 10452–10460. [Google Scholar] [CrossRef]
- Duval, F.; van Beek, T.A.; Zuilhof, H. Key steps towards the oriented immobilization of antibodies using boronic acids. Analyst 2015, 140, 6467–6472. [Google Scholar] [CrossRef]
- Ho, J.A.; Hsu, W.L.; Liao, W.C.; Chiu, J.K.; Chen, M.L.; Chang, H.C.; Li, C.C. Ultrasensitive electrochemical detection of biotin using electrically addressable site-oriented antibody immobilization approach via aminophenyl boronic acid. Biosens. Bioelectron. 2010, 26, 1021–1027. [Google Scholar] [CrossRef]
- Springsteen, G.; Wang, B. A detailed examination of boronic acid–diol complexation. Tetrahedron 2002, 58, 5291–5300. [Google Scholar] [CrossRef]
- Lim, S.A.; Ahmed, M.U. A carbon nanofiber-based label free immunosensor for high sensitive detection of recombinant bovine somatotropin. Biosens. Bioelectron. 2015, 70, 48–53. [Google Scholar] [CrossRef]
- Moreno-Guzmán, M.; Ojeda, I.; Villalonga, R.; González-Cortés, A.; Yáñez-Sedeño, P.; Pingarrón, J.M. Ultrasensitive detection of adrenocorticotropin hormone (ACTH) using disposable phenylboronic-modified electrochemical immunosensors. Biosens. Bioelectron. 2012, 35, 82–86. [Google Scholar] [CrossRef]
- Liao, W.C.; Annie Ho, J.A. Improved activity of immobilized antibody by paratope orientation controller: Probing paratope orientation by electrochemical strategy and surface plasmon resonance spectroscopy. Biosens. Bioelectron. 2014, 55, 32–38. [Google Scholar] [CrossRef]
- Zhao, J.; Mo, R.; Tian, L.-M.; Song, L.-J.; Luan, S.-F.; Yin, J.-H.; Ren, L.-Q. Oriented Antibody Immobilization and Immunoassay Based on Boronic Acid-containing Polymer Brush. Chin. J. Polym. Sci. 2018, 36, 472–478. [Google Scholar] [CrossRef]
- Adak, A.K.; Huang, K.T.; Li, P.J.; Fan, C.Y.; Lin, P.C.; Hwang, K.C.; Lin, C.C. Regioselective S(N)2-Type Reaction for the Oriented and Irreversible Immobilization of Antibodies to a Glass Surface Assisted by Boronate Formation. ACS Appl. Bio Mater. 2020, 3, 6756–6767. [Google Scholar] [CrossRef] [PubMed]
- Tacias-Pascacio, V.G.; Ortiz, C.; Rueda, N.; Berenguer-Murcia, Á.; Acosta, N.; Aranaz, I.; Civera, C.; Fernandez-Lafuente, R.; Alcántara, A.R. Dextran Aldehyde in Biocatalysis: More Than a Mere Immobilization System. Catalysts 2019, 9, 622. [Google Scholar] [CrossRef]
- Agrahari, A.K.; Bose, P.; Jaiswal, M.K.; Rajkhowa, S.; Singh, A.S.; Hotha, S.; Mishra, N.; Tiwari, V.K. Cu(I)-Catalyzed Click Chemistry in Glycoscience and Their Diverse Applications. Chem. Rev. 2021, 121, 7638–7956. [Google Scholar] [CrossRef]
- Brückner, M.; Simon, J.; Landfester, K.; Mailänder, V. The conjugation strategy affects antibody orientation and targeting properties of nanocarriers. Nanoscale 2021, 13, 9816–9824. [Google Scholar] [CrossRef]
- Shen, M.; Rusling, J.; Dixit, C.K. Site-selective orientated immobilization of antibodies and conjugates for immunodiagnostics development. Methods 2017, 116, 95–111. [Google Scholar] [CrossRef]
- Palmer, D.A.; French, M.T.; Miller, J.N. Use of protein A as an immunological reagent and its application using flow injection. A review. Analyst 1994, 119, 2769–2776. [Google Scholar] [CrossRef]
- Saha, K.; Bender, F.; Gizeli, E. Comparative study of IgG binding to proteins G and A: Nonequilibrium kinetic and binding constant determination with the acoustic waveguide device. Anal. Chem. 2003, 75, 835–842. [Google Scholar] [CrossRef]
- Caroselli, R.; García Castelló, J.; Escorihuela, J.; Bañuls, M.J.; Maquieira, Á.; García-Rupérez, J. Experimental Study of the Oriented Immobilization of Antibodies on Photonic Sensing Structures by Using Protein A as an Intermediate Layer. Sensors 2018, 18, 1012. [Google Scholar] [CrossRef]
- Mao, X.; Yu, B.; Li, Z.; Li, Z.; Shi, G. Comparison of lateral flow immunoassays based on oriented and nonoriented immobilization of antibodies for the detection of aflatoxin B1. Anal. Chim. Acta 2022, 1221, 340135. [Google Scholar] [CrossRef]
- Björk, I.; Petersson, B.A.; Sjöquist, J. Some physiochemical properties of protein A from Staphylococcus aureus. Eur. J. Biochem. 1972, 29, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Guisán, J.M.; Rocha-Martin, J. Oriented immobilization of antibodies onto sensing platforms—A critical review. Anal. Chim. Acta 2022, 1189, 338907. [Google Scholar] [CrossRef]
- Sun, X.; Ye, Y.; He, S.; Wu, Z.; Yue, J.; Sun, H.; Cao, X. A novel oriented antibody immobilization based voltammetric immunosensor for allergenic activity detection of lectin in kidney bean by using AuNPs-PEI-MWCNTs modified electrode. Biosens. Bioelectron. 2019, 143, 111607. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Gao, N.; Dai, P.; Zhu, Z.; You, H.; Han, W.; Li, L. Facile engineered polymeric microdevice via co-coupling of phenylboronic acid and Protein A for oriented antibody immobilization enables substantial signal enhancement for an enhanced fluorescence immunoassay. Sens. Actuator B-Chem. 2021, 346, 130444. [Google Scholar] [CrossRef]
- Shen, G.; Cai, C.; Wang, K.; Lu, J. Improvement of antibody immobilization using hyperbranched polymer and protein A. Anal. Biochem. 2011, 409, 22–27. [Google Scholar] [CrossRef]
- Park, M. Orientation Control of the Molecular Recognition Layer for Improved Sensitivity: A Review. BioChip J. 2019, 13, 82–94. [Google Scholar] [CrossRef]
- Kim, E.S.; Shim, C.K.; Lee, J.W.; Park, J.W.; Choi, K.Y. Synergistic effect of orientation and lateral spacing of protein G on an on-chip immunoassay. Analyst 2012, 137, 2421–2430. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Q.; Liu, X.; Yang, Y.; Yang, Y.; Liu, M.; Li, P.; Zhou, Y. Antibody-biotin-streptavidin-horseradish peroxidase (HRP) sensor for rapid and ultra-sensitive detection of fumonisins. Food Chem. 2020, 316, 126356. [Google Scholar] [CrossRef]
- Murillo, A.M.M.; Holgado, M.; Laguna, M. Reports on the sensitivity enhancement in interferometric based biosensors by biotin-streptavidin system. Heliyon 2023, 9, e23123. [Google Scholar] [CrossRef]
- Cho, I.H.; Paek, E.H.; Lee, H.; Kang, J.Y.; Kim, T.S.; Paek, S.H. Site-directed biotinylation of antibodies for controlled immobilization on solid surfaces. Anal. Biochem. 2007, 365, 14–23. [Google Scholar] [CrossRef]
- Yang, H.M.; Bao, R.M.; Yu, C.M.; Lv, Y.N.; Zhang, W.F.; Tang, J.B. Fc-specific biotinylation of antibody using an engineered photoactivatable Z-Biotin and its biosensing application. Anal. Chim. Acta 2017, 949, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Lawson, J.S.; Salmons, B.; Glenn, W.K. Oncogenic Viruses and Breast Cancer: Mouse Mammary Tumor Virus (MMTV), Bovine Leukemia Virus (BLV), Human Papilloma Virus (HPV), and Epstein-Barr Virus (EBV). Front. Oncol. 2018, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Gopinath, S.C.B.; Firdous, S.M.; Ramanathan, S. Early detection of viral DNA in breast cancer using fingered aluminium interdigitated electrode modified by Streptavidin-biotin tetravalent complex. J. Indian Chem. Soc. 2022, 99, 100604. [Google Scholar] [CrossRef]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Kosaka, N.; Iguchi, H.; Ochiya, T. Circulating microRNA in body fluid: A new potential biomarker for cancer diagnosis and prognosis. Cancer Sci. 2010, 101, 2087–2092. [Google Scholar] [CrossRef]
- Ding, X.; Yan, Y.; Li, S.; Zhang, Y.; Cheng, W.; Cheng, Q.; Ding, S. Surface plasmon resonance biosensor for highly sensitive detection of microRNA based on DNA super-sandwich assemblies and streptavidin signal amplification. Anal. Chim. Acta 2015, 874, 59–65. [Google Scholar] [CrossRef]
- Zhai, Q.; He, Y.; Li, X.; Guo, J.; Li, S.; Yi, G. A simple and ultrasensitive electrochemical biosensor for detection of microRNA based on hybridization chain reaction amplification. J. Electroanal. Chem. 2015, 758, 20–25. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, X.; Zhang, Z.; Ma, P.; Wang, X.; Song, D.; Sun, Y. Biotin-streptavidin sandwich integrated PDA-ZnO@Au nanocomposite based SPR sensor for hIgG detection. Talanta 2022, 246, 123496. [Google Scholar] [CrossRef]
- Ozawa, M.; Ozawa, T.; Nishio, M.; Ueda, K. The role of CH/π interactions in the high affinity binding of streptavidin and biotin. J. Mol. Graph. Model. 2017, 75, 117–124. [Google Scholar] [CrossRef]
- Jung, Y.; Lee, J.M.; Jung, H.; Chung, B.H. Self-directed and self-oriented immobilization of antibody by protein G-DNA conjugate. Anal. Chem. 2007, 79, 6534–6541. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Song, P.; Zhou, G.; Zuo, X.; Aldalbahi, A.; Lou, X.; Shi, J.; Fan, C. Electrochemical detection of nucleic acids, proteins, small molecules and cells using a DNA-nanostructure-based universal biosensing platform. Nat. Protoc. 2016, 11, 1244–1263. [Google Scholar] [CrossRef] [PubMed]
- Konč, J.; Brown, L.; Whiten, D.R.; Zuo, Y.; Ravn, P.; Klenerman, D.; Bernardes, G.J.L. A Platform for Site-Specific DNA-Antibody Bioconjugation by Using Benzoylacrylic-Labelled Oligonucleotides. Angew. Chem.-Int. Edit. 2021, 60, 25905–25913. [Google Scholar] [CrossRef]
- Kazane, S.A.; Sok, D.; Cho, E.H.; Uson, M.L.; Kuhn, P.; Schultz, P.G.; Smider, V.V. Site-specific DNA-antibody conjugates for specific and sensitive immuno-PCR. Proc. Natl. Acad. Sci. USA 2012, 109, 3731–3736. [Google Scholar] [CrossRef]
- Bano, F.; Fruk, L.; Sanavio, B.; Glettenberg, M.; Casalis, L.; Niemeyer, C.M.; Scoles, G. Toward multiprotein nanoarrays using nanografting and DNA directed immobilization of proteins. Nano Lett. 2009, 9, 2614–2618. [Google Scholar] [CrossRef]
- Arrabito, G.; Reisewitz, S.; Dehmelt, L.; Bastiaens, P.I.; Pignataro, B.; Schroeder, H.; Niemeyer, C.M. Biochips for cell biology by combined dip-pen nanolithography and DNA-directed protein immobilization. Small 2013, 9, 4243–4249. [Google Scholar] [CrossRef]
- Shen, H.; Song, J.; Yang, Y.; Su, P.; Yang, Y. DNA-directed enzyme immobilization on Fe3O4 modified with nitrogen-doped graphene quantum dots as a highly efficient and stable multi-catalyst system. J. Mater. Sci. 2019, 54, 2535–2551. [Google Scholar] [CrossRef]
- Yang, L.; Fan, D.; Zhang, Y.; Ding, C.; Wu, D.; Wei, Q.; Ju, H. Ferritin-Based Electrochemiluminescence Nanosurface Energy Transfer System for Procalcitonin Detection Using HWRGWVC Heptapeptide for Site-Oriented Antibody Immobilization. Anal. Chem. 2019, 91, 7145–7152. [Google Scholar] [CrossRef]
- Wacker, R.; Schröder, H.; Niemeyer, C.M. Performance of antibody microarrays fabricated by either DNA-directed immobilization, direct spotting, or streptavidin-biotin attachment: A comparative study. Anal. Biochem. 2004, 330, 281–287. [Google Scholar] [CrossRef]
- Li, Y.; Ren, J.; Nakajima, H.; Soh, N.; Nakano, K.; Imato, T. Surface plasmon resonance immunosensor for IgE analysis using two types of anti-IgE antibodies with different active recognition sites. JSAC 2007, 23, 31–38. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Wu, K.-Y.; Su, H.-L.; Hung, H.-Y.; Hsieh, Y.-Z. Sensitive label-free electrochemical analysis of human IgE using an aptasensor with cDNA amplification. Biosens. Bioelectron. 2013, 39, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Maehashi, K.; Matsumoto, K. Aptamer-based label-free immunosensors using carbon nanotube field-effect transistors. In Proceedings of the SENSORS, 2009 IEEE, Christchurch, New Zealand, 25–28 October 2009; pp. 1868–1871. [Google Scholar]
- Hianik, T. Affinity Biosensors for Detection Immunoglobulin E and Cellular Prions. Antibodies vs. DNA Aptamers. Electroanalysis 2016, 28, 1764–1776. [Google Scholar] [CrossRef]
- Yao, C.; Chen, Q.; Chen, M.; Zhang, B.; Luo, Y.; Huang, Q.; Huang, J.; Fu, W. A novel piezoelectric quartz micro-array immunosensor for detection of immunoglobulinE. J. Nanosci. Nanotechnol. 2006, 6, 3828–3834. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, J.P.; Han, S.J.; Sim, S.J. Aptamer biosensor for lable-free detection of human immunoglobulin E based on surface plasmon resonance. Sens. Actuator B-Chem. 2009, 139, 471–475. [Google Scholar] [CrossRef]
- Röthlisberger, D.; Honegger, A.; Plückthun, A. Domain interactions in the Fab fragment: A comparative evaluation of the single-chain Fv and Fab format engineered with variable domains of different stability. J. Mol. Biol. 2005, 347, 773–789. [Google Scholar] [CrossRef]
- Fuchs, S.M.; Raines, R.T. Polyarginine as a multifunctional fusion tag. Protein Sci. 2005, 14, 1538–1544. [Google Scholar] [CrossRef]
- Korodi, M.; Rákosi, K.; Baibarac, M.; Fejer, S.N. Reusable on-plate immunoprecipitation method with covalently immobilized antibodies on a protein G covered microtiter plate. J. Immunol. Methods 2020, 483, 112812. [Google Scholar] [CrossRef]
- Jung, Y.; Kang, H.J.; Lee, J.M.; Jung, S.O.; Yun, W.S.; Chung, S.J.; Chung, B.H. Controlled antibody immobilization onto immunoanalytical platforms by synthetic peptide. Anal. Biochem. 2008, 374, 99–105. [Google Scholar] [CrossRef]
- Kishimoto, S.; Nakashimada, Y.; Yokota, R.; Hatanaka, T.; Adachi, M.; Ito, Y. Site-Specific Chemical Conjugation of Antibodies by Using Affinity Peptide for the Development of Therapeutic Antibody Format. Bioconjugate Chem. 2019, 30, 698–702. [Google Scholar] [CrossRef]
- Tanwar, N.; Ojha, R.; Aggarwal, S.; Prajapati, V.K.; Munde, M. Design of inhibitor peptide sequences based on the interfacial knowledge of the protein G-IgG crystallographic complex and their binding studies with IgG. Eur. Biophys. J. Biophys. Lett. 2024, 53, 159–170. [Google Scholar] [CrossRef]
- Yoo, R.-J.; Choi, S.-J. Identification of a peptide ligand for antibody immobilization on biosensor surfaces. BioChip J. 2016, 10, 88–94. [Google Scholar] [CrossRef]
- Seo, J.S.; Lee, S.; Poulter, C.D. Regioselective covalent immobilization of recombinant antibody-binding proteins A, G, and L for construction of antibody arrays. J. Am. Chem. Soc. 2013, 135, 8973–8980. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.; Le Xuan, H.; Völzke, J.L.; Weller, M.G. Preactivation Crosslinking—An Efficient Method for the Oriented Immobilization of Antibodies. Methods Protoc. 2019, 2, 35. [Google Scholar] [CrossRef]
- Jung, J.; Yi, S.Y.; Jang, H.H.; Lee, C.-S.; Chung, B.H. Facile and oriented antibody immobilization on α-cyclodextrin-modified sensors surfaces. Macromol. Res. 2013, 21, 130–133. [Google Scholar] [CrossRef]
- Liedberg, B.; Nylander, C.; Lundström, I. Biosensing with surface plasmon resonance--how it all started. Biosens. Bioelectron. 1995, 10, i–ix. [Google Scholar] [CrossRef]
- Malmqvist, M. Biospecific interaction analysis using biosensor technology. Nature 1993, 361, 186–187. [Google Scholar] [CrossRef]
- Luppa, P.B.; Sokoll, L.J.; Chan, D.W. Immunosensors--principles and applications to clinical chemistry. Clin. Chim. Acta 2001, 314, 1–26. [Google Scholar] [CrossRef]
- Vashist, S.K.; Dixit, C.K.; MacCraith, B.D.; O’Kennedy, R. Effect of antibody immobilization strategies on the analytical performance of a surface plasmon resonance-based immunoassay. Analyst 2011, 136, 4431–4436. [Google Scholar] [CrossRef]
- Ko, S.; Park, T.J.; Kim, H.S.; Kim, J.H.; Cho, Y.J. Directed self-assembly of gold binding polypeptide-protein A fusion proteins for development of gold nanoparticle-based SPR immunosensors. Biosens. Bioelectron. 2009, 24, 2592–2597. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, Y.-P.; Kim, I.-H.; Ko, S. Rapid detection of aflatoxin B1 by a bifunctional protein crosslinker-based surface plasmon resonance biosensor. Food Control 2014, 36, 183–190. [Google Scholar] [CrossRef]
- Dutta, P.; Sawoo, S.; Ray, N.; Bouloussa, O.; Sarkar, A. Engineering bioactive surfaces with Fischer carbene complex: Protein A on self-assembled monolayer for antibody sensing. Bioconjugate Chem. 2011, 22, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.N.; Mutharasan, R. pH effect on protein G orientation on gold surfaces and characterization of adsorption thermodynamics. Langmuir 2012, 28, 6928–6934. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.; Wang, J.; Zeng, C.; Wang, M.; Qi, W.; He, Z. AuNP array coated substrate for sensitive and homogeneous SERS-immunoassay detection of human immunoglobulin G. RSC Adv. 2021, 11, 22744–22750. [Google Scholar] [CrossRef] [PubMed]
- de Juan-Franco, E.; Caruz, A.; Pedrajas, J.R.; Lechuga, L.M. Site-directed antibody immobilization using a protein A-gold binding domain fusion protein for enhanced SPR immunosensing. Analyst 2013, 138, 2023–2031. [Google Scholar] [CrossRef]
- Liang, A.; Tang, S.; Liu, M.; Yi, Y.; Xie, B.; Hou, H.; Luo, A. A molecularly imprinted electrochemical sensor with tunable electrosynthesized Cu-MOFs modification for ultrasensitive detection of human IgG. Bioelectrochemistry 2022, 146, 108154. [Google Scholar] [CrossRef]
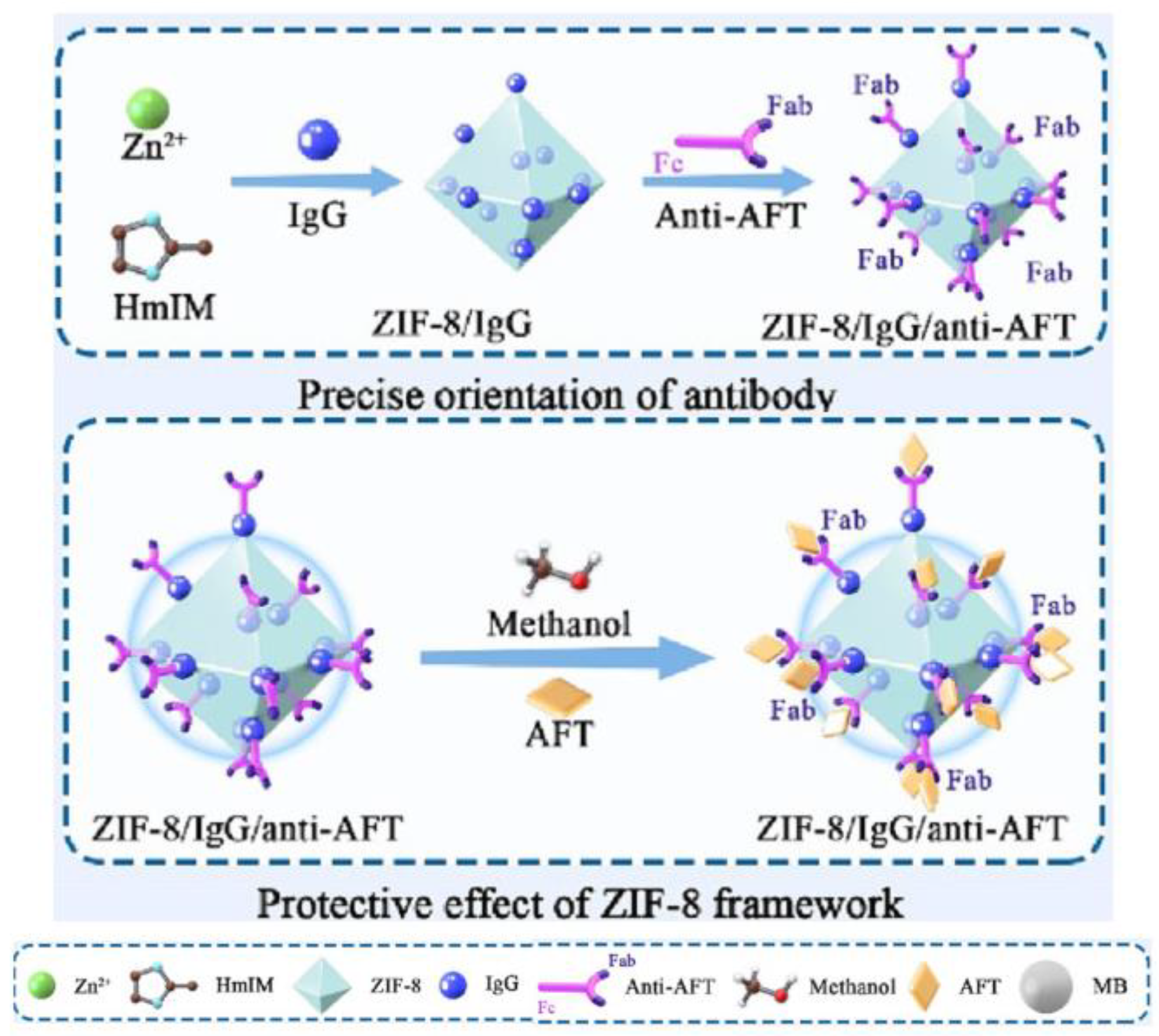
- Xuan, Z.; Shen, W.; Liu, H.; Ni, B.; Lian, Z.; Li, L.; Chen, J.; Guo, B.; Wang, S.; Ye, J. One-pot green synthesis of ZIF-8/IgG composite for the precise orientation and protection of antibody and its application in purification and detection of aflatoxins in peanut oil. Food Chem. 2024, 449, 139272. [Google Scholar] [CrossRef]
- Liao, B.-Y.; Chang, C.-J.; Wang, C.-F.; Lu, C.-H.; Chen, J.-K. Controlled antibody orientation on Fe3O4 nanoparticles and CdTe quantum dots enhanced sensitivity of a sandwich-structured electrogenerated chemiluminescence immunosensor for the determination of human serum albumin. Sens. Actuator B-Chem. 2021, 336, 129710. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, Z.; Zhang, Z.; Xu, H.; Jaffrezic-Renault, N.; Hu, J.; Guo, Z. Detection of parvovirus B19 genomic fragments using an electrochemical biosensor based on argonaute-assisted silver metallization. Microchem. J. 2025, 208, 112293. [Google Scholar] [CrossRef]
- Leibl, N.; Haupt, K.; Gonzato, C.; Duma, L. Molecularly Imprinted Polymers for Chemical Sensing: A Tutorial Review. Chemosensors 2021, 9, 123. [Google Scholar] [CrossRef]
- Weber, P.C.; Ohlendorf, D.H.; Wendoloski, J.J.; Salemme, F.R. Structural Origins of High-Affinity Biotin Binding to Streptavidin. Science 1989, 243, 85–88. [Google Scholar] [CrossRef]
- Niemeyer, C.M.; Sano, T.; Smith, C.L.; Cantor, C.R. Oligonucleotide-directed self-assembly of proteins: Semisynthetic DNA—Streptavidin hybrid molecules as connectors for the generation of macroscopic arrays and the construction of supramolecular bioconjugates. Nucleic Acids Res. 1994, 22, 5530–5539. [Google Scholar] [CrossRef] [PubMed]
- Hultschig, C.; Kreutzberger, J.; Seitz, H.; Konthur, Z.; Büssow, K.; Lehrach, H. Recent advances of protein microarrays. Curr. Opin. Chem. Biol. 2006, 10, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.L.; Reichert, J.M. Development trends for therapeutic antibody fragments. Nat. Biotechnol. 2009, 27, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Abe, A.; Ishikawa, N.; Rafique, A.; Ito, Y. A novel site-specific chemical conjugation of IgG antibodies by affinity peptide for immunoassays. J. Biochem. 2021, 169, 35–42. [Google Scholar] [CrossRef]
- Neundorf, I. Metal Complex-Peptide Conjugates: How to Modulate Bioactivity of Metal-Containing Compounds by the Attachment to Peptides. Curr. Med. Chem. 2017, 24, 1853–1861. [Google Scholar] [CrossRef]
- Liedberg, B.; Nylander, C.; Lunström, I. Surface plasmon resonance for gas detection and biosensing. Sens. Actuators B Chem. 1983, 4, 299–304. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Ma, M.; Aisa, H.A.; Chen, L. Oriented Immobilization of IgG for Immunosensor Development. Chemosensors 2025, 13, 50. https://doi.org/10.3390/chemosensors13020050
Zhang Y, Ma M, Aisa HA, Chen L. Oriented Immobilization of IgG for Immunosensor Development. Chemosensors. 2025; 13(2):50. https://doi.org/10.3390/chemosensors13020050
Chicago/Turabian StyleZhang, Yihan, Mingjie Ma, Haji Akber Aisa, and Longyi Chen. 2025. "Oriented Immobilization of IgG for Immunosensor Development" Chemosensors 13, no. 2: 50. https://doi.org/10.3390/chemosensors13020050
APA StyleZhang, Y., Ma, M., Aisa, H. A., & Chen, L. (2025). Oriented Immobilization of IgG for Immunosensor Development. Chemosensors, 13(2), 50. https://doi.org/10.3390/chemosensors13020050






