Progress in Layered Double Hydroxide-Based Materials for Gas and Electrochemical Sensing Applications
Abstract
1. Introduction
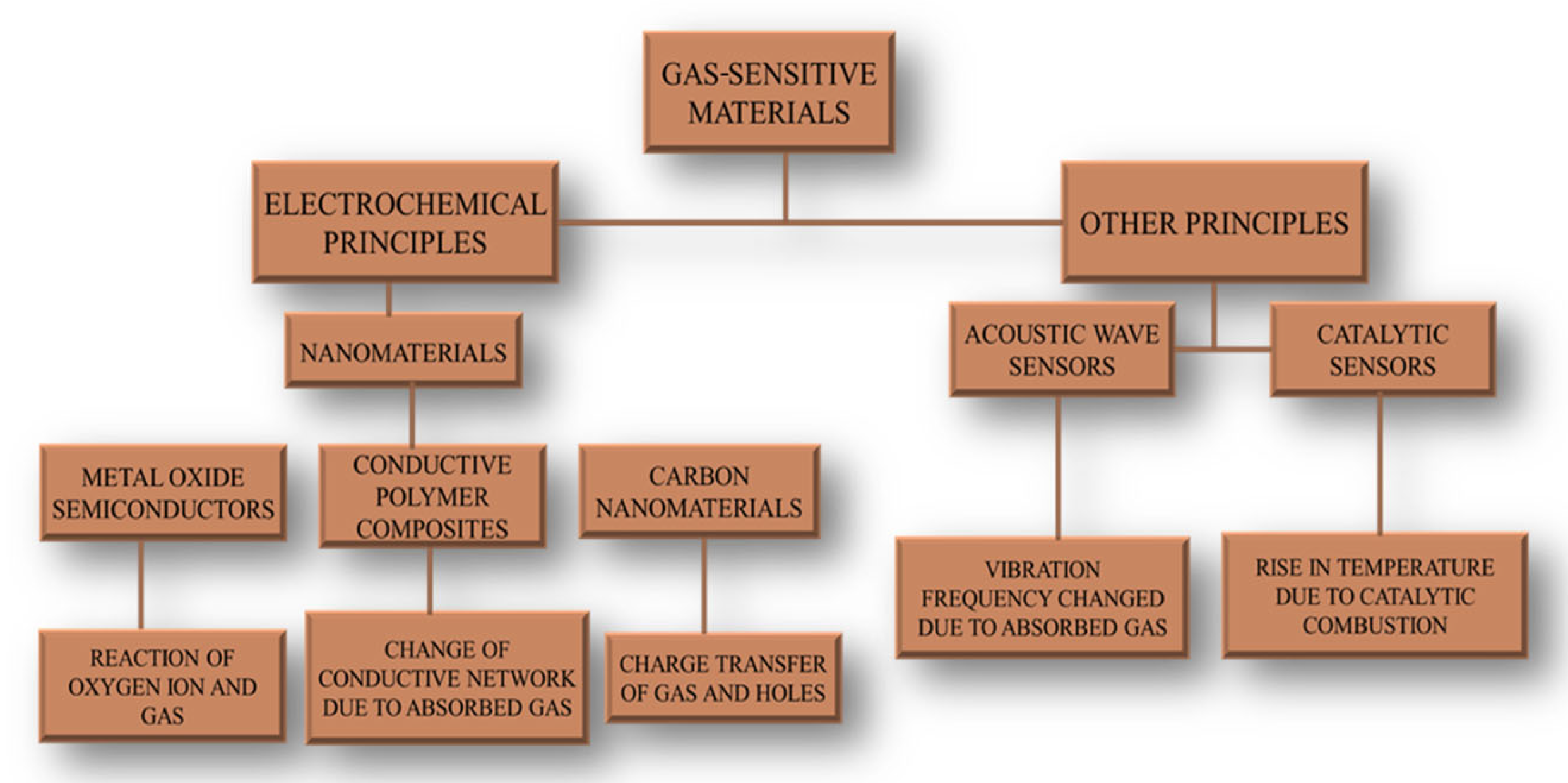
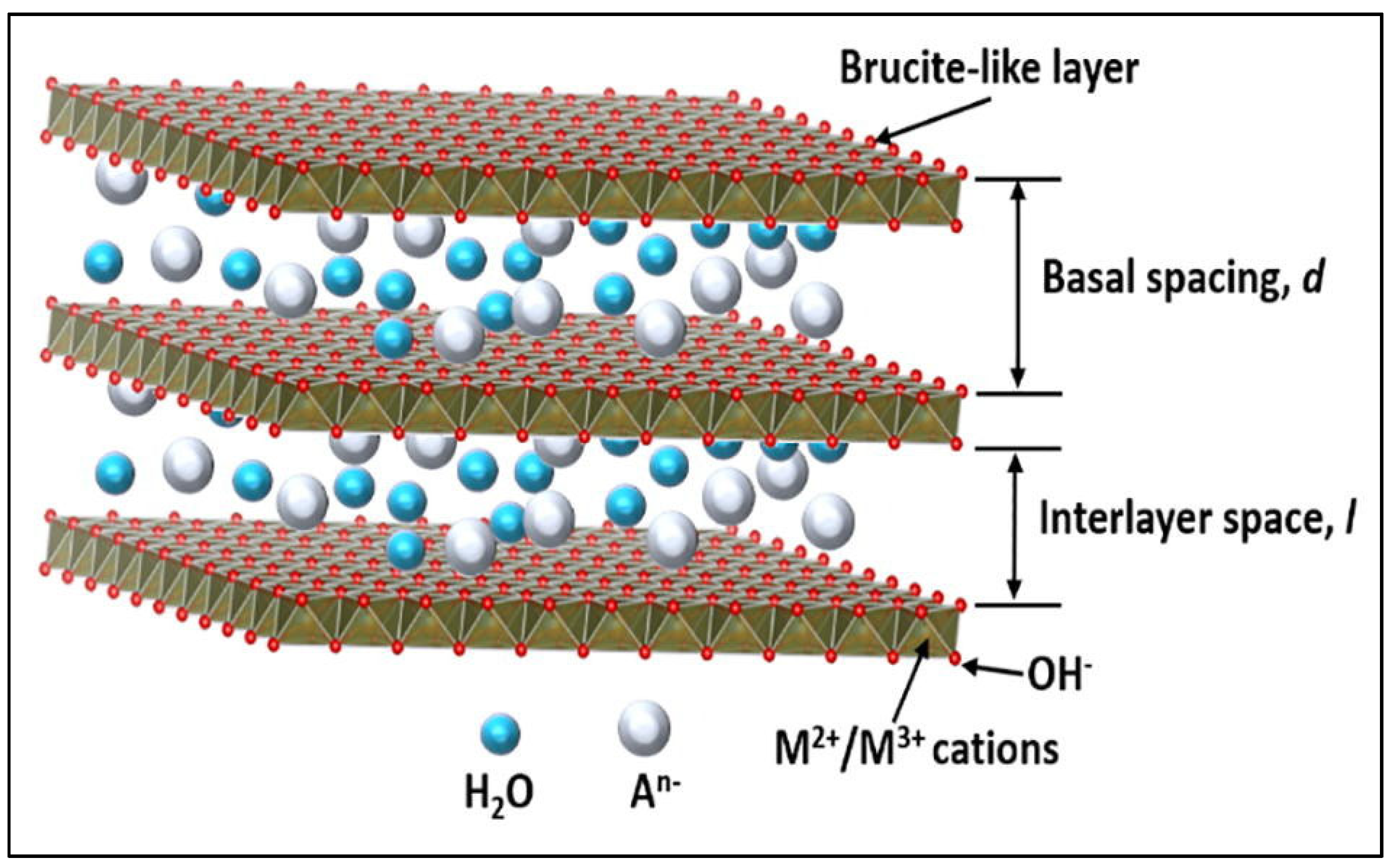
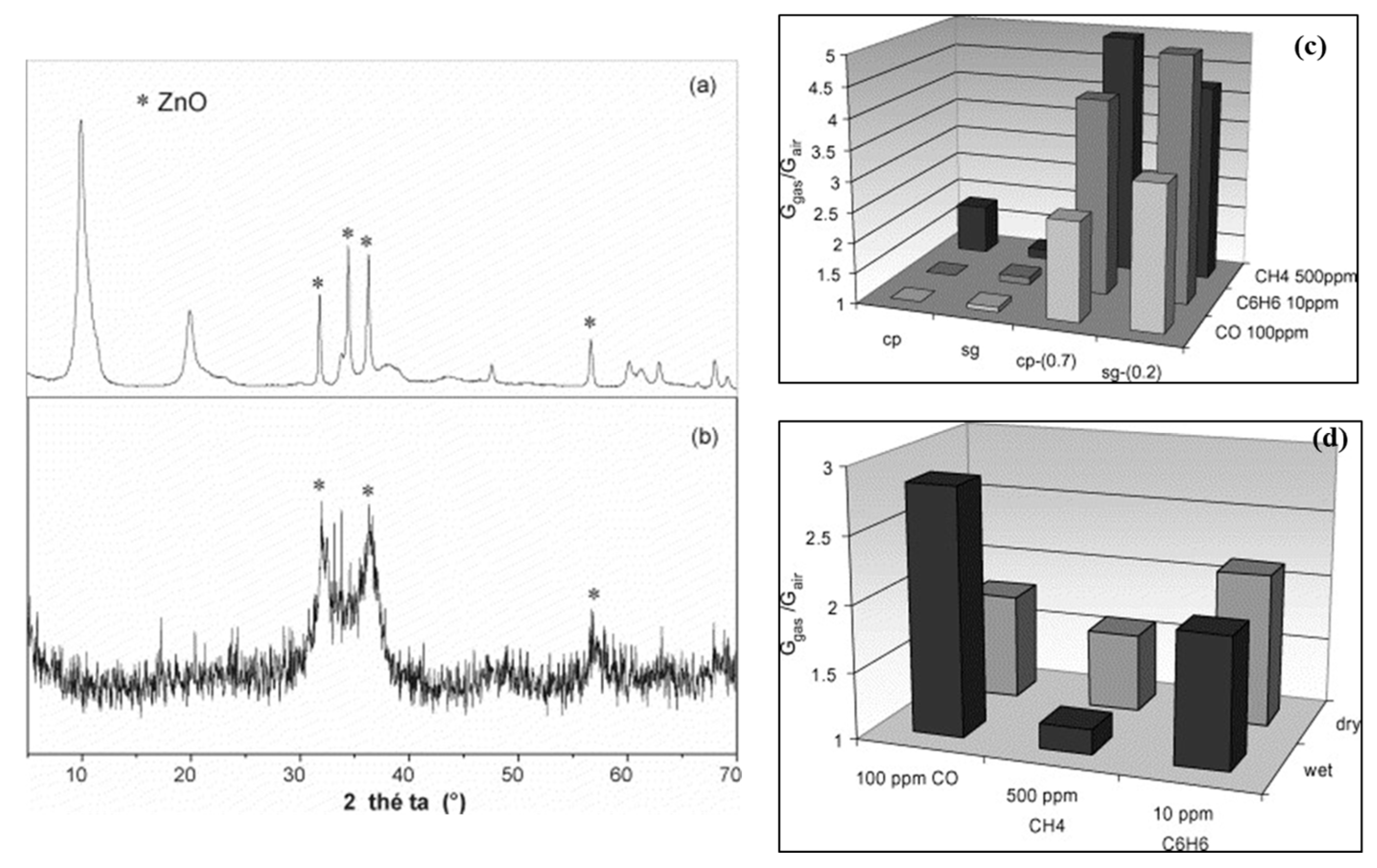
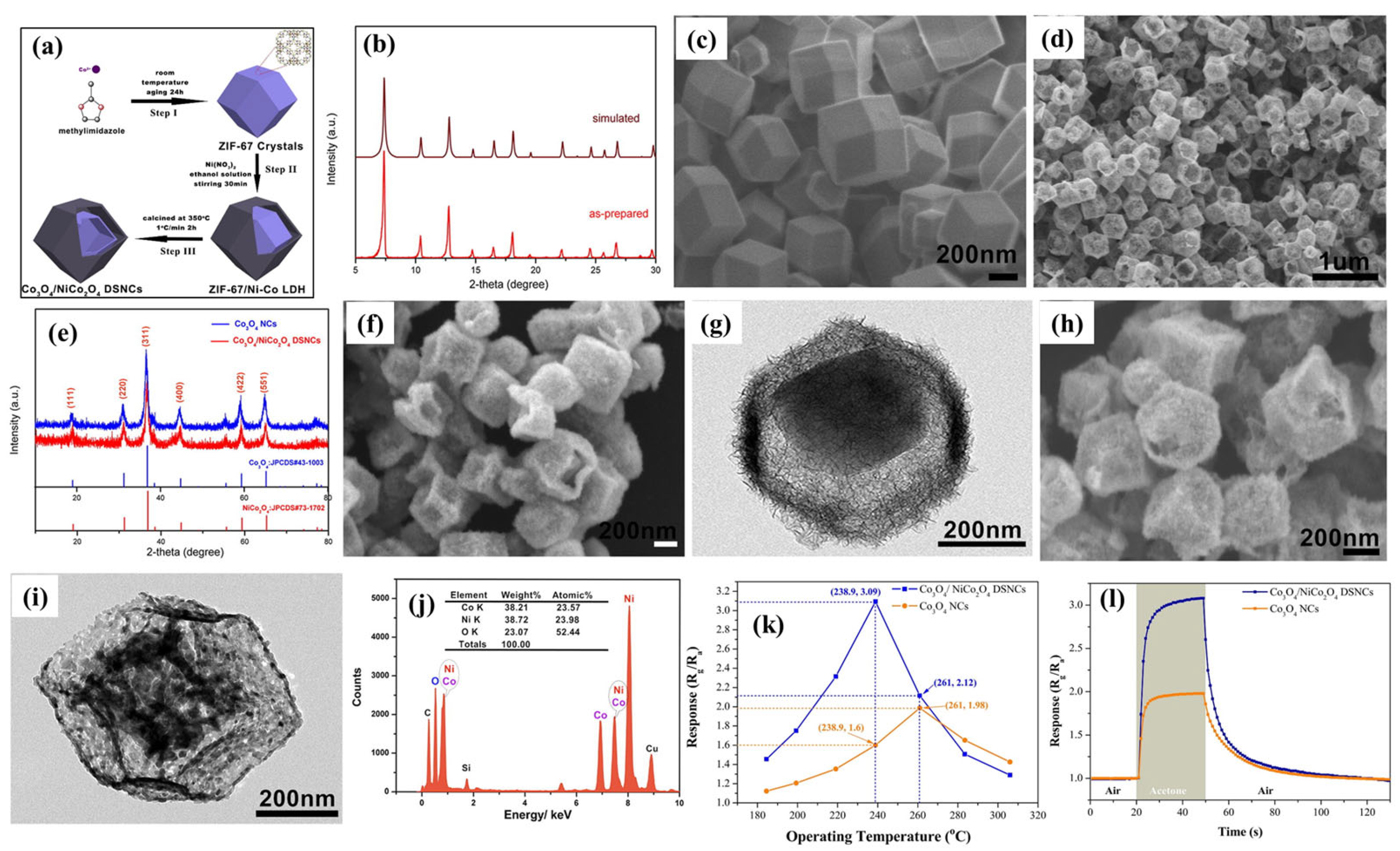
2. LDH-Based Materials in Gas Sensors
3. LDH-Based Electrochemical Sensors
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, H.; Deng, G.; Liu, Y. Monitoring the Influence of Industrialization and Urbanization on Spatiotemporal Variations of AQI and PM2.5 in Three Provinces, China. Atmosphere 2022, 13, 1377. [Google Scholar] [CrossRef]
- Nakhjiri, A.; Kakroodi, A.A. Air pollution in industrial clusters: A comprehensive analysis and prediction using multi-source data. Ecol. Inform. 2024, 80, 102504. [Google Scholar] [CrossRef]
- Afifa; Arshad, K.; Hussain, N.; Ashraf, M.H.; Saleem, M.Z. Air pollution and climate change as grand challenges to sustainability. Sci. Total Environ. 2024, 928, 172370. [Google Scholar] [CrossRef]
- Cuerda-Correa, E.M.; Alexandre-Franco, M.F.; Fernández-González, C. Advanced Oxidation Processes for the Removal of Antibiotics from Water. An Overview. Water 2020, 12, 102. [Google Scholar] [CrossRef]
- Hashim, H.S.; Fen, Y.W.; Omar, N.A.S.; Fauzi, N.I.M. Sensing Methods for Hazardous Phenolic Compounds Based on Graphene and Conducting Polymers-Based Materials. Chemosensors 2021, 9, 291. [Google Scholar] [CrossRef]
- Silva, J.A. Wastewater Treatment and Reuse for Sustainable Water Resources Management: A Systematic Literature Review. Sustainability 2023, 15, 10940. [Google Scholar] [CrossRef]
- Negahdary, M.; Ameku, W.A.; Santos, B.G.; Lima, I.S.; de Oliveira, T.G.; França, M.C.; Angnes, L. Recent electrochemical sensors and biosensors for toxic agents based on screen-printed electrodes equipped with nanomaterials. Microchem. J. 2023, 185, 108281. [Google Scholar] [CrossRef]
- Nishida, C.; Yatera, K. The Impact of Ambient Environmental and Occupational Pollution on Respiratory Diseases. Int. J. Environ. Res. Public Health 2022, 19, 2788. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, R.; Meng, F.; Zhang, J.; Zuo, K.; Han, E. Approaches to Enhancing Gas Sensing Properties: A Review. Sensors 2019, 19, 1495. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, S.; Liu, H.; Hu, S.; Zhang, D.; Ning, H. A Survey on Gas Sensing Technology. Sensors 2012, 12, 9635–9665. [Google Scholar] [CrossRef]
- Delfino, I.; Diano, N.; Lepore, M. Advanced Optical Sensing of Phenolic Compounds for Environmental Applications. Sensors 2021, 21, 7563. [Google Scholar] [CrossRef] [PubMed]
- Kuş, E.; Altındemir, G.; Bostan, Y.K.; Taşaltın, C.; Erol, A.; Wang, Y.; Sarcan, F. A Dual-Channel MoS2-Based Selective Gas Sensor for Volatile Organic Compounds. Nanomaterials 2024, 14, 633. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.H.; Rao, M.V.; Li, Q. Recent Advances in Electrochemical Sensors for Detecting Toxic Gases: NO2, SO2 and H2S. Sensors 2019, 19, 905. [Google Scholar] [CrossRef] [PubMed]
- Chakraborthy, A.; Nuthalapati, S.; Nag, A.; Afsarimanesh, N.; Alahi, M.E.E.; Altinsoy, M.E. A Critical Review of the Use of Graphene-Based Gas Sensors. Chemosensors 2022, 10, 355. [Google Scholar] [CrossRef]
- Idris, A.O.; Oseghe, E.O.; Msagati, T.A.M.; Kuvarega, A.T.; Feleni, U.; Mamba, B. Graphitic Carbon Nitride: A Highly Electroactive Nanomaterial for Environmental and Clinical Sensing. Sensors 2020, 20, 5743. [Google Scholar] [CrossRef]
- Dourandish, Z.; Tajik, S.; Beitollahi, H.; Jahani, P.M.; Nejad, F.G.; Sheikhshoaie, I.; Di Bartolomeo, A. A Comprehensive Review of Metal–Organic Framework: Synthesis, Characterization, and Investigation of Their Application in Electrochemical Biosensors for Biomedical Analysis. Sensors 2022, 22, 2238. [Google Scholar] [CrossRef]
- Zhao, Z.; Cao, J.; Zhu, B.; Li, X.; Zhou, L.; Su, B. Recent Advances in MXene-Based Electrochemical Sensors. Biosensors 2025, 15, 107. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, J.; Guo, X.; Li, Y.; Li, L.; Pan, L. Recent Advances in Conductive Polymers-Based Electrochemical Sensors for Biomedical and Environmental Applications. Polymers 2024, 16, 1597. [Google Scholar] [CrossRef]
- Tereshkov, M.; Dontsova, T.; Saruhan, B.; Krüger, S. Metal Oxide-Based Sensors for Ecological Monitoring: Progress and Perspectives. Chemosensors 2024, 12, 42. [Google Scholar] [CrossRef]
- Kim, A.; Varga, I.; Adhikari, A.; Patel, R. Recent Advances in Layered Double Hydroxide-Based Electrochemical and Optical Sensors. Nanomaterials 2021, 11, 2809. [Google Scholar] [CrossRef]
- Nemček, L.; Hagarová, I.; Matúš, P. Layered Double Hydroxides as Next-Generation Adsorbents for the Removal of Selenium from Water. Appl. Sci. 2024, 14, 8513. [Google Scholar] [CrossRef]
- Chubar, N.; Gilmour, R.; Gerda, V.; Mičušík, M.; Omastova, M.; Heister, K.; Man, P.; Fraissard, J.; Zaitsev, V. Layered double hydroxides as the next generation inorganic anion exchangers: Synthetic methods versus applicability. Adv. Colloid Interface Sci. 2017, 245, 62–80. [Google Scholar] [CrossRef] [PubMed]
- Bukhtiyarova, M.V.; Bulavchenko, O.A.; Bukhtiyarov, A.V.; Nuzhdin, A.L.; Bukhtiyarova, G.A. Selective Hydrogenation of 5-Acetoxymethylfurfural over Cu-Based Catalysts in a Flow Reactor: Effect of Cu-Al Layered Double Hydroxides Synthesis Conditions on Catalytic Properties. Catalysts 2022, 12, 878. [Google Scholar] [CrossRef]
- Li, C.; Bao, Y.; Liu, E.; Zhao, B.; Sun, T. Recent Advances of Modified Ni (Co, Fe)-Based LDH 2D Materials for Water Splitting. Molecules 2023, 28, 1475. [Google Scholar] [CrossRef]
- Huang, S.-H.; Chen, Y.-J.; Huang, W.-F.; Uan, J.-Y. Electrodeposition of a Li-Al Layered Double Hydroxide (LDH) on a Ball-like Aluminum Lathe Waste Strips in Structured Catalytic Applications: Preparation and Characterization of Ni-Based LDH Catalysts for Hydrogen Evolution. Catalysts 2022, 12, 520. [Google Scholar] [CrossRef]
- Li, Y.; Narducci, R.; Varone, A.; Kaciulis, S.; Bolli, E.; Pizzoferrato, R. Zn–Al Layered Double Hydroxides Synthesized on Aluminum Foams for Fluoride Removal from Water. Processes 2021, 9, 2109. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M.; Li, R.; Feng, X.; Pang, X.; Rao, J.; Cong, D.; Yin, C.; Zhang, Y. Active Corrosion Protection of Mg–Al Layered Double Hydroxide for Magnesium Alloys: A Short Review. Coatings 2021, 11, 1316. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, Y.q.; Zhang, Y.; Wang, B.; Yan, H.; Liu, W.; Lin, Y. Manganese-based layered double hydroxide nanoparticles as highly efficient ozone decomposition catalysts with tunable valence state. Nanoscale 2020, 12, 12817–12823. [Google Scholar] [CrossRef]
- Mureseanu, M.; Cioatera, N.; Carja, G. Fe-Ce/Layered Double Hydroxide Heterostructures and Their Derived Oxides: Electrochemical Characterization and Light-Driven Catalysis for the Degradation of Phenol from Water. Nanomaterials 2023, 13, 981. [Google Scholar] [CrossRef]
- Xia, G.; Zheng, Y.; Sun, Z.; Xia, S.; Ni, Z.; Yao, J. Fabrication of ZnAl-LDH mixed metal-oxide composites for photocatalytic degradation of 4-chlorophenol. Environ. Sci. Pollut. Res. 2022, 29, 39441–39450. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, C.; Rad, S.; He, H.; Qin, L. A Comprehensive Review of Layered Double Hydroxide-Based Carbon Composites as an Environmental Multifunctional Material for Wastewater Treatment. Processes 2022, 10, 617. [Google Scholar] [CrossRef]
- Ahmad, K.; Raza, W.; Alsulmi, A.; Kim, H. Fabrication of Electrochemical Sensor for Metronidazole Using MoS2/Graphite-like Carbon Nitride Composite Modified Glassy Carbon Electrode. Diam. Relat. Mater. 2023, 138, 110178. [Google Scholar] [CrossRef]
- Raza, W.; Ahmad, K. Room Temperature Gas Sensor Based on Reduce Graphene Oxide for Environmental Monitoring. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–19. [Google Scholar]
- Fanget, S.; Hentz, S.; Puget, P.; Arcamone, J.; Matheron, M.; Colinet, E.D. Gas sensors based on gravimetric detection—A review. Sens. Actuators B Chem. 2011, 160, 804–821. [Google Scholar] [CrossRef]
- Lin, T.; Lv, X.; Hu, Z.; Xu, A.; Feng, C. Semiconductor Metal Oxides as Chemoresistive Sensors for Detecting Volatile Organic Compounds. Sensors 2019, 19, 233. [Google Scholar] [CrossRef]
- Ghidotti, M.; Fabbri, D.; Torri, C. Determination of linear and cyclic volatile methyl siloxanes in biogas and biomethane by solid-phase microextraction and gas chromatography-mass spectrometry. Talanta 2019, 195, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Fois, M.; Cox, T.; Ratcliffe, N.; de Lacy Costello, B. Rare Earth Doped Metal Oxide Sensor for the Multimodal Detection of Volatile Organic Compounds (VOCs). Sens. Actuators B Chem. 2021, 330, 129264. [Google Scholar] [CrossRef]
- Saruhan, B.; Lontio Fomekong, R.; Nahirniak, S. Review: Influences of Semiconductor Metal Oxide Properties on Gas Sensing Characteristics. Front. Sens. 2021, 2, 657931. [Google Scholar] [CrossRef]
- Baig, N.; Sajid, M. Applications of Layered Double Hydroxides Based Electrochemical Sensors for Determination of Environmental Pollutants: A Review. Trends Environ. Anal. Chem. 2017, 16, 1–15. [Google Scholar] [CrossRef]
- Guo, Y.; Xiao, Y.; Zhang, L.; Song, Y.F. Fabrication of (Calcein-ZnS) n Ordered Ultrathin Films on the Basis of Layered Double Hydroxide and Its Ethanol Sensing Behavior. Ind. Eng. Chem. Res. 2012, 51, 8966–8973. [Google Scholar] [CrossRef]
- Jiménez-Cadena, G.; Riu, J.; Rius, F.X. Gas Sensors Based on Nanostructured Materials. Analyst 2007, 132, 1083–1099. [Google Scholar] [CrossRef]
- Wang, K.; Cao, Z.; Ding, Q.; Yoo, J.; Singh, N.; Kang, H.; Wang, L.; Xu, L.; Kim, J.S. Electronic Structure Tuning of Layered Double Hydroxides for Electrochemical Sensing and Its Biomedical Applications. Sci. China Chem. 2024, 67, 3614–3630. [Google Scholar] [CrossRef]
- Yunusa, Z.; Hamidon, M.N.; Kaiser, A.; Awang, Z. Gas Sensors: A Review. Sens. Transducers 2014, 168, 61–75. [Google Scholar]
- Feng, S.; Farha, F.; Li, Q.; Wan, Y.; Xu, Y.; Zhang, T.; Ning, H. Review on Smart Gas Sensing Technology. Sensors 2019, 19, 3760. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.Y.; Xu, D.M.; Song, Y.F.; Guo, Y. ZnO/ZnAl2O4 Prepared by Calcination of ZnAl Layered Double Hydroxides for Ethanol Sensing. Sens. Actuators B Chem. 2013, 188, 1148–1154. [Google Scholar] [CrossRef]
- Tang, S.; Yao, Y.; Chen, T.; Kong, D.; Shen, W.; Lee, H.K. Recent Advances in the Application of Layered Double Hydroxides in Analytical Chemistry: A Review. Anal. Chim. Acta 2020, 1103, 32–48. [Google Scholar] [CrossRef]
- Asif, M.; Aziz, A.; Azeem, M.; Wang, Z.; Ashraf, G.; Xiao, F.; Chen, X.; Liu, H. A Review on Electrochemical Biosensing Platform Based on Layered Double Hydroxides for Small Molecule Biomarkers Determination. Adv. Colloid Interface Sci. 2018, 262, 21–38. [Google Scholar] [CrossRef]
- Polese, D.; Mattoccia, A.; Giorgi, F.; Pazzini, L.; Di Giamberardino, L.; Fortunato, G.; Medaglia, P.G. A Phenomenological Investigation on Chlorine Intercalated Layered Double Hydroxides Used as Room Temperature Gas Sensors. J. Alloys Compd. 2017, 692, 915–922. [Google Scholar] [CrossRef]
- Zakaria, S.A.; Ahmadi, S.H.; Amini, M.H. Chemiresistive Gas Sensors Based on Layered Double Hydroxides (LDHs) Structures: A Review. Sens. Actuators A Phys. 2022, 346, 113827. [Google Scholar] [CrossRef]
- Karmakar, A.K.; Hasan, M.S.; Sreemani, A.; Das Jayanta, A.; Hasan, M.M.; Tithe, N.A.; Biswas, P. A Review on the Current Progress of Layered Double Hydroxide Application in Biomedical Sectors. Eur. Phys. J. Plus 2022, 137, 801. [Google Scholar] [CrossRef]
- Aziz, A.; Asif, M.; Ashraf, G.; Azeem, M.; Majeed, I.; Ajmal, M.; Wang, J.; Liu, H. Advancements in Electrochemical Sensing of Hydrogen Peroxide, Glucose and Dopamine by Using 2D Nanoarchitectures of Layered Double Hydroxides or Metal Dichalcogenides. A Review. Microchim. Acta 2019, 186, 671. [Google Scholar] [CrossRef]
- Boumeriame, H.; Da Silva, E.S.; Cherevan, A.S.; Chafik, T.; Faria, J.L.; Eder, D. Layered Double Hydroxide (LDH)-Based Materials: A Mini-Review on Strategies to Improve the Performance for Photocatalytic Water Splitting. J. Energy Chem. 2021, 64, 406–431. [Google Scholar] [CrossRef]
- Xiao, Y.P.; Zhang, L.M.; Guo, Y.; Song, Y.F. (Pyrenetetrasulfonate/ZnS)n Ordered Ultrathin Films with ZnAl Layered Double Hydroxide as Precursor and Ethanol-Sensing Properties. Eur. J. Inorg. Chem. 2013, 2013, 3348–3351. [Google Scholar] [CrossRef]
- Morandi, S.; Prinetto, F.; Di Martino, M.; Ghiotti, G.; Lorret, O.; Tichit, D.; Malagù, C.; Vendemiati, B.; Carotta, M.C. Synthesis and Characterisation of Gas Sensor Materials Obtained from Pt/Zn/Al Layered Double Hydroxides. Sens. Actuators B Chem. 2006, 118, 215–220. [Google Scholar] [CrossRef]
- Liu, S.R.; Guan, M.Y.; Li, X.Z.; Guo, Y. Light Irradiation Enhanced Triethylamine Gas Sensing Materials Based on ZnO/ZnFe2O4 Composites. Sens. Actuators B Chem. 2016, 236, 350–357. [Google Scholar] [CrossRef]
- Hong, D.; Zhang, J.; Rehman, A.U.; Gong, L.; Zhou, J.; Kan, K.; Li, L.; Shi, K. One-Step Synthesis of Hierarchical Ni-Fe-Al Layered Double Hydroxide with Excellent Sensing Properties for NO:X at Room Temperature. RSC Adv. 2016, 6, 103192–103198. [Google Scholar] [CrossRef]
- Sun, H.; Chu, Z.; Hong, D.; Zhang, G.; Xie, Y.; Li, L.; Shi, K. Three-Dimensional Hierarchical Flower-like Mg-Al-Layered Double Hydroxides: Fabrication, Characterization and Enhanced Sensing Properties to NOx at Room Temperature. J. Alloys Compd. 2016, 658, 561–568. [Google Scholar] [CrossRef]
- Qu, F.; Zhang, B.; Zhou, X.; Jiang, H.; Wang, C.; Feng, X.; Jiang, C.; Yang, M. Metal-Organic Frameworks-Derived Porous ZnO/Ni0.9Zn0.1O Double-Shelled Nanocages as Gas Sensing Material for Selective Detection of Xylene. Sens. Actuators B Chem. 2017, 252, 649–656. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, P.; Liu, T.; Feng, Y.; Blackman, C.; Li, D. Facile Synthesis of Mesoporous Hierarchical Co3O4-TiO2 p-n Heterojunctions with Greatly Enhanced Gas Sensing Performance. J. Mater. Chem. A 2017, 5, 10387–10397. [Google Scholar] [CrossRef]
- Kang, G.; Zhu, Z.; Tang, B.H.; Wu, C.H.; Wu, R.J. Rapid Detection of Ozone in the Parts per Billion Range Using a Novel Ni–Al Layered Double Hydroxide. Sens. Actuators B Chem. 2017, 241, 1203–1209. [Google Scholar] [CrossRef]
- Qu, F.; Jiang, H.; Yang, M. MOF-Derived Co3O4/NiCo2O4 Double-Shelled Nanocages with Excellent Gas Sensing Properties. Mater. Lett. 2017, 190, 75–78. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, F.; Gao, L.; Duan, G. Co3O4 Nanosheet-Built Hollow Spheres Containing Ultrafine Neck-Connected Grains Templated by PS@Co-LDH and Their Ppb-Level Gas-Sensing Performance. Sens. Actuators B Chem. 2018, 261, 553–565. [Google Scholar] [CrossRef]
- Qu, F.; Thomas, T.; Zhang, B.; Zhou, X.; Zhang, S.; Ruan, S.; Yang, M. Self-Sacrificing Templated Formation of Co3O4/ZnCo2O4 Composite Hollow Nanostructures for Highly Sensitive Detecting Acetone Vapor. Sens. Actuators B Chem. 2018, 273, 1202–1210. [Google Scholar] [CrossRef]
- Zhang, X.; Ikram, M.; Liu, Z.; Teng, L.; Xue, J.; Wang, D.; Li, L.; Shi, K. Expanded Graphite/NiAl Layered Double Hydroxide Nanowires for Ultra-Sensitive, Ultra-Low Detection Limits and Selective NO: X Gas Detection at Room Temperature. RSC Adv. 2019, 9, 8768–8777. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Wang, L.; Wang, X. A High Performance Sensor Based on PANI/ZnTi-LDHs Nanocomposite for Trace NH3 Detection. Org. Electron. 2019, 66, 102–109. [Google Scholar] [CrossRef]
- Liu, Z.; Teng, L.; Ma, L.; Liu, Y.; Zhang, X.; Xue, J.; Ikram, M.; Ullah, M.; Li, L.; Shi, K. Porous 3D Flower-like CoAl-LDH Nanocomposite with Excellent Performance for NO2 Detection at Room Temperature. RSC Adv. 2019, 9, 21911–21921. [Google Scholar] [CrossRef]
- Zhang, X.; Teng, L.; Liu, Y.; Liu, Z.; Xue, J.; Ikram, M.; Ullah, M.; Li, L.; Shi, K. 3D Flower-like NiZnAl Multimetal Oxide Constructed by Ultra-Thin Porous Nanosheets: A Long-Term and Stable Sensing Material for NOx at Room Temperature. Sens. Actuators B Chem. 2019, 300, 126899. [Google Scholar] [CrossRef]
- Hong, Y.; Wang, D.; Lin, C.; Luo, S.; Pan, Q.; Li, L.; Shi, K. Room-Temperature Efficient NO2 gas Sensors Fabricated by Porous 3D Flower-like ZnAl-Layered Double Hydroxides. New J. Chem. 2020, 44, 18469–18480. [Google Scholar] [CrossRef]
- He, L.; Zhang, W.; Zhang, X.; Bai, X.; Chen, J.; Ikram, M.; Zhang, G.; Shi, K. 3D flower-like NiCo-LDH composites for a high-performance NO2 gas sensor at room temperature. Colloids Surf. A Physicochem. Eng. Aspects 2020, 603, 125142. [Google Scholar] [CrossRef]
- Gheibi, S.O.; Fallah Shojaei, A.; Khorshidi, A.; Hosseini-Golgoo, S.M. Synthesis, characterization, and gas sensing properties of Ni-Cr-Al LDH. Appl. Phys. A 2021, 127, 614. [Google Scholar] [CrossRef]
- Nie, L.; Fan, G.; Wang, A.; Zhang, L.; Guan, J.; Han, N.; Chen, Y. Finely Dispersed and Highly Toluene Sensitive NiO/NiGa2O4 Heterostructures Prepared from Layered Double Hydroxides Precursors. Sens. Actuators B Chem. 2021, 345, 130412. [Google Scholar] [CrossRef]
- Vigna, L.; Nigro, A.; Verna, A.; Ferrari, I.V.; Marasso, S.L.; Bocchini, S.; Fontana, M.; Chiodoni, A.; Pirri, C.F.; Cocuzza, M. Layered Double Hydroxide-Based Gas Sensors for Voc Detection at Room Temperature. ACS Omega 2021, 6, 20205–20217. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Xu, X.; Xia, Q.; Wang, X. Layered Double Oxides for Sensing Reducing Volatile Organic Compounds: The Effect of Local Charge Region Modulation. Chempluschem 2021, 86, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Ding, W.; Zhao, R. ZIF-8-Derived ZnTi-LDHs with Unique Self-Supported Architecture and Corresponding LDHs/RGO Hybrid for Gas Sensor Applications. Chem. Phys. Lett. 2021, 781, 138965. [Google Scholar] [CrossRef]
- Zhong, M.; Lao, Z.; Tan, J.; Yu, G.; Liu, Y.; Liang, Y. Synthesis of CoNi-Layered Double Hydroxide on Graphene Oxide as Adsorbent and Construction of Detection Method for Taste and Odor Compounds in Smelling Water. J. Hazard. Mater. 2022, 428, 128227. [Google Scholar] [CrossRef]
- Shinde, R.B.; Padalkar, N.S.; Sadavar, S.V.; Patil, A.S.; Kale, S.B.; Magdum, V.V.; Chitare, Y.M.; Kulkarni, S.P.; Patil, U.M.; Parale, V.G.; et al. Lattice Engineering Route for Self-Assembled Nanohybrids of 2D Layered Double Hydroxide with 0D Isopolyoxovanadate: Chemiresistive SO2 Sensor. Mater. Today Chem. 2022, 24, 100801. [Google Scholar] [CrossRef]
- Shinde, R.B.; Padalkar, N.S.; Sadavar, S.V.; Kale, S.B.; Magdum, V.V.; Chitare, Y.M.; Kulkarni, S.P.; Patil, U.M.; Parale, V.G.; Park, H.H.; et al. 2D–2D Lattice Engineering Route for Intimately Coupled Nanohybrids of Layered Double Hydroxide and Potassium Hexaniobate: Chemiresistive SO2 Sensor. J. Hazard. Mater. 2022, 432, 128734. [Google Scholar] [CrossRef]
- Sadrolhosseini, A.R.; Ghasemi, E.; Pirkarimi, A.; Hamidi, S.M.; Taheri Ghahrizjani, R. Highly Sensitive Surface Plasmon Resonance Sensor for Detection of Methylene Blue and Methylene Orange Dyes Using NiCo-Layered Double Hydroxide. Opt. Commun. 2023, 529, 129057. [Google Scholar] [CrossRef]
- Gupta, N.K.; Leyva, C.; Viltres, H.; Dhavale, R.P.; Kim, K.S.; Romero-Galarza, A.; Park, H.H. Zinc-Aluminum Layered Double Hydroxide and Double Oxide for Room-Temperature Oxidation of Sulfur Dioxide Gas. Chemosphere 2023, 338, 139503. [Google Scholar] [CrossRef]
- Selvaraj, A.K.; Suresh, I.; Nesakumar, N.; Rayappan, J.B.B.; Kulinich, S.A.; Kulandaiswamy, A.J. Highly Sensitive Cu/Fe Calcined Layered Double Hydroxide Sensor to Detect Imidacloprid, a Neurotransmission Blocker. Colloids Surf. A Physicochem. Eng. Asp. 2024, 687, 133540. [Google Scholar] [CrossRef]
- Zakaria, S.A.; Ahmadi, S.H.; Amini, M.H. Investigation the AMOST Effect on the Sensitivity of CeO2/Ni-Al Layered Double Hydroxide Composite Based Sensors. Microchem. J. 2024, 207, 112128. [Google Scholar] [CrossRef]
- Zakaria, S.A.; Amini, M.H.; Ahmadi, S.H. CeO2/Ni–Al Layered Double Hydroxide Composite Decorated with Ag Nanoparticles as a Gas Sensor. Mater. Sci. Semicond. Process. 2024, 178, 108391. [Google Scholar] [CrossRef]
- Zakaria, S.A.; Ahmadi, S.H.; Amini, M.H. Investigation of Ag Nanoparticles Decorated Ni-Al Layered Double Hydroxide as a Gas Sensor. Mater. Chem. Phys. 2024, 318, 129216. [Google Scholar] [CrossRef]
- Zakaria, S.A.; Ahmadi, S.H.; Amini, M.H. CeO2/Ni-Al Layered Double Hydroxide Composite as a Gas Sensor for Volatile Organic Compounds Detection. Mater. Today Commun. 2024, 40, 109677. [Google Scholar] [CrossRef]
- Fu, Z.; Lu, J.; Zhu, J.; Wang, R.; Feng, L. Effect of Laminate Structure on Morphology and Ethanol Sensing Properties of Hierarchical Zinc–Cobalt Layered Double Hydroxides. New J. Chem. 2024, 48, 8963–8973. [Google Scholar] [CrossRef]
- Zhan, T.; Song, Y.; Li, X.; Hou, W. Electrochemical sensor for bisphenol A based on ionic liquid functionalized Zn-Al layered double hydroxide modified electrode. Mater. Sci. Eng. C 2016, 64, 354–361. [Google Scholar] [CrossRef]
- Heli, H.; Pishahang, J.; Amiri, H.B. Synthesis of hexagonal CoAl-layered double hydroxide nanoshales/carbon nanotubes composite for the non-enzymatic detection of hydrogen peroxide. J. Electroanal. Chem. 2016, 768, 134–144. [Google Scholar] [CrossRef]
- Asadpour-Zeynali, K.; Amini, R. A novel voltammetric sensor for mercury(II) based on mercaptocarboxylic acid intercalated layered double hydroxide nanoparticles modified electrode. Sens. Actuators B Chem. 2017, 246, 961–968. [Google Scholar] [CrossRef]
- Asif, M.; Wang, H.; Dong, S.; Aziz, A.; Zhang, G.; Xiao, F.; Liu, H. Metal oxide intercalated layered double hydroxide nanosphere: With enhanced electrocatalyic activity towards H2O2 for biological applications. Sens. Actuators B Chem. 2017, 239, 243–252. [Google Scholar] [CrossRef]
- Li, S.-S.; Jiang, M.; Jiang, T.-J.; Liu, J.-H.; Guo, Z.; Huang, X.-J. Competitive adsorption behavior toward metal ions on nano-Fe/Mg/Ni ternary layered double hydroxide proved by XPS: Evidence of selective and sensitive detection of Pb(II). J. Hazard. Mater. 2017, 338, 1–10. [Google Scholar] [CrossRef]
- Qiao, X.; Wei, M.; Tian, D.; Xia, F.; Chen, P.; Zhou, C. One-step electrosynthesis of cadmium/aluminum layered double hydroxides composite as electrochemical probe for voltammetric detection of anthracene. J. Electroanal. Chem. 2018, 808, 35–40. [Google Scholar] [CrossRef]
- Tao, Y.; Chang, Q.; Liu, Q.; Yang, G.; Guan, H.; Chen, G.; Dong, C. Highly sensitive nonenzymatic H2O2 sensor based on NiFe-layered double hydroxides nanosheets grown on Ni foam. Surf. Interfaces 2018, 12, 102–107. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Y.; Xie, D.; Gu, Y.; Zhu, X.; Zhang, H.; Wang, G.; Zhang, Y.; Zhao, H. Hierarchical MgFe-layered double hydroxide microsphere/graphene composite for simultaneous electrochemical determination of trace Pb(II) and Cd(II). Chem. Eng. J. 2018, 347, 953–962. [Google Scholar] [CrossRef]
- Zhan, T.; Song, Y.; Tan, Z.; Hou, W. Electrochemical bisphenol A sensor based on exfoliated Ni2Al-layered double hydroxide nanosheets modified electrode. Sens. Actuators B Chem. 2017, 238, 962–971. [Google Scholar] [CrossRef]
- Xiang, X.; Pan, F.; Du, Z.; Feng, X.; Gao, C.; Li, Y. MgAl-layered double hydroxide flower arrays grown on carbon paper for efficient electrochemical sensing of nitrite. J. Electroanal. Chem. 2019, 855, 113632. [Google Scholar] [CrossRef]
- Vilian, A.T.E.; Ranjith, K.S.; Lee, S.J.; Umapathi, R.; Hwang, S.-K.; Oh, C.W.; Huh, Y.S.; Han, Y.-K. Hierarchical dense Ni−Co layered double hydroxide supported carbon nanofibers for the electrochemical determination of metronidazole in biological samples. Electrochim. Acta 2020, 354, 136723. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, P.; Zhan, T.; Yu, X.; Wen, Y.; Liu, X.; Gao, H.; Wang, P.; She, X. In situ growth of ZIF-67 on ultrathin CoAl layered double hydroxide nanosheets for electrochemical sensing toward naphthol isomers. J. Colloid Interface Sci. 2020, 576, 313–321. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, R.; Wang, H.; Yu, X.; Liu, X.; He, P.; She, X.; Zhan, T. Electrochemical sensing for naphthol isomers based on the in situ growth of zeolitic imidazole framework-67 on ultrathin CoAl layered double hydroxide nanosheets by a reaction–diffusion technique. J. Colloid Interface Sci. 2021, 599, 762–772. [Google Scholar] [CrossRef]
- Joseph, X.B.; Sherlin, V.A.; Wang, S.-F.; George, M. Integration of iron–manganese layered double hydroxide/tungsten carbide composite: An electrochemical tool for diphenylamine H•+ analysis in environmental samples. Environ. Res. 2022, 212, 113291. [Google Scholar] [CrossRef]
- Joseph, X.B.; Stanley, M.M.; Wang, S.-F.; George, M. Growth of 2D-layered double hydroxide nanorods heterojunction with 2D tungsten carbide nanocomposite: Improving the electrochemical sensing of norfloxacin. J. Ind. Eng. Chem. 2022, 110, 434–446. [Google Scholar] [CrossRef]
- Karuppiah, C.; Babulal, S.M.; Chen, T.-W.; Chen, S.-M.; Hsu, L.-F.; Al Farraj, D.A.; Ramaraj, S.K.; Elshikh, M.S.; Yang, C.-C. A novel ammonium zinc molybdate layered double hydroxide nanoflakes/vapor grown carbon fibers nanomaterials based electrocatalyst for the monitoring of dimetridazole drug in real samples. J. Environ. Chem. Eng. 2022, 10, 108227. [Google Scholar] [CrossRef]
- Liu, Z.; Liao, D.; Yu, J.; Jiang, X. An electrochemical sensor based on oxygen-vacancy cobalt–aluminum layered double hydroxides and hydroxylated multiwalled carbon nanotubes for catechol and hydroquinone detection. Microchem. J. 2022, 175, 107216. [Google Scholar] [CrossRef]
- Kokulnathan, T.; Wang, T.-J.; Kumar, E.A.; Ahmed, F. Construction of nickel cobalt-layered double hydroxide/functionalized–halloysite nanotubes composite for electrochemical detection of organophosphate insecticide. Chem. Eng. J. 2022, 433, 133639. [Google Scholar] [CrossRef]
- Lv, J.; Wu, M.; Fan, M.; Zhang, Q.; Chang, Z.; Wang, X.; Zhou, Q.; Wang, L.; Chong, R.; Zhang, L. Insights into the multirole CoAl layered double hydroxide on boosting photoelectrochemical activity of hematite: Application to hydrogen peroxide sensing. Talanta 2023, 262, 124681. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, Q.; Xiao, Y.; Li, D.; Jiang, X.; Wu, P. Cobalt-based layered double hydroxide nanosheet-supported AuNPs for high performance electrochemical H2O2 detection. Appl. Surf. Sci. 2023, 630, 157463. [Google Scholar] [CrossRef]
- Joseph, X.B.; Baby, J.N.; Wang, S.-F.; George, M. Emerging carbonate anion intercalated- ZnCr-layered double hydroxide/vanadium carbide nanocomposite: A electrochemical sensor for diethofencarb fungicide monitoring. Chemosphere 2023, 335, 139099. [Google Scholar] [CrossRef]
- Beitollahi, H.; Nejad, F.G.; Dourandish, Z.; Aflatoonian, M.R. Electrochemical detection of carmoisine in the presence of tartrazine on the surface of screen printed graphite electrode modified with nickel-cobalt layered double hydroxide ultrathin nanosheets. Chemosphere 2023, 337, 139369. [Google Scholar] [CrossRef]
- Kokulnathan, T.; Wang, T.-J.; Ahmed, F.; Arshi, N. Fabrication of flower-like nickel cobalt-layered double hydroxide for electrochemical detection of carbendazim. Surf. Interfaces 2023, 36, 102570. [Google Scholar] [CrossRef]
- Singh, A.K.; Gautam, R.K.; Agrahari, S.; Tiwari, I. Oxidized g-C3N4 decorated with Cu–Al layered double hydroxide as a sustainable electrochemical sensing material for quantification of diclofenac. Mater. Chem. Phys. 2023, 294, 127002. [Google Scholar] [CrossRef]
- Karuppiah, C.; Babulal, S.M.; Chen, S.-M.; Palanisamy, S.; Hsu, L.-F.; Yang, C.-C.; Chiesa, M. Simultaneous electrochemical determination of benzenediol compounds in environmental samples using nano architectures of hydrogen ammonium zinc molybdate layered double hydroxides integrated with carbon black modified electrode. J. Water Process Eng. 2023, 55, 104202. [Google Scholar] [CrossRef]
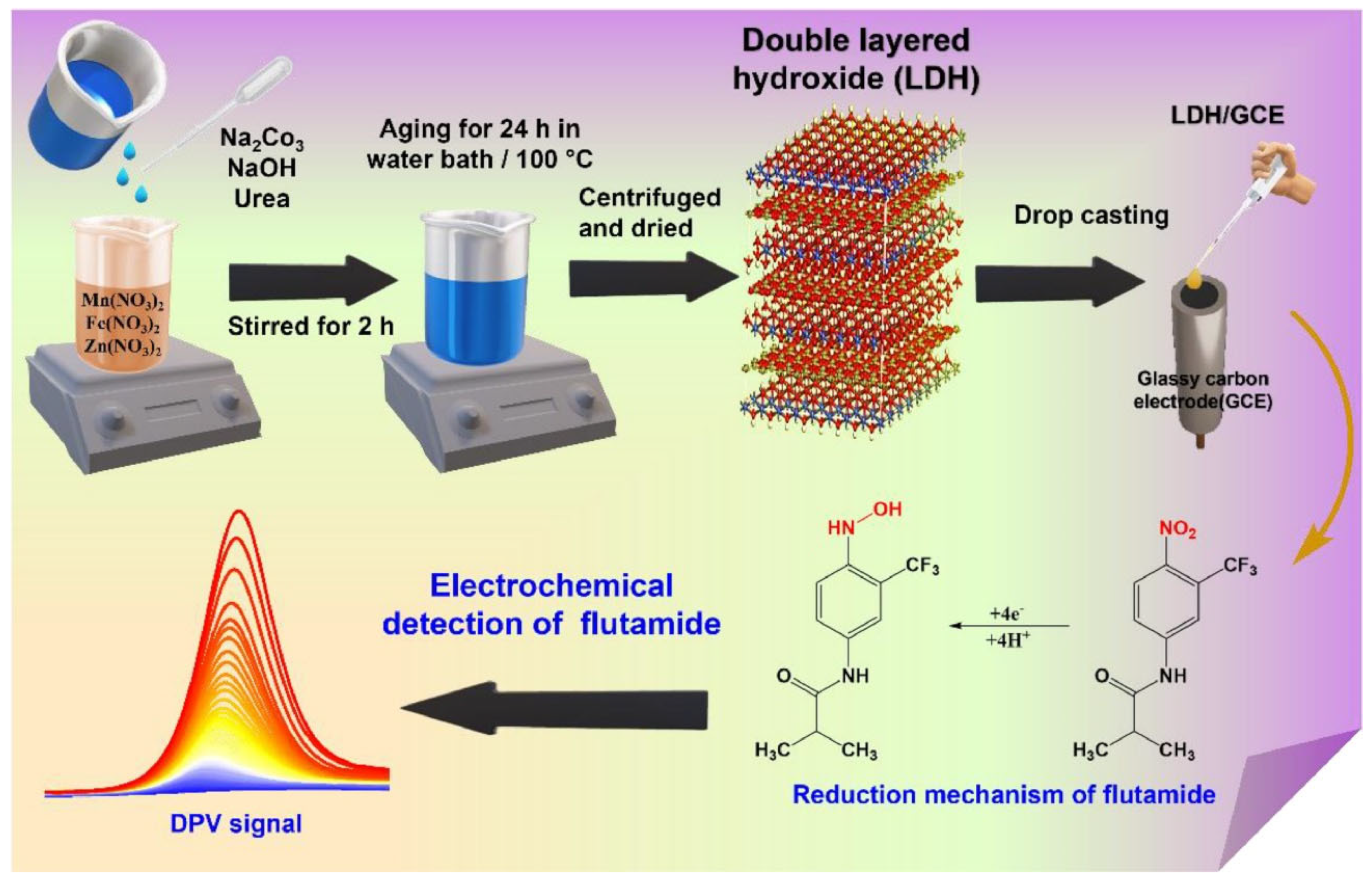
- Tharuman, S.; Nataraj, N.; Chen, S.-M.; Sasirekha, V.; Ragumoorthy, C. Ternary MnFeZn layered double hydroxides as an efficient material for the electrochemical sensing of flutamide. Surf. Interfaces 2023, 41, 103195. [Google Scholar] [CrossRef]
- He, Z.; Jin, Y.; Yuan, X.; Xue, K.; Hu, J.; Xiong, X. ZIF in situ transformation hollow porous self-supporting CoZn-LDH@CuO electrode for electrochemical sensing of glucose and H2O2. Microchem. J. 2023, 195, 109457. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Mohammadi, S.Z.; Nejad, F.G.; Dourandish, Z. Voltammetric sensor based on a Ni-Co-layered double hydroxide/multi-walled carbon nanotubes nanocomposite for 4-aminophenol determination. Chem. Phys. Impact 2024, 8, 100653. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, S.; Li, J.; Chen, H.; Gao, Y.; Xiong, X. Construction of hollow NiCo-LDH nanocages for electrochemical sensing of hydrazine based on template etching strategy. Microchem. J. 2024, 207, 111803. [Google Scholar] [CrossRef]
- Khan, M.M.; Shaikh, H.; Al Souwaileh, A.; Khan, M.Y.; Batool, M.; Memon, S.Q.; Solangi, A.R. A highly selective nickel-aluminum layered double hydroxide nanostructures based electrochemical sensor for detection of pentachlorophenol. Arab. J. Chem. 2024, 17, 105604. [Google Scholar] [CrossRef]
- Ashry, A.; Rabia, M.; Mostafa, S.M.; Korany, M.A.; Farghali, A.A.; Khalil, M.M. β-Cyclodextrin/Zn–Fe layered double hydroxides/graphitic carbon nitride nanomaterials based potentiometric sensor for paroxetine determination in environmental water samples. RSC Adv. 2024, 14, 34791–34803. [Google Scholar] [CrossRef]
- Ragumoorthy, C.; Nataraj, N.; Chen, S.-M. Cobalt aluminum layered double hydroxide as a superior electrochemical probe for the sensitive detection of anti-inflammatory agent mesalazine. J. Alloys Compd. 2024, 989, 174352. [Google Scholar] [CrossRef]
- Stanley, M.M.; Sriram, B.; Wang, S.-F.; Sherlin, A.; Kogularasu, S.; Lee, Y.-Y. Mary George, Rational confinement of ternary layered double hydroxide within three-dimensional graphene aerogel: An electrochemical tool for propyl gallate sensing. Chem. Eng. J. 2024, 485, 149682. [Google Scholar] [CrossRef]
- Stanley, M.M.; Sriram, B.; Wang, S.-F.; Sherlin, V.A.; Kogularasu, S.; George, M. Preserving food quality: Electrochemical detection of synthetic food antioxidant, propyl gallate in processed foods using ternary component layered double hydroxide/graphene aerogel synergy. Mater. Today Sustain. 2025, 29, 101061. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, F.; Wu, C.; Hu, K.; Yang, Q.; Huang, K. Preparation of Ni/Co layered double hydroxide hollow cakes as an electrochemical sensing platform for monitoring the production of hydrogen peroxide in formulated beverages. Microchem. J. 2024, 202, 110793. [Google Scholar] [CrossRef]
- Guo, J.; Wu, J.; Xu, L.; Yuan, X.; Tan, C.; Wang, Q.; Xiong, X. Microplasma-assisted construction of cross-linked network hierarchical structure of NiMoO4 nanorods @NiCo-LDH nanosheets for electrochemical sensing of non-enzymatic H2O2 in food. Food Chem. 2024, 461, 140940. [Google Scholar] [CrossRef]

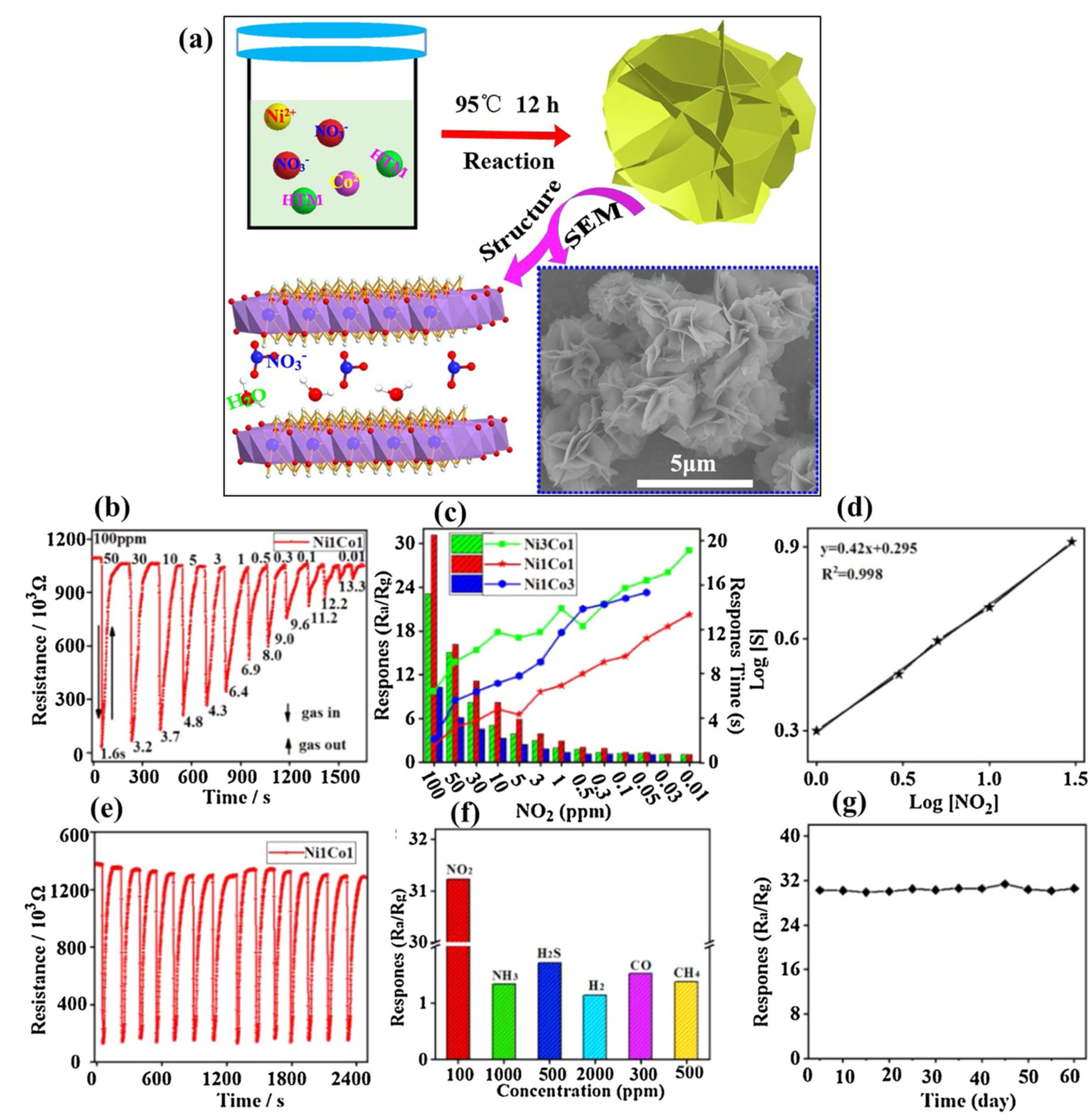
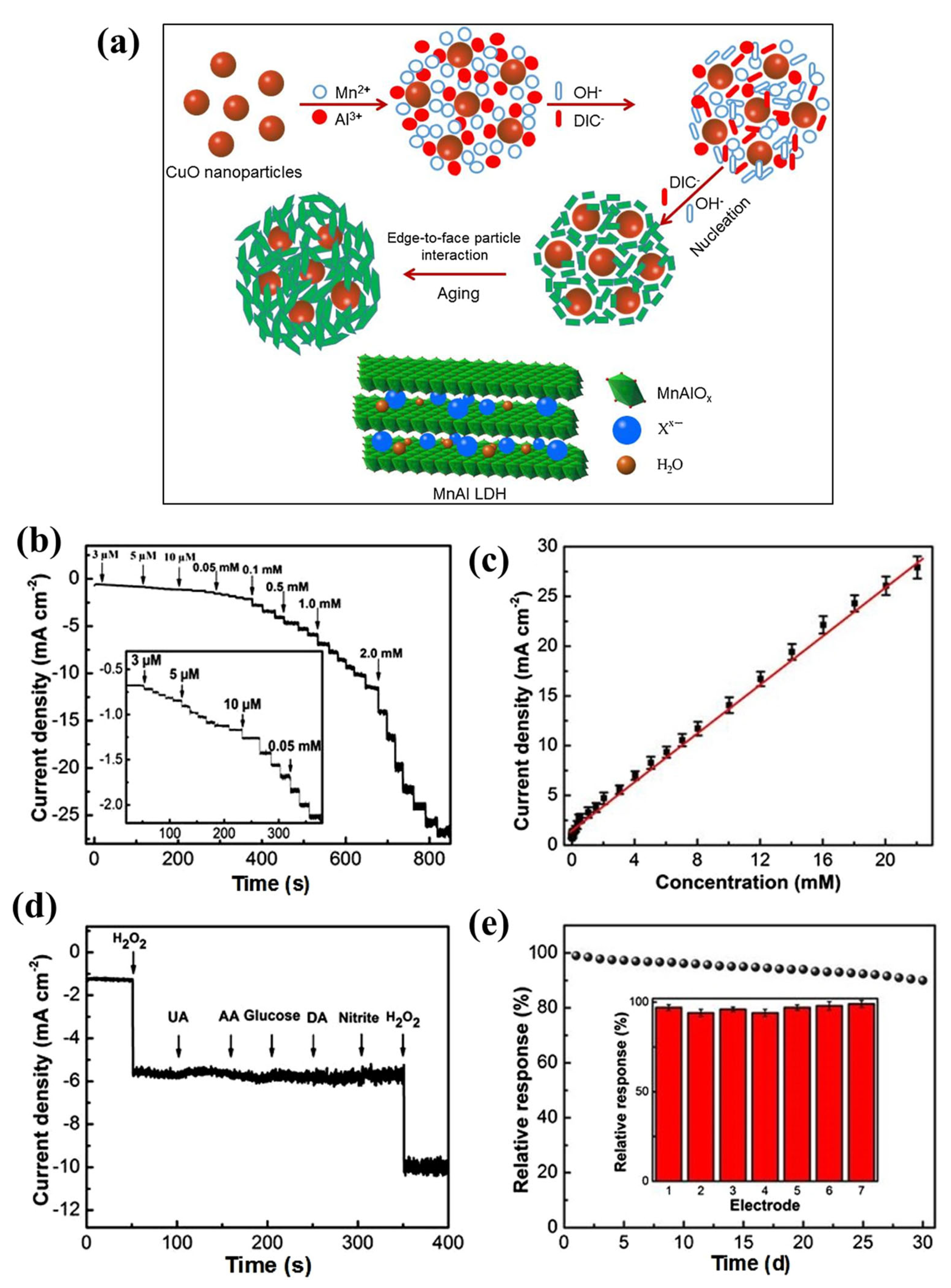
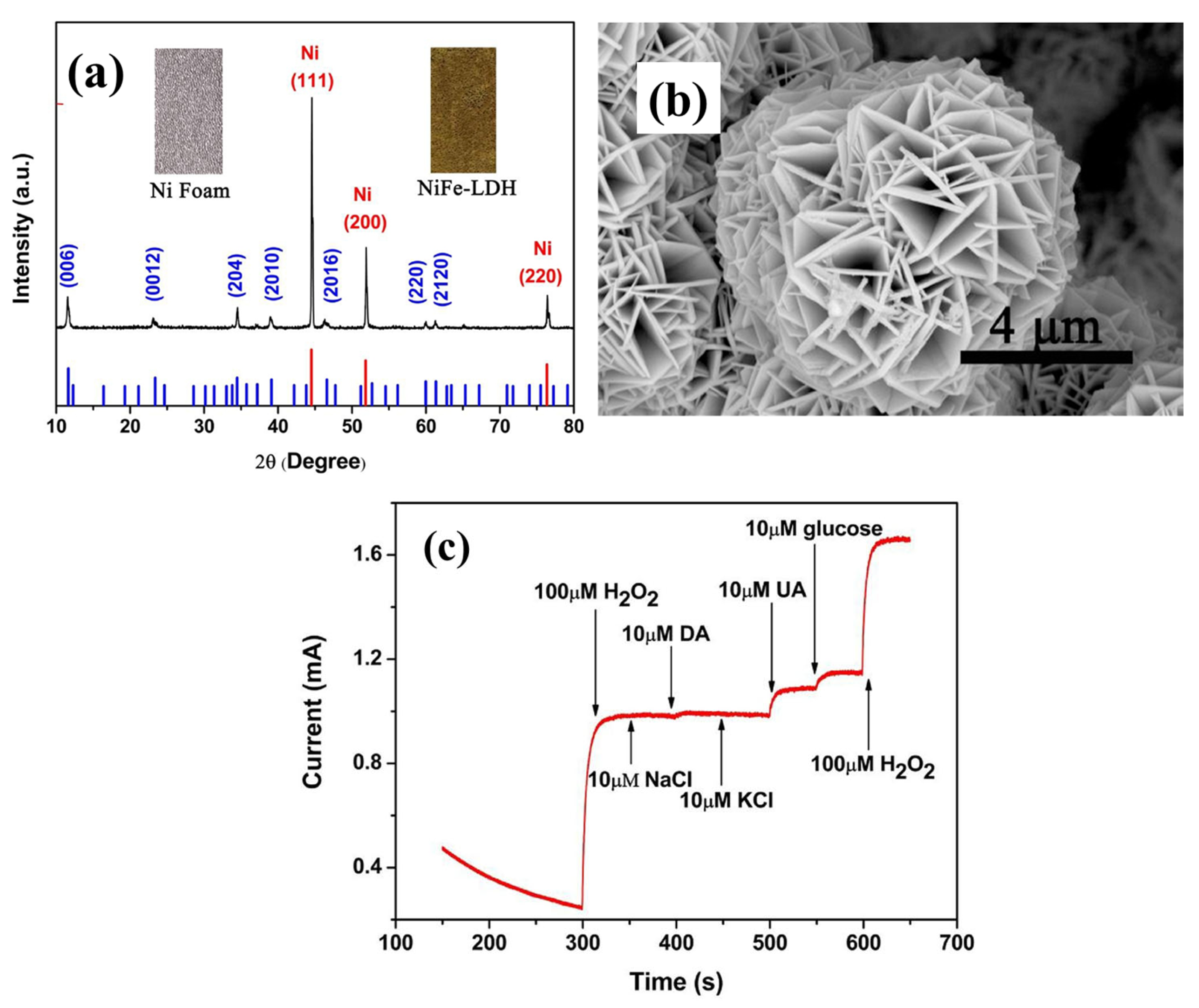
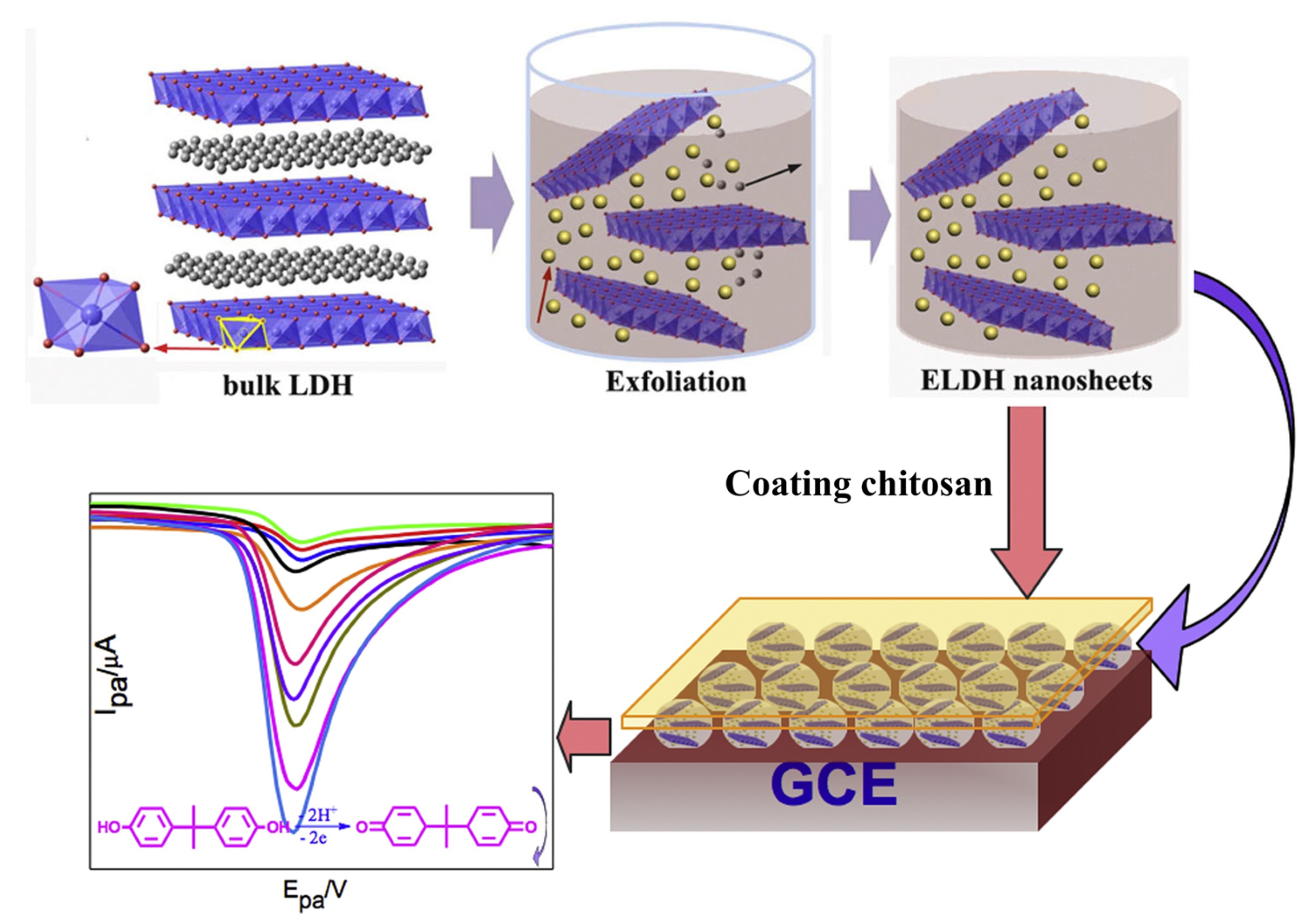
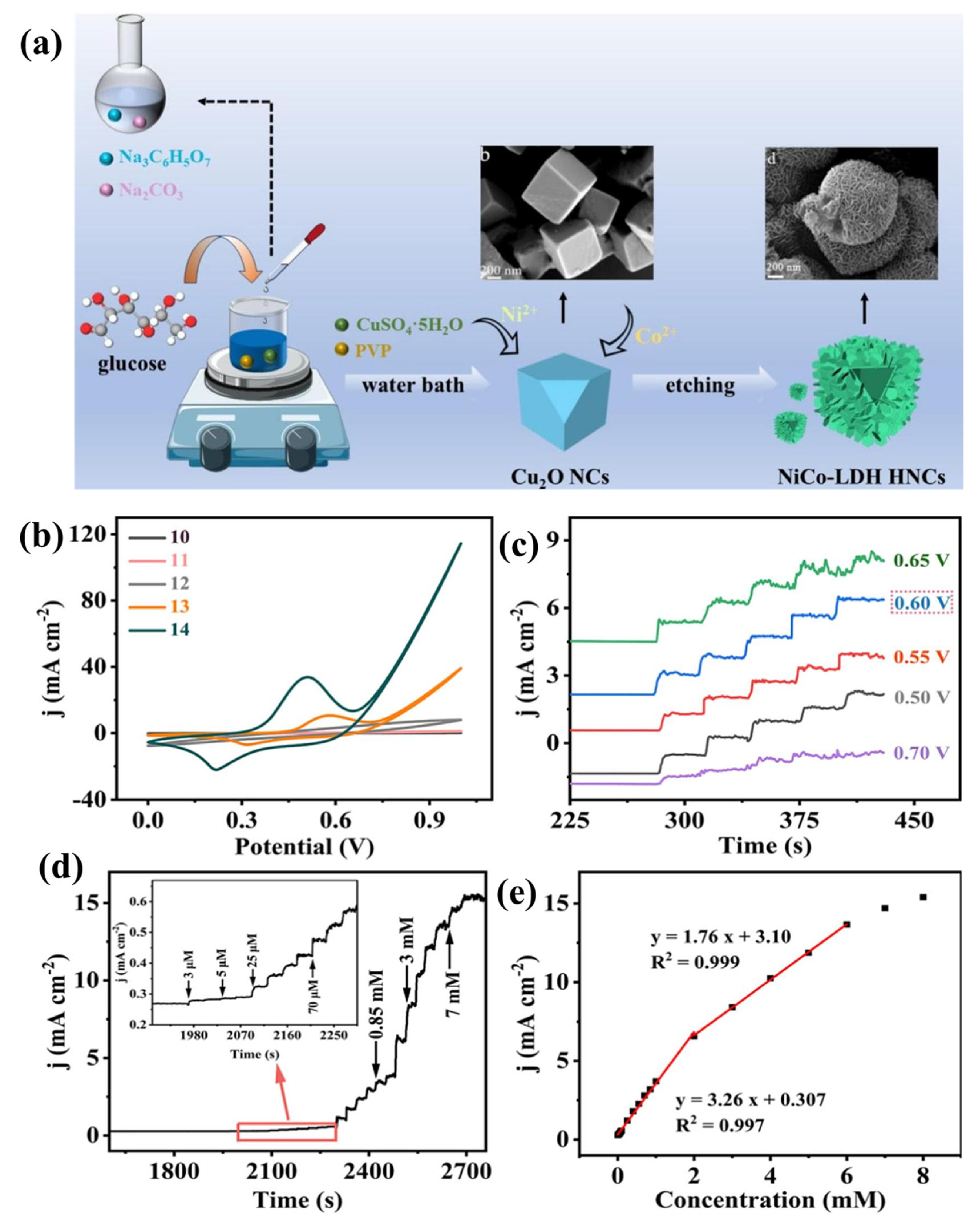
| Sensors | Analytes | Detection Range (ppm) | Response Time (min) | Recovery Time (min) | Response (S) | References |
|---|---|---|---|---|---|---|
| Ni-Cr-AL-LDH | Acetone, ethanol | 500–5000 | 6, 1 | 3, 1 | Rg−Ra/Ra × 100 | [70] |
| NiO/NiGa2O4-LDH | Toluene | 0.1–5 | 4.7 | 6.3 | Rg/Ra | [71] |
| ZnAl−Cl, ZnFe−Cl, ZnAl− NO3, and MgAl−NO3-LDH | Acetone, ethanol, ammonia, chlorine | Fixed concentrations of 10% of saturated vapors | 3.1, 3.1, 3.9, 2.3 | 2.3, 4.05, 3.7, 3.2 | Rg−Ra/Ra × 100 | [72] |
| CuCr-, ZnCr-, and ZnTi-LDH | Methanol, ethanol, acetone | 1–40 | --- | 0.61, 0.71, 1.48 | Rg−Ra/Ra | [73] |
| ZnTi-LDHs/rGO | NO2 | 0.05–20 | 0.033 | 0.86 | Rg−Ra/Ra × 100 | [74] |
| CoNi- LDH on GO (GO@LDH) | 2,4-Dimethyl benzaldehyde | 10–300 µg/L | ---- | ----- | ----- | [75] |
| zinc–chromium-LDH | SO2 | 0.1–100 | 0.33 | 5.12 | Rg−Ra/Ra × 100 | [76] |
| zinc–chromium-LDH | SO2 | 0.1–100 | 0.33 | 2.8 | Rg−Ra/Ra × 100 | [77] |
| NiCo-LDH | Methylene Blue, Methylene orange | 0.005–10 | 4.5, 2.2 | --- | Rg−Ra/Ra | [78] |
| Zn–Al-LDH | SO2 | - | - | - | Rgas/Rair | [79] |
| Cu/Fe -LDH | Imidacloprid | - | - | - | Rg−Ra/Ra | [80] |
| (CeO2)x/Ni-Al LDH | Methanol, ethanol, and acetone | 25–300 | 1.01, 0.666, 0.42 | 3.45, 0.91, 2.13 | Rair/Rgas | [81] |
| CeO2/Ni–Al-LDH | Ethanol | 25–300 | 0.666 | 1.27 | Rair/Rgas | [82] |
| Ni-Al LDH | Methanol, ethanol, and acetone | 20- 300 | 0.2, 0.83,0.33 | 2.25, 1.95,1.9 | Rair/Rgas | [83] |
| (CeO2)x/Ni-Al–LDH | Methanol, ethanol, and acetone | 25–300 | 1.01, 0.666, 0.41 | 3.45, 0.92, 2.13 | Rair/Rgas | [84] |
| ZnCo-LDHs | Ethanol | 50–250 | --- | ---- | Rg/Ra | [85] |
| Electrode Material | Analyte | Detection Limit (µM) | Linear Range (µM) | Sensitivity (µA/µM·cm2) | Sensing Method | References |
|---|---|---|---|---|---|---|
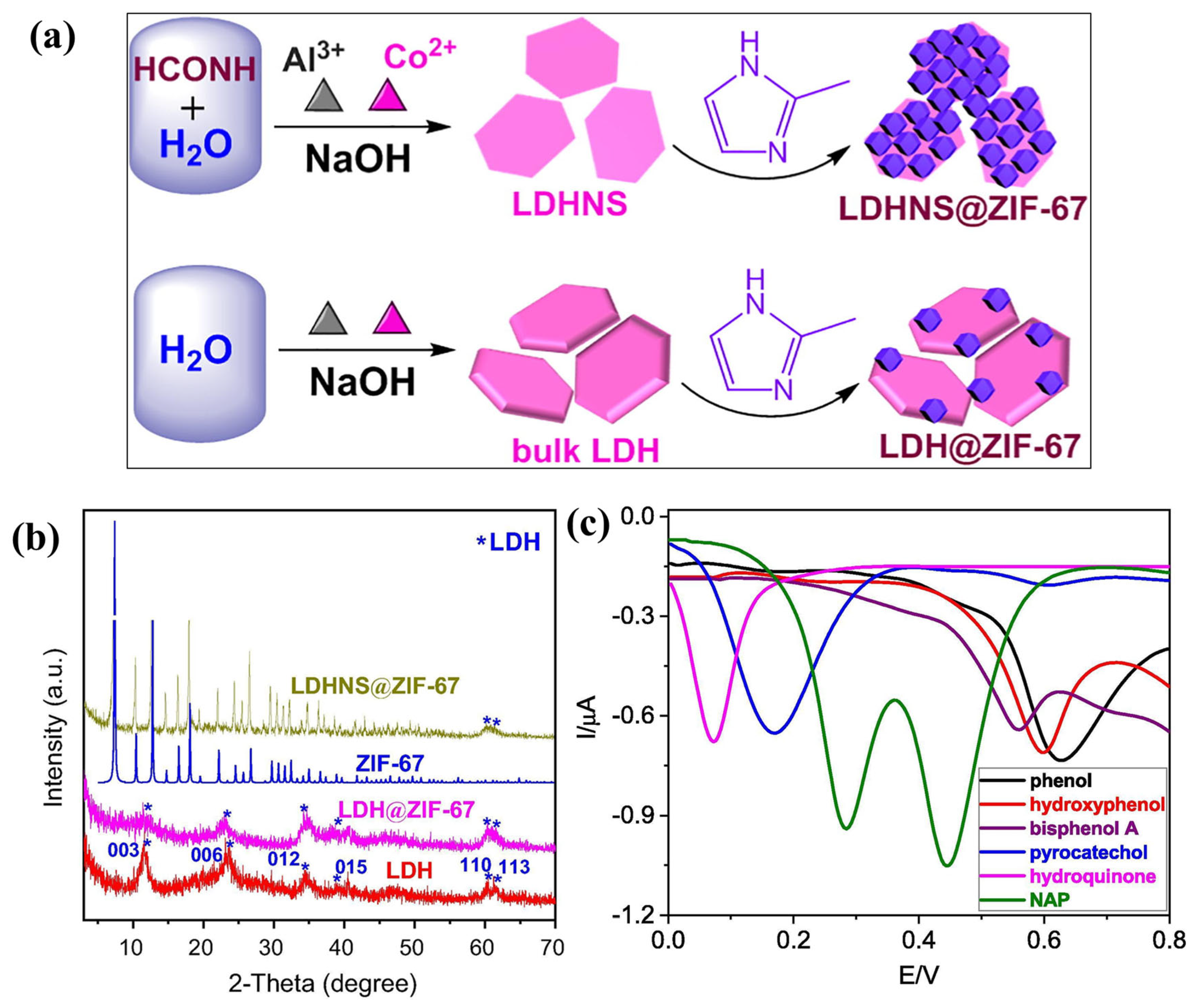
| ILs-LDH modified GCE | BPA | 0.0046 | 0.01–3.0 | - | DPV | [86] |
| CoAl-LDH/MWCNTs | H2O2 | 5 μmol dm−3 | 100–4000 μmol dm−3 | 118 mA dm3 mol−1 cm−2 | CA | [87] |
| Mg–Al–TGA LDH/GCE | Hg | 0.8 nM | 2–800 nM | - | SWASV | [88] |
| CuO@MnAl/GCE | H2O2 | 0.126 | 6 μM to 22 mM | - | Amperometry | [89] |
| Fe/Mg/Ni LDH/GCE | Pb | 0.032 | 0.03–1 | 68.1 μA μM−1 | SWASV | [90] |
| Cd/Al/GCE | Anthracene | 0.5 fM | 0.1–100.0 pM | - | DPV | [91] |
| NiFe LDH/nickel foam | H2O2 | 0.5 | 5 × 10−4–0.84 mM | - | Amperometry | [92] |
| MgFe LDH/GCE | Cd (II) | 5.9 nM | 0.1–1.0 | - | SWASV | [93] |
| MgFe LDH/GCE | Pb (II) | 2.7 nM | 0.1–1.0 | - | SWASV | [93] |
| Exfoliated Ni2P/Al LDH/GCE | BPA | 6.8 nM | 0.02 to 1.51 μM | - | DPV | [94] |
| MgAl LDH/CP | Nitrite | 0.03 | 14.8–222 μM | - | Amperometry | [95] |
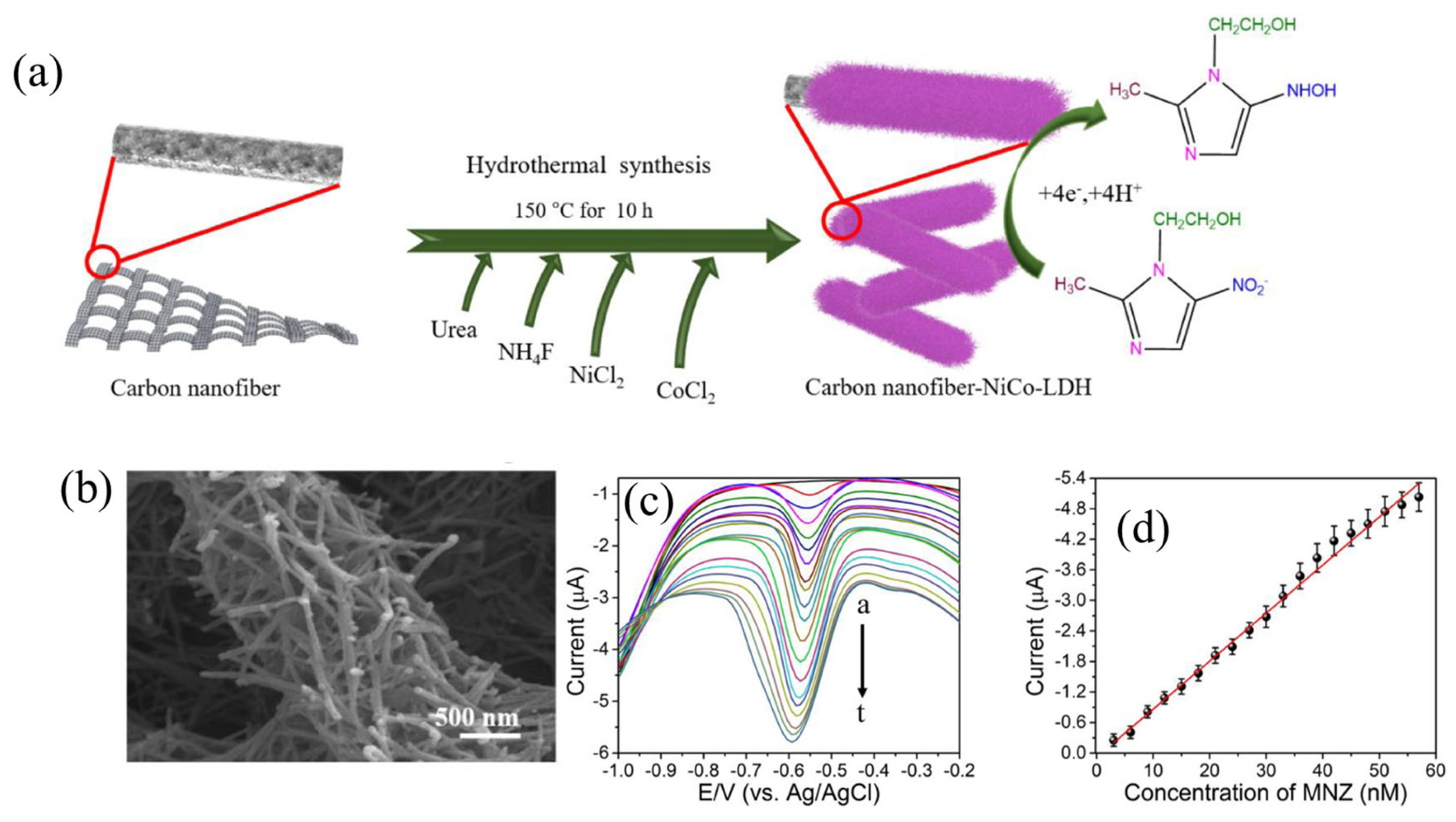
| CNF–NiCo-LDH-GCE | MNZ | 0.13 nM | 3 to 57 nM | 1.294 μA nM−1cm−2. | DPV | [96] |
| WC@NiCo-LDH | NRF | 0.005 | 0.02–83.4 | 6.53 μA μM−1cm2 | DPV | [97] |
| WC@NiCo-LDH | NRF | 0.002 | 0.002–346 | 40.81 μA μM−1cm2 | Amperometry | [98] |
| WC@FeMn-LDH | DPA | 0.0011 | 0.01–183.34 | - | DPV | [99] |
| WC@NiCo–LDH | NRF | 0.005 | 0.02–83.4 | - | DPV | [100] |
| AZnMo-LDHs@VGCF | DMZ | 0.021 | 0.25–570 | 1 | DPV | [101] |
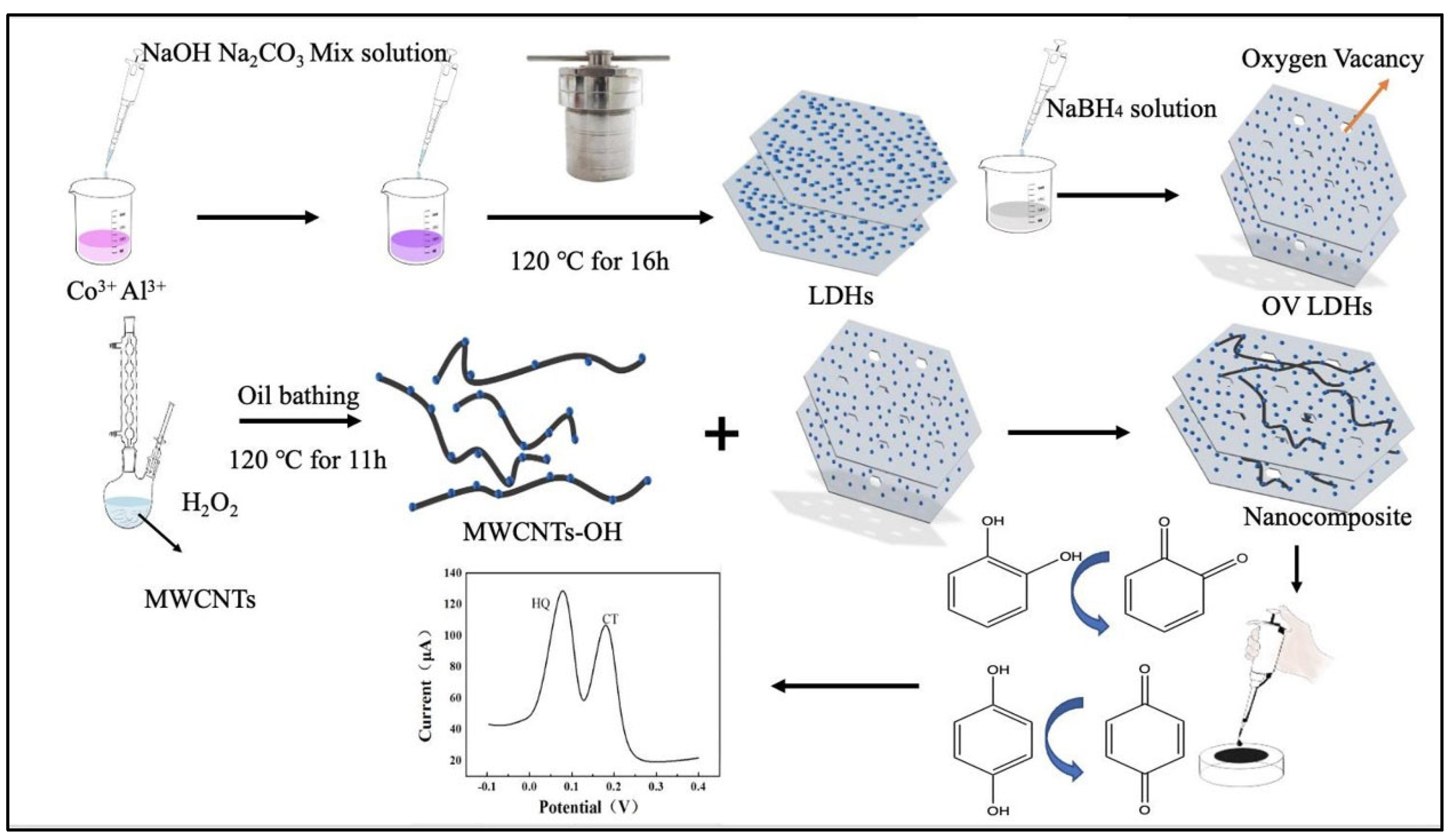
| OV-LDHs/H-MWCNTs/GCE | CT | 0.074 | 0.5–150 | - | DPV | [102] |
| OV-LDHs/H-MWCNTs/GCE | HQ | 0.076 | 0.5–150 | - | DPV | [[102] |
| NiCo-LDH/F-HNTs | PT | 0.003 | 0.012–24.5 | - | DPV | [[103] |
| CoAl-LDH/α-Fe2O3 | H2O2 | 0.04 | 0.001–2 | - | PEC | [104] |
| AuNPs/Co-LDH | H2O2 | 0.19 | 4 μM–16 mM | 406.61 μA mM−1cm−2 | Amperometry | [105] |
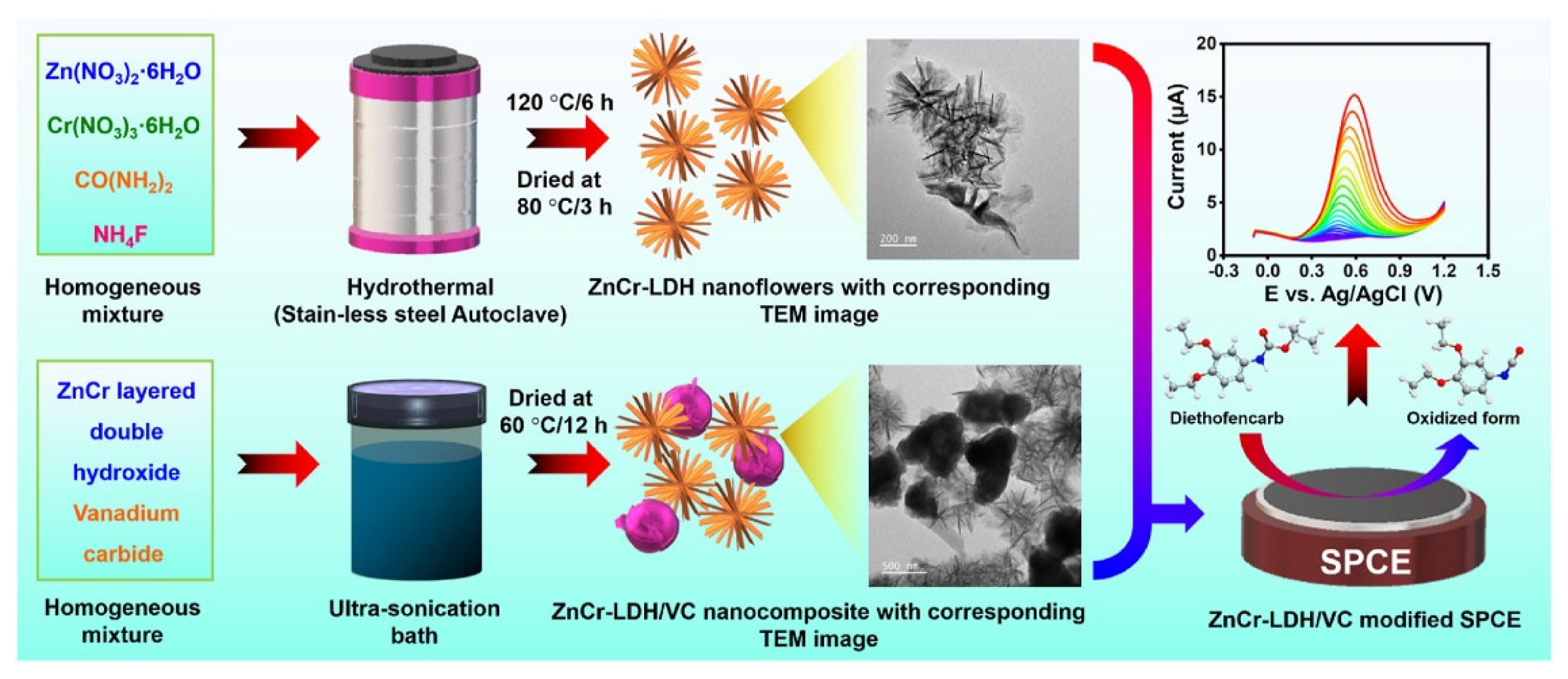
| ZnCr-LDH/VC | DFC | 0.002 | 0.01–228 | - | DPV | [106] |
| Ni–Co LDH NSs/SPGE | Carmoisine | 0.09 | 0.3–125 | - | DPV | [107] |
| NiCo-LDH | CDM | 0.001 | 0.006–14.1 | 3.38 | DPV | [108] |
| o-g-C3N4/CuAl-LDH/GCE | DS | 0.64 | 0.5–60 | - | DPV | [109] |
| AZnMo-LDHs/CB | HQ | 0.0054 | 0.05–971 | - | DPV | [110] |
| AZnMo-LDHs/CB | CC | 0.0018 | 0.1–1036 | - | DPV | [110] |
| AZnMo-LDHs/CB | RC | 0.075 | 0.5–1408.5 | - | DPV | [110] |
| MnFeZn-LDH | FLA | 0.012 | 0.0199–2735.7 | 5.04 | DPV | [111] |
| CoZn-LDH@CuO NSA/CF | H2O2 | 0.17 | 0.8 μM–3.5 mM | 4585 μA mM−1cm | Amperometry | [112] |
| Ni-Co-LDH/MWCNTs/CPE | 4-AP | 0.01 | 0.02–700 | 0.076 µA/µM | DPV | [113] |
| NiCo-LDH | Hydrazine | 0.135 | 3–2 × 103 | 3260 μA mM−1cm−2 | Amperometry | [114] |
| Ni-Al-LDH | PCP | 0.004 | 0.05–50 | - | DPV | [115] |
| CoAl-LDH/GCE | MLZ | 0.029 | 0.049–665.1 | - | DPV | [117] |
| NiFeCo-LDH/GA | PG | 0.00087 | 0.001–297.43 | - | DPV | [118] |
| NiFeCu-LDH/GA | PG | 0.004 | 0.02–279.1 | - | DPV | [119] |
| NiCo-LDH HC | H2O2 | 0.22 | - | 7050 μA mM−1cm−2 | Amperometry | [120] |
| NiMoO4 NRs@NiCo-LDH NSs/CC | H2O2 | 0.112 | 1–9000 | - | Amperometry | [121] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raza, W.; Ahmad, K.; Oh, T.H. Progress in Layered Double Hydroxide-Based Materials for Gas and Electrochemical Sensing Applications. Chemosensors 2025, 13, 115. https://doi.org/10.3390/chemosensors13030115
Raza W, Ahmad K, Oh TH. Progress in Layered Double Hydroxide-Based Materials for Gas and Electrochemical Sensing Applications. Chemosensors. 2025; 13(3):115. https://doi.org/10.3390/chemosensors13030115
Chicago/Turabian StyleRaza, Waseem, Khursheed Ahmad, and Tae Hwan Oh. 2025. "Progress in Layered Double Hydroxide-Based Materials for Gas and Electrochemical Sensing Applications" Chemosensors 13, no. 3: 115. https://doi.org/10.3390/chemosensors13030115
APA StyleRaza, W., Ahmad, K., & Oh, T. H. (2025). Progress in Layered Double Hydroxide-Based Materials for Gas and Electrochemical Sensing Applications. Chemosensors, 13(3), 115. https://doi.org/10.3390/chemosensors13030115