TiO2 Ceramic Nanotubes—Conducting Polymer Assemblies with Embedded Gold Particles for Potential Use as Chemosensors in the Detection of Oral Diseases
Abstract
:1. Introduction
2. Technology for Obtaining Hybrid Nanostructures from Ceramic Nanotubes and Conducting Polymers with Embedded Gold Nanoparticles
Materials and Methods of Preparation
3. Results and Discussion
3.1. Characterization Equipment
3.2. TEM and Selected Area (Electron) Diffraction (SAED) Analysis of TiO2—Conducting Polymers Composites
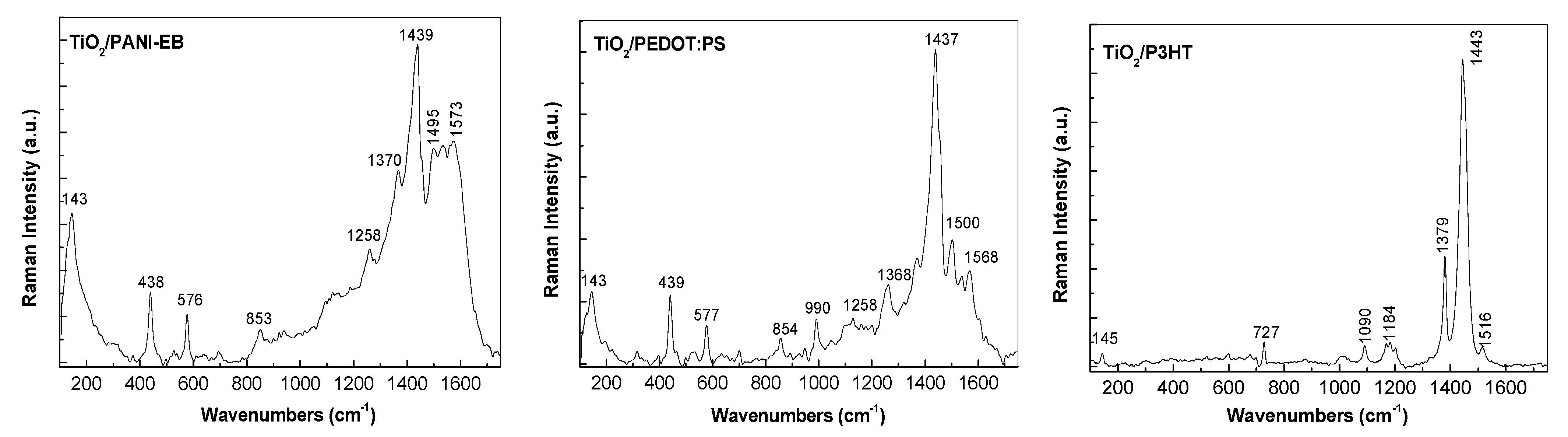
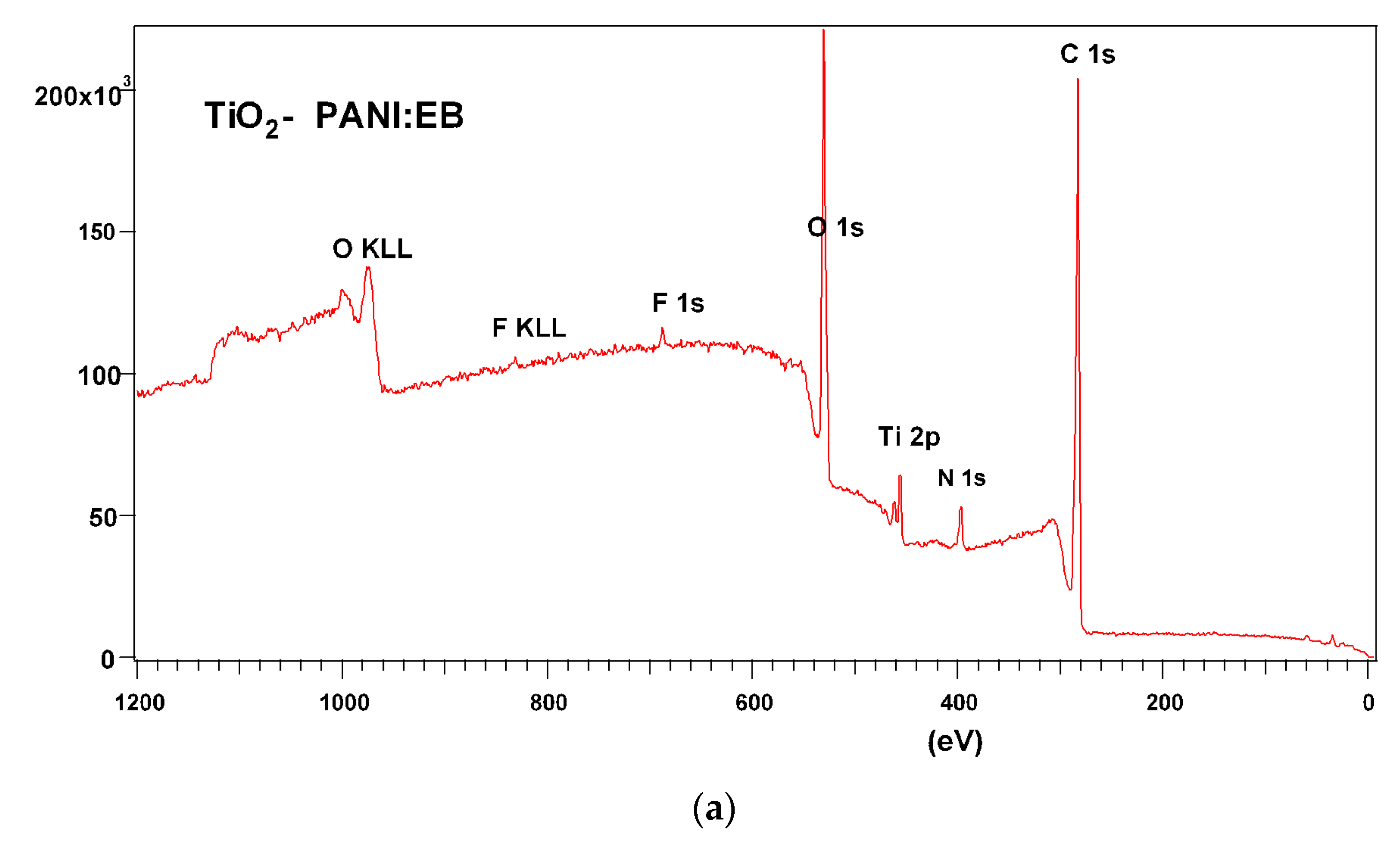
3.3. Raman and FTIR, XPS, and EDS Analysis of TiO2—Conducting Polymers Composites
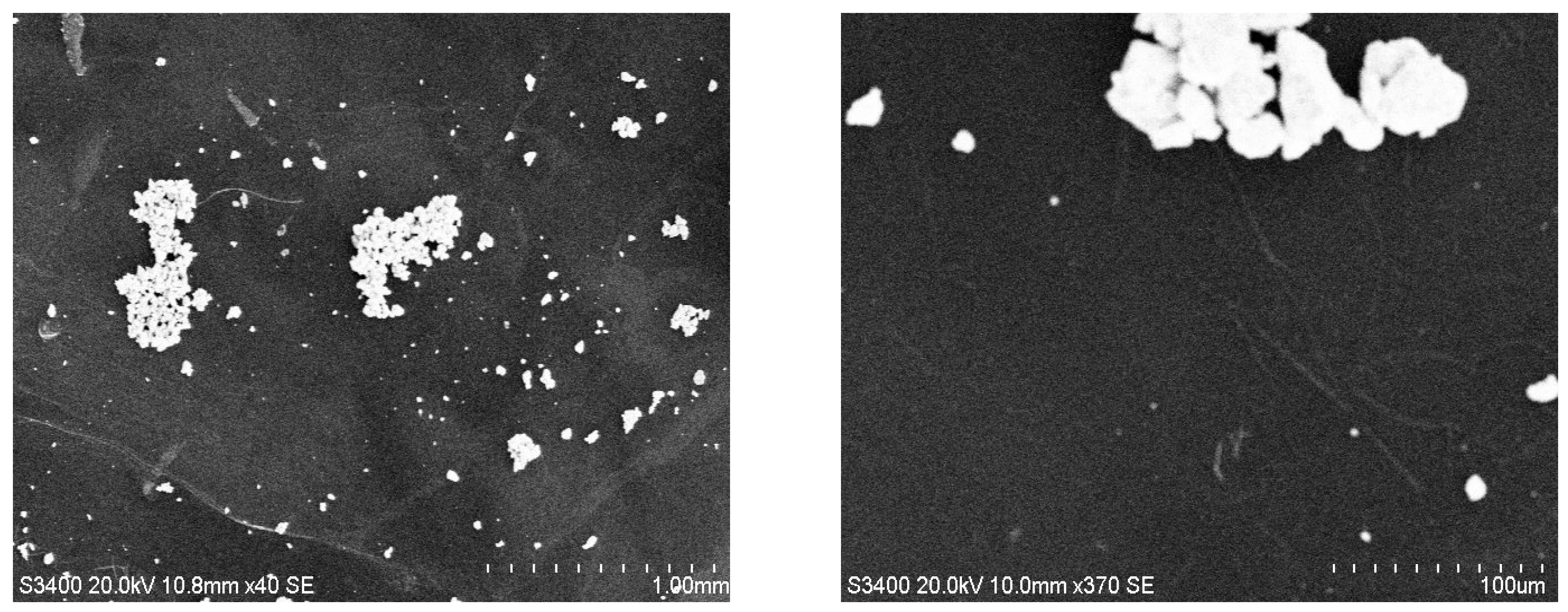
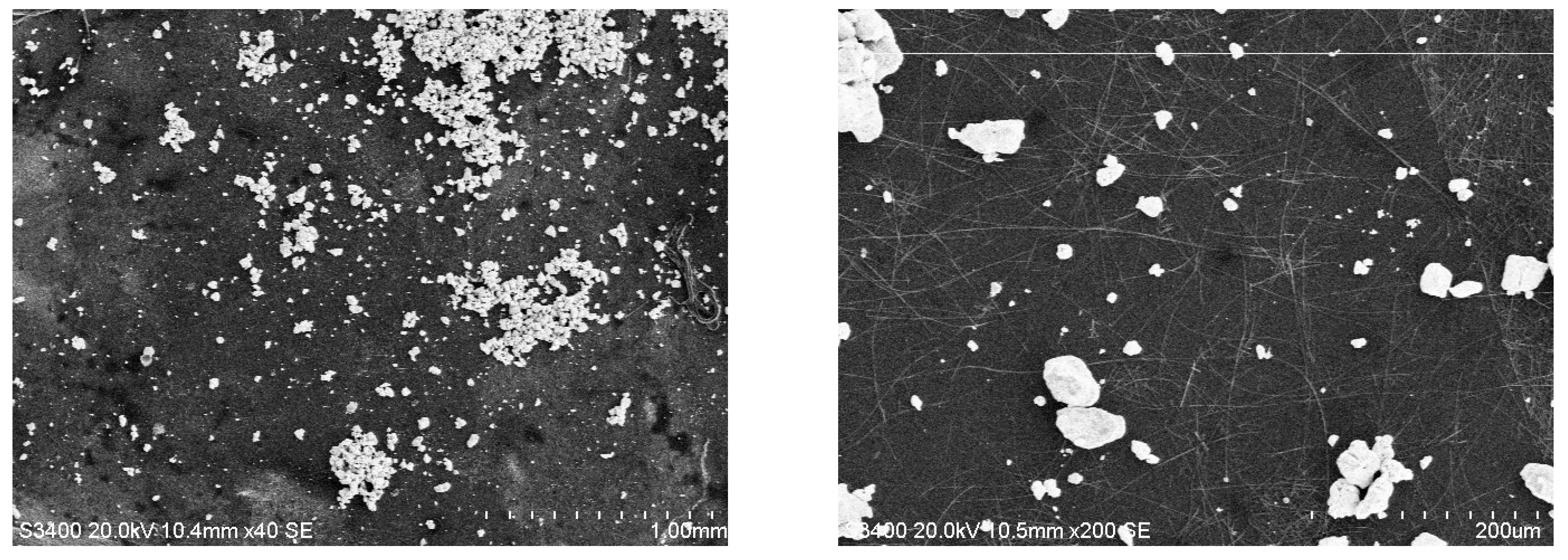
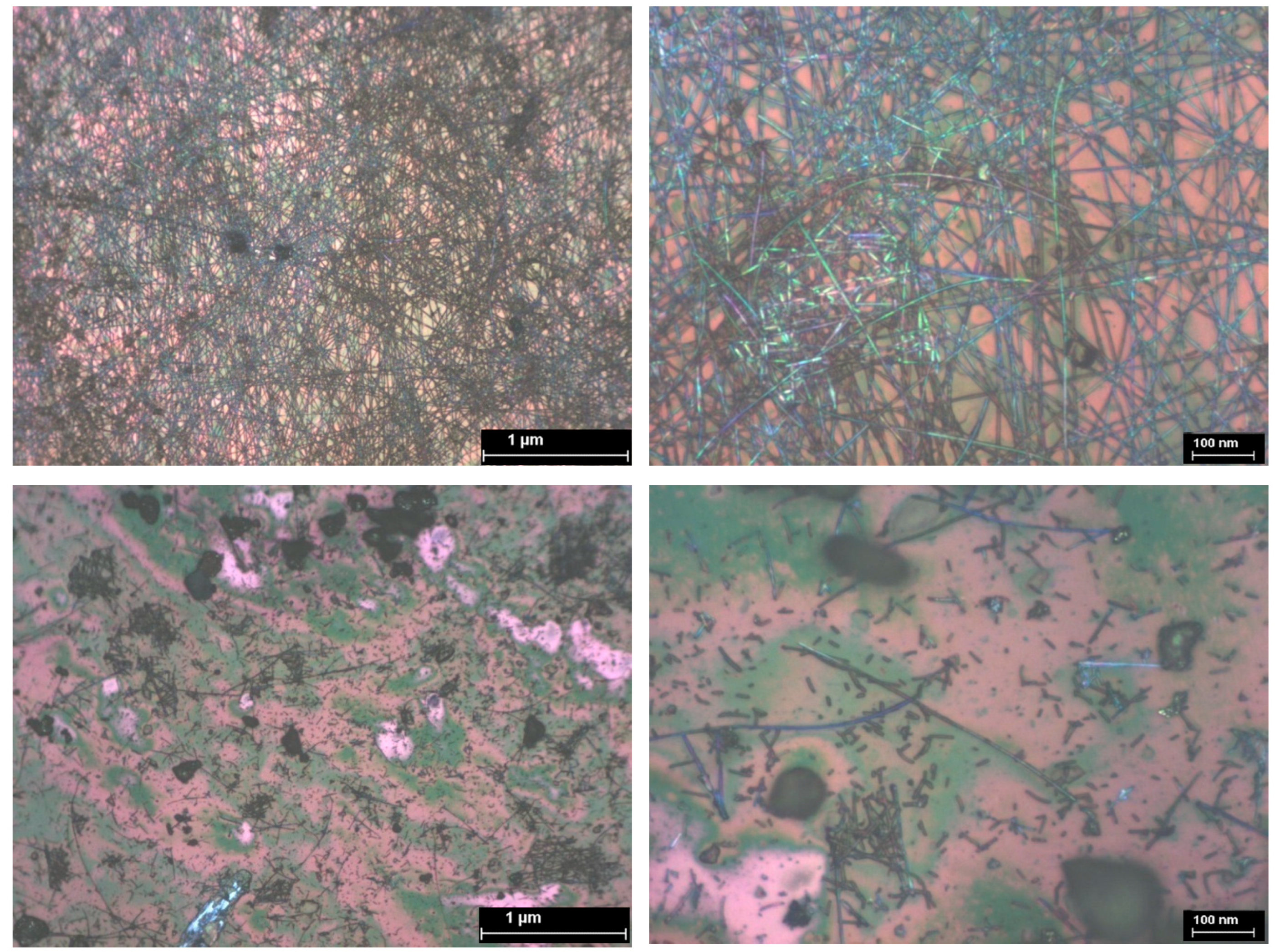
3.4. SEM Analysis of TiO2—Conducting Polymers Composites with Au Addition
3.5. AFM Analysis of TiO2—Conducting Polymers Composites with Au Addition
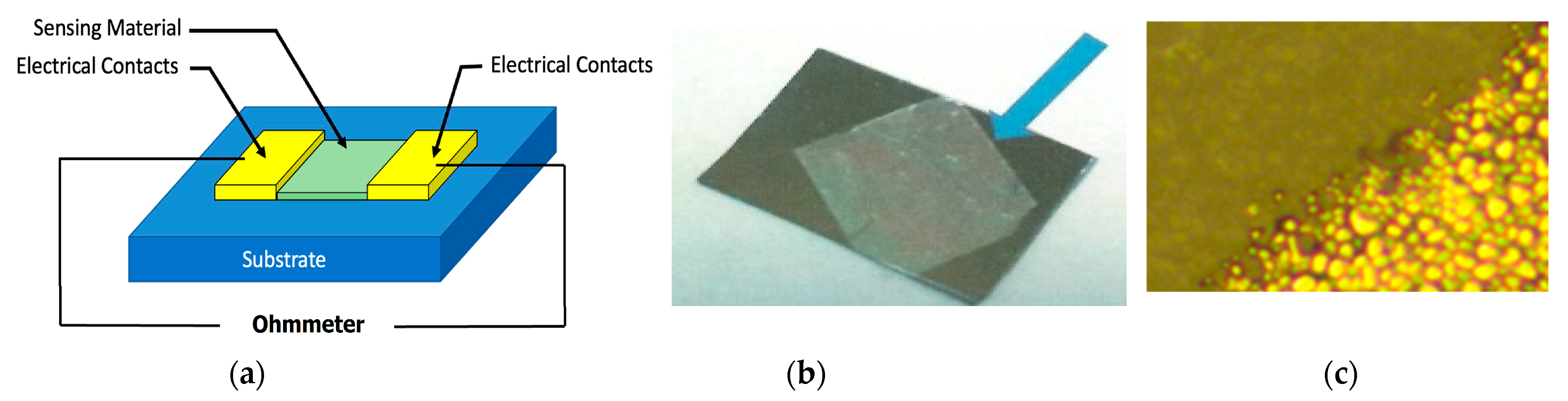
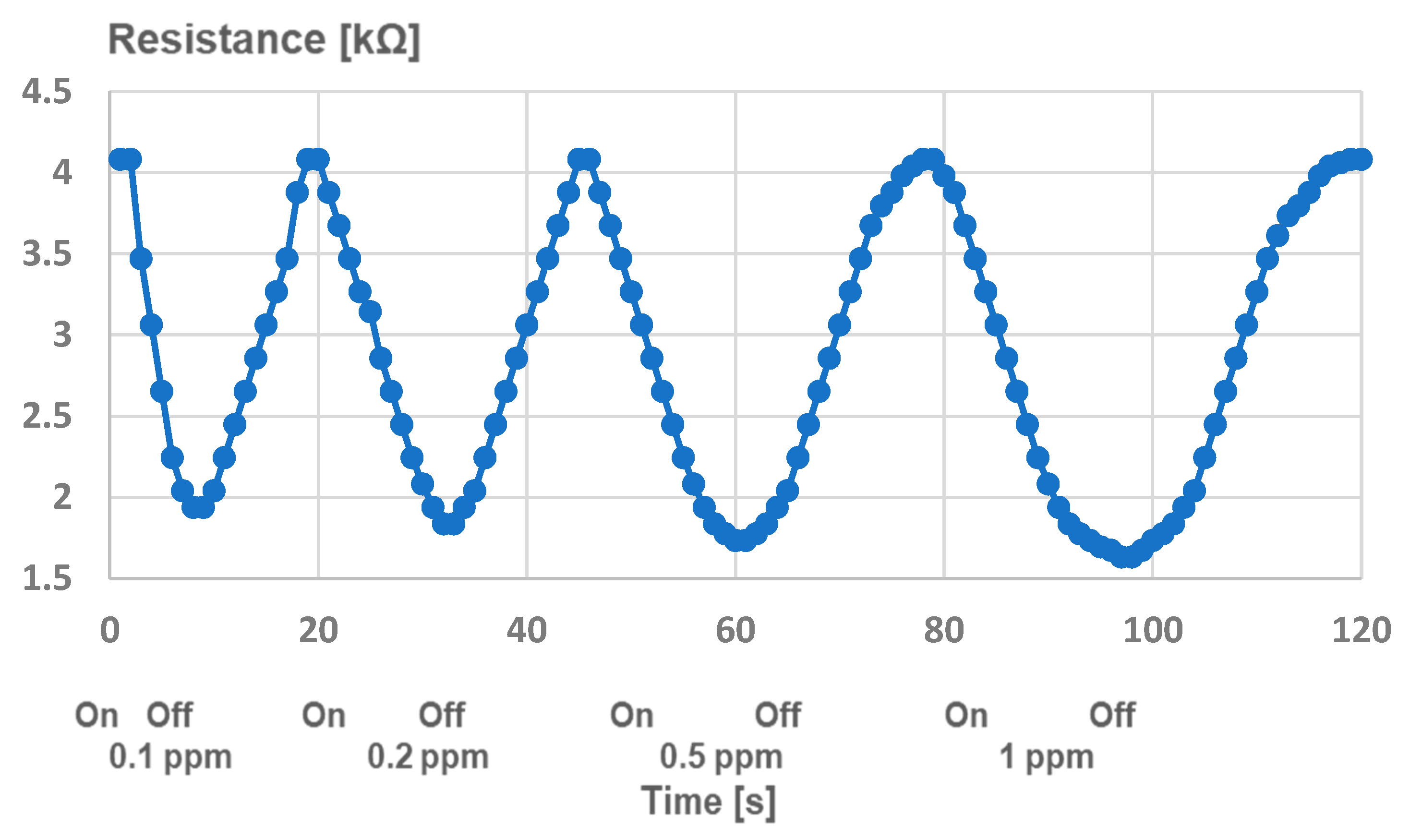
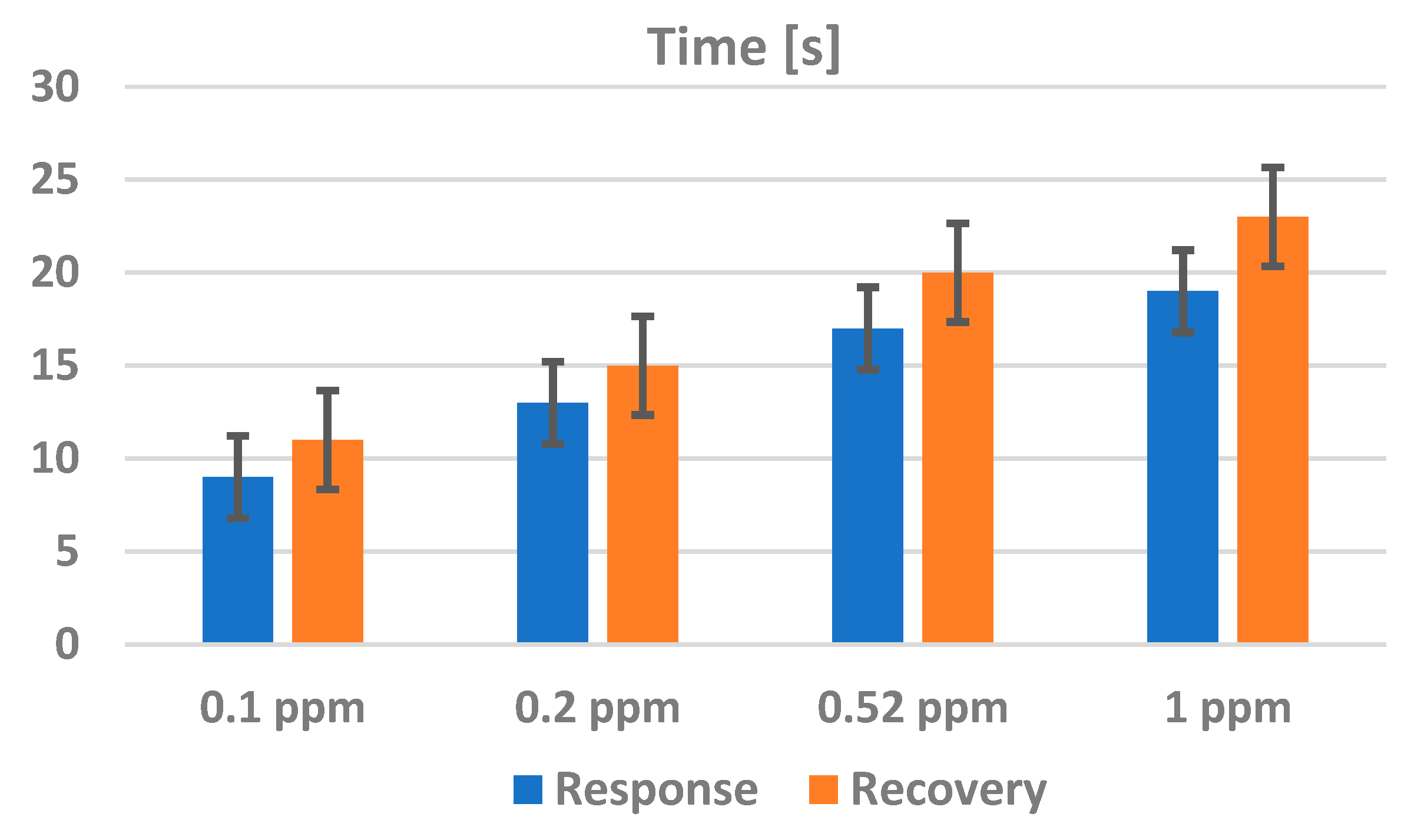
4. Analysis of Functionality as Gas Sensors for H2s of TiO2–PANI-EB Composites with the Addition of Gold Nanoparticles
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tanda, N.; Hoshikawa, Y.; Ishida, N.; Sato, T.; Takahashi, N.; Hosokawa, R.; Koseki, T. Oral malodorous gases and oral microbiota: From halitosis to carcinogenesis. J. Oral. Biosci. 2015, 57, 175–178. [Google Scholar] [CrossRef]
- Wu, D.; Ngowi, E.; Zhai, Y.; Wang, Y.; Khan, N.; Kombo, A.; Khattak, S.; Li, T.; Ji, R. Role of Hydrogen Sulfide in Oral Disease. Oxid. Med. Cell Longev. 2022, 25, 1886277. [Google Scholar] [CrossRef]
- Yoneda, M.; Suzuki, N.; Hirofuji, T. Current Status of the Techniques Used for Halitosis Analysis. Austin Chromatogr. 2015, 2, 1024. [Google Scholar]
- Lee, Y.; Shin, S.; Hong, J. Investigation of volatile sulfur compound level and halitosis in patients with gingivitis and periodontitis. Sci. Rep. 2023, 13, 13175. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.breathinstitute.co.uk/medical-library-bad-breath/methods-of-diagnosis-and-causes-of-bad-breath/breath-gas-measurement-methods/ (accessed on 18 December 2024).
- Nakhleh, M.K.; Quatredeniers, M.; Haick, H. Detection of Halitosis in Breath: Between the Past, Present and Future. Oral Dis. 2017, 24, 685–695. [Google Scholar] [CrossRef]
- Available online: https://www.glsciencesinc.com/oralchroma?srsltid=AfmBOooEzSxQkIdBPg5LPXkIQN8xrjUGIM8hVsLnPX13nm0qTWZXdUXJ (accessed on 18 December 2024).
- Available online: https://www.halimeter.com/ (accessed on 18 December 2024).
- Salako, N.; Philip, L. Comparison of the use of the Halimeter and the Oral Chroma™ in the assessment of the ability of common cultivable oral anaerobic bacteria to produce malodorous volatile sulfur compounds from cysteine and methionine. Med. Princ. Pract. 2011, 20, 75. [Google Scholar] [CrossRef]
- Hanada, M.; Koda, H.; Onaga, K.; Tanaka, K.; Okabayashi, T.; Itoh, T.; Miyazaki, H. Portable oral malodor analyzer using highly sensitive In2O3 gas sensor combined with a simple gas chromatography system. Analytica Chimica Acta 2003, 475, 27–35. [Google Scholar] [CrossRef]
- Ikava, K.; Iwakura, M.; Washio, J.; Kusano, A.; Tanda, N.; Koseki, T. Circadian changes of volatile sulfur compounds measured by Breathtron™. Int. Congr. Ser. 2005, 1284, 89–90. [Google Scholar] [CrossRef]
- Zhang, T.; Xue, Y.; Chen, Y.; Wan, H.; Wang, P. Research on Intelligent Electronic Nose Based on Exhaled Breath to Detect Halitosis. ECS Meet. Abstr. 2021, MA 2021-01, 1348. [Google Scholar] [CrossRef]
- Umapathy, V.; Natarajan, P.; Swamikannu, B.; Moses, J.; Jones, S.; Chandran, M.; Anbumozhi, M. Emerging Biosensors for Oral Cancer Detection and Diagnosis—A Review Unravelling Their Role in Past and Present Advancements in the Field of Early Diagnosis. Biosensors 2022, 12, 498. [Google Scholar] [CrossRef]
- Li, Y.; Tang, H.; Liu, Y.; Qiao, Y.; Xia, H.; Zhou, J. Oral wearable sensors: Health management based on the oral cavity. Biosens. Bioelectron. 2022, 10, 100135. [Google Scholar] [CrossRef]
- EP0819940A2; Method of Detecting Halitosis and Detector for Use in the Method. European Patent Office: Munich, Germany, 1997.
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Wang, T.; Sun, P.; Liu, F.; Lu, G. Revealing the correlation between gas selectivity and semiconductor energy band structure derived from off-stoichiometric spinel CdGa2O4. Sens. Actuators B 2022, 352, 131039. [Google Scholar] [CrossRef]
- He, H.; Zhao, C.; Xu, J.; Qu, K.; Jiang, Z.; Gao, Z.; Song, Y. Exploiting Free-Standing p-CuO/n-TiO2 Nanochannels as a Flexible Gas Sensor with High Sensitivity for H2S at Room Temperature. ACS Sens. 2021, 6, 3387–3397. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, N.; Lin, Z.; Wang, J.; Liu, W.; Sun, K.; Fu, Y.; Wang, Z. Room-Temperature High-Performance H2S Sensor Based on Porous CuO Nanosheets Prepared by Hydrothermal Method. ACS Appl. Mater. Interfaces 2016, 8, 20962–20968. [Google Scholar] [CrossRef]
- Han, G.; Liu, C.; Guan, C.; Li, I.; Wang, Y.; Li, Z. Advances in electrochemical biosensors for the detection of common oral diseases. Authorea. 2023. [Google Scholar] [CrossRef]
- Se, A.; Albarella, J.; Carey, J.; Kim, P.; McNamara, W. Low-cost colorimetric sensor for the quantitative detection of gaseous hydrogen sulfide. Sens. Actuators B Chem. 2008, 134, 234–237. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, D.; Lv, W.; Xia, Y.; Ou, K.; Li, Y.; Du, Z.; He, Y.; Dai, J.; Wu, S.; et al. Surface acoustic wave hydrogen sulfide gas sensors based on porous SnO2-SiO2 composite films. Sens. Actuators B Chem. 2024, 417, 136117. [Google Scholar] [CrossRef]
- Zhao, L.; Che, J.; Cao, Q.; Shen, S.; Tang, Y. Highly Sensitive Surface Acoustic Wave H2S Gas Sensor Using Electron-beam-evaporated CuO as Sensitive Layer. Sens. Mater. 2023, 35, 2293–2304. Available online: https://sensors.myu-group.co.jp/sm_pdf/SM3323.pdf (accessed on 18 December 2024). [CrossRef]
- Ohatkar, S.; Rane, S.; Ambesange, A.; More, A.; Rane, S. Nanostructured Metal Oxide Based Thick Film Sensors. Int. J. Latest Technol. Eng. Manag. Appl. Sci. 2016, 5, 93–97. Available online: https://www.ijltemas.in/DigitalLibrary/Vol.5Issue5/93-97.pdf (accessed on 18 December 2024).
- Ayyala, S.; Covington, J. Nickel-Oxide Based Thick-Film Gas Sensors for Volatile Organic Compound Detection. Chemosensors 2021, 9, 247. [Google Scholar] [CrossRef]
- Shin, H.; Kim, D.; Jung, W.; Jang, J.; Kim, Y.; Lee, Y.; Chang, K.; Lee, J.; Park, J.; Namkoong, K.; et al. Surface Activity-Tuned Metal Oxide Chemiresistor: Toward Direct and Quantitative Halitosis Diagnosis. ACS Nano 2021, 15, 9. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, L.; Wang, J.; Liu, F.; You, R.; Lv, S.; Yang, Z.; He, J.; Liu, A.; Yan, X.; et al. Mixed potential type H2S sensor based on stabilized zirconia and a Co2SnO4 sensing electrode for halitosis monitoring. Sens. Actuators B Chem. 2020, 321, 128587. [Google Scholar] [CrossRef]
- Ma, S.; Jia, J.; Tian, Y.; Cao, L.; Shi, S.; Li, X.; Wang, Z. Improved H2S sensing properties of Ag/TiO2 nanofibers. Ceram. Int. 2016, 42, 2041. [Google Scholar]
- Shirsat, M.; Bangar, M.; Deshusses, M.; Myung, N.; Mulchandani, A. Polyaniline nanowires-gold nanoparticles hybrid network based chemiresistive hydrogen sulfide sensor. Appl. Phys. Lett. 2009, 94, 083502. [Google Scholar] [CrossRef]
- Feng, D.; Du, L.; Xing, X.; Wang, C.; Chen, J.; Zhu, Z.; Tian, Y.; Yang, D. Highly Sensitive and Selective NiO/WO3 Composite Nanoparticles in Detecting H2S Biomarker of Halitosis. ACS Sens. 2021, 6, 733–741. [Google Scholar] [CrossRef]
- Panes-Ruiz, L.; Riemenschneider, L.; Chawa, M.; Loffler, M.; Rellinghaus, B.; Tetzlaff, R.; Bezugly, V.; Ibarlucea, B.; Cuniberti, G. Selective and self-validating breath-level detection of hydrogen sulfide in humid air by gold nanoparticle-functionalized nanotube arrays. Nano Res. 2022, 15, 2512–2521. [Google Scholar] [CrossRef]
- Kato, T.; Tanaka, T.; Uchida, K. Detection of PPB-Level H2S Concentrations in Exhaled Breath Using Au Nanosheet Sensors with Small Variability, High Selectivity, and Long-Term Stability. ACS Sens. 2024, 9, 708–716. [Google Scholar] [CrossRef]
- Ramgir, N.; Sharma, P.; Datta, N.; Kaur, M.; Debnath, A.; Aswal, D.; Gupta, S. Room temperature H2S sensor based on Au modified ZnO nanowires. Sens. Actuators B Chem. 2013, 186, 718–726. [Google Scholar] [CrossRef]
- Kaiser, A.; Ceja, E.; Huber, F.; Herr, U.; Thonke, K. Highly Sensitive H2S Sensing with Gold and Platinum Surface-Modified ZnO Nanowire ChemFETs. Proceedings 2020, 60, 7. [Google Scholar] [CrossRef]
- Vuong, N.; Kim, D.; Kim, H. Porous Au-embedded WO3 Nanowire Structure for Efficient Detection of CH4 and H2S. Sci. Rep. 2015, 5, 11040. Available online: https://www.nature.com/articles/srep11040 (accessed on 18 December 2024).
- Punginsang, M.; Liewhiran, C.; Wisitsoraat, A.; Zappa, D.; Comini, E.; Sberveglieri, G. Selective H2S Gas Sensors Based on Au-functionalized WO3 Nanowires. In Proceedings of the 17th International Meeting on Chemical Sensors—IMCS 2018, Vienna, Austria, 15–19 July 2018; pp. 853–854. [Google Scholar] [CrossRef]
- Hitchman, M.; Saffell, J. Considerations of Thermodynamics and Kinetics for the Effects of Relative Humidity on the Electrolyte in Electrochemical Toxic Gas Sensors. ACS Sens. 2021, 6, 3985–3993. [Google Scholar] [CrossRef]
- Xu, Q.; Zong, B.; Li, Q.; Fang, X.; Mao, S.; Ostrikov, K. H2S sensing under various humidity conditions with Ag nanoparticle functionalized Ti3C2Tx MXene field-effect transistors. J. Hazard. Mater. 2022, 424, 127492. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Wu, Z.; Liu, X.; Chen, Z.; Sun, Y.; Dai, N.; Ge, M. Humidity-tolerant and highly sensitive gas sensor for hydrogen sulfide based on WO3 nanocubes modified with CeO2. RSC Adv. 2024, 14, 15039–15047. [Google Scholar] [CrossRef]
- Enculescu, M.; Costas, A.; Evanghelidis, A.; Enculescu, I. Fabrication of ZnO and TiO2 Nanotubes via Flexible Electrospun Nanofibers for Photocatalytic Applications. Nanomaterials 2021, 11, 1305. [Google Scholar] [CrossRef] [PubMed]
- Trandabat, A.F.; Ciobanu, R.C.; Schreiner, O.D.; Aradoaei, M.; Aradoaei, S. Manufacturing of TiO2, Al2O3 and Y2O3 Ceramic Nanotubes for Application as Electrodes for Printable Electrochemical Sensors. Crystals 2024, 14, 454. [Google Scholar] [CrossRef]
- Trandabat, A.F.; Ciobanu, R.C.; Schreiner, O.D.; Schreiner, T.G.; Aradoaei, S. Ceramic Nanotubes—Conducting Polymer Assemblies with Potential Application as Chemosensors for Breath Hydrogen sulfide Detection in Chronic Kidney Disease. Chemosensors 2024, 12, 198. [Google Scholar] [CrossRef]
- Umeda, H.; Mezaki, Y.; Oshio, A.; Kaneko, Y.; Okamoto, R.; Kusumoto, S.; Kunimura, S. Gold Nanoparticles Produced by Low-temperature Heating of the Dry Residue of a Droplet of an HCl Acidic Solution of HAuCl4·4H2O in a Low Vacuum. Anal. Sci. 2021, 37, 1427–1432. [Google Scholar] [CrossRef]
- Ii, K.; Kurita, Y.; Kida, N.; Kunimura, S. Preparation of gold nanoparticles using low-temperature heating of the dry residue of a droplet of an HAuCl4 solution in air. Anal. Sci. 2024, 40, 213–217. [Google Scholar] [CrossRef]
- Surface Roughness Parameters. Available online: https://www.keyence.eu/ss/products/microscope/roughness/line/tab03_b.jsp (accessed on 18 December 2024).
- ISO 21920-2:2021; Geometrical Product specifications (GPS)—Surface Texture: Profile. ISO: Geneva, Switzerland. Available online: https://www.iso.org/standard/72226.html (accessed on 18 December 2024).
- Taudul, B.; Tielens, F.; Calatayud, M. On the Origin of Raman Activity in Anatase TiO2 (Nano)Materials: An Ab Initio Investigation of Surface and Size Effects. Nanomaterials 2023, 13, 1856. [Google Scholar] [CrossRef]
- Quillard, S.; Louarn, G.; Lefrant, S.; Macdiarmid, A.G. Vibrational analysis of polyaniline: A comparative study of leucoemeraldine, emeraldine, and pernigraniline bases. Phys. Rev. B 1994, 50, 12496. [Google Scholar]
- Garreau, S.; Louarn, G.; Buisson, J.P.; Froyer, G.; Lefrant, S. In Situ Spectroelectrochemical Raman Studies of Poly(3,4-ethylenedioxythiophene) (PEDT). Macromolecules 1999, 32, 6807–6812. [Google Scholar]
- Trznadel, M.; Zagórska, M.; Lapkowski, M.; Louarn, G.; Lefrant, S.; Pron, A. UV–VIS–NIR and Raman spectroelectrochemistry of regioregular poly(3-octylthiophene): Comparison with its non-regioregular analogue. J. Chem. Soc. Faraday Trans. 1998, 24, 3527–3780. [Google Scholar]
- Santos, E.; Catto, E.; Peterline, A.; Avansi, W. Transition metal (Nb and W) doped TiO2 nanostructures: The role of metal doping in their photocatalytic activity and ozone gas-sensing performance. Appl. Surf. Sci. 2022, 579, 152146. [Google Scholar] [CrossRef]
- Orizu, G.; Ugwuoke, P.; Asogwa, P.; Offiah, S. A review on the inference of doping TiO2 with metals/nonmetals to improve its photocatalytic activities. IOP Conf. Ser. Earth Environ. Sci. 2023, 1178, 012008. [Google Scholar] [CrossRef]
- Gartner, M.; Szekeres, A.; Stroescu, H.; Mitrea, D.; Covei, M. Advanced Nanostructured Coatings Based on Doped TiO2 for Various Applications. Molecules 2023, 28, 7828. [Google Scholar] [CrossRef] [PubMed]
- McGarrity, M.; Zhao, F. Graphene-Based Chemiresistor Sensors for Drinking Water Quality Monitoring. Sensors 2023, 23, 9828. [Google Scholar] [CrossRef]
- Lin, Y.; Manalili, D.; Khodabakhsh, A.; Cristescu, S.M. Real-Time Measurement of CH4 in Human Breath Using a Compact CH4/CO2 Sensor. Sensors 2024, 24, 1077. [Google Scholar] [CrossRef]
- Yanxin, C.; Yi, J.; Wenzhao, L.; Rongchao, J.; Shaozhen, T.; Wenbin, H. Adsorption and interaction of H2S/SO2 on TiO2. Catal. Today 1999, 50, 39–47. [Google Scholar] [CrossRef]
- Junkaew, A.; Maitarad, P.; Arróyave, R.; Kungwan, N.; Zhang, D.; Shib, L.; Namuangruk, S. The complete reaction mechanism of H2S desulfurization on an anatase TiO2 (001) surface: A density functional theory investigation. Catal. Sci. Technol. 2017, 7, 356–365. [Google Scholar] [CrossRef]
- Shahzad, N.; Hussain, A.; Mustafa, N.; Ali, N.; Kanounf, M.; Goumri-Said, S. First principles study of the adsorption and dissociation mechanisms of H2S on a TiO2 anatase (001) surface. RSC Adv. 2016, 6, 7941–7949. [Google Scholar] [CrossRef]
- Hwang, J.; Kim, M.; Jin, C.; Mirzaei, A.; Choi, S.; Choi, M.; Lee, H. Surface Reaction Mechanism and Characteristics of 2-Dimensional TiO2 and 0-Dimensional Ag Nanocomposites Specialized for H2S Gas Sensing at Room Temperature. Sens. Actuators Rep. 2025, 9, 100290. [Google Scholar] [CrossRef]
- Rodiawan; Wang, S.; Suhdi. Gold-nanoparticle-decorated Tin Oxide of a Gas Sensor Material for Detecting Low Concentrations of Hydrogen Sulfide. Sens. Mater. 2023, 35, 1121–1130. [Google Scholar] [CrossRef]
- Nagmani; Pravarthana, D.; Tyagi, A.; Jagadale, T.C.; Prellier, W.; Aswal, D.K. Highly sensitive and selective H2S gas sensor based on TiO2 thin films. Appl. Surf. Sci. 2021, 549, 149281. [Google Scholar] [CrossRef]
- Alaya, I.; Madani, M.; Bouguila, N.; El Mir, L.; Fazio, E.; Corsaro, C.; Neri, G. Conductometric H2S Sensors Based on TiO2 Nanoparticles. Materials 2024, 17, 3283. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Shen, W.; Chen, X.; Corriou, J. A fast response and recovery H2S gas sensor based on free-standing TiO2 nanotube array films prepared by one-step anodization method. Ceram. Int. 2017, 43, 14200–14209. [Google Scholar] [CrossRef]
- Marquardt, N.; Dahlke, M.; Schaate, A. Cu-MOF-808 as a Sensing Material for Gaseous Hydrogen Sulfide. ChemPlusChem 2023, 88, e202300109. [Google Scholar] [CrossRef]
- Yu, Q.; Gao, P.; Zhang, K.; Tong, X.; Yang, H.; Liu, S.; Du, J.; Zhao, Q.; Huang, W. Luminescent gold nanocluster-based sensing platform for accurate H2S detection in vitro and in vivo with improved anti-interference. Light Sci. Appl. 2017, 6, e17107. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, Y.; Wang, K.; Luo, C.; Xie, M.; Shi, X.; Lin, X. Review of Chemical Sensors for Hydrogen Sulfide Detection in Organisms and Living Cells. Sensors 2023, 23, 3316. [Google Scholar] [CrossRef]
- Nie, X.; Han, X.; Yu, S.; Liu, W.; Yang, Y. Embedding gold nanoclusters in metal-organic frameworks as a dual-channel hydrogel optical sensor for hydrogen sulfide detection. J. Solid State Chem. 2025, 346, 125283. [Google Scholar] [CrossRef]
- Wang, Z.J.; Li, Q.; Tan, L.L.; Liu, C.; Shang, L. Metal–Organic Frameworks-Mediated Assembly of Gold Nanoclusters for Sensing Applications. J. Anal. Test. 2022, 6, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Alzamly, A.; Greish, Y.; Bakiro, M.; Nguyen, H.; Mahmoud, S. A Highly Sensitive and Flexible Metal–Organic Framework Polymer-Based H2S Gas Sensor. ACS Omega 2021, 6, 17690–17697. [Google Scholar] [CrossRef] [PubMed]
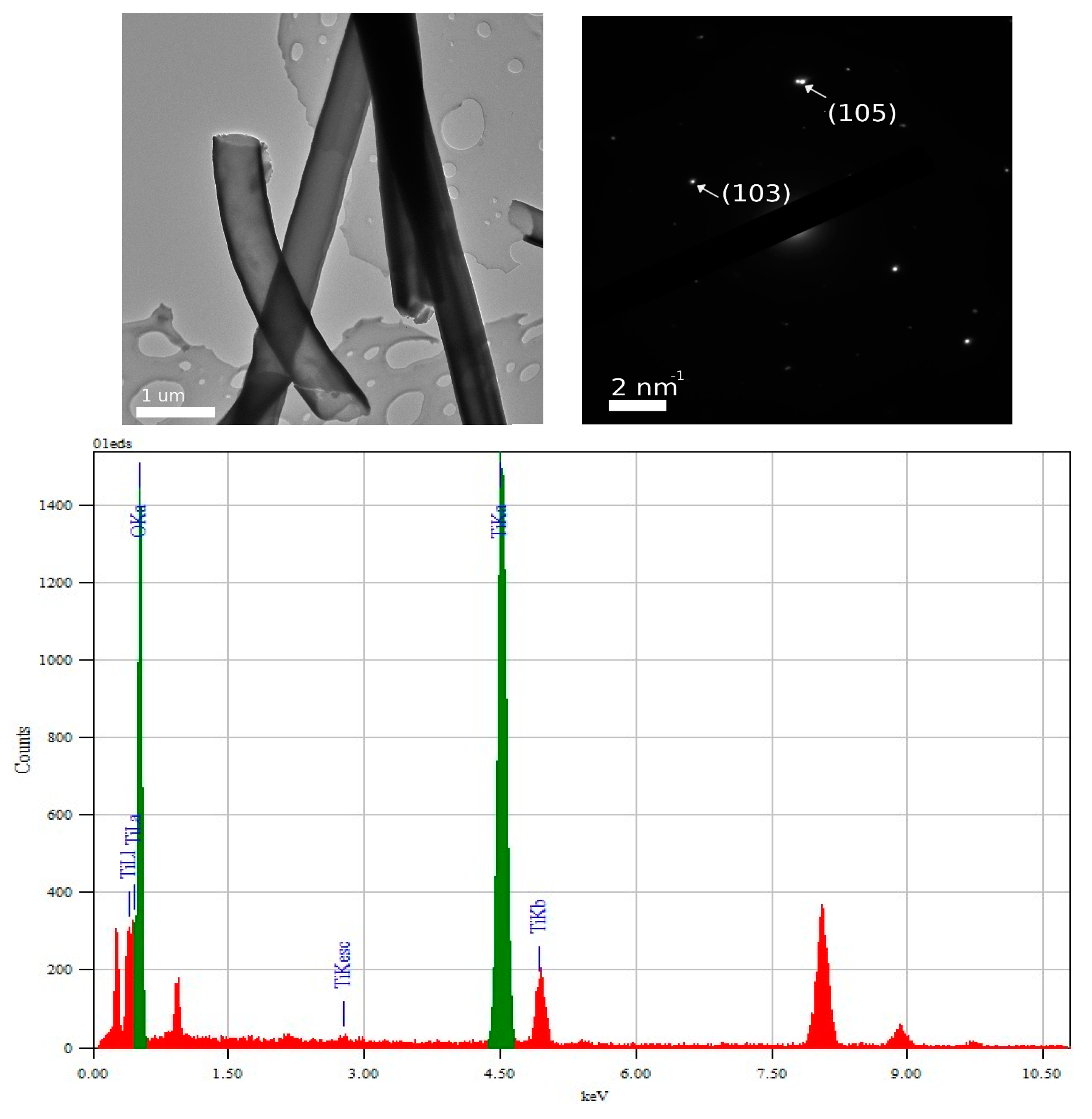
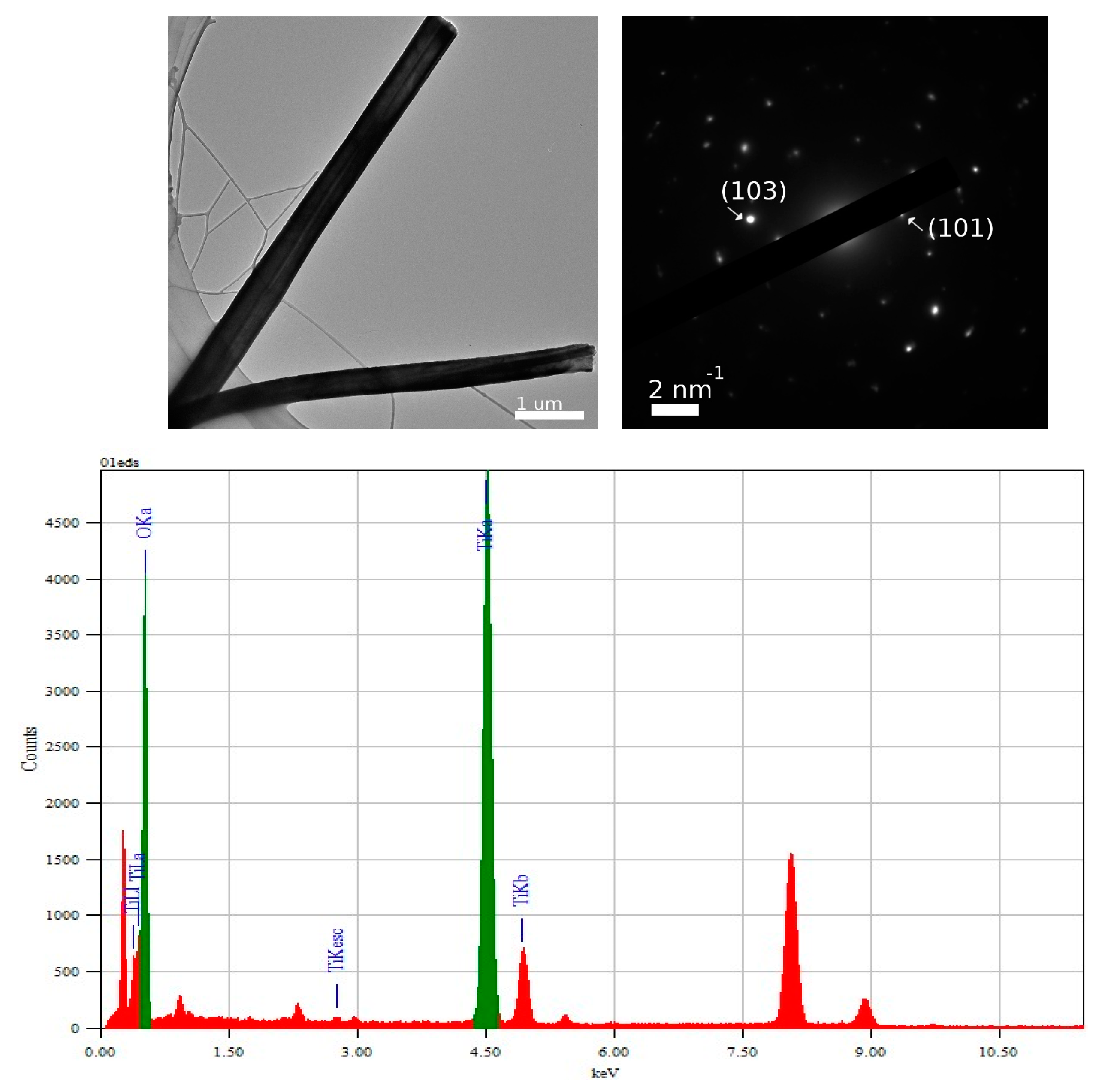
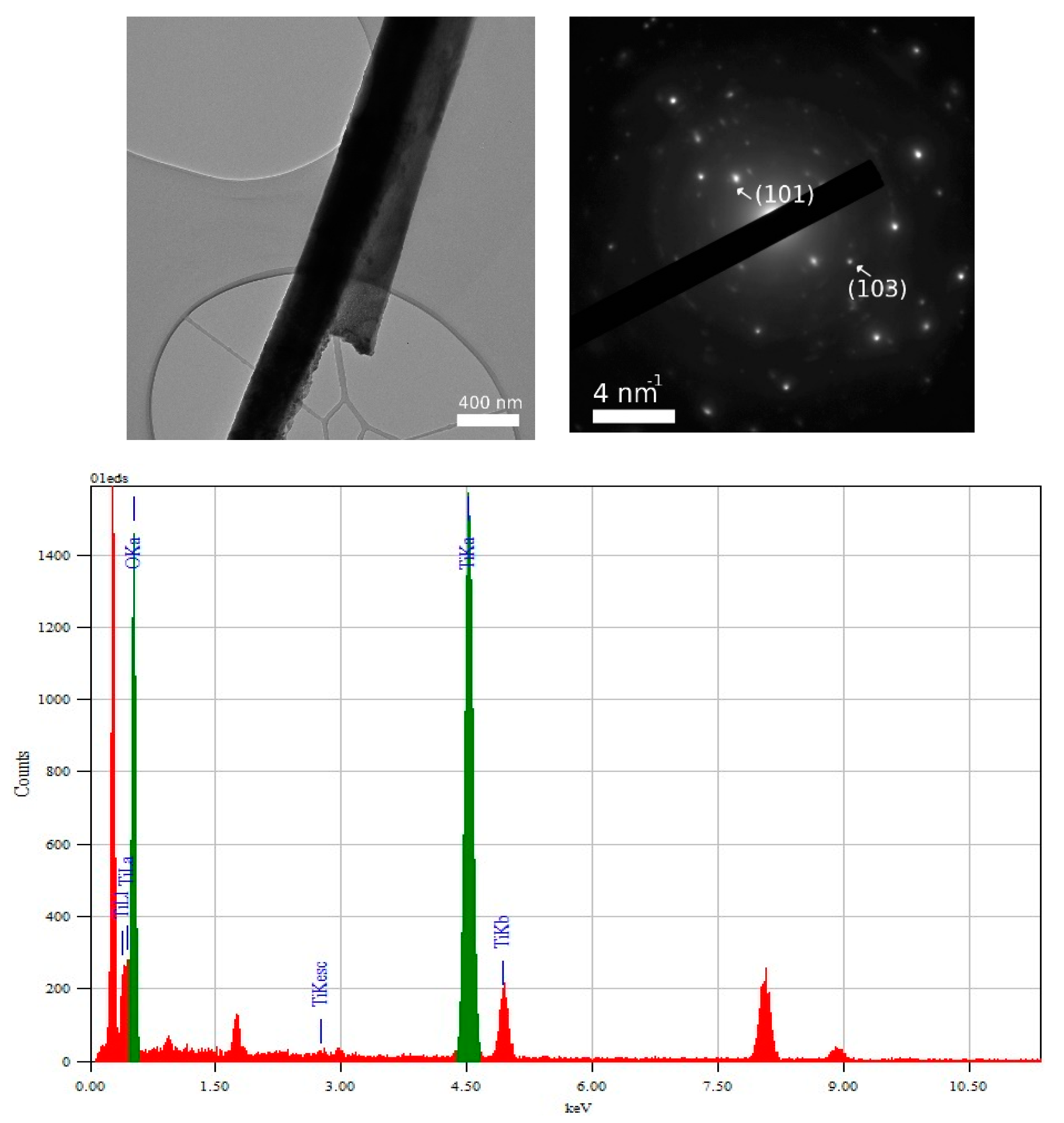



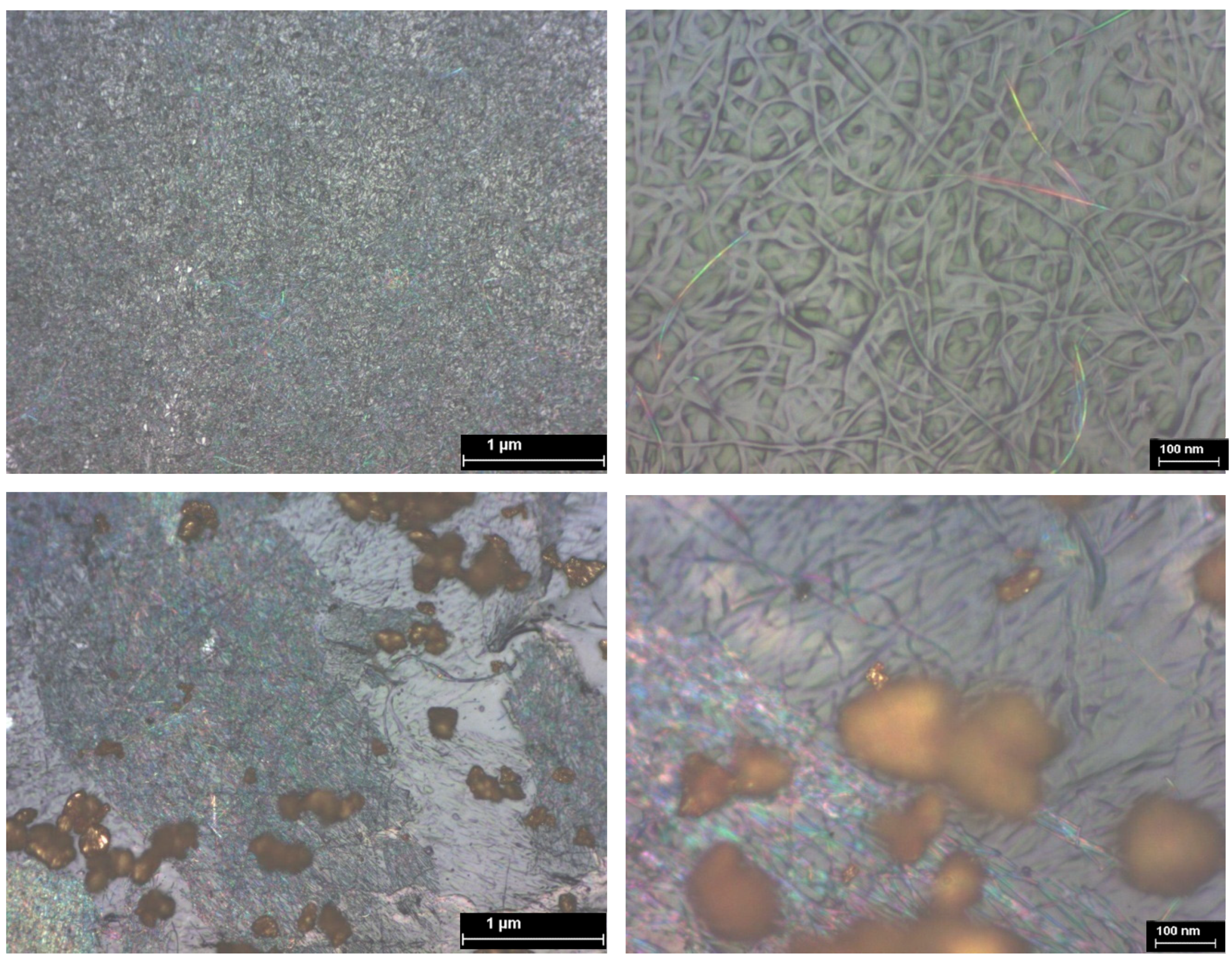

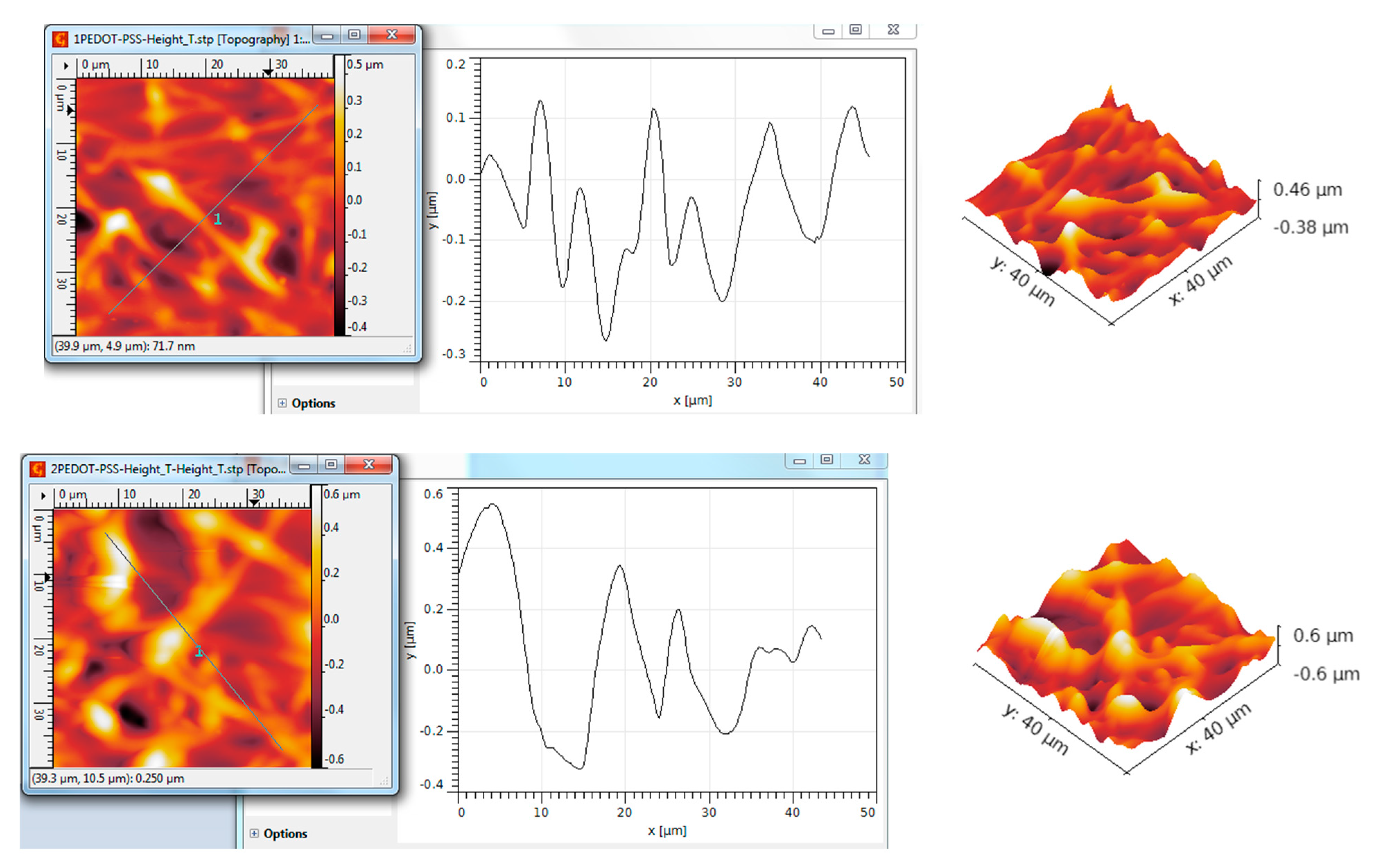
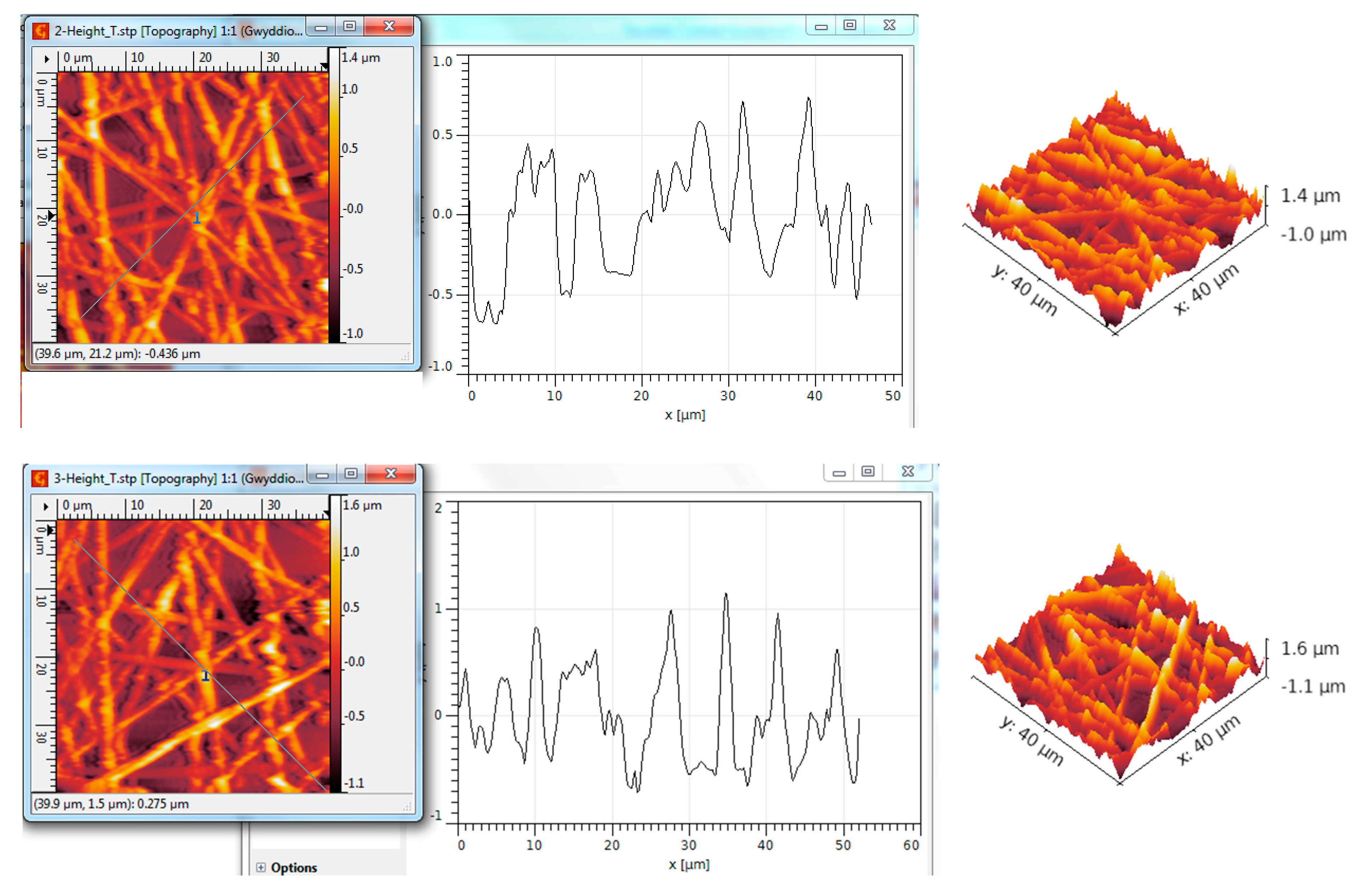

| Scanned Material | RMS (nm) | Ra (nm) | RSk | RKu |
|---|---|---|---|---|
| TiO2 nanotubes–PANI-EB | 947 | 715 | −0.18 | 4.04 |
| TiO2 nanotubes–PANI-EB/Au | 1197 | 948 | −0.01 | 2.97 |
| TiO2 nanotubes–PEDOT: PS | 131 | 105 | 0.38 | 3.04 |
| TiO2 nanotubes–PEDOT: PS/Au | 107 | 79 | 0.56 | 4.42 |
| TiO2 nanotubes–P3HT | 362 | 298 | 0.17 | 2.53 |
| TiO2 nanotubes–P3HT/Au | 420 | 341 | 0.42 | 2.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schreiner, O.D.; Trandabat, A.F.; Ciobanu, R.C.; Schreiner, T.G. TiO2 Ceramic Nanotubes—Conducting Polymer Assemblies with Embedded Gold Particles for Potential Use as Chemosensors in the Detection of Oral Diseases. Chemosensors 2025, 13, 117. https://doi.org/10.3390/chemosensors13040117
Schreiner OD, Trandabat AF, Ciobanu RC, Schreiner TG. TiO2 Ceramic Nanotubes—Conducting Polymer Assemblies with Embedded Gold Particles for Potential Use as Chemosensors in the Detection of Oral Diseases. Chemosensors. 2025; 13(4):117. https://doi.org/10.3390/chemosensors13040117
Chicago/Turabian StyleSchreiner, Oliver Daniel, Alexandru F. Trandabat, Romeo Cristian Ciobanu, and Thomas Gabriel Schreiner. 2025. "TiO2 Ceramic Nanotubes—Conducting Polymer Assemblies with Embedded Gold Particles for Potential Use as Chemosensors in the Detection of Oral Diseases" Chemosensors 13, no. 4: 117. https://doi.org/10.3390/chemosensors13040117
APA StyleSchreiner, O. D., Trandabat, A. F., Ciobanu, R. C., & Schreiner, T. G. (2025). TiO2 Ceramic Nanotubes—Conducting Polymer Assemblies with Embedded Gold Particles for Potential Use as Chemosensors in the Detection of Oral Diseases. Chemosensors, 13(4), 117. https://doi.org/10.3390/chemosensors13040117





