Validation of an LC-MS Method for Quantification of Mycotoxins and Characterization of Fungal Strains Occurring in Food and Feed
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standards, Reagents and Consumables
2.2. Instrumentation and LC-MS Conditions
2.3. Preparation of Calibrants and Quality Control Standards
2.4. Fungal Strains Selection and Production of Contaminated Material
2.5. Sample Preparation
2.6. Method Validation
3. Results and Discussion
3.1. Optimization of LC-MS/MS Conditions and LC-MS/MS Use
3.2. Optimization of Sample Preparation
3.3. Method Validation
3.3.1. Specificity and Selectivity
3.3.2. Linearity and Matrix Effect
| Analyte | Matrix Effect | Linearity | |||||
|---|---|---|---|---|---|---|---|
| TV | Tcrit | Effect | TV | Tcrit | Best Model | R² | |
| AFB1 | 38.66 | 2.04 | Yes | 0.44 | 8.68 | Linear | 0.99 |
| AFB2 | 30.19 | 2.04 | Yes | 5.56 | 8.68 | Linear | 0.99 |
| AFG1 | 8.85 | 2.04 | Yes | 0.08 | 8.68 | Linear | 0.99 |
| AFG2 | 28.18 | 2.04 | Yes | 0.14 | 8.68 | Linear | 0.99 |
| OTA | 27.73 | 2.04 | Yes | 2.38 | 8.68 | Linear | 0.99 |
| FB1 | 35.66 | 2.04 | Yes | 0.57 | 8.68 | Linear | 0.99 |
| FB2 | 33.65 | 2.04 | Yes | 0.04 | 8.68 | Linear | 0.99 |
| FB3 | 63.43 | 2.04 | Yes | 0.65 | 8.68 | Linear | 0.99 |
| HT2 | 1.74 | 2.04 | No | 8.05 | 8.68 | Linear | 0.99 |
| DON | 5.99 | 2.04 | Yes | 0.06 | 8.68 | Linear | 0.99 |
| ZEN | 20.02 | 2.04 | Yes | 0.44 | 8.68 | Linear | 0.99 |
| CIT | 43.23 | 2.04 | Yes | 4.89 | 8.68 | Linear | 0.99 |
| ALT | 6.41 | 2.04 | Yes | 2.82 | 8.68 | Linear | 0.99 |
| TEN | 53.85 | 2.04 | Yes | 3.70 | 8.68 | Linear | 0.99 |
| 3/15AcDON | 16.73 | 2.04 | Yes | 0.92 | 8.68 | Linear | 0.99 |
| BEA | 5.65 | 2.04 | Yes | 1.62 | 8.68 | Linear | 0.99 |
| ENN A | 0.08 | 2.04 | No | 5.23 | 8.68 | Linear | 0.99 |
| ENN A1 | 12.15 | 2.04 | Yes | 0.27 | 8.68 | Linear | 0.99 |
| ENN B | 6.04 | 2.04 | Yes | 0.01 | 8.68 | Linear | 0.99 |
| ENN B1 | 15.10 | 2.04 | Yes | 0.20 | 8.68 | Linear | 0.99 |
3.3.3. Limit of Detection and Limit of Quantification
3.3.4. Apparent Recovery, Repeatability, Reproducibility, and Measurement Uncertainty
| Analyte | Concentration Levels (µg kg−1) | LOD (µg kg−1) | LOQ (µg kg−1) | Recovery (%) | Repeatability (RSDr, %) | Acceptance Criteria for RSDr (%) | Reproducibility (RSDwR, %) | Acceptance Criteria for RSDwR (%) | Measurement Uncertainty (%) |
|---|---|---|---|---|---|---|---|---|---|
| AFB1 | 1 | 99 | 2.95 | 20 | 4.57 | 30 | 10.26 | ||
| 5 | 0.5 | 1 | 97 | 2.47 | 20 | 3.41 | 30 | 9.66 | |
| 10 | 97 | 3.12 | 16.6 | 3.13 | 25 | 8.92 | |||
| AFB2 | 1 | 104 | 1.52 | 20 | 9.24 | 30 | 22.29 | ||
| 5 | 0.5 | 1 | 99 | 1.87 | 20 | 4.40 | 30 | 10.23 | |
| 10 | 98 | 2.22 | 16.6 | 3.89 | 25 | 10.09 | |||
| AFG1 | 1 | 105 | 3.49 | 20 | 3.49 | 30 | 11.81 | ||
| 5 | 0.5 | 1 | 101 | 1.76 | 20 | 1.78 | 30 | 4.19 | |
| 10 | 102 | 1.38 | 16.6 | 3.15 | 25 | 8.40 | |||
| AFG2 | 1 | 106 | 4.53 | 20 | 5.89 | 30 | 17.59 | ||
| 5 | 0.5 | 1 | 97 | 1.27 | 20 | 1.72 | 30 | 6.34 | |
| 10 | 98 | 1.68 | 16.6 | 1.68 | 25 | 5.74 | |||
| OTA | 5 | 104 | 2.61 | 20 | 3.52 | 30 | 11.41 | ||
| 25 | 2.5 | 5 | 98 | 1.63 | 16.6 | 1.90 | 25 | 5.52 | |
| 50 | 98 | 1.23 | 16.6 | 1.23 | 25 | 4.04 | |||
| FB1 | 100 | 100 | 3.01 | 16.6 | 10.89 | 25 | 21.99 | ||
| 500 | 50 | 100 | 97 | 0.99 | 14.6 | 3.49 | 22 | 10.46 | |
| 1000 | 98 | 4.25 | 14.6 | 5.23 | 22 | 12.59 | |||
| FB2 | 100 | 101 | 4.01 | 16.6 | 4.72 | 25 | 10.26 | ||
| 500 | 50 | 100 | 98 | 1.78 | 14.6 | 3.69 | 22 | 9.59 | |
| 1000 | 98 | 1.54 | 14.6 | 2.22 | 22 | 6.08 | |||
| FB3 | 100 | 101 | 4.46 | 16.6 | 7.16 | 25 | 15.10 | ||
| 500 | 50 | 100 | 97 | 1.38 | 14.6 | 2.75 | 22 | 10.96 | |
| 1000 | 97 | 1.47 | 14.6 | 3.96 | 22 | 8.93 | |||
| HT2 | 400 | 84 | 3.52 | 14.6 | 6.92 | 22 | 29.42 | ||
| 2000 | 200 | 400 | 105 | 2.04 | 10.6 | 5.80 | 16 | 16.89 | |
| 4000 | 99 | 3.29 | 10.6 | 4.83 | 16 | 10.61 | |||
| DON | 400 | 100 | 4.35 | 14.6 | 4.42 | 22 | 9.14 | ||
| 2000 | 200 | 400 | 101 | 1.56 | 10.6 | 5.14 | 16 | 10.79 | |
| 4000 | 100 | 1.94 | 10.6 | 5.06 | 16 | 10.59 | |||
| ZEN | 200 | 100 | 6.94 | 14.6 | 6.94 | 22 | 13.89 | ||
| 1000 | 100 | 200 | 95 | 2.36 | 14.6 | 2.61 | 22 | 10.12 | |
| 2000 | 98 | 6.48 | 10.6 | 7.37 | 16 | 16.29 | |||
| CIT | 100 | 105 | 2.26 | 16.6 | 2.49 | 25 | 10.48 | ||
| 500 | 50 | 100 | 90 | 2.73 | 14.6 | 5.36 | 22 | 20.90 | |
| 1000 | 90 | 1.12 | 14.6 | 5.68 | 22 | 21.21 | |||
| ALT | 200 | 93 | 4.96 | 14.6 | 7.13 | 22 | 21.27 | ||
| 1000 | 100 | 200 | 95 | 4.52 | 14.6 | 4.88 | 22 | 14.66 | |
| 2000 | 95 | 2.96 | 10.6 | 3.70 | 16 | 12.12 | |||
| TEN | 5 | 103 | 5.11 | 20 | 5.11 | 30 | 13.06 | ||
| 25 | 2.5 | 5 | 97 | 1.39 | 16.6 | 1.39 | 25 | 5.93 | |
| 50 | 97 | 0.70 | 16.6 | 0.70 | 25 | 4.49 | |||
| 3/15AcDON | 200 | 106 | 4.23 | 14.6 | 4.23 | 22 | 14.04 | ||
| 1000 | 100 | 200 | 98 | 1.58 | 14.6 | 2.20 | 22 | 6.54 | |
| 2000 | 99 | 1.48 | 10.6 | 1.69 | 16 | 4.65 | |||
| BEA | 2.56 | 74 | 14.41 | 20 | 14.41 | 30 | 54.70 | ||
| 12.76 | 1.3 | 2.56 | 100 | 6.95 | 16.6 | 7.74 | 25 | 15.82 | |
| 25.60 | 97 | 4.35 | 16.6 | 5.66 | 25 | 14.56 | |||
| ENN A | 2 | 95 | 4.64 | 20 | 4.64 | 30 | 13.90 | ||
| 10 | 1 | 2 | 97 | 1.62 | 16.6 | 2.30 | 25 | 7.75 | |
| 20 | 98 | 1.70 | 16.6 | 1.70 | 25 | 5.10 | |||
| ENN A1 | 2 | 92 | 3.38 | 20 | 6.86 | 30 | 21.44 | ||
| 10 | 1 | 2 | 96 | 0.75 | 16.6 | 2.61 | 25 | 9.43 | |
| 20 | 97 | 0.98 | 16.6 | 2.09 | 25 | 6.93 | |||
| ENN B | 2 | 96 | 9.13 | 20 | 16.22 | 30 | 36.29 | ||
| 10 | 1 | 2 | 95 | 4.14 | 16.6 | 4.99 | 25 | 14.79 | |
| 20 | 94 | 1.67 | 16.6 | 4.35 | 25 | 14.58 | |||
| ENN B1 | 2 | 94 | 11.02 | 20 | 14.83 | 30 | 35.37 | ||
| 10 | 1 | 2 | 97 | 1.47 | 16.6 | 1.65 | 25 | 9.72 | |
| 20 | 96 | 2.83 | 16.6 | 3.05 | 25 | 6.77 |
3.3.5. Application for Characterization of Toxigenic Abilities upon Artificial Inoculation of Maize Grains
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, R.; Anwar, F.; Ghazali, F.M. A Comprehensive Review of Mycotoxins: Toxicology, Detection, and Effective Mitigation Approaches. Heliyon 2024, 10, e28361. [Google Scholar] [CrossRef] [PubMed]
- Furlong, E.B.; Buffon, J.G.; Cerqueira, M.B.; Kupski, L. Mitigation of Mycotoxins in Food—Is It Possible? Foods 2024, 13, 1112. [Google Scholar] [CrossRef] [PubMed]
- Neme, K.; Mohammed, A. Mycotoxin Occurrence in Grains and the Role of Postharvest Management as a Mitigation Strategies. A Review. Food Control 2017, 78, 412–425. [Google Scholar] [CrossRef]
- Pitt, J.I.; Taniwaki, M.H.; Cole, M.B. Mycotoxin Production in Major Crops as Influenced by Growing, Harvesting, Storage and Processing, with Emphasis on the Achievement of Food Safety Objectives. Food Control 2013, 32, 205–215. [Google Scholar] [CrossRef]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef]
- Bartholomew, H.P.; Bradshaw, M.; Jurick, W.M.; Fonseca, J.M. The Good, the Bad, and the Ugly: Mycotoxin Production During Postharvest Decay and Their Influence on Tritrophic Host–Pathogen–Microbe Interactions. Front. Microbiol. 2021, 12, 611881. [Google Scholar] [CrossRef]
- Gavahian, M.; Sheu, S.; Magnani, M.; Mousavi Khaneghah, A. Emerging Technologies for Mycotoxins Removal from Foods: Recent Advances, Roles in Sustainable Food Consumption, and Strategies for Industrial Applications. Food Process. Preserv. 2022, 46, e15922. [Google Scholar] [CrossRef]
- Zhang, C.; Qu, Z.; Hou, J.; Yao, Y. Contamination and Control of Mycotoxins in Grain and Oil Crops. Microorganisms 2024, 12, 567. [Google Scholar] [CrossRef]
- Mihalache, O.A.; De Boevre, M.; Dellafiora, L.; De Saeger, S.; Moretti, A.; Pinson-Gadais, L.; Ponts, N.; Richard-Forget, F.; Susca, A.; Dall’Asta, C. The Occurrence of Non-Regulated Mycotoxins in Foods: A Systematic Review. Toxins 2023, 15, 583. [Google Scholar] [CrossRef]
- Mastanjević, K.; Kovačević, D.; Nešić, K.; Krstanović, V.; Habschied, K. Traditional Meat Products—A Mycotoxicological Review. Life 2023, 13, 2211. [Google Scholar] [CrossRef]
- Pleadin, J.; Lešić, T.; Milićević, D.; Markov, K.; Šarkanj, B.; Vahčić, N.; Kmetič, I.; Zadravec, M. Pathways of Mycotoxin Occurrence in Meat Products: A Review. Processes 2021, 9, 2122. [Google Scholar] [CrossRef]
- Becker-Algeri, T.A.; Castagnaro, D.; de Bortoli, K.; de Souza, C.; Drunkler, D.A.; Badiale-Furlong, E. Mycotoxins in Bovine Milk and Dairy Products: A Review. J. Food Sci. 2016, 81, R544–R552. [Google Scholar] [CrossRef] [PubMed]
- Flores-Flores, M.E.; Lizarraga, E.; López de Cerain, A.; González-Peñas, E. Presence of Mycotoxins in Animal Milk: A Review. Food Control 2015, 53, 163–176. [Google Scholar] [CrossRef]
- Jallow, A.; Xie, H.; Tang, X.; Qi, Z.; Li, P. Worldwide Aflatoxin Contamination of Agricultural Products and Foods: From Occurrence to Control. Comp. Rev. Food Sci. Food Safe 2021, 20, 2332–2381. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide Contamination of Food-Crops with Mycotoxins: Validity of the Widely Cited ‘FAO Estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Magnoli, A.P.; Poloni, V.L.; Cavaglieri, L. Impact of Mycotoxin Contamination in the Animal Feed Industry. Curr. Opin. Food Sci. 2019, 29, 99–108. [Google Scholar] [CrossRef]
- Hussein, H. Toxicity, Metabolism, and Impact of Mycotoxins on Humans and Animals. Toxicology 2001, 167, 101–134. [Google Scholar] [CrossRef]
- El-Sayed, R.A.; Jebur, A.B.; Kang, W.; El-Demerdash, F.M. An Overview on the Major Mycotoxins in Food Products: Characteristics, Toxicity, and Analysis. J. Future Foods 2022, 2, 91–102. [Google Scholar] [CrossRef]
- Gurikar, C.; Shivaprasad, D.P.; Sabillón, L.; Nanje Gowda, N.A.; Siliveru, K. Impact of Mycotoxins and Their Metabolites Associated with Food Grains. Grain Oil Sci. Technol. 2023, 6, 1–9. [Google Scholar] [CrossRef]
- Armendáriz, C.R.; Fernández, Á.J.G.; Gironés, M.C.L.R.; de la Torre, A.H. Mycotoxins. In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 424–427. ISBN 978-0-12-386455-0. [Google Scholar]
- Ramdas Kodape, A.; Raveendran, A.; Shivegowda Vivek Babu, C. Aflatoxins: A Postharvest Associated Challenge and Mitigation Opportunities. In Aflatoxins-Occurrence, Detection and Novel Detoxification Strategies; Claude Assaf, J., Ed.; IntechOpen: Rijeka, Croatia, 2022; ISBN 978-1-80356-884-3. [Google Scholar]
- Eaton, D.L.; Beima, K.M.; Bammler, T.K.; Riley, R.T.; Voss, K.A. Hepatotoxic Mycotoxins. In Comprehensive Toxicology; Elsevier: Amsterdam, The Netherlands, 2010; pp. 527–569. ISBN 978-0-08-046884-6. [Google Scholar]
- Wang, L.; Hua, X.; Shi, J.; Jing, N.; Ji, T.; Lv, B.; Liu, L.; Chen, Y. Ochratoxin A: Occurrence and Recent Advances in Detoxification. Toxicon 2022, 210, 11–18. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.; Nebbia, C.S.; et al. Risk Assessment of Ochratoxin A in Food. EFS2 2020, 18, e06113. [Google Scholar] [CrossRef]
- Mwabulili, F.; Xie, Y.; Li, Q.; Sun, S.; Yang, Y.; Ma, W. Research Progress of Ochratoxin a Bio-Detoxification. Toxicon 2023, 222, 107005. [Google Scholar] [CrossRef] [PubMed]
- Ringot, D.; Chango, A.; Schneider, Y.-J.; Larondelle, Y. Toxicokinetics and Toxicodynamics of Ochratoxin A, an Update. Chem.-Biol. Interact. 2006, 159, 18–46. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.W. Fumonisins. In Veterinary Toxicology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1003–1018. ISBN 978-0-12-811410-0. [Google Scholar]
- Chen, J.; Wei, Z.; Wang, Y.; Long, M.; Wu, W.; Kuca, K. Fumonisin B1: Mechanisms of Toxicity and Biological Detoxification Progress in Animals. Food Chem. Toxicol. 2021, 149, 111977. [Google Scholar] [CrossRef]
- Gao, Z.; Luo, K.; Zhu, Q.; Peng, J.; Liu, C.; Wang, X.; Li, S.; Zhang, H. The Natural Occurrence, Toxicity Mechanisms and Management Strategies of Fumonisin B1: A Review. Environ. Pollut. 2023, 320, 121065. [Google Scholar] [CrossRef]
- Kamle, M.; Mahato, D.K.; Devi, S.; Lee, K.E.; Kang, S.G.; Kumar, P. Fumonisins: Impact on Agriculture, Food, and Human Health and Their Management Strategies. Toxins 2019, 11, 328. [Google Scholar] [CrossRef]
- Tola, M.; Kebede, B. Occurrence, Importance and Control of Mycotoxins: A Review. Cogent Food Agric. 2016, 2, 1191103. [Google Scholar] [CrossRef]
- Jia, H.; Liu, N.; Zhang, Y.; Wang, C.; Yang, Y.; Wu, Z. 3-Acetyldeoxynivalenol Induces Cell Death through Endoplasmic Reticulum Stress in Mouse Liver. Environ. Pollut. 2021, 286, 117238. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Grasl-Kraupp, B.; et al. Risks to Human and Animal Health Related to the Presence of Deoxynivalenol and Its Acetylated and Modified Forms in Food and Feed. EFS2 2017, 15, e04718. [Google Scholar] [CrossRef]
- Meneely, J.; Greer, B.; Kolawole, O.; Elliott, C. T-2 and HT-2 Toxins: Toxicity, Occurrence and Analysis: A Review. Toxins 2023, 15, 481. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Leblanc, J.; Nielsen, E.; et al. Assessment of Information as Regards the Toxicity of T-2 and HT-2 Toxin for Ruminants. EFS2 2022, 20, e07564. [Google Scholar] [CrossRef]
- Hasuda, A.L.; Bracarense, A.P.F.R.L. Toxicity of the Emerging Mycotoxins Beauvericin and Enniatins: A Mini-Review. Toxicon 2024, 239, 107534. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the Risks to Human and Animal Health Related to the Presence of Beauvericin and Enniatins in Food and Feed. EFS2 2014, 12, 3802. [Google Scholar] [CrossRef]
- Nagda, A.; Meena, M. Alternaria Mycotoxins in Food and Feed: Occurrence, Biosynthesis, Toxicity, Analytical Methods, Control and Detoxification Strategies. Food Control 2024, 158, 110211. [Google Scholar] [CrossRef]
- Cinar, A.; Onbaşı, E. Mycotoxins: The Hidden Danger in Foods. In Mycotoxins and Food Safety; Sabuncuoğlu, S., Ed.; IntechOpen: Rijeka, Croatia, 2020; ISBN 978-1-78984-874-8. [Google Scholar]
- Raters, M.; Matissek, R. Thermal Stability of Aflatoxin B1 and Ochratoxin A. Mycotox Res. 2008, 24, 130–134. [Google Scholar] [CrossRef]
- Gbashi, S.; Madala, N.E.; De Saeger, S.; De Boevre, M.; Njobeh, P.B. Numerical Optimization of Temperature-Time Degradation of Multiple Mycotoxins. Food Chem. Toxicol. 2019, 125, 289–304. [Google Scholar] [CrossRef]
- Paterson, R.R.M.; Lima, N. Toxicology of Mycotoxins. In Molecular, Clinical and Environmental Toxicology; Luch, A., Ed.; Experientia Supplementum; Birkhäuser Basel: Basel, Switzerland, 2010; Volume 100, pp. 31–63. ISBN 978-3-7643-8337-4. [Google Scholar]
- Muñoz-Solano, B.; Lizarraga Pérez, E.; González-Peñas, E. Monitoring Mycotoxin Exposure in Food-Producing Animals (Cattle, Pig, Poultry, and Sheep). Toxins 2024, 16, 218. [Google Scholar] [CrossRef]
- Awuchi, C.G.; Ondari, E.N.; Nwozo, S.; Odongo, G.A.; Eseoghene, I.J.; Twinomuhwezi, H.; Ogbonna, C.U.; Upadhyay, A.K.; Adeleye, A.O.; Okpala, C.O.R. Mycotoxins’ Toxicological Mechanisms Involving Humans, Livestock and Their Associated Health Concerns: A Review. Toxins 2022, 14, 167. [Google Scholar] [CrossRef]
- Liew, W.-P.-P.; Mohd-Redzwan, S. Mycotoxin: Its Impact on Gut Health and Microbiota. Front. Cell. Infect. Microbiol. 2018, 8, 60. [Google Scholar] [CrossRef]
- Sobrova, P.; Adam, V.; Vasatkova, A.; Beklova, M.; Zeman, L.; Kizek, R. Deoxynivalenol and Its Toxicity. Interdiscip. Toxicol. 2010, 3, 94. [Google Scholar] [CrossRef]
- Saricaoglu, B.; Gültekin Subaşı, B.; Karbancioglu-Guler, F.; Lorenzo, J.M.; Capanoglu, E. Phenolic Compounds as Natural Microbial Toxin Detoxifying Agents. Toxicon 2023, 222, 106989. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.A.A.; Tabana, Y.M.; Musa, K.B.; Sandai, D.A. Effects of Different Mycotoxins on Humans, Cell Genome and Their Involvement in Cancer. Oncol. Rep. 2017, 37, 1321–1336. [Google Scholar] [CrossRef] [PubMed]
- Claeys, L.; Romano, C.; De Ruyck, K.; Wilson, H.; Fervers, B.; Korenjak, M.; Zavadil, J.; Gunter, M.J.; De Saeger, S.; De Boevre, M.; et al. Mycotoxin Exposure and Human Cancer Risk: A Systematic Review of Epidemiological Studies. Comp. Rev. Food Sci. Food Safe 2020, 19, 1449–1464. [Google Scholar] [CrossRef]
- Ekwomadu, T.; Mwanza, M.; Musekiwa, A. Mycotoxin-Linked Mutations and Cancer Risk: A Global Health Issue. Int. J. Environ. Res. Public Health 2022, 19, 7754. [Google Scholar] [CrossRef]
- Kraft, S.; Buchenauer, L.; Polte, T. Mold, Mycotoxins and a Dysregulated Immune System: A Combination of Concern? Int. J. Mol. Sci. 2021, 22, 12269. [Google Scholar] [CrossRef]
- Marchese, S.; Polo, A.; Ariano, A.; Velotto, S.; Costantini, S.; Severino, L. Aflatoxin B1 and M1: Biological Properties and Their Involvement in Cancer Development. Toxins 2018, 10, 214. [Google Scholar] [CrossRef]
- The European Commission. COMMISSION REGULATION (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006; The European Commission: Brussels, Belgium, 2023. [Google Scholar]
- The European Commission. COMMISSION REGULATION (EU) 2024/1022 of 8 April 2024 Amending Regulation (EU) 2023/915 as Regards Maximum Levels of Deoxynivalenol in Food; The European Commission: Brussels, Belgium, 2024. [Google Scholar]
- The European Commission. COMMISSION REGULATION (EU) 2024/1038 of 9 April 2024 Amending Regulation (EU) 2023/915 as Regards Maximum Levels of T-2 and HT-2 Toxins in Food; The European Commission: Brussels, Belgium, 2024. [Google Scholar]
- Publications Office of the European Union. Commission Implementing Regulation (EU) 2021/808 of 22 March 2021 on the Performance of Analytical Methods for Residues of Pharmacologically Active Substances Used in Food-Producing Animals and on the Interpretation of Results as Well as on the Methods to Be Used for Sampling and Repealing Decisions 2002/657/EC and 98/179/EC; Publications Office of the European Union: Luxembourg, 2009; pp. 4535–4539. [Google Scholar]
- Scauflaire, J.; Gourgue, M.; Callebaut, A.; Munaut, F. Fusarium Temperatum, a Mycotoxin-Producing Pathogen of Maize. Eur. J. Plant Pathol. 2012, 133, 911–922. [Google Scholar] [CrossRef]
- Han, Z.; Tangni, E.K.; Huybrechts, B.; Munaut, F.; Scauflaire, J.; Wu, A.; Callebaut, A. Screening Survey of Co-Production of Fusaric Acid, Fusarin C, and Fumonisins B1, B2 and B3 by Fusarium Strains Grown in Maize Grains. Mycotoxin Res. 2014, 30, 231–240. [Google Scholar] [CrossRef]
- Guo, W.; Yang, J.; Niu, X.; Tangni, E.K.; Zhao, Z.; Han, Z. A Reliable and Accurate UHPLC-MS/MS Method for Screening of Aspergillus, Penicillium and Alternaria Mycotoxins in Orange, Grape and Apple Juices. Anal. Methods 2021, 13, 192–201. [Google Scholar] [CrossRef]
- Kovač, M.; Nevistić, A.; Kovač, T.; Babić, J.; Šarić, A.; Miličević, B.; Panjičko, M.; Šarkanj, B. Development and Validation of an UHPLC-MS/MS Method for the Simultaneous Determination of 11 EU-Regulated Mycotoxins in Selected Cereals. J. Fungi 2022, 8, 665. [Google Scholar] [CrossRef]
- Haydari, G.; Hashemi Hazaveh, S.J.; Daraei, B.; Bayat, M. Validation of an UHPLC-MS/MS Method for Simultaneous Analysis of 11 Mycotoxins in Wheat Flour Using Immunoaffinity Column. Iran. J. Pharm. Res. IJPR 2019, 18, 182. [Google Scholar] [CrossRef]
- Yapo, A.E.; Strub, C.; Durand, N.; Ahoua, A.R.C.; Schorr-Galindo, S.; Bonfoh, B.; Fontana, A.; Koussémon, M. Mass Spectrometry-Based Detection and Risk Assessment of Mycotoxin Contamination of ‘Kankankan’ Used for Roasted Meat Consumption in Abidjan, Côte d’Ivoire. Food Addit. Contam. Part A 2020, 37, 1564–1578. [Google Scholar] [CrossRef] [PubMed]
- Mol, H.G.J.; Plaza-Bolaños, P.; Zomer, P.; de Rijk, T.C.; Stolker, A.A.M.; Mulder, P.P.J. Toward a Generic Extraction Method for Simultaneous Determination of Pesticides, Mycotoxins, Plant Toxins, and Veterinary Drugs in Feed and Food Matrixes. Anal. Chem. 2008, 80, 9450–9459. [Google Scholar] [CrossRef]
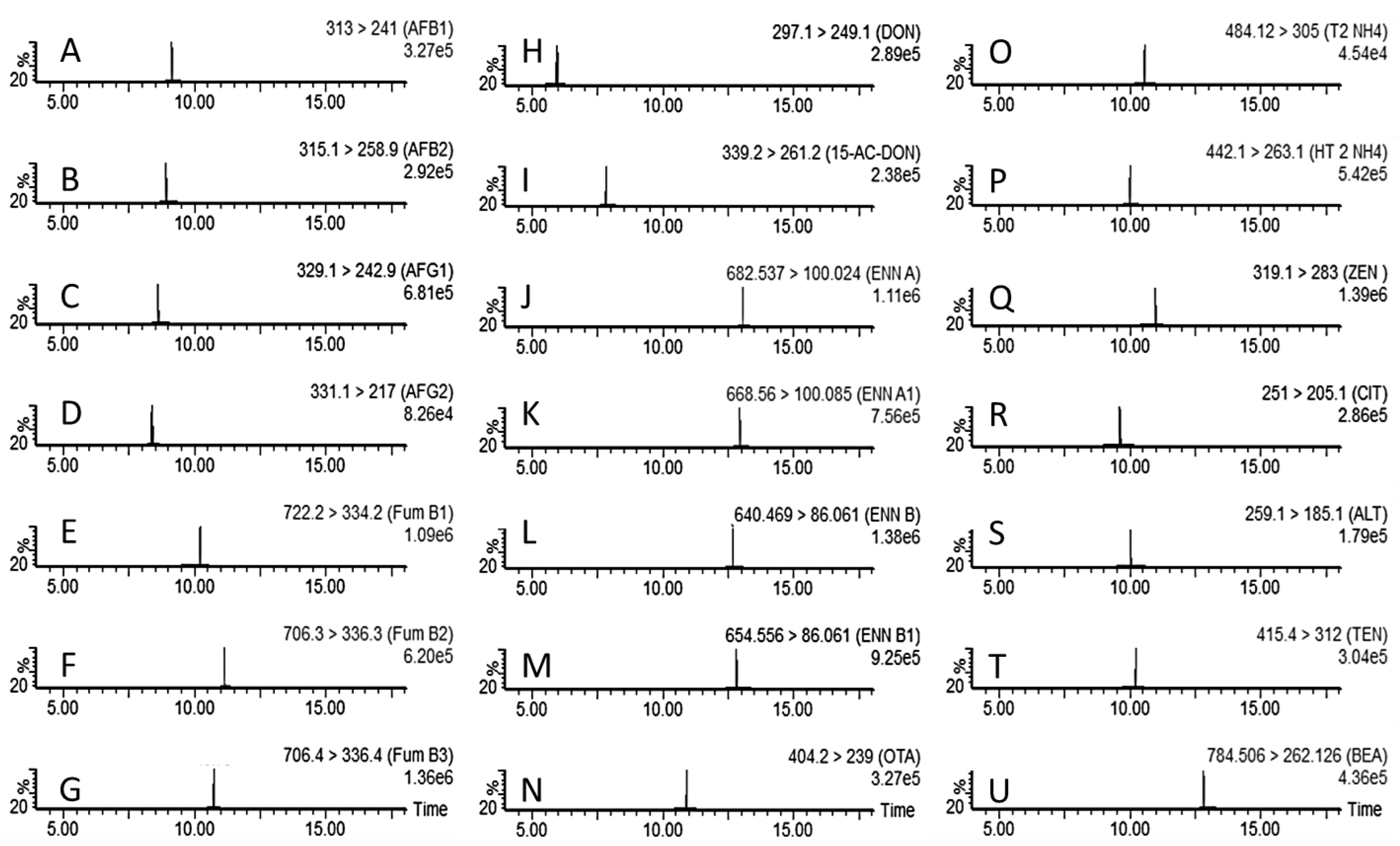
| Analyte | Precursor Ion (m/z) | Product Ion (m/z) * | Cone Voltage (V) | Collision Energy (eV) | Retention Time (min) |
|---|---|---|---|---|---|
| AFB1 | 313.0 | 241.0 | 30 | 37 | 9.1 |
| 313.0 | 213.0 | 30 | 44 | ||
| AFB2 | 315.1 | 258.9 | 30 | 27 | 8.9 |
| 315.1 | 243.1 | 30 | 37 | ||
| AFG1 | 329.1 | 242.9 | 30 | 27 | 8.6 |
| 329.1 | 199.7 | 30 | 41 | ||
| AFG2 | 331.1 | 217.0 | 45 | 40 | 8.4 |
| 331.1 | 285.0 | 45 | 30 | ||
| FB1 | 722.2 | 334.2 | 30 | 38 | 10.2 |
| 722.2 | 352.2 | 30 | 34 | ||
| FB2 | 706.3 | 336.3 | 30 | 36 | 11.1 |
| 706.3 | 318.2 | 30 | 37 | ||
| FB3 | 706.3 | 336.4 | 30 | 37 | 10.7 |
| 706.3 | 318.2 | 30 | 39 | ||
| DON | 297.1 | 249.1 | 30 | 10 | 5.9 |
| 297.1 | 203.0 | 30 | 12 | ||
| 3/15AcDON | 339.2 | 261.2 | 30 | 10 | 7.8 |
| 339.2 | 137.0 | 30 | 10 | ||
| ENN A | 682.5 | 100.0 | 30 | 60 | 13.1 |
| 682.5 | 210.1 | 30 | 19 | ||
| ENN A1 | 668.6 | 100.1 | 30 | 60 | 12.9 |
| 668.6 | 210.0 | 30 | 23 | ||
| ENN B | 640.5 | 86.1 | 30 | 50 | 12.6 |
| 640.5 | 196.0 | 30 | 25 | ||
| ENN B1 | 654.6 | 86.1 | 30 | 59 | 12.8 |
| 654.6 | 196.0 | 30 | 29 | ||
| OTA | 404.2 | 239.0 | 30 | 24 | 10.9 |
| 404.2 | 192.8 | 30 | 43 | ||
| T2 | 484.1 | 305.0 | 30 | 15 | 10.5 |
| 484.1 | 215.0 | 30 | 20 | ||
| HT2 | 442.1 | 263.1 | 25 | 12 | 10.0 |
| 442.1 | 245.1 | 25 | 13 | ||
| ZEN | 319.1 | 283.0 | 30 | 15 | 11.0 |
| 319.1 | 187.1 | 30 | 20 | ||
| CIT | 251.1 | 205.1 | 25 | 25 | 9.7 |
| 251.1 | 191.0 | 25 | 30 | ||
| ALT | 259.1 | 185.1 | 30 | 30 | 10.0 |
| 259.1 | 213.2 | 30 | 25 | ||
| TEN | 415.4 | 312.0 | 30 | 20 | 10.2 |
| 415.4 | 171.1 | 30 | 20 | ||
| BEA | 784.5 | 262.1 | 30 | 10 | 12.8 |
| 784.5 | 137.0 | 30 | 10 |
| Fungal Strain Species | Strain N° | AFB1 | AFB2 | AFG1 | AFG2 | OTA | FB1 | FB2 | FB3 | HT2 | DON | ZEN | CIT | ALT | TEN | 3/15 AcDON | BEA | ENN A | ENN A1 | ENN B | ENN B1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alternaria arborescens | IHEM 1367 | - | - | - | - | - | - | - | - | - | - | - | - | 2411.65 | 1.03 | - | - | - | - | - | - |
| Alternaria arborescens | IHEM 10114 | - | - | - | - | - | - | - | - | - | - | - | - | 1872.26 | 16.37 | - | - | - | - | - | - |
| Alternaria alternata | IHEM 3121 | - | - | - | - | - | - | - | - | - | - | - | - | 131.94 | - | - | - | - | - | - | - |
| Aspergillus flavus | IHEM 4388 | 0.01 | - | 0.01 | - | - | 0.15 | 0.13 | 0.12 | - | - | - | - | - | - | - | - | - | - | - | - |
| Aspergillus flavus | IHEM 23903 | 4.54 | 0.12 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Aspergillus nomius | IHEM 2262 | 0.08 | - | 0.08 | - | - | 0.56 | 0.40 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Aspergillus parasiticus | IHEM 4387 | 98.97 | 3.58 | 27.31 | 1.28 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Aspergillus parasiticus | IHEM 4383 | 284.20 | 16.52 | 155.99 | 12.00 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Fusarium avenaceum | IHEM 14084 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | <LOQ | 1.22 | 127.61 | 18.68 |
| Fusarium cerealis | IHEM 14083 | - | - | - | - | - | - | - | - | - | - | 466.30 | - | - | - | - | - | - | - | - | - |
| Fusarium culmorum | IHEM 3322 | - | - | - | - | - | - | - | - | - | - | 220.79 | - | - | - | - | - | - | - | - | - |
| Fusarium graminearum | IHEM 2995 | - | - | - | - | - | - | - | - | - | 122.93 | 65.01 | - | - | - | 53.11 | - | - | - | - | - |
| Fusarium graminearum | MUCL 53186 | - | - | - | - | - | - | - | - | - | 384.57 | 1401.21 | - | - | - | 204.26 | - | - | - | - | - |
| Fusarium oxysporum | IHEM 3798 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 9.85 | - | - | - | - |
| Fusarium oxysporum | IHEM 13830 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 13.10 | - | - | - | - |
| Fusarium poae | IHEM 13813 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 45.72 | - | - | - | - |
| Fusarium poae | IHEM 15929 | - | - | - | - | - | - | - | - | 472.13 | - | - | - | - | - | - | 49.12 | - | - | - | - |
| Fusarium proliferatum | IHEM 10152 | - | - | - | - | - | 652.58 | 107.30 | 57.32 | - | - | - | - | - | - | - | 4.65 | - | - | - | - |
| Fusarium subglutinans | IHEM 3820 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Fusarium verticillioides | IHEM 10153 | - | - | - | - | - | 396.86 | 219.52 | 70.08 | - | - | - | - | - | - | - | - | - | - | - | - |
| Fusarium verticillioides | IHEM 23528 | - | - | - | - | - | 1928.38 | 1438.83 | 604.43 | - | - | - | - | - | - | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masquelier, J.; Tangni, E.K.; Becker, P.; Sanders, J.; Laporte, J.; Mertens, B. Validation of an LC-MS Method for Quantification of Mycotoxins and Characterization of Fungal Strains Occurring in Food and Feed. Chemosensors 2025, 13, 106. https://doi.org/10.3390/chemosensors13030106
Masquelier J, Tangni EK, Becker P, Sanders J, Laporte J, Mertens B. Validation of an LC-MS Method for Quantification of Mycotoxins and Characterization of Fungal Strains Occurring in Food and Feed. Chemosensors. 2025; 13(3):106. https://doi.org/10.3390/chemosensors13030106
Chicago/Turabian StyleMasquelier, Julien, Emmanuel K. Tangni, Pierre Becker, Julie Sanders, Joëlle Laporte, and Birgit Mertens. 2025. "Validation of an LC-MS Method for Quantification of Mycotoxins and Characterization of Fungal Strains Occurring in Food and Feed" Chemosensors 13, no. 3: 106. https://doi.org/10.3390/chemosensors13030106
APA StyleMasquelier, J., Tangni, E. K., Becker, P., Sanders, J., Laporte, J., & Mertens, B. (2025). Validation of an LC-MS Method for Quantification of Mycotoxins and Characterization of Fungal Strains Occurring in Food and Feed. Chemosensors, 13(3), 106. https://doi.org/10.3390/chemosensors13030106





