The Development of Rapid Test Strips for Alexandrum tamarense
Abstract
1. Introduction
2. Materials and Methods
2.1. Alexandrium tamarense Cultivation Method
2.2. Preparation of Monoclonal and Polyclonal Antibodies
2.2.1. Immunization of BALB/c Mice
2.2.2. Cell Fusion Assays and Monoclonal Screening
2.3. Preparation of Test Strip Methods
2.3.1. Synthesis of Colloidal Gold Nano-Particles
2.3.2. Assembly of the Test Strip
2.4. Methods for Validation of Test Strip Performance
2.4.1. Effectiveness
2.4.2. Sensitivity
2.4.3. Specificity
2.5. Field Application of Test Strips
3. Results
3.1. The Titer Results of the Polyclonal Antibodies and Monoclonal Antibodies
3.2. The Structure of a Test Strip
3.3. Performance Verification of Test Strips
3.3.1. Effectiveness of the Test Strips
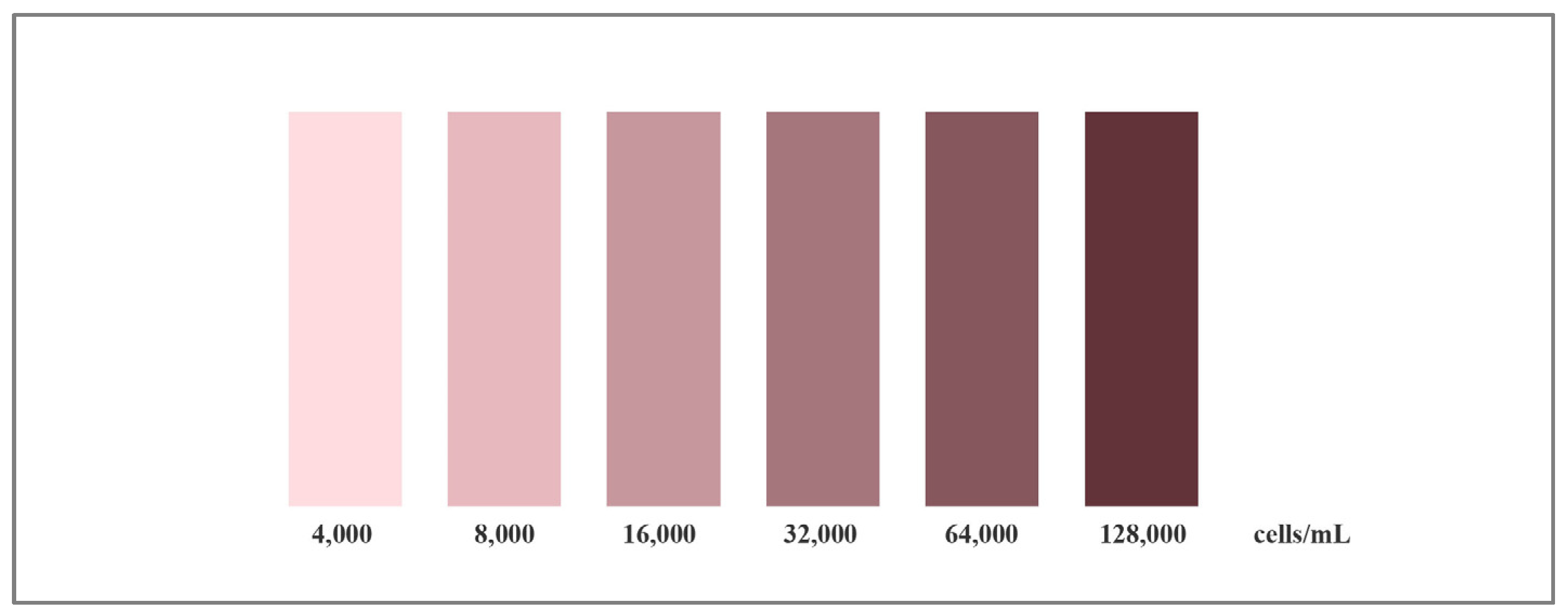
3.3.2. Sensitivity of the Test Strips
3.3.3. Specificity of the Test Strips
3.3.4. Field Application of the Test Strips
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deeds, J.R.; Landsberg, J.H.; Etheridge, S.M.; Pitcher, G.C.; Longan, S.W. Non-Traditional Vectors for Paralytic Shellfish Poisoning. Mar. Drugs 2008, 6, 308–348. [Google Scholar] [CrossRef]
- Anderson, D.M.; Alpermann, T.J.; Cembella, A.D.; Collos, Y.; Masseret, E.; Montresor, M. The Globally Distributed Genus Alexandrium: Multifaceted Roles in Marine Ecosystems and Impacts on Human Health. Harmful Algae 2012, 14, 10–35. [Google Scholar] [CrossRef] [PubMed]
- Genovesi, B.; Shin-Grzebyk, M.-S.; Grzebyk, D.; Laabir, M.; Gagnaire, P.-A.; Vaquer, A.; Pastoureaud, A.; Lasserre, B.; Collos, Y.; Berrebi, P.; et al. Assessment of Cryptic Species Diversity within Blooms and Cyst Bank of the Alexandrium tamarense Complex (Dinophyceae) in a Mediterranean Lagoon Facilitated by Semi-Multiplex PCR. J. Plankton Res. 2011, 33, 405–414. [Google Scholar] [CrossRef]
- Lilly, E.L.; Halanych, K.M.; Anderson, D.M. Species Boundaries and Global Biogeography of the Alexandrium tamarense Complex (Dinophyceae). J. Phycol. 2007, 43, 1329–1338. [Google Scholar] [CrossRef]
- Song, H.W.; Lee, S.; Park, Y.K.; Woo, S.Y. Quantitative Sasang Constitution Diagnosis Method for Distinguishing between Tae-eumin and Soeumin Types Based on Elasticity Measurements of the Skin of the Human Hand. Evid.-Based Complement. Altern. Med. 2009, 6, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Soto-Liebe, K.; Méndez, M.A.; Fuenzalida, L.; Krock, B.; Cembella, A.; Vásquez, M. PSP Toxin Release from the Cyanobacterium Raphidiopsis brookii D9 (Nostocales) Can Be Induced by Sodium and Potassium Ions. Toxicon 2012, 60, 1324–1334. [Google Scholar] [CrossRef]
- Hosoi-Tanabe, S.; Sako, Y. Species-Specific Detection and Quantification of Toxic Marine Dinoflagellates Alexandrium tamarense and A. catenella ByReal-Time PCR Assay. Mar. Biotechnol. 2005, 7, 506–514. [Google Scholar] [CrossRef]
- Nagai, S.; Itakura, S. Specific Detection of the Toxic Dinoflagellates Alexandrium tamarense and Alexandrium catenella from Single Vegetative Cells by a Loop-Mediated Isothermal Amplification Method. Mar. Genom. 2012, 7, 43–49. [Google Scholar] [CrossRef]
- Ruvindy, R.; Bolch, C.J.; MacKenzie, L.; Smith, K.F.; Murray, S.A. QPCR Assays for the Detection and Quantification of Multiple Paralytic Shellfish Toxin-Producing Species of Alexandrium. Front. Microbiol. 2018, 9, 3153. [Google Scholar] [CrossRef]
- Watanabe, R.; Matsushima, R.; Harada, T.; Oikawa, H.; Murata, M.; Suzuki, T. Quantitative Determination of Paralytic Shellfish Toxins in Cultured Toxic Algae by LC-MS/MS. Food Addit. Contam. Part A 2013, 30, 1351–1357. [Google Scholar] [CrossRef]
- Chen, G.F.; Zhang, C.Y.; Wang, Y.Y.; Chen, W. Application of Reverse Dot Blot Hybridization to Simultaneous Detection and Identification of Harmful Algae. Environ. Sci. Pollut. Res. 2015, 22, 10516–10528. [Google Scholar] [CrossRef] [PubMed]
- Chin Chwan Chuong, J.J.; Rahman, M.; Ibrahim, N.; Heng, L.Y.; Tan, L.L.; Ahmad, A. Harmful Microalgae Detection: Biosensors versus Some Conventional Methods. Sensors 2022, 22, 3144. [Google Scholar] [CrossRef] [PubMed]
- Gas, F.; Baus, B.; Pinto, L.; Compere, C.; Tanchou, V.; Quéméneur, E. One Step Immunochromatographic Assay for the Rapid Detection of Alexandrium minutum. Biosens. Bioelectron. 2010, 25, 1235–1239. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tsao, Z.-J.; Liao, Y.-C.; Liu, B.-H.; Su, C.-C.; Yu, F.-Y. Development of a Monoclonal Antibody against Domoic Acid and Its Application in Enzyme-Linked Immunosorbent Assay and Colloidal Gold Immunostrip. J. Agric. Food Chem. 2007, 55, 4921–4927. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Pan, F.-G.; Li, Y.-S.; Zhang, Y.-Y.; Zhang, J.-H.; Lu, S.-Y.; Ren, H.-L.; Liu, Z.-S. Colloidal Gold Probe-Based Immunochromatographic Assay for the Rapid Detection of Brevetoxins in Fishery Product Samples. Biosens. Bioelectron. 2009, 24, 2744–2747. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Meng, K.; Yu, Q.; Chen, C.; Chen, J.; Li, J.; Wang, X.; Wang, Y.; Li, M.; Chen, C.; et al. Rapid Detection Method of Skeletonema pseudocostatum and Preparation of Test Strip. Environ. Sci. Pollut. Res. 2022, 29, 70202–70208. [Google Scholar] [CrossRef]
- Hnasko, R. (Ed.) ELISA: Methods and Protocols; Springer: New York, NY, USA, 2015; Volume 1318, ISBN 978-1-4939-2741-8. [Google Scholar]
- Wu, Q.; Guo, X.; Huang, Q.; Xie, Y.; Guo, L.; Yang, X.; Sun, M.; Yin, D. Development of a Colloidal Gold Immunochromatographic Test Strip for Detecting the Smooth Brucella. Sci. Rep. 2024, 14, 25068. [Google Scholar] [CrossRef]
- Zhang, C. Hybridoma Technology for the Generation of Monoclonal Antibodies. In Antibody Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2012; pp. 117–135. [Google Scholar]
- Chuanlai, X.; Huting, W.; Chifang, P.; Zhengyu, J.; Liqiang, L. Colloidal Gold-Based Immumochromatographic Assay for Detection of Diethylstilbestrol Residues. Biomed. Chromatogr. 2006, 20, 1390–1394. [Google Scholar] [CrossRef]
- Omidfar, K.; Kia, S.; Larijani, B. Development of a Colloidal Gold-Based Immunochromatographic Test Strip for Screening of Microalbuminuria. Hybridoma 2011, 30, 117–124. [Google Scholar] [CrossRef]
- Guo, L.; Song, S.; Liu, L.; Peng, J.; Kuang, H.; Xu, C. Comparsion of an Immunochromatographic Strip with ELISA for Simultaneous Detection of Thiamphenicol, Florfenicol and Chloramphenicol in Food Samples. Biomed. Chromatogr. 2015, 29, 1432–1439. [Google Scholar] [CrossRef]
- Lin, L.; Song, S.; Wu, X.; Liu, L.; Kuang, H. A Colloidal Gold Immunochromatography Test Strip Based on a Monoclonal Antibody for the Rapid Detection of Triadimefon and Triadimenol in Foods. Food Agric. Immunol. 2020, 31, 447–462. [Google Scholar] [CrossRef]
- John, U.; Litaker, R.W.; Montresor, M.; Murray, S.; Brosnahan, M.L.; Anderson, D.M. Formal Revision of the Alexandrium tamarense Species Complex (Dinophyceae) Taxonomy: The Introduction of Five Species with Emphasis on Molecular-Based (RDNA) Classification. Protist 2014, 165, 779–804. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, Z.; Mu, J.; Fang, J.; Zhang, C.; Zhou, K. The Development of Rapid Test Strips for Alexandrum tamarense. Chemosensors 2025, 13, 53. https://doi.org/10.3390/chemosensors13020053
Kang Z, Mu J, Fang J, Zhang C, Zhou K. The Development of Rapid Test Strips for Alexandrum tamarense. Chemosensors. 2025; 13(2):53. https://doi.org/10.3390/chemosensors13020053
Chicago/Turabian StyleKang, Zhang, Jiahang Mu, Junhua Fang, Changgong Zhang, and Kefu Zhou. 2025. "The Development of Rapid Test Strips for Alexandrum tamarense" Chemosensors 13, no. 2: 53. https://doi.org/10.3390/chemosensors13020053
APA StyleKang, Z., Mu, J., Fang, J., Zhang, C., & Zhou, K. (2025). The Development of Rapid Test Strips for Alexandrum tamarense. Chemosensors, 13(2), 53. https://doi.org/10.3390/chemosensors13020053



