Chemosensors for H2O2 Detection: Principles, Active Materials, and Applications
Abstract
1. Introduction
- (1)
- High Reactivity: H2O2 is colorless, odorless, and volatile, making it prone to decomposition, particularly under light, heat, or in the presence of catalysts like metal ions. This instability complicates accurate detection, as decomposition products (H2O and O2) often interfere with measurements. Additionally, the strong oxidizing nature of H2O2 may lead to undesired reactions with other substances during detection.
- (2)
- Limited Selectivity: The presence of other oxidative chemicals (e.g., O2, O3) in real-world samples can interfere with H2O2 detection, leading to false positives or negatives by producing similar signals or reacting with detection reagents.
- (3)
- Environmental Sensitivity: H2O2 coexists with H2O, a challenge for gas sensors. Its volatility and sensitivity to environmental conditions, such as temperature and light, demand strict control during testing to ensure accuracy.
- (4)
- Sample Preparation: Preventing H2O2 decomposition, removing interferences, and enriching analyte concentrations require labor-intensive preparation, making detection methods prone to noise or susceptible to missed low concentrations.
- (5)
- Instrumental Limitations: Analytical instruments may face constraints in detection range, precision, and reproducibility, affecting result reliability.
- (6)
- Cost and Accessibility: Traditional methods, such as chemical titration, are time-consuming, require skilled operators, and are often restricted to research settings. Advanced methods may demand expensive equipment, reagents, and expertise, limiting accessibility.
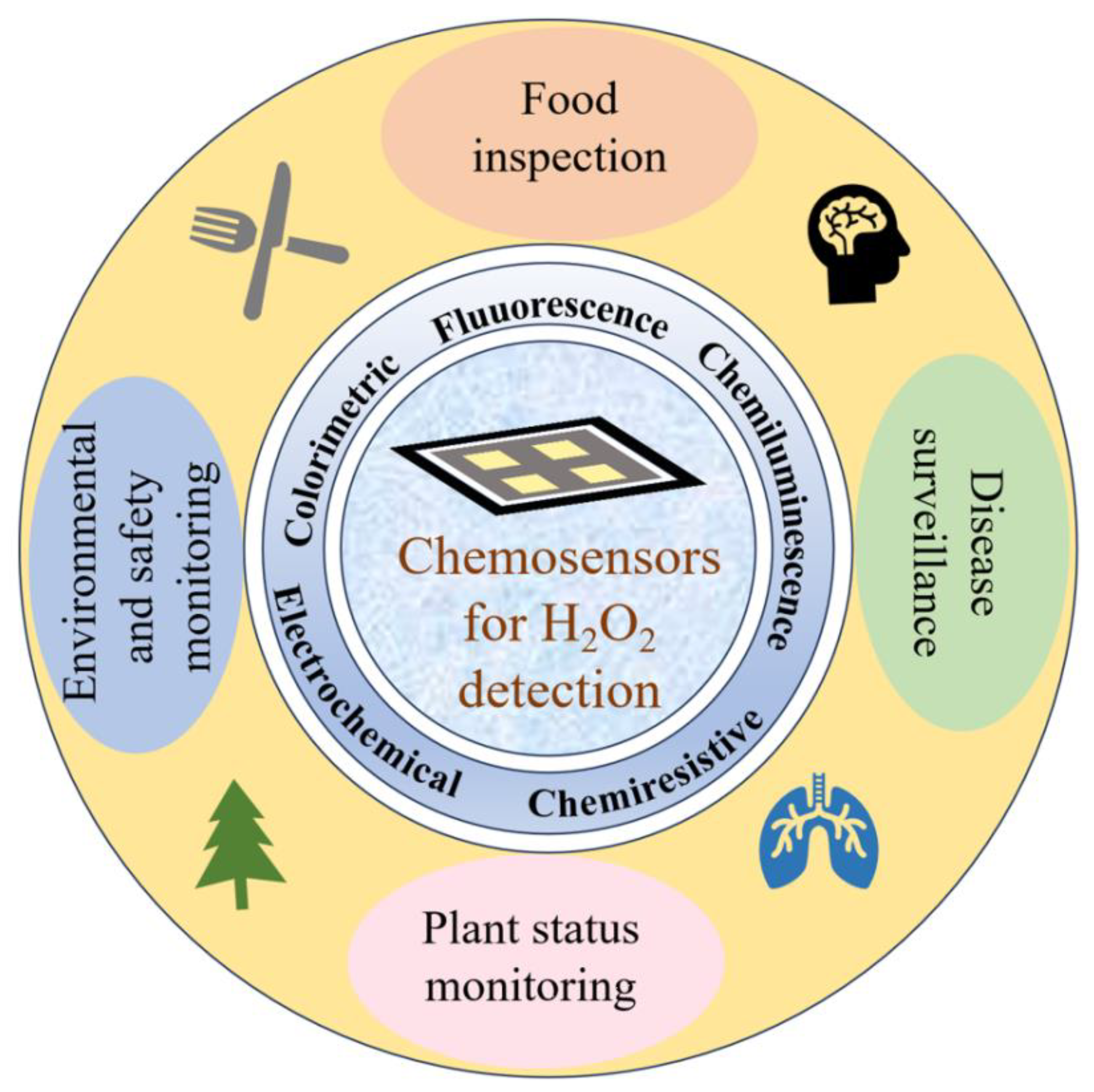
2. Mechanisms of Chemosensors for H2O2 Detection
2.1. Optical Chemosensors
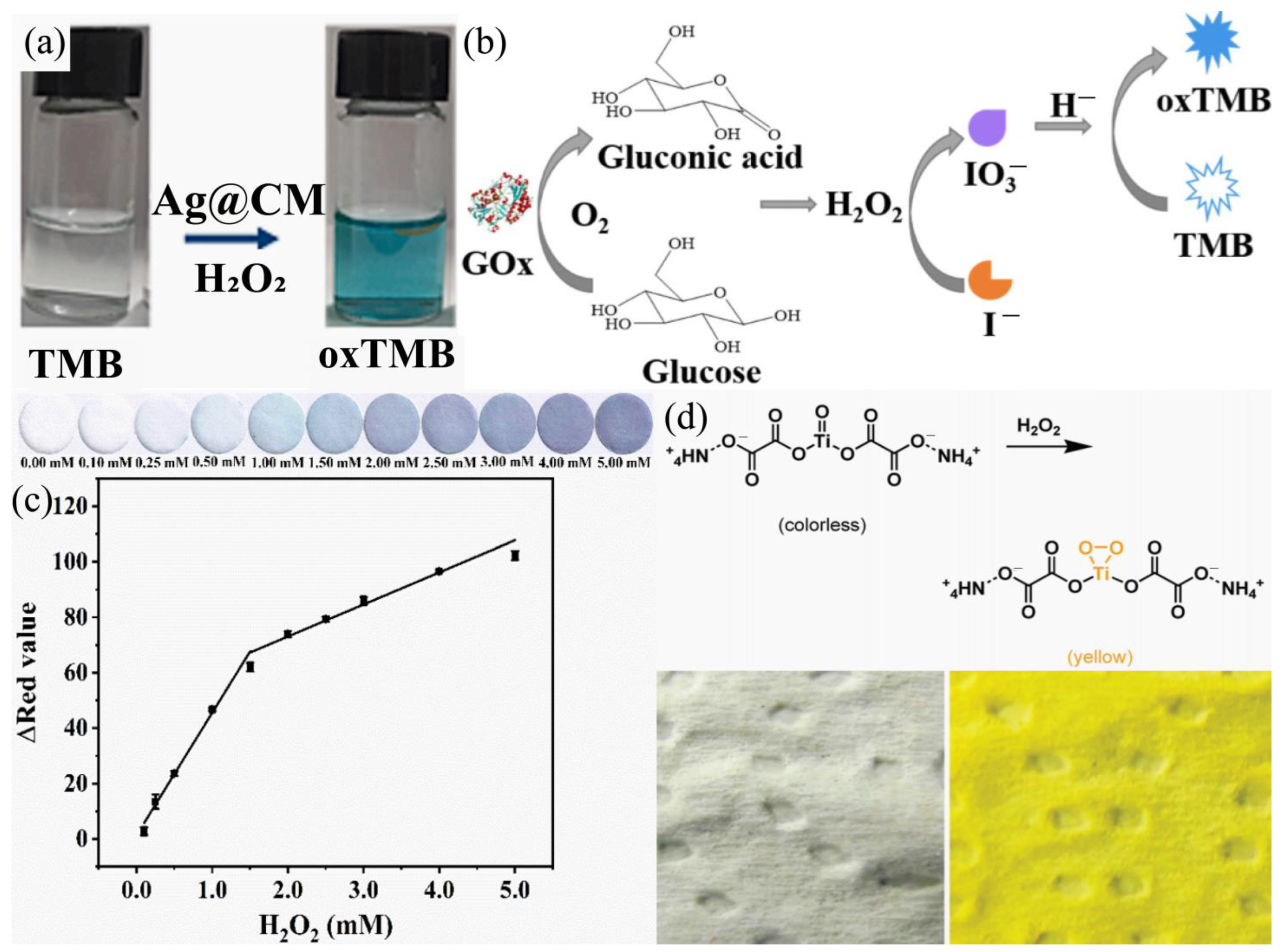
2.1.1. Colorimetric Sensor
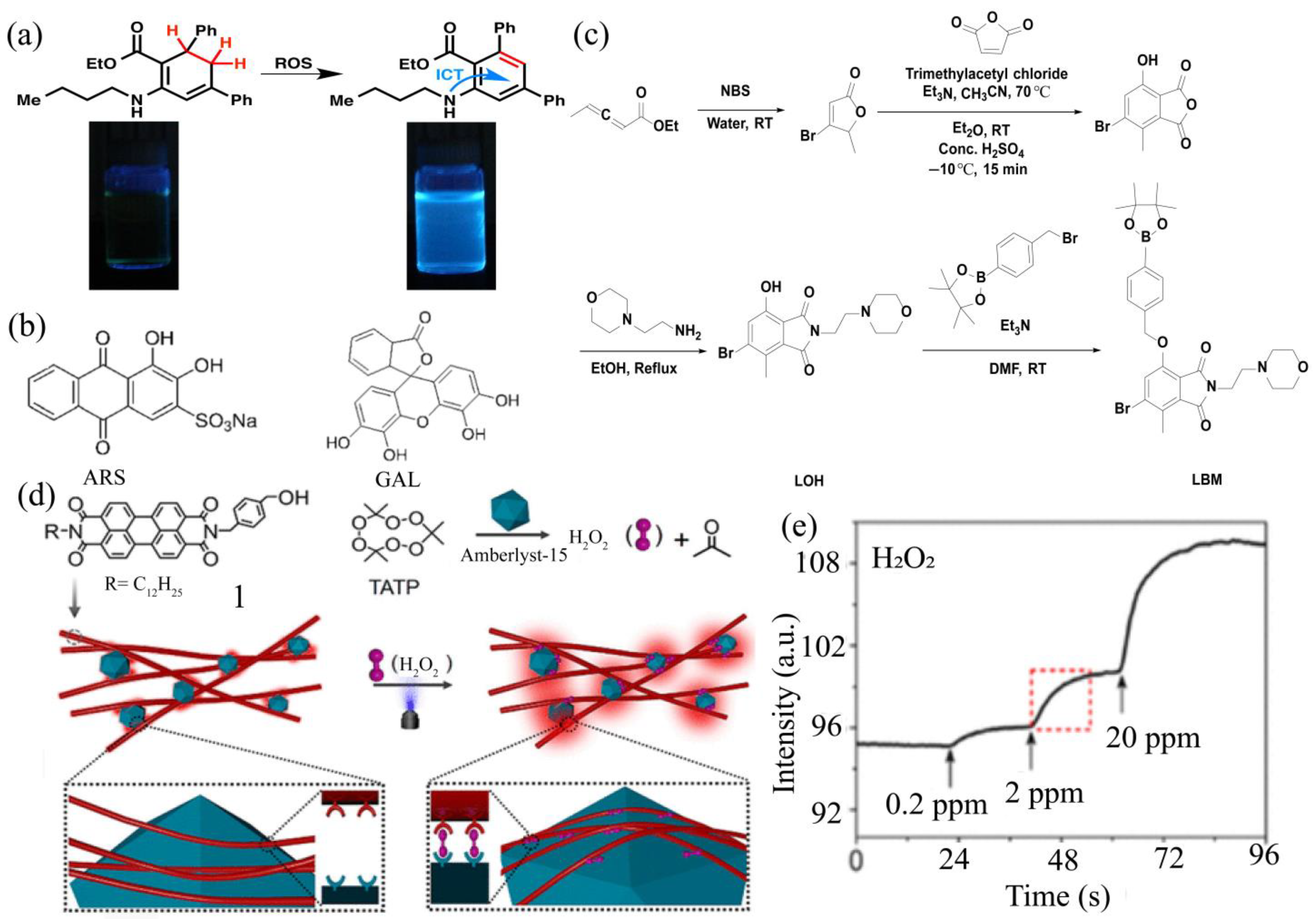
2.1.2. Fluorescent Sensor
2.1.3. Chemiluminescent Sensor
2.2. Electrical Chemosensors
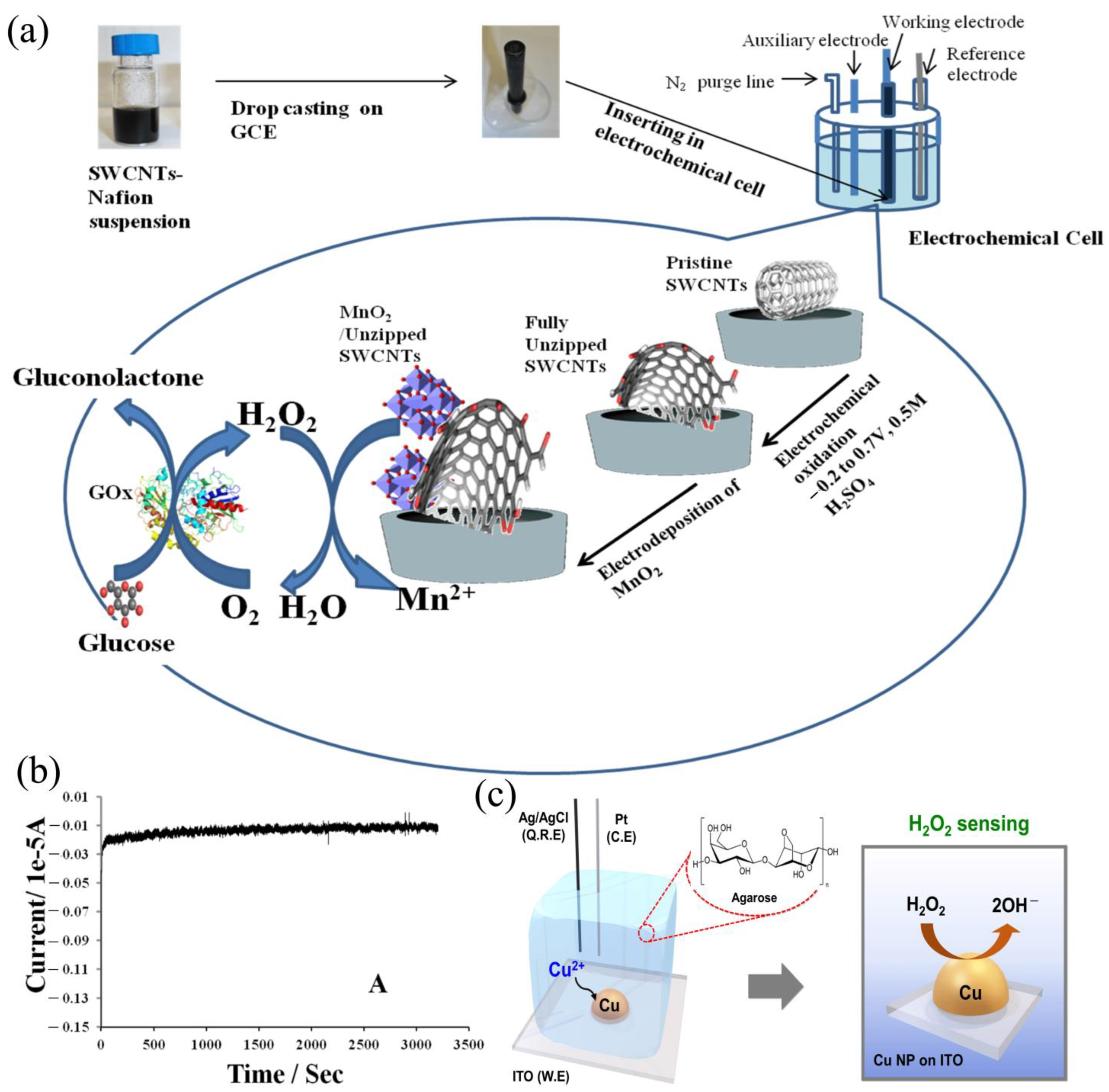
2.2.1. Electrochemical Sensor
2.2.2. Chemiresistive Sensor
3. Applications of Chemosensors for H2O2 Detection
3.1. Food Inspection
3.2. Environmental and Safety Monitoring
3.3. Disease Surveillance
3.4. Plant Status Monitoring
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rampon, C.; Volovitch, M.; Joliot, A.; Vriz, S. Hydrogen peroxide and redox regulation of developments. Antioxidants 2018, 7, 159. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Albanese, L.; Meneguzzo, F.; Pagliaro, M. Hydrogen peroxide: A key chemical for today’s sustainable development. ChemSusChem 2016, 9, 3374–3381. [Google Scholar] [CrossRef]
- Chen, Y.; Zaman, F.; Jia, Y.; Huang, Y.; Li, T.; Bai, F.; Song, L.; Li, J. Harmful cyanobacterial bloom control with hydrogen peroxide: Mechanism, affecting factors, development, and prospects. Curr. Pollut. Rep. 2024, 10, 566–579. [Google Scholar] [CrossRef]
- Rigoletto, M.; Laurenti, E.; Tummino, M.L. An overview of environmental catalysis mediated by hydrogen peroxide. Catalysts 2024, 14, 267. [Google Scholar] [CrossRef]
- Ullah, N.; Khan, S.; Barton, B.; Guziejewski, D.; Smarzewska, S.; Koszelska, K.; Mirceski, V. A mini review on topographical and structural insight of metal carbides for electrocatalytic hydrogen peroxide detection. Microchem. J. 2024, 200, 109961. [Google Scholar] [CrossRef]
- Xing, L.; Zhang, W.; Fu, L.; Lorenzo, J.M.; Hao, Y. Fabrication and application of electrochemical sensor for analyzing hydrogen peroxide in food system and biological samples. Food Chem. 2022, 385, 132555. [Google Scholar] [CrossRef] [PubMed]
- Pirsa, S.; Purghorbani, F. An overview of hydrogen peroxide sensors and their applications in food quality control. Sens. Rev. 2024, 44, 159–170. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Biological production, detection, and fate of hydrogen peroxide. Antioxid. Redox Signal. 2018, 29, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Burek, B.O.; Bormann, S.; Hollmann, F.; Bloh, J.Z.; Holtmann, D. Hydrogen peroxide driven biocatalysis. Green Chem. 2019, 21, 3232–3249. [Google Scholar] [CrossRef]
- Ruan, S.; Liu, W.; Wang, W.; Lu, Y. Research progress of SERS sensors based on hydrogen peroxide and related substances. Crit. Rev. Anal. Chem. 2023, 54, 3570–3591. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, K.B.; Migliorini, F.L.; Christinelli, W.A.; Correa, D.S. Detection of hydrogen peroxide (H2O2) using a colorimetric sensor based on cellulose nanowhiskers and silver nanoparticles. Carbohydr. Polym. 2019, 212, 235–241. [Google Scholar] [CrossRef]
- Liu, J.; Li, M.; Liu, W.; Hao, Z.; Zhang, F.; Pang, H.; Zhang, R.; Zhang, L. Advances in non-enzymatic electrochemical materials for H2O2 sensing. J. Electroanal. Chem. 2024, 954, 118060. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, L.; Chen, B.; Zhang, Q.; Zhu, R. Detecting hydrogen peroxide reliably in water via ion chromatography: A method evaluation update and comparison in the presence of interfering components. Environ. Sci. Water Res. Technol. 2020, 6, 2396–2404. [Google Scholar] [CrossRef]
- Chen, L.; Wei, L.; Ru, Y.; Weng, M.; Wang, L.; Dai, Q. A mini-review of the electro-peroxone technology for wastewaters: Characteristics, mechanism and prospect. Chin. Chem. Lett. 2023, 34, 108162. [Google Scholar] [CrossRef]
- Schnabel, T.; Mehling, S.; Londong, J.; Springer, C. Hydrogen peroxide-assisted photocatalytic water treatment for the removal of anthropogenic trace substances from the effluent of wastewater treatment plants. Water Sci. Technol. 2020, 82, 2019–2028. [Google Scholar] [CrossRef]
- Siddique, I. Unveiling the power of high-performance liquid chromatography: Techniques, applications, and innovations. Eur. J. Adv. Eng. Technol. 2021, 8, 79–84. [Google Scholar] [CrossRef]
- Jawad, A.; Chen, Z.; Yin, G. Bicarbonate activation of hydrogen peroxide: A new emerging technology for wastewater treatment. Chin. J. Catal. 2016, 37, 810–825. [Google Scholar] [CrossRef]
- Ahmad, T.; Iqbal, A.; Halim, S.A.; Uddin, J.; Khan, A.; El Deeb, S.; Al-Harrasi, A. Recent advances in electrochemical sensing of hydrogen peroxide (H2O2) released from cancer cells. Nanomaterials 2022, 12, 1475. [Google Scholar] [CrossRef]
- Song, Y.; Wang, C.; Sha, J.; Liu, X.; Han, L.; Li, L. Photoelectrochemical sensor based on the sensitive interface of photosensitive electrode for the detection of hydrogen peroxide in dried bean curds. J. Food Compos. Anal. 2023, 119, 105237. [Google Scholar] [CrossRef]
- Shamkhalichenar, H.; Choi, J.W. Non-enzymatic hydrogen peroxide electrochemical sensors based on reduced graphene oxide. J. Electrochem. Soc. 2020, 167, 037531. [Google Scholar] [CrossRef]
- Saranchina, N.V.; Bazhenova, O.A.; Bragina, S.K.; Gavrilenko, N.A.; Volgina, T.N.; Gavrilenko, M.A. Stabilization of silver nanoparticles in a transparent polymethacrylate matrix while maintaining the capabilities of a colorimetric hydrogen peroxide sensor. Chem. Pap. 2024, 78, 8219–8224. [Google Scholar] [CrossRef]
- Tang, J.; Li, F.; Liu, C.; Shu, J.; Yue, J.; Xu, B.; Liu, X.; Zhang, K.; Jiang, W. Attractive benzothiazole-based fluorescence probe for the highly efficient detection of hydrogen peroxide. Anal. Chim. Acta 2022, 1214, 339939. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.L.; Zheng, G.S.; Wu, M.Y.; Wei, J.Y.; Lou, Q.; Ye, Y.L.; Shan, C.X. Chemiluminescent carbon nanodots as sensors for hydrogen peroxide and glucose. Nanophotonics 2020, 9, 3597–3604. [Google Scholar] [CrossRef]
- Shen, H.; Liu, H.; Wang, X. Surface construction of catalase-immobilized Au/PEDOT nanocomposite on phase-change microcapsules for enhancing electrochemical biosensing detection of hydrogen peroxide. Appl. Surf. Sci. 2023, 612, 155816. [Google Scholar] [CrossRef]
- Clausmeyer, J.; Actis, P.; Córdoba, A.L.; Korchev, Y.; Schuhmann, W. Nanosensors for the detection of hydrogen peroxide. Electrochem. Commun. 2014, 40, 28–30. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, Z.; Shen, T.; Qin, Y. Graphene/Gold nanoparticle composite-based paper sensor for electrochemical detection of hydrogen peroxide. Fuller. Nanotubes Carbon Nanostruct. 2019, 27, 23–27. [Google Scholar] [CrossRef]
- Gu, S.; Hsieh, C.T.; Mallick, B.C.; Fu, C.C.; Juang, R.S.; Gandomif, Y.A.; Kihm, K.D. Novel electrochemical sensor based on highly amidized graphene quantum dots to electrochemically detect hydrogen peroxide. Appl. Surf. Sci. 2020, 528, 146936. [Google Scholar] [CrossRef]
- Feizabadi, M.; Soleymanpour, A.; Faridnouri, H.; Ajloo, D. Improving stability of biosensor based on covalent immobilization of horseradish peroxidase by γ-aminobutyric acid and application in detection of H2O2. Int. J. Biol. Macromol. 2019, 136, 597–606. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, Y.; He, Y.; Wang, Y. Mitochondria and lysosome-targetable fluorescent probes for hydrogen peroxide. J. Mater. Chem. B 2021, 9, 908–920. [Google Scholar] [CrossRef] [PubMed]
- Alruwais, R.S.; Adeosun, W.A. Electrochemical detection of hydrogen peroxide using copper-based metal–organic frameworks: Nanoarchitectonics and sensing performance. J. Inorg. Organomet. Polym. Mater. 2024, 34, 14–37. [Google Scholar] [CrossRef]
- Peng, M.; Zhao, Y.; Chen, D.; Tan, Y. Free-standing 3D electrodes for electrochemical detection of hydrogen peroxide. ChemCatChem 2019, 11, 4222–4237. [Google Scholar] [CrossRef]
- da Silva Neves, M.M.P.; González-García, M.B.; Hernández-Santos, D.; Fanjul-Bolado, P. Future trends in the market for electrochemical biosensing. Curr. Opin. Electrochem. 2018, 10, 107–111. [Google Scholar] [CrossRef]
- Baye, A.F.; Nguyen, H.T.; Kim, H. FeO/Fe3C-assisted Fe3O4 redox sites as robust peroxidase mimics for colorimetric detection of H2O2. Sens. Actuators B-Chem. 2023, 377, 133097. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Zhao, L.; Han, H.; Zhang, S.; Huang, Y.; Wang, Y.; Song, D.; Ma, P.; Ren, P.; et al. A neoteric dual-signal colorimetric fluorescent probe for detecting endogenous/exogenous hydrogen peroxide in cells and monitoring drug-induced hepatotoxicity. Talanta 2021, 233, 122578. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, J.M.; Jiang, Y.S.; Wen, L.; He, M.J.; Xiao, Y.; Chen, J.Y. Silver nanoparticles@ metal-organic framework as peroxidase mimics for colorimetric determination of hydrogen peroxide and blood glucose. Chin. J. Anal. Chem. 2022, 50, 100187. [Google Scholar] [CrossRef]
- Parveen, S.; Islam, S.N.; Ahmad, A. Mycological synthesis of ruthenium oxide quantum dots and their application in the colorimetric detection of H2O2. Adv. Powder. Technol. 2022, 33, 103861. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Yang, C.; Ma, C.; Tang, J. A novel smartphone-based colorimetric biosensor for reliable quantification of hydrogen peroxide by enzyme-inorganic hybrid nanoflowers. Biochem. Eng. J. 2021, 167, 107925. [Google Scholar] [CrossRef]
- Peng, L.J.; Zhang, C.Y.; Zhang, W.Y.; Zhou, H.Y.; Yang, F.Q. The peroxidase-like catalytic activity of in situ prepared cobalt carbonate and its applications in colorimetric detection of hydrogen peroxide, glucose and ascorbic acid. Colloids Surf. A 2022, 651, 129744. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, X.; Wan, G.; Shi, S.; Wang, G. NiFe2O4/CNTs fabricated by atomic layer deposition as highly stable peroxidase mimics for sensitive colorimetric detection of hydrogen peroxide and glucose. Mater. Res. Bull. 2022, 147, 111637. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Zeng, M.; Zhao, Y.; Zuo, X.; Meng, F.; Lu, Y. Zr (IV)-based metal-organic framework nanocomposites with enhanced peroxidase-like activity as a colorimetric sensing platform for sensitive detection of hydrogen peroxide and phenol. Environ. Res. 2022, 203, 111818. [Google Scholar] [CrossRef]
- Zhang, H.; Ren, Q.; Lu, J.; Xu, Y.; Wang, Y.; Liu, S.; Luo, X. Simultaneous generation and immobilization of silver nanoparticles on cellulose membranes by hydrogen bond-assisted in-situ reaction for colorimetric detection of mercury ions and hydrogen peroxide. Chem. Eng. J. 2023, 478, 147264. [Google Scholar] [CrossRef]
- Yu, K.; Li, M.; Chai, H.; Liu, Q.; Hai, X.; Tian, M.; Qu, L.; Xu, T.; Zhang, G.; Zhang, X. MOF-818 nanozyme-based colorimetric and electrochemical dual-mode smartphone sensing platform for in situ detection of H2O2 and H2S released from living cells. Chem. Eng. J. 2023, 451, 138321. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Zhang, H.; Yang, F.Q. An economical and portable paper-based colorimetric sensor for the determination of hydrogen peroxide-related biomarkers. Chemosensors 2022, 10, 335. [Google Scholar] [CrossRef]
- Xu, M.; Bunes, B.R.; Zang, L. Paper-based vapor detection of hydrogen peroxide: Colorimetric sensing with tunable interface. ACS Appl. Mater. Interfaces 2011, 3, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.S.; Ramakrishnappa, T. Green synthesized uncapped Ag colloidal nanoparticles for selective colorimetric sensing of divalent Hg and H2O2. J. Environ. 2021, 9, 105365. [Google Scholar] [CrossRef]
- Ghohestani, E.; Tashkhourian, J.; Hemmateenejad, B. Colorimetric determination of peroxide value in vegetable oils using a paper based analytical device. Food Chem. 2023, 403, 134345. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Lin, T.; Huang, J.; Chen, Y.; Zhong, J.; Guo, L.; Fu, F. Boron-and phenyl-doped graphitic carbon nitride (g-C3N4) nanosheets for colorimetric detection of hydrogen peroxide in soaked foods. J. Nanosci. Nanotechnol. 2019, 19, 4220–4227. [Google Scholar] [CrossRef]
- Mu, Z.; Guo, J.; Li, M.; Wu, S.; Zhang, X.; Wang, Y. A sensitive fluorescence detection strategy for H2O2 and glucose by using aminated Fe–Ni bimetallic MOF as fluorescent nanozyme. Microchim. Acta 2023, 190, 81. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Park, S.; Jain, N.; Kim, Y.; Nimse, S.B.; Churchill, D.G. Novel mycophenolic acid precursor-based fluorescent probe for intracellular H2O2 detection in living cells and daphnia magna and zebrafish model systems. Analyst 2024, 149, 4477–4486. [Google Scholar] [CrossRef] [PubMed]
- Fagadar-Cosma, E.; Plesu, N.; Lascu, A.; Anghel, D.; Cazacu, M.; Ianasi, C.; Fagadar-Cosma, G.; Fratilescu, I.; Epuran, C. Novel platinum-porphyrin as sensing compound for efficient fluorescent and electrochemical detection of H2O2. Chemosensors 2020, 8, 29. [Google Scholar] [CrossRef]
- González-Ruiz, V.; Rajesh, J.; Olives, A.I.; Rocchi, D.; Gómez-Carpintero, J.; González, J.F.; Sridharan, V.; Martín, M.A.; Menéndez, J.C. Antioxidants as molecular probes: Structurally novel dihydro-m-terphenyls as turn-on fluorescence chemodosimeters for biologically relevant oxidants. Antioxidants 2020, 9, 605. [Google Scholar]
- Olenin, A.Y. Methods of nonenzymatic determination of hydrogen peroxide and related reactive oxygen species. J. Anal. Chem. 2017, 72, 243–255. [Google Scholar] [CrossRef]
- Li, M.; Lei, P.; Song, S.; Shuang, S.; Dong, C. Alizarin-based molecular probes for the detection of hydrogen peroxide and peroxynitrite. Analyst 2021, 146, 509–514. [Google Scholar] [CrossRef]
- Yu, X.; Gong, Y.; Xiong, W.; Li, M.; Zhao, J.; Che, Y. Turn-on fluorescent detection of hydrogen peroxide and triacetone triperoxide via enhancing interfacial interactions of a blended system. Anal. Chem. 2019, 91, 6967–6970. [Google Scholar] [CrossRef]
- Teniou, A.; Madi, I.A.; Mouhoub, R.; Marty, J.L.; Rhouati, A. One-step chemiluminescent assay for hydrogen peroxide analysis in water. Chemosensors 2023, 11, 455. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, K.H.; Seo, S.E.; il Kim, M.; Park, S.J.; Kwon, O.S. Cytochrome C-decorated graphene field-effect transistor for highly sensitive hydrogen peroxide detection. J. Ind. Eng. Chem. 2020, 83, 29–34. [Google Scholar] [CrossRef]
- Stoikov, D.; Shafigullina, I.; Shurpik, D.; Stoikov, I.; Evtugyn, G. A flow-through biosensor system based on pillar [3] arene [2] quinone and ferrocene for determination of hydrogen peroxide and uric acid. Chemosensors 2024, 12, 98. [Google Scholar] [CrossRef]
- Xu, W.; Fei, J.; Yang, W.; Zheng, Y.; Dai, Y.; Sakran, M.; Zhang, J.; Zhu, W.; Hong, J.; Zhou, X. A colorimetric/electrochemical dual-mode sensor based on Fe3O4@MoS2-Au NPs for high-sensitivity detection of hydrogen peroxide. Microchem. J. 2022, 181, 107825. [Google Scholar] [CrossRef]
- Guo, X.; Cao, Q.; Liu, Y.; He, T.; Liu, J.; Huang, S.; Tang, H.; Ma, M. Organic electrochemical transistor for in situ detection of H2O2 released from adherent cells and its application in evaluating the in vitro cytotoxicity of nanomaterial. Anal. Chem. 2019, 92, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Wu, D.; Wang, T.; Xiao, J.; Yu, L. A label-free colorimetric assay based on gold nanoparticles for the detection of H2O2 and glucose. Chemosensors 2022, 10, 100. [Google Scholar] [CrossRef]
- Zakaria, A.B.M.; Leszczynska, D. Electrochemically prepared unzipped single walled carbon nanotubes-MnO2 nanostructure composites for hydrogen peroxide and glucose sensing. Chemosensors 2019, 7, 1. [Google Scholar] [CrossRef]
- Shringi, A.K.; Kumar, R.; Dennis, N.F.; Yan, F. Two-dimensional tellurium nanosheets for the efficient nonenzymatic electrochemical detection of H2O2. Chemosensors 2024, 12, 17. [Google Scholar] [CrossRef]
- Spanu, D.; Dhahri, A.; Binda, G.; Monticelli, D.; Pinna, M.; Recchia, S. Ultrafast electrochemical self-doping of anodic titanium dioxide nanotubes for enhanced electroanalytical and photocatalytic performance. Chemosensors 2023, 11, 560. [Google Scholar] [CrossRef]
- Wiedemair, J.; van Dorp, H.D.; Olthuis, W.; van den Berg, A. Developing an amperometric hydrogen peroxide sensor for an exhaled breath analysis system. Electrophoresis 2012, 33, 3181–3186. [Google Scholar] [CrossRef] [PubMed]
- Purwidyantri, A.; Tian, Y.C.; Saputra, G.M.A.; Prabowo, B.A.; Liu, H.L.; Yang, C.M.; Lai, C.S. Gold nanoframe array electrode for straightforward detection of hydrogen peroxide. Chemosensors 2021, 9, 37. [Google Scholar] [CrossRef]
- Mihailova, I.; Krasovska, M.; Sledevskis, E.; Gerbreders, V.; Mizers, V.; Ogurcovs, A. Assessment of oxidative stress by detection of H2O2 in Rye samples using a CuO-and Co3O4-nanostructure-based electrochemical sensor. Chemosensors 2023, 11, 532. [Google Scholar] [CrossRef]
- Han, J.; Kim, J.; Kim, B.K.; Park, K. Hydrogel-based electrodeposition of copper nanoparticles for selective detection for hydrogen peroxide. Chemosensors 2023, 11, 384. [Google Scholar] [CrossRef]
- Lee, S.; Lee, Y.J.; Kim, J.H.; Lee, G.J. Electrochemical detection of H2O2 released from prostate cancer cells using Pt nanoparticle-decorated Rgo-CNT nanocomposite-modified screen-printed carbon electrodes. Chemosensors 2020, 8, 63. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, S.; Xiang, X.; Shu, J.; Fei, J. Fabricating process-electrochemical property correlation of laser-scribed graphene and smartphone-based electrochemical platform for portable and sensitive biosensing. Biosens. Bioelectron. 2023, 237, 115525. [Google Scholar] [CrossRef]
- Kader, M.A.; Azmi, N.S.; Kafi, A.K.M.; Hossain, M.S.; Masri, M.F.B.; Ramli, A.N.M.; Tan, C.S. Synthesis and characterization of a multiporous SnO2 nanofibers-supported Au nanoparticles-based amperometric sensor for the nonenzymatic detection of H2O2. Chemosensors 2023, 11, 130. [Google Scholar] [CrossRef]
- Gligor, D.; Maicaneanu, S.A.; Varodi, C. Low-cost carbon paste Cu (II)-exchanged zeolite amperometric sensor for hydrogen peroxide detection. Chemosensors 2024, 12, 23. [Google Scholar] [CrossRef]
- Zanoni, J.; Moura, J.P.; Santos, N.F.; Carvalho, A.F.; Fernandes, A.J.; Monteiro, T.; Rodrigues, J. Dual transduction of H2O2 detection using ZnO/laser-induced graphene composites. Chemosensors 2021, 9, 102. [Google Scholar] [CrossRef]
- Xie, X.; Gao, N.; Zhu, L.; Hunter, M.; Chen, S.; Zang, L. PEDOT:PSS/PEDOT film chemiresistive sensors for hydrogen peroxide vapor detection under ambient conditions. Chemosensors 2023, 11, 124. [Google Scholar] [CrossRef]
- Xie, X.; Gao, N.; Hunter, M.; Zhu, L.; Yang, X.; Chen, S.; Zang, L. PEDOT films doped with titanyl oxalate as chemiresistive and colorimetric dual-mode sensors for the detection of hydrogen peroxide vapor. Sensors 2023, 23, 3120. [Google Scholar] [CrossRef] [PubMed]
- Karamoschos, N.; Tasis, D. Photocatalytic evolution of hydrogen peroxide: A minireview. Energies 2022, 15, 6202. [Google Scholar] [CrossRef]
- Kim, G.; Lee, Y.E.K.; Kopelman, R. Hydrogen peroxide (H2O2) detection with nanoprobes for biological applications: A mini-review. In Oxidative Stress Nanotechnology: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2013; pp. 101–114. [Google Scholar]
- Żamojć, K.; Zdrowowicz, M.; Jacewicz, D.; Wyrzykowski, D.; Chmurzyński, L. Fluorescent probes used for detection of hydrogen peroxide under biological conditions. Crit. Rev. Anal. Chem. 2016, 46, 171–200. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.H.; Shim, H.S.; Ha, J.; Moon, H.R. MOF-on-MOF architectures: Applications in separation, catalysis, and sensing. Bull. Korean Chem. Soc. 2021, 42, 956–969. [Google Scholar] [CrossRef]
- Schäferling, M.; Grögel, D.B.; Schreml, S. Luminescent probes for detection and imaging of hydrogen peroxide. Microchim. Acta 2011, 174, 1–18. [Google Scholar] [CrossRef]
- Zheng, D.J.; Yang, Y.S.; Zhu, H.L. Recent progress in the development of small-molecule fluorescent probes for the detection of hydrogen peroxide. TrAC Trend. Anal. Chem. 2019, 118, 625–651. [Google Scholar] [CrossRef]
- Rezende, F.; Brandes, R.P.; Schröder, K. Detection of hydrogen peroxide with fluorescent dyes. Antioxid. Redox Signal. 2018, 29, 585–602. [Google Scholar] [CrossRef]
- Qiu, X.; Xin, C.; Qin, W.; Li, Z.; Zhang, D.; Zhang, G.; Huang, W. A novel pyrimidine based deep-red fluorogenic probe for detecting hydrogen peroxide in Parkinson’s disease models. Talanta 2019, 199, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiao, W.; Xie, S.; Fan, G.; Shi, X.; Meng, H.; Yang, H. Low-density pt nanoarray-based hydrogen peroxide sensing platform and its application in trace sarcosine detection. Electrochim. Acta 2023, 443, 141952. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Gatto, G.; Broncova, G.; Kumar, A. Electrochemical sensors and their applications: A review. Chemosensors 2022, 10, 363. [Google Scholar] [CrossRef]
- Xiao, Q.; Xu, C. Research progress on chemiluminescence immunoassay combined with novel technologies. TrAC Trend. Anal. Chem. 2020, 124, 115780. [Google Scholar] [CrossRef]
- Hey, Y.Y.; Bak, Y.; Seung, B.P.; Subba, R.C.; Kyung, H.S.; Kim, S.; Lee, J. Dual-modal iridium-based self-immolative chemosensors for differential responses against reactive oxygen species and their applications to detect diabetes. Adv. Mater. Interfaces 2023, 10, 2202408. [Google Scholar]
- Riaz, M.A.; Chen, Y. Electrodes and electrocatalysts for electrochemical hydrogen peroxide sensors: A review of design strategies. Nanoscale Horiz. 2022, 7, 463–479. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Cai, S.; Ren, Q.Q.; Wen, W.; Zhao, Y.D. Recent advances in electrochemical sensing for hydrogen peroxide: A review. Analyst 2012, 137, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yuan, R.; Chai, Y.; Hu, F. Electrochemical sensing of hydrogen peroxide using metal nanoparticles: A review. Microchim. Acta 2013, 180, 15–32. [Google Scholar] [CrossRef]
- Prakash, P.A.; Yogeswaran, U.; Chen, S.M. A review on direct electrochemistry of catalase for electrochemical sensors. Sensors 2009, 9, 1821–1844. [Google Scholar] [CrossRef] [PubMed]
- Caval, M.; Sanna, C.; Marceddu, S.; Rocchitta, G.; Serra, P.A. The platinization of graphite composites turns widespread and low-cost materials into hydrogen peroxide sensors and high-value biosensor transducers. Chemosensors 2023, 11, 153. [Google Scholar] [CrossRef]
- Mohan, S.; Dinesha, P.; Kumar, S. NOx reduction behaviour in copper zeolite catalysts for ammonia SCR systems: A review. Chem. Eng. J. 2020, 384, 123253. [Google Scholar] [CrossRef]
- Vijayaram, S.; Razafindralambo, H.; Sun, Y.Z.; Vasantharaj, S.; Ghafarifarsani, H.; Hoseinifar, S.H.; Raeeszadeh, M. Applications of green synthesized metal nanoparticles—A review. Biol. Trace Elem. Res. 2024, 202, 360–386. [Google Scholar] [CrossRef] [PubMed]
- Zango, Z.U.; Khoo, K.S.; Garba, A.; Garba, Z.N.; Danmallam, U.N.; Aldaghri, O.; Ramesh, M.D. A review on titanium oxide nanoparticles modified metal-organic frameworks for effective CO2 conversion and efficient wastewater remediation. Environ. Res. 2024, 252, 119024. [Google Scholar] [CrossRef]
- Wang, W.; Liu, L.; Feng, J.; Yin, Y. Photocatalytic reversible color switching based on titania nanoparticles. Small Methods 2018, 2, 1700273. [Google Scholar] [CrossRef]
- Li, X.; Shi, F.; Wang, L.; Zhang, S.; Yan, L.; Zhang, X.; Sun, W. Electrochemical biosensor based on horseradish peroxidase and black phosphorene quantum dot modified electrode. Molecules 2023, 28, 6151. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Ai, Y.; Li, X.; Wang, L.; Zhang, Z.; Ding, W.; Sun, W. Preparation of electrochemical horseradish peroxidase biosensor with black phosphorene–zinc oxide nanocomposite and their applications. RSC Adv. 2023, 13, 32028–32038. [Google Scholar] [CrossRef]
- Zhao, L.; Li, C.; Qi, H.; Gao, Q.; Zhang, C. Electrochemical lectin-based biosensor array for detection and discrimination of carcinoembryonic antigen using dual amplification of gold nanoparticles and horseradish peroxidase. Sens. Actuators B Chem. 2016, 235, 575–582. [Google Scholar] [CrossRef]
- Rahimi, P.; Joseph, Y. Enzyme-based biosensors for choline analysis: A review. TrAC Trends Anal. Chem. 2019, 110, 367–374. [Google Scholar] [CrossRef]
- Sun, M.; Cui, C.; Chen, H.; Wang, D.; Zhang, W.; Guo, W. Enzymatic and non-enzymatic uric acid electrochemical biosensors: A Review. ChemPlusChem 2023, 88, e202300262. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.S.; Aziz, M.A.; Oyama, M.; Al-Betar, A.R.F. Controlled-potential-based electrochemical sulfide sensors: A review. Chem. Rec. 2021, 21, 204–238. [Google Scholar] [CrossRef] [PubMed]
- Kajal, N.; Singh, V.; Gupta, R.; Gautam, S. Metal organic frameworks for electrochemical sensor applications: A review. Environ. Res. 2022, 204, 112320. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xu, W.; Liang, X.; Yang, Y.; Zhou, Y. Carbon nanotubes in electrochemical, colorimetric, and fluorimetric immunosensors and immunoassays: A review. Microchim. Acta 2020, 187, 206. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Vidic, J.; Jiang, D.; Loget, G.; Sojic, N. Photoinduced electrochemiluminescence immunoassays. Anal. Chem. 2024, 96, 18262–18268. [Google Scholar] [CrossRef]
- Liu, H.; Weng, L.; Yang, C. A review on nanomaterial-based electrochemical sensors for H2O2, H2S and NO inside cells or released by cells. Microchim. Acta 2017, 184, 1267–1283. [Google Scholar] [CrossRef]
- Sohrabi, H.; Maleki, F.; Khaaki, P.; Kadhom, M.; Kudaibergenov, N.; Khataee, A. Electrochemical-based sensing platforms for detection of glucose and H2O2 by porous metal–organic frameworks: A review of status and prospects. Biosensors 2023, 13, 347. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, H.; Li, H.; Yao, C. Recent developments in electrochemical sensors based on nanomaterials for determining glucose and its byproduct H2O2. J. Mater. Sci. 2017, 52, 10455–10469. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, W. Recent advances in graphene-based nanomaterials for fabricating electrochemical hydrogen peroxide sensors. Biosens. Bioelectron. 2017, 89, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Luo, X.; Liu, Y.; Zhao, Y.; Cui, Y. Development of an arduino-based integrated system for sensing of hydrogen peroxide. Sens. Actuators Rep. 2021, 3, 100045. [Google Scholar] [CrossRef]
- Yang, X.; Qiu, W.; Gao, R.; Wang, Y.; Bai, Y.; Xu, Z.; Bao, S.J. MIL-47 (V) catalytic conversion of H2O2 for sensitive H2O2 detection and tumor cell inhibition. Sens. Actuators B-Chem. 2022, 354, 131201. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, Y.; Song, W.J. Zeolitic imidazolate frameworks for use in electrochemical and optical chemical sensing and biosensing: A review. Microchim. Acta 2020, 187, 234. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Liang, D.W. Novel electrochemical advanced oxidation processes with H2O2 generation cathode for water treatment: A review. J. Environ. 2022, 10, 107896. [Google Scholar] [CrossRef]
- Oliveira, M.R.F.; Herrasti, P.; Furtado, R.F.; Melo, A.M.A.; Alves, C.R. Polymeric composite including magnetite nanoparticles for hydrogen peroxide detection. Chemosensors 2023, 11, 323. [Google Scholar] [CrossRef]
- Mikołajczak, N.; Tańska, M.; Ogrodowska, D. Phenolic compounds in plant oils: A review of composition, analytical methods, and effect on oxidative stability. Trends Food Sci. Technol. 2021, 113, 110–138. [Google Scholar] [CrossRef]
- Stepanov, E.V.; Shcherbakov, I.A. Physicochemical methods of studying hydrogen peroxide for biomedical applications. Phys. Wave Phenom. 2023, 31, 92–97. [Google Scholar] [CrossRef]
- Kakeshpour, T.; Metaferia, B.; Zare, R.N.; Bax, A. Quantitative detection of hydrogen peroxide in rain, air, exhaled breath, and biological fluids by NMR spectroscopy. Proc. Natl. Acad. Sci. USA 2022, 119, e2121542119. [Google Scholar] [CrossRef]
- Vahidpour, F.; Alghazali, Y.; Akca, S.; Hommes, G.; Schöning, M.J. An enzyme-based interdigitated electrode-type biosensor for detecting low concentrations of H2O2 vapor/aerosol. Chemosensors 2022, 10, 202. [Google Scholar] [CrossRef]
- Michel, P.; Boudenne, J.L.; Robert-Peillard, F.; Coulomb, B. Analysis of homemade peroxide-based explosives in water: A review. TrAC Trend. Anal. Chem. 2023, 158, 116884. [Google Scholar] [CrossRef]
- Romolo, F.S.; Connell, S.; Ferrari, C.; Suarez, G.; Sauvain, J.J.; Hopf, N.B. Locating bomb factories by detecting hydrogen peroxide. Talanta 2016, 160, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Jiang, J.; He, L.; Chen, L. Mitochondrial superoxide/hydrogen peroxide: An emerging therapeutic target for metabolic diseases. Free Radic. Biol. Med. 2020, 152, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hou, X.; Fang, H.; Yang, X. Boronate-based fluorescent probes as a prominent tool for H2O2 sensing and recognition. Curr. Med. Chem. 2022, 29, 2476–2489. [Google Scholar] [CrossRef]
- Li, J.; Xu, N.; Zhang, Y.; Dong, H.; Li, C. Research progress of heterogeneous photocatalyst for H2O2 production: A mini review. Chin. Chem. Lett. 2024, 110470. [Google Scholar] [CrossRef]
- Jarnda, K.V.; Wang, D.; Anaman, R.; Johnson, V.E.; Roberts, G.P.L.; Johnson, P.S.; Jallawide, B.W.; Kai, T.; Ding, P. Recent advances in electrochemical non-enzymatic glucose sensor for the detection of glucose in tears and saliva: A review. Sens. Actuators A-Phys. 2023, 363, 114778. [Google Scholar] [CrossRef]
- Do, H.H.; Kim, S.Y.; Van Le, Q. Development of non-precious metal oxide-based electrodes for enzyme-free glucose detection: A review. Microchem. J. 2023, 193, 109202. [Google Scholar] [CrossRef]
- Lee, W.C.; Kim, K.B.; Gurudatt, N.G.; Hussain, K.K.; Choi, C.S.; Park, D.S.; Shim, Y.B. Comparison of enzymatic and non-enzymatic glucose sensors based on hierarchical Au-Ni alloy with conductive polymer. Biosens. Bioelectron. 2019, 130, 48–54. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Asif, M.; Liu, Q.; Xiao, F.; Liu, H.; Xia, B.Y. Noble metal construction for electrochemical nonenzymatic glucose detection. Adv. Mater. Technol. 2023, 8, 2200272. [Google Scholar] [CrossRef]
- Xue, M.; Mao, W.; Chen, J.; Zheng, F.; Chen, W.; Shen, W.; Tang, S. Application of Au or Ag nanomaterials for colorimetric detection of glucose. Analyst 2021, 146, 6726–6740. [Google Scholar] [CrossRef] [PubMed]
- Okoye, C.N.; Chinnappareddy, N.; Stevens, D.; Kamunde, C. Factors affecting liver mitochondrial hydrogen peroxide emission. Comp. Biochem. Phys. B 2022, 259, 110713. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.K.; Gawroński, P.; Munir, S.; Jindal, S.; Kerchev, P. Hydrogen peroxide-induced stress acclimation in plants. Cell. Mol. Life Sci. 2022, 79, 129. [Google Scholar] [CrossRef]
- Smirnoff, N.; Arnaud, D. Hydrogen peroxide metabolism and functions in plants. New Phytol. 2019, 221, 1197–1214. [Google Scholar] [CrossRef] [PubMed]


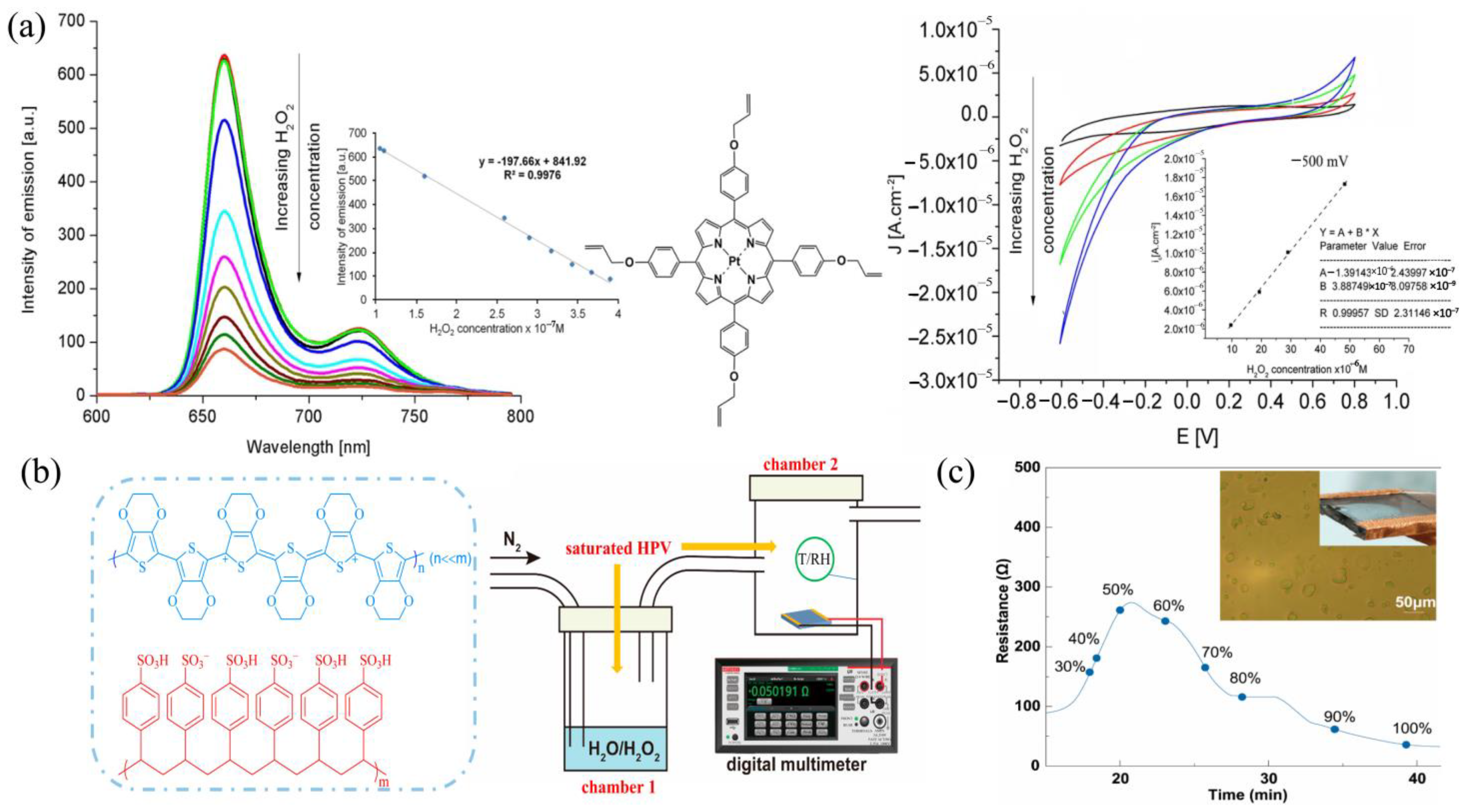

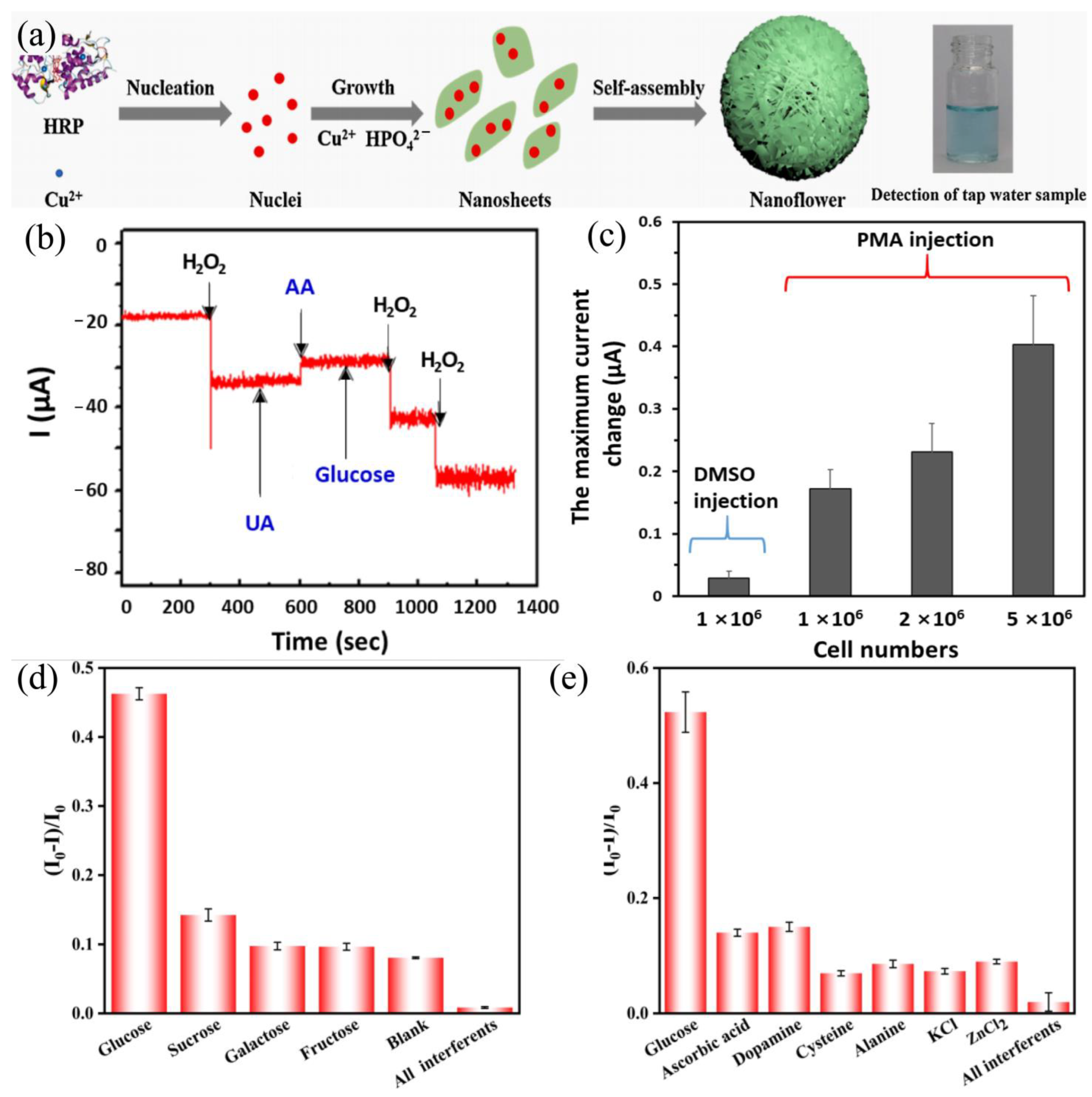
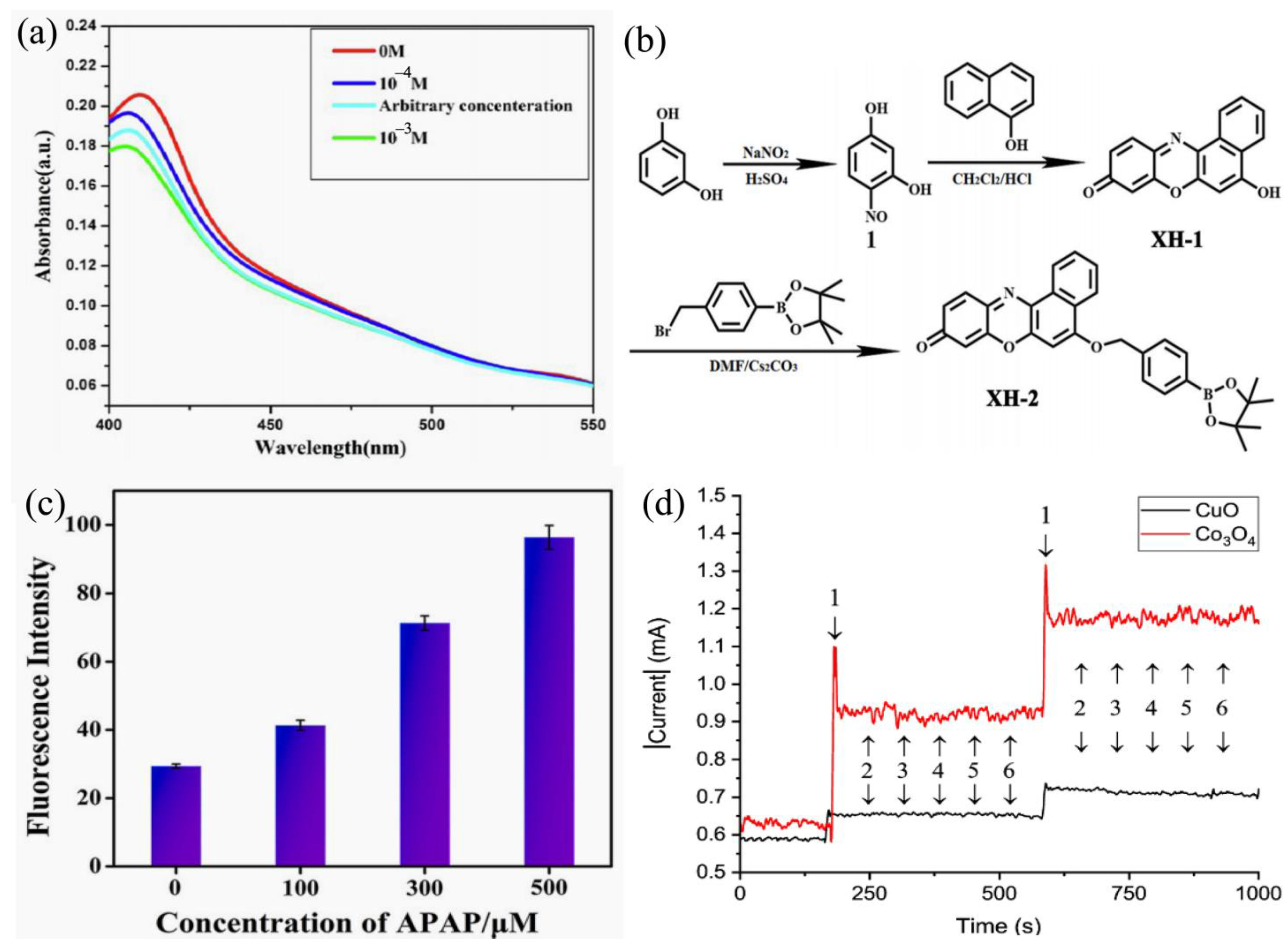
| Mechanism | Sensor Materials | LDL | H2O2 (Physical State) | Linear Range | Ref. |
|---|---|---|---|---|---|
| Colorimetric | Fe3O4-Fe0/Fe3C | 67.1 pM | liquid | 0.01–0.25 μM | [33] |
| Colorimetric | XH-2 | 0.091 μM | liquid | 0–120 μM | [34] |
| Colorimetric | AgNPs@MOF | 0.17 μM | liquid | 0.5–50 μM | [35] |
| Colorimetric | RuO2 NPs | 0.39 μM | liquid | 1–10,000 μM | [36] |
| Colorimetric | HRP/Cu3(PO4)2·3H2O | 0.5 μM | liquid | 5–500 μM | [37] |
| Colorimetric | CoCO3/TMB | 1.39 μM | liquid | 5.0–75.0 μM | [38] |
| Colorimetric | NiFe2O4/CNTs | 2.2 μM | liquid | 5–60 μM | [39] |
| Colorimetric | Zr/MOF/PVP | 2.76 μM | liquid | 10–800 μM | [40] |
| Colorimetric | Ag@CMs | 5 μM | liquid | 5–200 µM | [41] |
| Colorimetric | MOF-818 | 9.02 μM | liquid | 13.3–10,000 μM | [42] |
| Colorimetric | Gox/TMB | 30 μM | liquid | 500–6000 μM | [43] |
| Colorimetric | Ti (IV) oxo complexes | 0.1 ppm | gaseous | 0–1.0 ppm | [44] |
| Colorimetric | AgNPs | 0.216 ppm | gaseous | 0–300 ppm | [45] |
| Colorimetric | Paper/KI | 0.015 meq/Kg | liquid | 0.01–30 meq/Kg | [46] |
| Colorimetric | BPCN NSs | 1.0 μM | liquid | 0–1000 μM | [47] |
| Fluorescent | Fe3Ni-MOF-NH2 | 0.005 μM | liquid | 0.01–16 μM | [48] |
| Fluorescent | LBM | 0.013 μM | Liquid | 0–50 µM | [49] |
| Fluorescent Electrochemical | TAPP | 0.03 μM 0.3 μM | liquid | 0.105–0.39 μM 1–50 μM | [50] |
| Fluorescent | chalcones, primary amines, and β-ketoesters | 1.08 μM | liquid | 0–50 µM | [51] |
| Fluorescent | (dfppy)2Ir-bpy-NH2 | 3.084 µM | liquid | 0–500 µM | [52] |
| Fluorescent | ARS/GAL | 7.4 μM | liquid | 60–500 µM | [53] |
| Fluorescent | TATP | 0.2 ppm | gaseous | - | [54] |
| Chemiluminescent | Hemoglobin/luminol | 308 μM | liquid | 500–12,000 μM | [55] |
| Electrochemical | FET/Cyt c | 100 fM | liquid | 1 × 102–1 × 1014 fM | [56] |
| Electrochemical | pillar[3]arene[2]quinone/ferrocene | 0.0003 μM | liquid | 0.001–100 μM | [57] |
| Electrochemical | Fe3O4@MoS2-AuNPs | 0.08 μM | liquid | 1–120 μM | [58] |
| Electrochemical | PtNPs/MWCNTs | 0.2 μM | liquid | 0.5–100 μM | [59] |
| Electrochemical | AuNPs/CeO2 | 0.21 μM | liquid | 0.01–100,000 μM | [60] |
| Electrochemical | SWCNTs/MnO2 | 0.31 μM | liquid | 2–5000 μM | [61] |
| Electrochemical | Te NSs | 0.47 μM | liquid | 0.2–5 µM | [62] |
| Electrochemical | TiO2 NTs | 0.98 µM | liquid | 3–200 µM | [63] |
| Electrochemical | Ta/Pt/Ti | 1 μM 42 ppb | Liquid gaseous | - | [64] |
| Electrochemical | BGN/GNA | 1.183 μM | liquid | 10–100,000 μM | [65] |
| Electrochemical | CuO Co3O4 | 1.34 μM 1.05 μM | liquid liquid | 20–7000 μM | [66] |
| Electrochemical | CuNPs/ITO | 1.73 µM | liquid | 1–500 µM | [67] |
| Electrochemical | PtNP/rGO–CNT/PtNP/SPCE | 4.3 μM | liquid | 25–1000 μM | [68] |
| Electrochemical | LSG | 4.6 μM | liquid | 20–3400 μM | [69] |
| Electrochemical | AuNPs/SnO2NFs | 6.67 μM | liquid | 49.98–3937.21 μM | [70] |
| Electrochemical | Cu-exchanged zeolitic volcanic tuff | 10 μM | liquid | 10–30,000 µM | [71] |
| Electrochemical | ZnO/laser-induced graphene | 190 μM | liquid | 800–14,600 μM | [72] |
| Chemiresistive | PEDOT:PSS/PEDOT | 1.0 ppm | gaseous | 0–10.5 ppm | [73] |
| Chemiresistive | PEDOT:PSS-ATO/PEDOT | 1.0 ppm | gaseous | 1.0–10.5 ppm | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, M.; Sun, H.; Chen, S.; Yang, M.; Dong, R.; Yang, X.; Zang, L. Chemosensors for H2O2 Detection: Principles, Active Materials, and Applications. Chemosensors 2025, 13, 54. https://doi.org/10.3390/chemosensors13020054
Zhou M, Sun H, Chen S, Yang M, Dong R, Yang X, Zang L. Chemosensors for H2O2 Detection: Principles, Active Materials, and Applications. Chemosensors. 2025; 13(2):54. https://doi.org/10.3390/chemosensors13020054
Chicago/Turabian StyleZhou, Meng, Hui Sun, Shuai Chen, Mingna Yang, Rongqing Dong, Xiaomei Yang, and Ling Zang. 2025. "Chemosensors for H2O2 Detection: Principles, Active Materials, and Applications" Chemosensors 13, no. 2: 54. https://doi.org/10.3390/chemosensors13020054
APA StyleZhou, M., Sun, H., Chen, S., Yang, M., Dong, R., Yang, X., & Zang, L. (2025). Chemosensors for H2O2 Detection: Principles, Active Materials, and Applications. Chemosensors, 13(2), 54. https://doi.org/10.3390/chemosensors13020054







