Gas Sensitivity Improvements of Nanowire Hexadecafluorinated Iron Phthalocyanines by Thermal Vacuum Annealing
Abstract
:1. Introduction
2. Materials and Methods
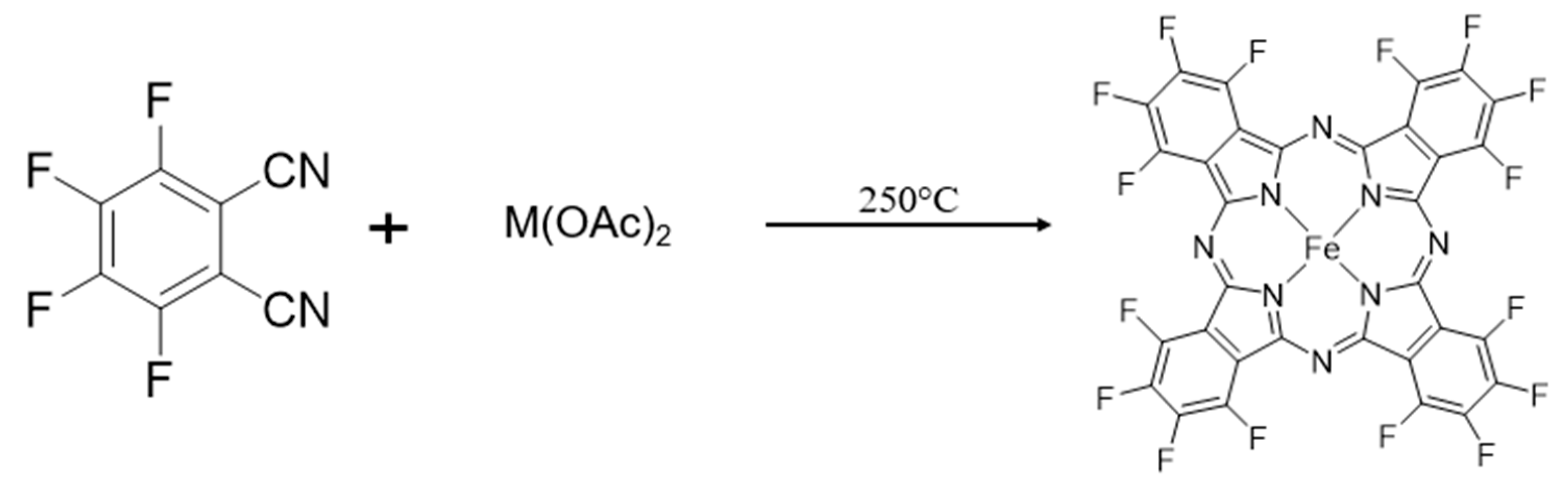
2.1. Synthesis of FePcF16 Powder
2.2. Synthesis of MPcF16 Nanowires
3. Results and Discussion
3.1. SEM Images of FePcF16 Nanowires
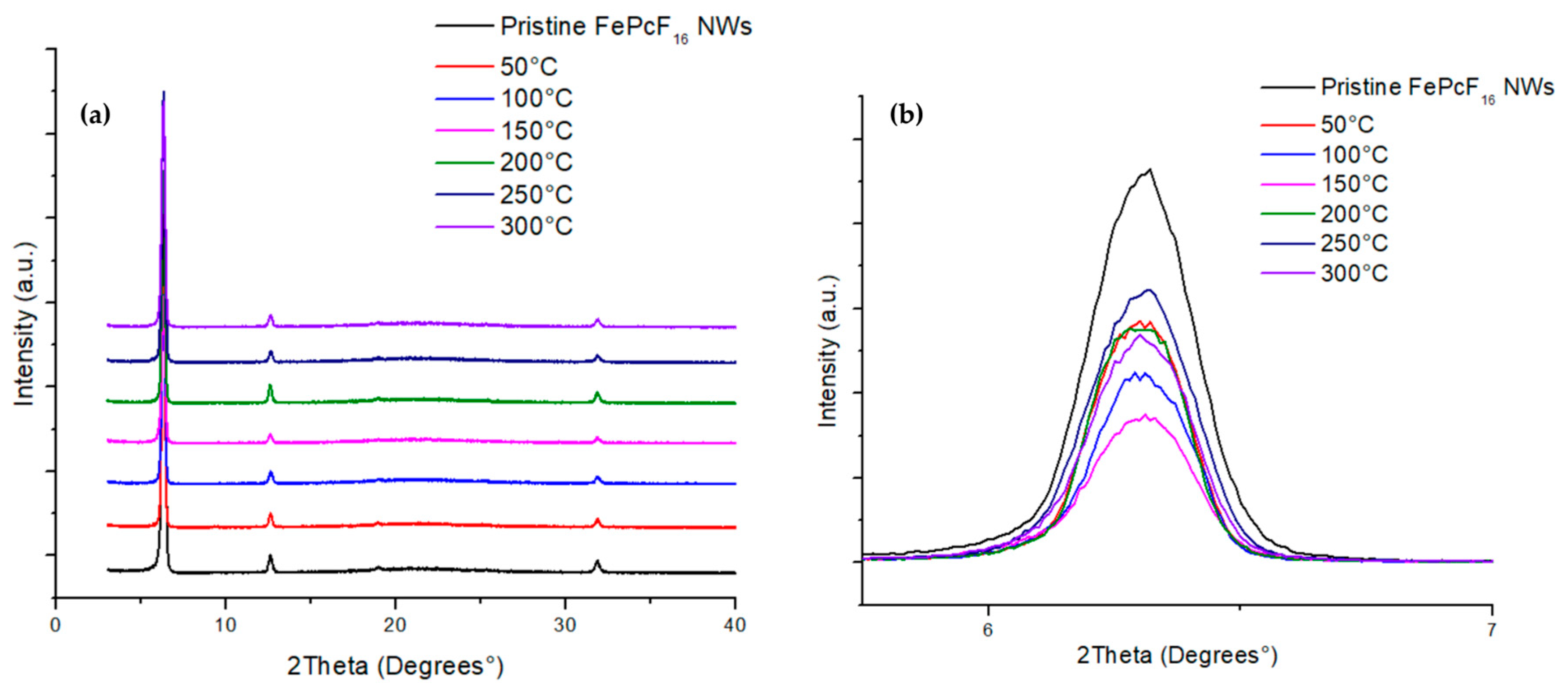
3.2. Analysis of XRD Patterns
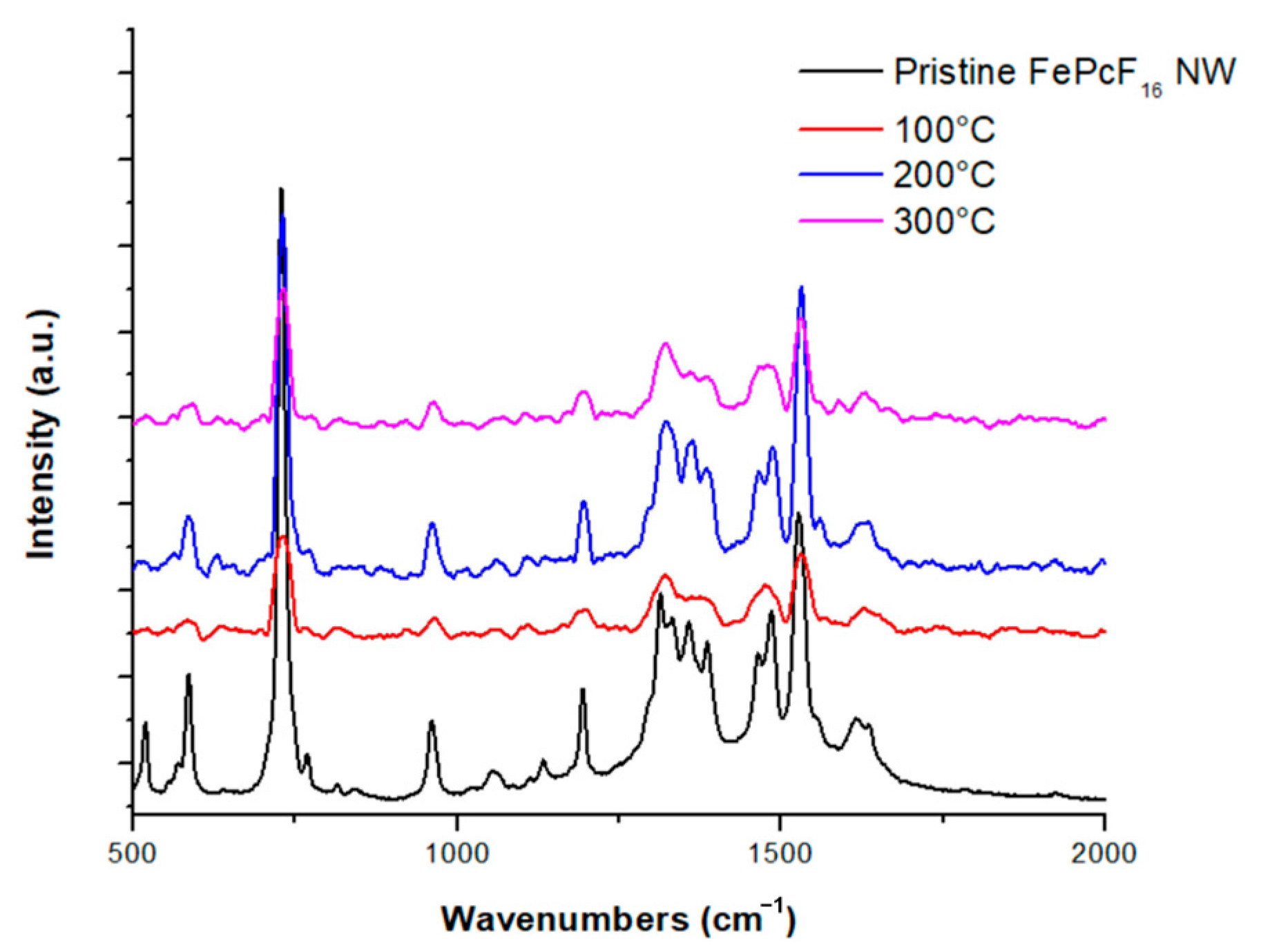
3.3. Raman of FePcF16 NW
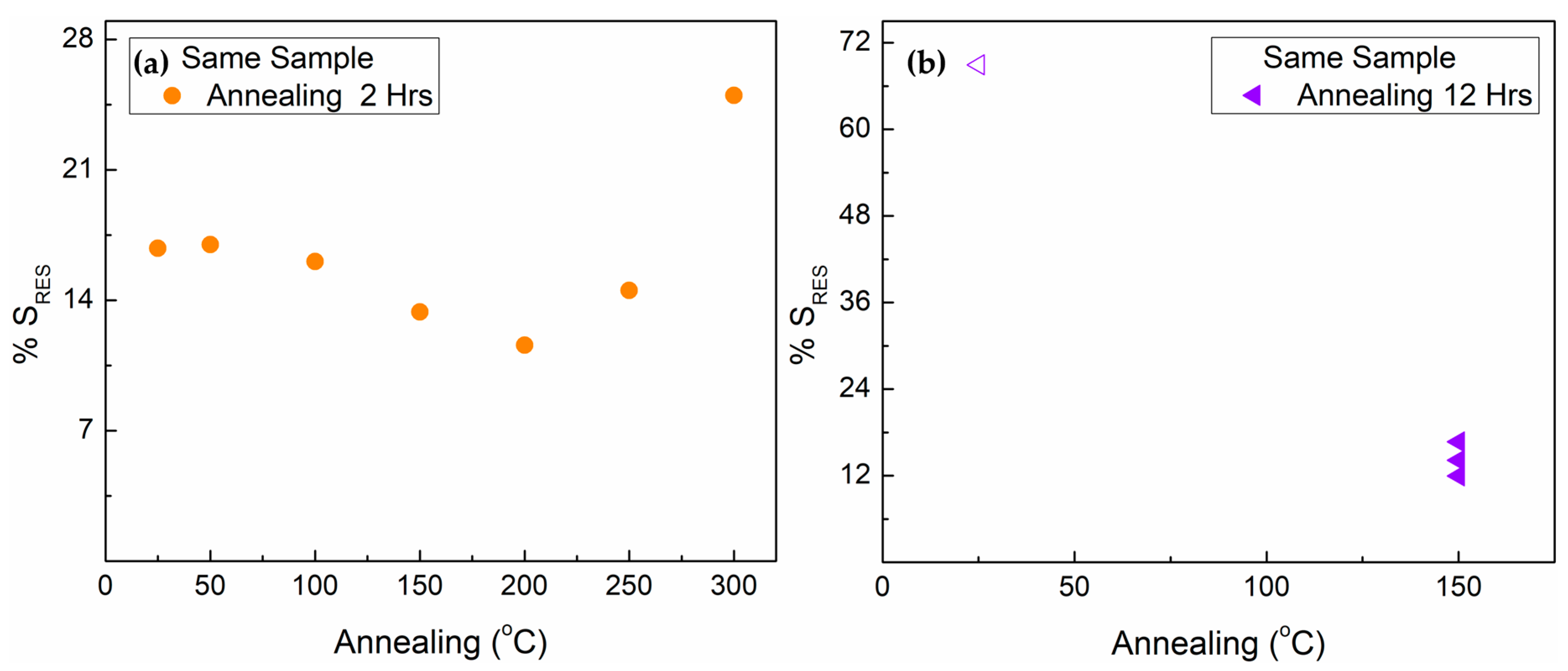
3.4. Effect of Annealing on the FePcF16 NWs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- How Air Pollution is Destroying Our Health. Available online: https://www.who.int/news-room/spotlight/how-air-pollution-is-destroying-our-health (accessed on 20 May 2022).
- Pogány, A.; Balslev-Harder, D.; Braban, C.F.; Cassidy, N.; Ebert, V.; Ferracci, V.; Hieta, T.; Leuenberger, D.; Martin, N.A.; Pascale, C.; et al. A Metrological Approach to Improve Accuracy and Reliability of Ammonia Measurements in Ambient Air. Meas. Sci. Technol. 2016, 27, 110512. [Google Scholar] [CrossRef]
- Permissible Exposure Limits—Annotated Tables. Available online: https://www.osha.gov/annotated-pels/table-z-1 (accessed on 23 September 2023).
- Padappayil, R.P.; Borger, J. Ammonia Toxicity. Available online: https://www.ncbi.nlm.nih.gov/books/NBK546677/ (accessed on 23 January 2024).
- Jung, H.T. The Present and Future of Gas Sensors. ACS Sens. 2022, 7, 912–913. [Google Scholar] [CrossRef] [PubMed]
- Dey, A. Semiconductor Metal Oxide Gas Sensors: A Review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Kwak, D.; Lei, Y.; Maric, R. Ammonia Gas Sensors: A Comprehensive Review. Talanta 2019, 204, 713–730. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.D. Gas Adsorption on Phthalocyanines and Its Effects on Electrical Properties. Prog. Surf. Sci. 1989, 31, 1–60. [Google Scholar] [CrossRef]
- Meng, Z.; Aykanat, A.; Mirica, K.A. Welding Metallophthalocyanines into Bimetallic Molecular Meshes for Ultrasensitive, Low-Power Chemiresistive Detection of Gases. J. Am. Chem. Soc. 2019, 141, 2046–2053. [Google Scholar] [CrossRef]
- Myers, J.F.; Rayner Canham, G.W.; Lever, B.P. Higher Oxidation Level Phthalocyanine Complexes of Chromium, Iron, Cobalt, and Zinc. Phthalocyanine Radical Species. Inorg. Chem. 1975, 14, 461–468. [Google Scholar] [CrossRef]
- Achar, B.N.; Jayasree, P.K. Novel “synthetic Metals” Based on Symmetrically Tetrasubstituted Nickel Phthalocyanines. Synth. Met. 1999, 104, 101–106. [Google Scholar] [CrossRef]
- Debnath, A.K.; Samanta, S.; Singh, A.; Aswal, D.K.; Gupta, S.K.; Yakhmi, J.V. Parts-per-Billion Level Chlorine Sensors with Fast Kinetics Using Ultrathin Cobalt Phthalocyanine Films. Chem. Phys. Lett. 2009, 480, 185–188. [Google Scholar] [CrossRef]
- Parkhomenko, R.G.; Sukhikh, A.S.; Klyamer, D.D.; Krasnov, P.O.; Gromilov, S.; Kadem, B.; Hassan, A.K.; Basova, T.V. Thin Films of Unsubstituted and Fluorinated Palladium Phthalocyanines: Structure and Sensor Response toward Ammonia and Hydrogen. J. Phys. Chem. C 2017, 121, 1200–1209. [Google Scholar] [CrossRef]
- Flores, S.Y.; Gonzalez-Espiet, J.; Cintrón, J.; Villanueva, N.D.J.; Camino, F.E.; Kisslinger, K.; Cruz, D.M.P.; Rivera, R.D.; Fonseca, L.F. Fluorinated Iron and Cobalt Phthalocyanine Nanowire Chemiresistors for Environmental Gas Monitoring at Parts-per-Billion Levels. ACS Appl. Nano Mater. 2022, 5, 4688–4699. [Google Scholar] [CrossRef]
- Kong, X.; Dong, Z.; Wu, Y.; Li, X.; Chen, Y.; Jiang, J. High Sensitive Ambipolar Response towards Oxidizing NO2 and Reducing NH3 Based on Bis(Phthalocyaninato) Europium Semiconductors. Chin. J. Chem. 2016, 34, 975–982. [Google Scholar] [CrossRef]
- Jiang, H.; Ye, J.; Hu, P.; Wei, F.; Du, K.; Wang, N.; Ba, T.; Feng, S.; Kloc, C. Fluorination of Metal Phthalocyanines: Single-Crystal Growth, Efficient N-Channel Organic Field-Effect Transistors, and Structure-Property Relationships. Sci. Rep. 2014, 4, 7573. [Google Scholar] [CrossRef]
- Alonzo-Medina, G.M.; González-González, A.; Sacedón, J.L.; Oliva, A.I. Understanding the Thermal Annealing Process on Metallic Thin Films. IOP Conf. Ser. Mater. Sci. Eng. 2013, 45, 012013. [Google Scholar] [CrossRef]
- Shen, J.; Chen, B.; Wan, J.; Shen, J.; Li, J. Materials Science & Engineering A Effect of Annealing on Microstructure and Mechanical Properties of an Al-Mg-Sc-Zr Alloy. Mater. Sci. Eng. A 2022, 838, 142821. [Google Scholar] [CrossRef]
- Yang, Y.; Maeng, B.; Jung, D.G.; Lee, J.; Kim, Y.; Kwon, J.B.; An, H.K.; Jung, D. Annealing Effects on SnO2 Thin Film for H2 Gas Sensing. Nanomaterials 2022, 12, 3227. [Google Scholar] [CrossRef]
- Zhang, H.; He, F.; Che, Y.; Song, Y.; Zhou, M.; Ding, D. Effect of Annealing Treatment on Response Characteristics of Pd-Ni Alloy Based Hydrogen Sensor. Surf. Interfaces 2023, 36, 102597. [Google Scholar] [CrossRef]
- Ramola, R.C.; Negi, S.; Rawat, M.; Singh, R.C.; Singh, F. Annealing Effects on Gas Sensing Response of Ga-Doped ZnO Thin Films. ACS Omega 2021, 6, 11660–11668. [Google Scholar] [CrossRef]
- Liu, C.J.; Hsieh, J.C.; Ju, Y.H. Response Characteristics of Lead Phthalocyanine Gas Sensor: Effect of Operating Temperature and Postdeposition Annealing. J. Vac. Sci. Technol. A Vac. Surf. Film. 1996, 14, 753–756. [Google Scholar] [CrossRef]
- Elesh, E.; Mohammed, Z. Morphological, Linear and Nonlinear Properties of Gallium Phthalocyanine Chloride Annealed Thin Films. Optik 2020, 219, 165176. [Google Scholar] [CrossRef]
- Abdelhamid, S.M.; Dongol, M.; Elhady, A.F.; Abuelwafa, A.A. Role of Vacuum Annealing on the Structural, Optical Properties and DC Conductivity of Titanium (IV) Phthalocyanine Dichloride Thin Films. Phys. Scr. 2023, 98, 125960. [Google Scholar] [CrossRef]
- Kheiri, F.; Soleimanian, V.; Ghasemi, M.; Mokhtari, A. The Microstructure, Optical and Gas Sensing Properties of Bilayer TiO2/ZnO Systems in Terms of Annealing Temperature. Mater. Sci. Semicond. Process. 2021, 121, 105462. [Google Scholar] [CrossRef]
- Chen, L.Y.; Hunter, G.W.; Neudeck, P.G.; Knight, D. Surface and Interface Properties of PdCr/SiC Schottky Diode Gas Sensor Annealed at 425°C. Solid. State. Electron. 1998, 42, 2209–2214. [Google Scholar] [CrossRef]
- Stegmann, C.; Muench, F.; Rauber, M.; Hottes, M.; Brötz, J.; Kunz, U.; Lauterbach, S.; Kleebe, H.J.; Ensinger, W. Platinum Nanowires with Pronounced Texture, Controlled Crystallite Cize and Excellent Growth Homogeneity Fabricated by Optimized Pulsed Electrodeposition. RSC Adv. 2014, 4, 4804–4810. [Google Scholar] [CrossRef]
- Kumar, N.; Kumar, R.; Kumar, S.; Chakarvarti, S.K. Structural and Electrical Studies of Template Synthesized Copper Nanowires. Curr. Appl. Phys. 2014, 14, 1547–1552. [Google Scholar] [CrossRef]
- Štengl, V.; Bakardjieva, S.; Murafa, N.; Večerníková, E.; Šubrt, J.; Balek, V. Preparation and Characterization of Titania Based Nanowires. J. Nanoparticle Res. 2007, 9, 455–470. [Google Scholar] [CrossRef]
- Kurtulus, O.; Li, Z.; Mews, A.; Pietsch, U. X-Ray Investigation of CdSe Nanowires. Phys. Status Solidi Appl. Mater. Sci. 2009, 206, 1752–1756. [Google Scholar] [CrossRef]
- Datir, A.; Koinkar, P.; Chakane, S. Effects of Heat Annealing on the Gas Sensing Properties of Spin Coated Unsubstituted Copper Phthalocyanine Films. Adv. Mater. Res. 2015, 1110, 241–245. [Google Scholar] [CrossRef]
- Klyamer, D.; Sukhikh, A.; Bonegardt, D.; Krasnov, P.O.; Popovetskiy, P.; Basova, T. Thin Films of Chlorinated Vanadyl Phthalocyanines as Active Layers of Chemiresistive Sensors for the Detection of Ammonia. Micromachines 2023, 14, 1773. [Google Scholar] [CrossRef]
- Jafari, M.J.; Azim-Araghi, M.E.; Barhemat, S.; Riyazi, S. Effect of Post-Deposition Annealing on Surface Morphology and Gas Sensing Properties of Palladium Phthalocyanine Thin Films. Surf. Interface Anal. 2012, 44, 601–608. [Google Scholar] [CrossRef]
- Popielarski, P.; Mosińska, L.; Skowronski, L.; Szczesny, R.; Figà, V.; Naparty, M.; Derkowska-Zielinska, B. Influence of Heat Treatment on Surface, Structural and Optical Properties of Nickel and Copper Phthalocyanines Thin Films. Int. J. Mol. Sci. 2022, 23, 11055. [Google Scholar] [CrossRef] [PubMed]
- King, B.; Moorthy, S.G.; Lesniewska, E.; Meunier-Prest, R.; Bouvet, M.; Lessard, B.H. Modulating the Majority Charge Carrier Type and Performance of Organic Heterojunction Ammonia Sensors by Increasing Peripheral Fluorination of the Silicon Phthalocyanine Sublayer. Sens. Actuators B Chem. 2024, 408, 135507. [Google Scholar] [CrossRef]

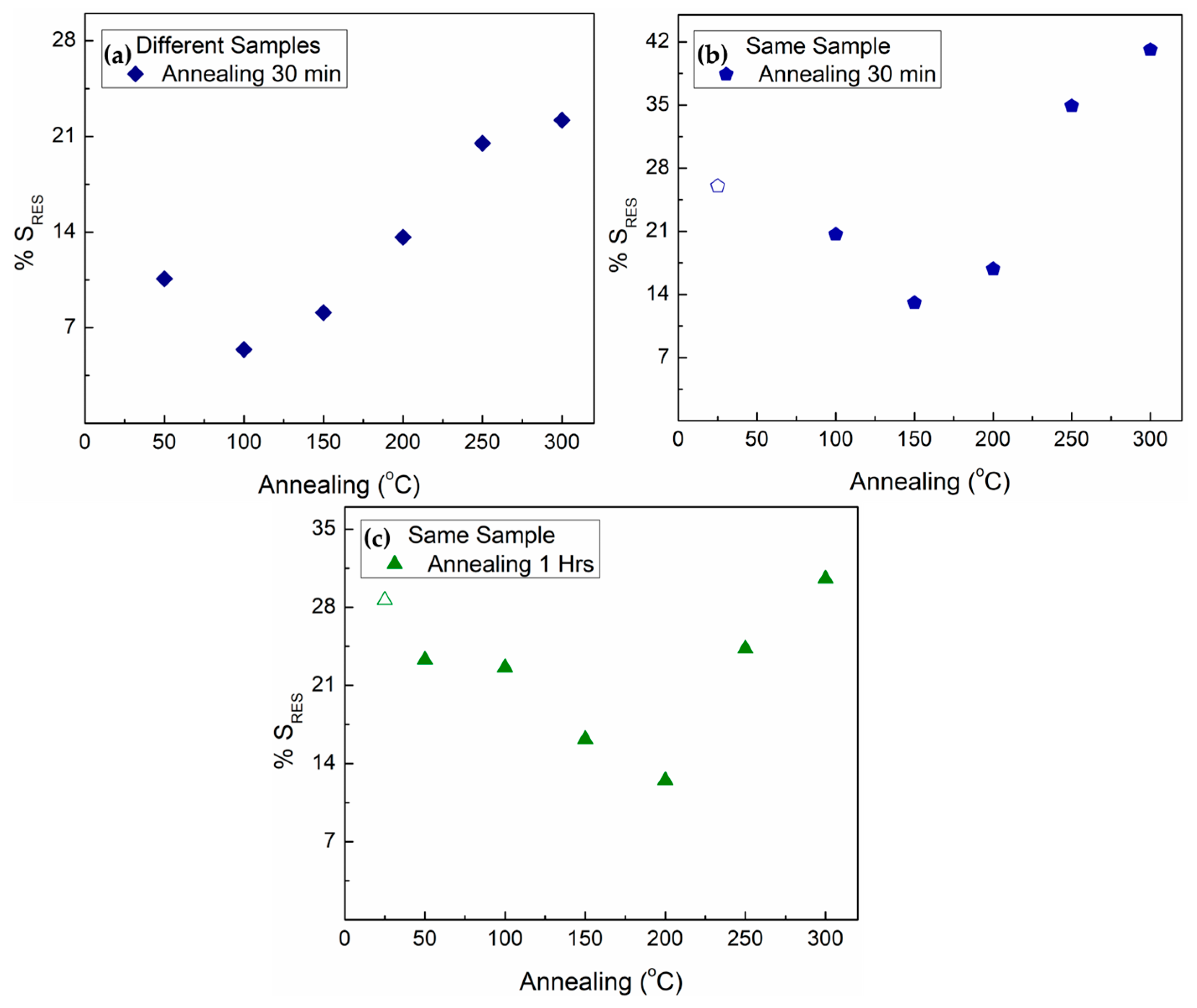

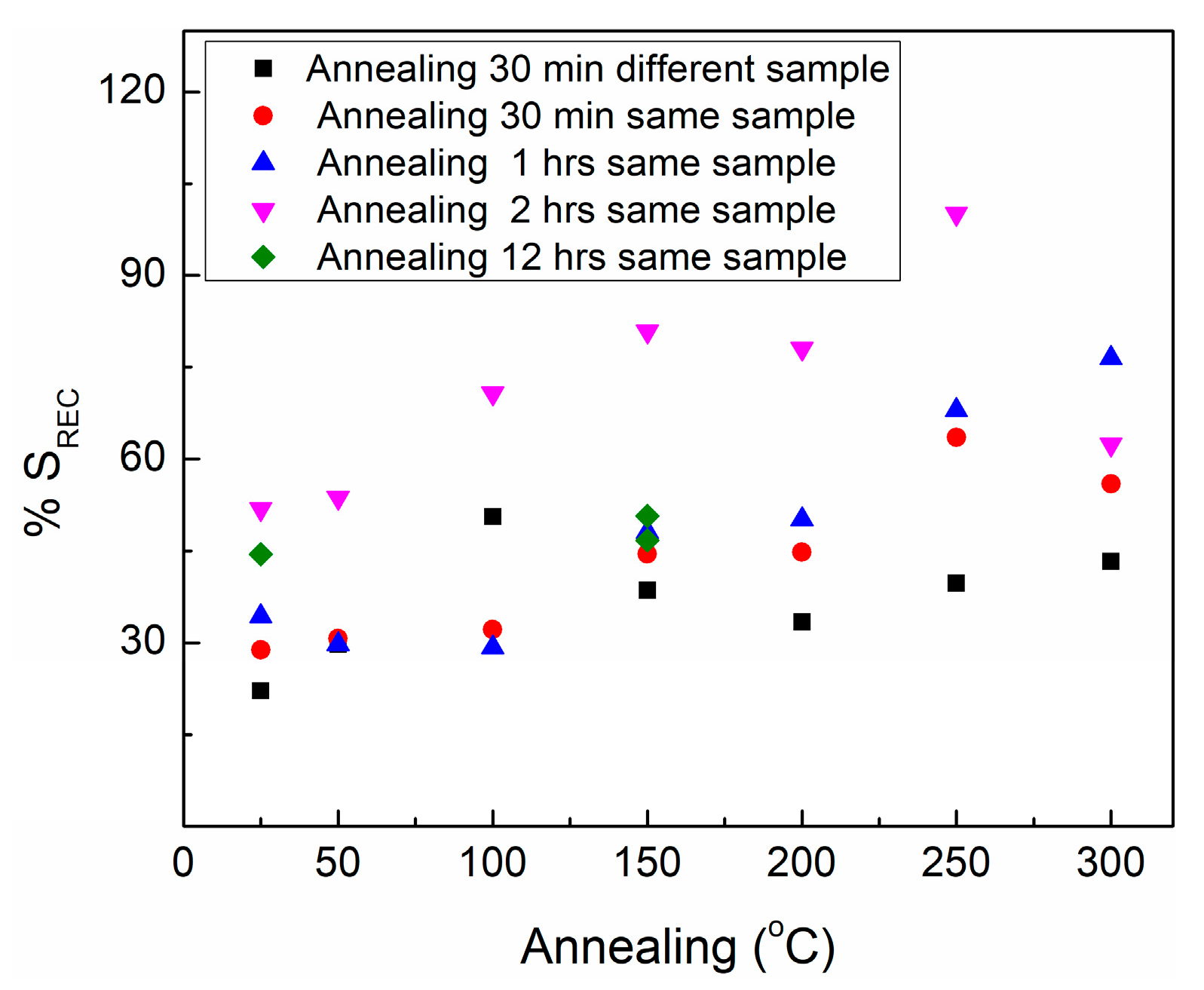
| Annealing Temperature | 30 Min Annealing | 1 h Annealing | 2 h Annealing | 12 h Annealing |
|---|---|---|---|---|
| 50 °C | −15.5% | −18.2% | 3.4% | - |
| 100 °C | −21.11% | −20.6% | −3.6% | - |
| 150 °C | −50.2% | −43.7% | −19.4% | −76.9% |
| 200 °C | −36.18% | −56.5% | −30.5% | - |
| 250 °C | 32.1% | −15.9% | −12.8% | - |
| 300 °C | 57.7% | 6.5% | 51.3% | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metzler, C.L.; Flores, S.Y.; Cruz Lozada, J.; González, J.; Suárez Schmidt, S.; Barrionuevo, D.; Feng, P.; Otaño, W.; Fonseca, L.; Piñero Cruz, D.M. Gas Sensitivity Improvements of Nanowire Hexadecafluorinated Iron Phthalocyanines by Thermal Vacuum Annealing. Chemosensors 2025, 13, 95. https://doi.org/10.3390/chemosensors13030095
Metzler CL, Flores SY, Cruz Lozada J, González J, Suárez Schmidt S, Barrionuevo D, Feng P, Otaño W, Fonseca L, Piñero Cruz DM. Gas Sensitivity Improvements of Nanowire Hexadecafluorinated Iron Phthalocyanines by Thermal Vacuum Annealing. Chemosensors. 2025; 13(3):95. https://doi.org/10.3390/chemosensors13030095
Chicago/Turabian StyleMetzler, Carmen L., Soraya Y. Flores, John Cruz Lozada, Jean González, Sebastián Suárez Schmidt, Danilo Barrionuevo, Peter Feng, Wilfredo Otaño, Luis Fonseca, and Dalice M. Piñero Cruz. 2025. "Gas Sensitivity Improvements of Nanowire Hexadecafluorinated Iron Phthalocyanines by Thermal Vacuum Annealing" Chemosensors 13, no. 3: 95. https://doi.org/10.3390/chemosensors13030095
APA StyleMetzler, C. L., Flores, S. Y., Cruz Lozada, J., González, J., Suárez Schmidt, S., Barrionuevo, D., Feng, P., Otaño, W., Fonseca, L., & Piñero Cruz, D. M. (2025). Gas Sensitivity Improvements of Nanowire Hexadecafluorinated Iron Phthalocyanines by Thermal Vacuum Annealing. Chemosensors, 13(3), 95. https://doi.org/10.3390/chemosensors13030095







