Silica Optical Fibers Connected via a Micro MIP-Core Waveguide to Build Optical-Chemical Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical
2.2. MIP and NIP Preparation
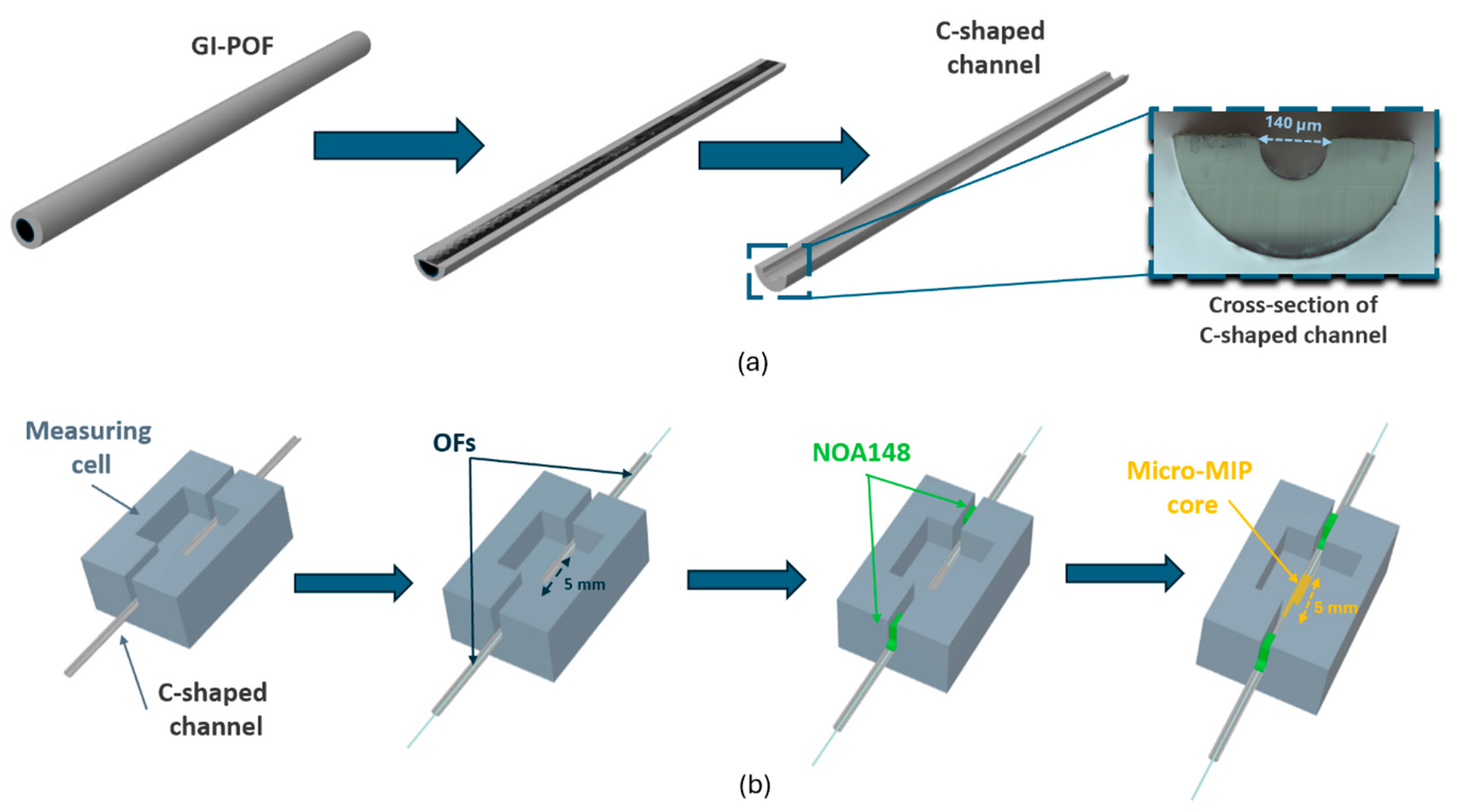
2.3. Fabrication Steps of Optical-Chemical Sensors
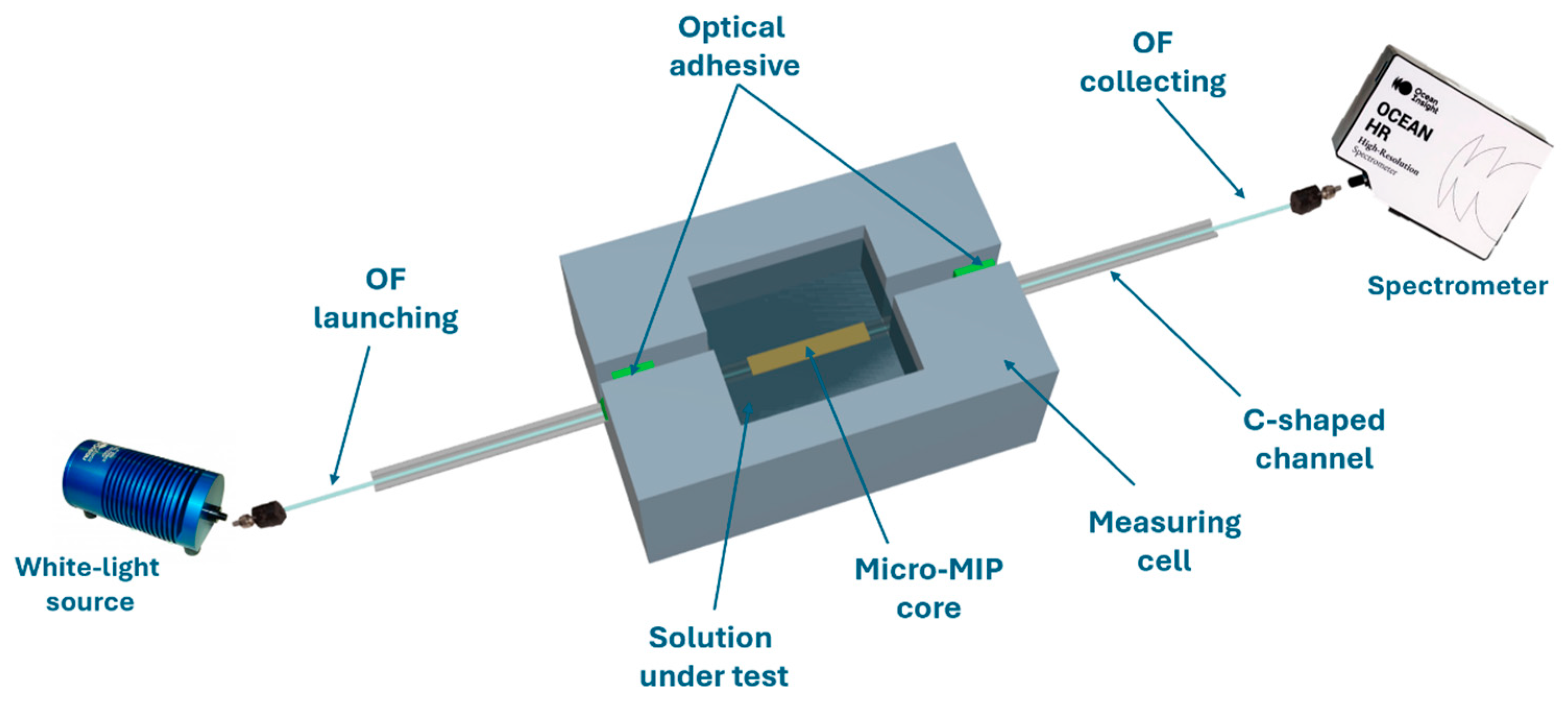
2.4. Experimental Setup
2.5. Measurement Protocol
3. Micro OF-MIP-OF Sensor Experimental Results
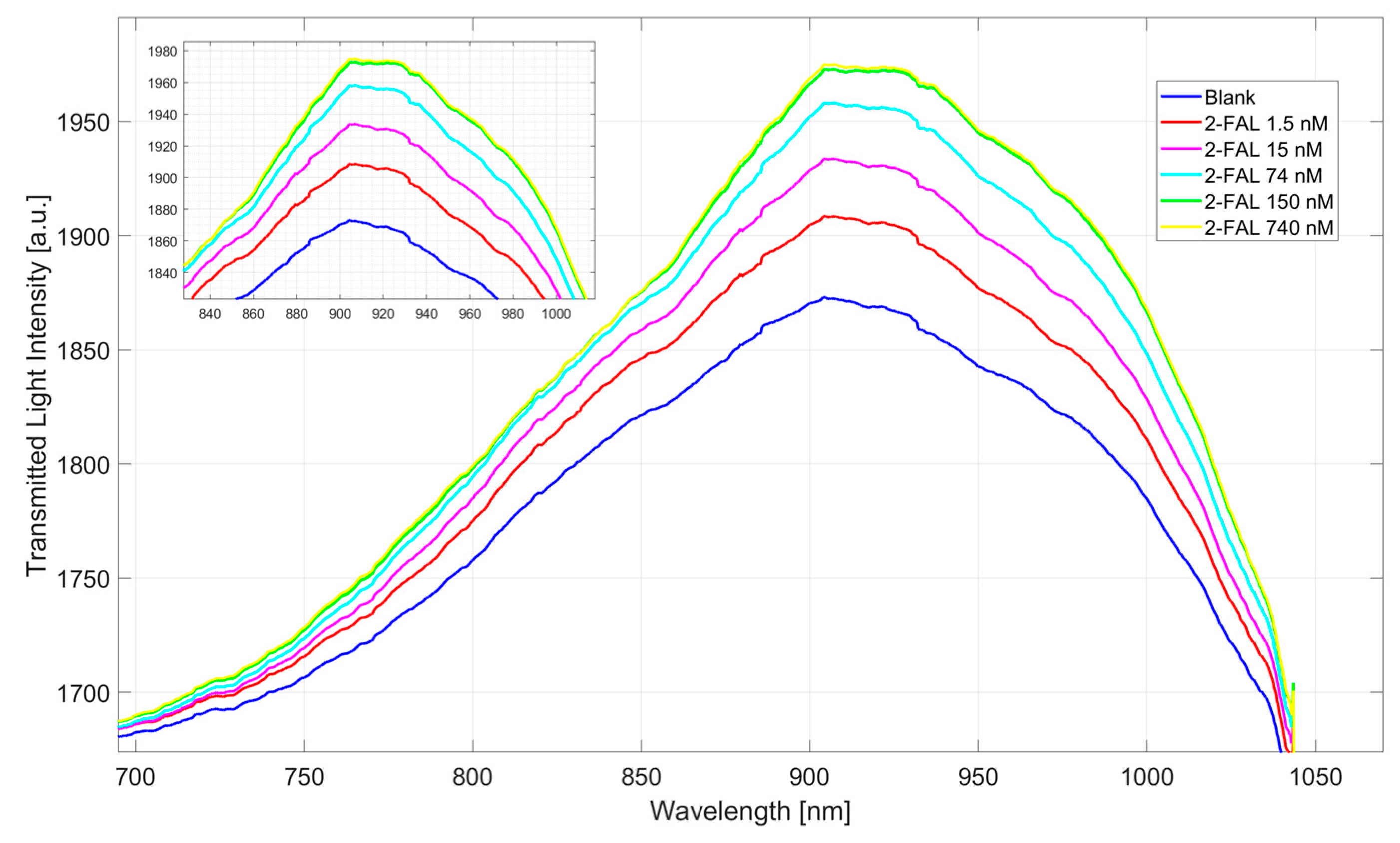
3.1. Dose–Response Curve for 2-FAL Detection
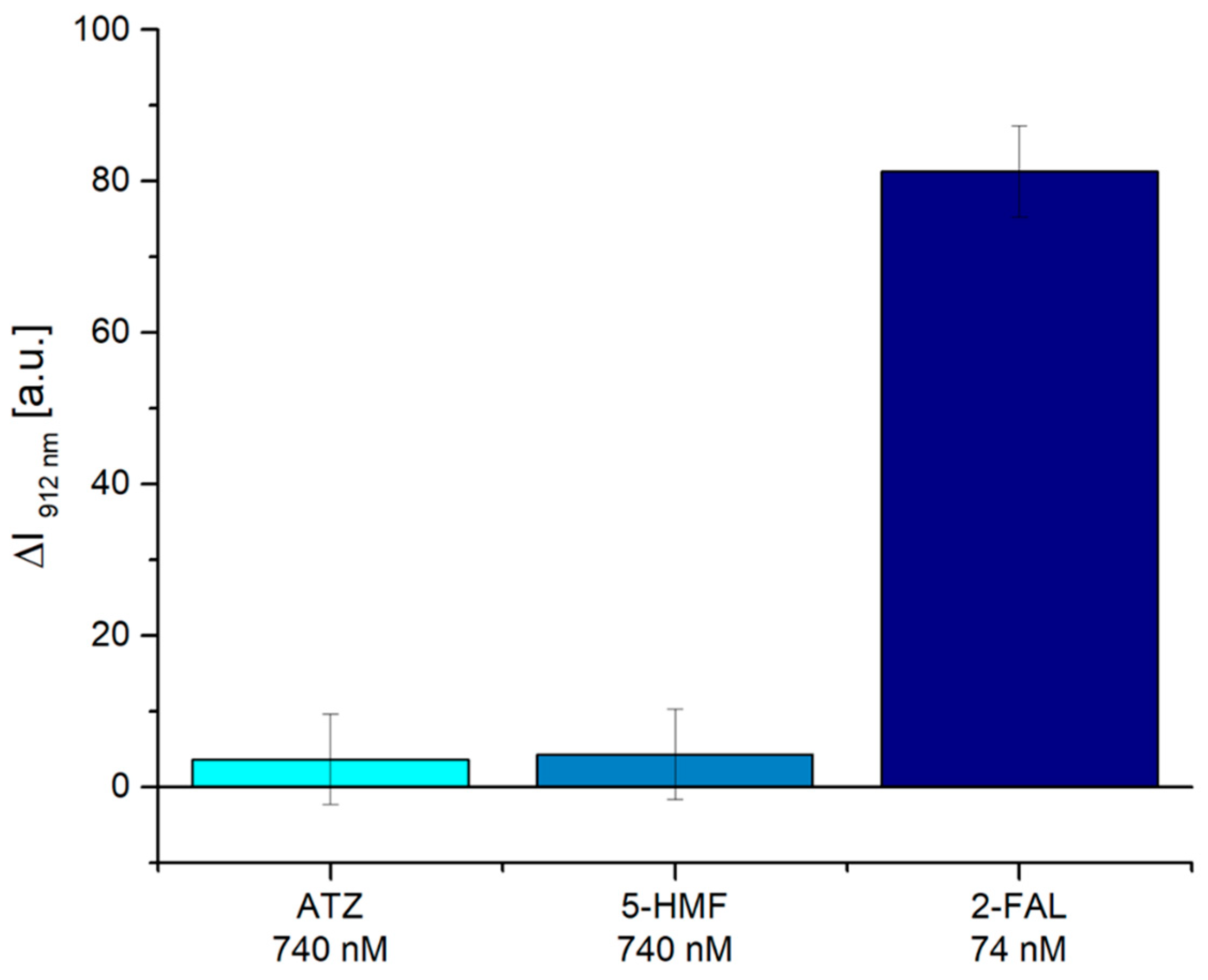
3.2. Selectivity Tests
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoydonckx, H.E.; Van Rhijn, W.M.; Van Rhijn, W.; De Vos, D.E.; Jacobs, P.A. Furfural and Derivatives. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Cocchi, M.; Durante, C.; Lambertini, P.; Manzini, S.; Marchetti, A.; Sighinolfi, S.; Totaro, S. Evolution of 5-(Hydroxymethyl)Furfural and Furfural in the Production Chain of the Aged Vinegar Aceto Balsamico Tradizionale Di Modena. Food Chem. 2011, 124, 822–832. [Google Scholar] [CrossRef]
- Câmara, J.S.; Alves, M.A.; Marques, J.C. Changes in Volatile Composition of Madeira Wines During Their Oxidative Ageing. Anal. Chim. Acta 2006, 563, 188–197. [Google Scholar] [CrossRef]
- Pereira, V.; Albuquerque, F.M.; Ferreira, A.C.; Cacho, J.; Marques, J.C. Evolution of 5-Hydroxymethylfurfural (HMF) and Furfural (F) in Fortified Wines Submitted to Overheating Conditions. Food Res. Int. 2011, 44, 71–76. [Google Scholar] [CrossRef]
- Ortu, E.; Caboni, P. Levels of 5-Hydroxymethylfurfural, Furfural, 2-Furoic Acid in Sapa Syrup, Marsala Wine and Bakery Products. Int. J. Food Prop. 2017, 20, S2543–S2551. [Google Scholar] [CrossRef]
- Lago, L.O.; Welke, J.E. Carbonyl Compounds in Wine: Factors Related to Presence and Toxic Effects. Cienc. Rural 2019, 49, e20190349. [Google Scholar] [CrossRef]
- Pérez-Sariñana, B.Y.; Saldaña-Trinidad, S. Chemistry and Biotransformation of Coffee By-Products to Biofuels. In The Question of Caffeine; InTech: London, UK, 2017. [Google Scholar]
- Chaichi, M.; Ghasemzadeh-Mohammadi, V.; Hashemi, M.; Mohammadi, A. Furanic Compounds and Furfural in Different Coffee Products by Headspace Liquid-Phase Micro-Extraction Followed by Gas Chromatography–Mass Spectrometry: Survey and Effect of Brewing Procedures. Food Addit. Contam. Part B 2015, 8, 73–80. [Google Scholar] [CrossRef]
- Akıllıoglu, H.G.; Gökmen, V. Mitigation of Acrylamide and Hydroxymethyl Furfural in Instant Coffee by Yeast Fermentation. Food Res. Int. 2014, 61, 252–256. [Google Scholar] [CrossRef]
- Veríssimo, M.I.S.; Gamelas, J.A.F.; Evtuguin, D.V.; Gomes, M.T.S.R. Determination of 5-Hydroxymethylfurfural in Honey, Using Headspace-Solid-Phase Microextraction Coupled with a Polyoxometalate-Coated Piezoelectric Quartz Crystal. Food Chem. 2017, 220, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Türköz Acar, E.; Helvacioğlu, S.; Charehsaz, M.; Aydin, A. Determination and Safety Evaluation of Furfural and Hydroxymethylfurfural in Some Honey Samples by Using a Validated HPLC-DAD Method. J. Res. Pharm. 2018, 22, 189–197. [Google Scholar] [CrossRef]
- Jeuring, H.J.; Kuppers, F.J.E.M. High Performance Liquid Chromatography of Furfural and Hydroxymethylfurfural in Spirits and Honey. J. AOAC Int. 1980, 63, 1215–1218. [Google Scholar] [CrossRef]
- Li, M.; Shen, M.; Lu, J.; Yang, J.; Huang, Y.; Liu, L.; Fan, H.; Xie, J.; Xie, M. Maillard Reaction Harmful Products in Dairy Products: Formation, Occurrence, Analysis, and Mitigation Strategies. Food Res. 2022, 151, 110839. [Google Scholar] [CrossRef] [PubMed]
- López-Mercado, J.; Nambo, A.; Toribio-Nava, M.-E.; Melgoza-Sevilla, O.; Cázarez-Barragán, L.; Cajero-Zul, L.; Guerrero-Ramírez, L.-G.; Handy, B.E.; Cardenas-Galindo, M.-G. High and Low Esterification Degree Pectins Decomposition by Hydrolysis and Modified Maillard Reactions for Furfural Production. Clean Technol. Environ. Policy 2018, 20, 1413–1422. [Google Scholar] [CrossRef]
- Kus, S.; Gogus, F.; Eren, S. Hydroxymethyl Furfural Content of Concentrated Food Products. Int. J. Food Prop. 2005, 8, 367–375. [Google Scholar] [CrossRef]
- Huang, H.; Chen, J.; Zheng, M.; Zhang, L.; Ji, H.; Cao, H.; Dai, F.; Wang, L. Precursors and Formation Pathways of Furfural in Sugarcane Juice during Thermal Treatment. Food Chem. 2023, 402, 134318. [Google Scholar] [CrossRef] [PubMed]
- Es-Safi, N.-E.; Cheynier, V.; Moutounet, M. Study of the Reactions between (+)-Catechin and Furfural Derivatives in the Presence or Absence of Anthocyanins and Their Implication in Food Color Change. J. Agric. Food Chem. 2000, 48, 5946–5954. [Google Scholar] [CrossRef]
- Merli, D.; Cutaia, A.; Prosperi, S.; Zanoni, C.; Alberti, G. Molecularly Imprinted Polypyrrole-Modified Screen-Printed Electrode: Toward a Sensor for Furfural Derivatives Determination. Sens. Actuators B Chem. 2025, 9, 100299. [Google Scholar] [CrossRef]
- Durmaz, G.; Gökmen, V. Determination of 5-Hydroxymethyl-2-Furfural and 2-Furfural in Oils as Indicators of Heat Pre-Treatment. Food Chem. 2010, 123, 912–916. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Song, Y.; Hu, X.S.; Liao, X.J.; Ni, Y.Y.; Li, Q.H. Determination of 5-Hydroxymethylfurfural and Furfural in Soft Beverages by HPLC. Adv. Mater. Res. 2012, 550–553, 1959–1966. [Google Scholar] [CrossRef]
- Gaspar, E.M.S.M.; Lucena, A.F.F. Improved HPLC Methodology for Food Control—Furfurals and Patulin as Markers of Quality. Food Chem. 2009, 114, 1576–1582. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, J.; Xu, Y.; Wang, Y.; Wu, L.; Weng, X.; You, C.; Feng, J. A One-Step Electrochemically Reduced Graphene Oxide Based Sensor for Sensitive Voltammetric Determination of Furfural in Milk Products. Anal. Methods 2021, 13, 56–63. [Google Scholar] [CrossRef]
- Norazhar, A.B.; Abu-Siada, A.; Islam, S. A Review on Chemical Diagnosis Techniques for Transformer Paper Insulation Degradation. In Proceedings of the Australasian Universities Power Engineering Conference (AUPEC), Hobart, Australia, 29 September–3 October 2013. [Google Scholar]
- Lundgaard, L.E.; Hansen, W.; Linhjell, D.; Painter, T.J. Aging of Oil-Impregnated Paper in Power Transformers. IEEE Trans. Power Deliv. 2004, 19, 230–239. [Google Scholar] [CrossRef]
- Lessard, M.C.; Van Nifterik, L.; Masse, M.; Penneau, J.F.; Grob, R. Thermal Aging Study of Insulating Papers Used in Power Transformers. In Proceedings of the Conference on Electrical Insulation and Dielectric Phenomena—CEIDP, Millbrae, CA, USA, 23–23 October 1996; pp. 854–859. [Google Scholar]
- Ali, M.S.; Abu Bakar, A.H.; Omar, A.; Abdul Jaafar, A.S.; Mohamed, S.H. Conventional Methods of Dissolved Gas Analysis Using Oil-Immersed Power Transformer for Fault Diagnosis: A Review. Electr. Power Syst. Res. 2023, 216, 109064. [Google Scholar] [CrossRef]
- Homola, J.; Yee, S.S.; Gauglitz, G. Surface Plasmon Resonance Sensors: Review. Sens. Actuators B Chem. 1999, 54, 3–15. [Google Scholar] [CrossRef]
- Yang, W.; Ma, Y.; Sun, H.; Huang, C.; Shen, X. Molecularly Imprinted Polymers Based Optical Fiber Sensors: A Review. TrAC—Trends Anal. 2022, 152, 116608. [Google Scholar] [CrossRef]
- Yang, B.; Jin, L.; Xu, Z.; Wang, W.; Wang, G.; Wu, J.; Sun, D.; Ma, J. A High Performance Differential Sensing System Based on Molecularly Imprinted Polymer for Specific Propofol Concentration Monitoring. Microchem. J. 2024, 204, 111121. [Google Scholar] [CrossRef]
- Xu, Z.; Jin, L.; Yang, B.; Wang, W.; Yang, Y.; Wang, G.; Wu, J.; Sun, D.; Ma, J. An Advanced Optic-Fiber Differential Sensing System Enhanced by Molecularly Imprinted Polymer for Specific Sodium Benzoate Detection. Food Chem. 2024, 455, 139773. [Google Scholar] [CrossRef]
- Pesavento, M.; Zeni, L.; De Maria, L.; Alberti, G.; Cennamo, N. SPR-Optical Fiber-Molecularly Imprinted Polymer Sensor for the Detection of Furfural in Wine. Biosensors 2021, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Cennamo, N.; Arcadio, F.; Zeni, L.; Alberti, G.; Pesavento, M. Optical-Chemical Sensors Based on Plasmonic Phenomena Modulated via Micro-Holes in Plastic Optical Fibers Filled by Molecularly Imprinted Polymers. Sens. Actuators B Chem. 2022, 372, 132672. [Google Scholar] [CrossRef]
- Cennamo, N.; Zeni, L.; Pesavento, M.; Marchetti, S.; Marletta, V.; Baglio, S.; Graziani, S.; Pistorio, A.; Ando, B. A Novel Sensing Methodology to Detect Furfural in Water, Exploiting MIPs, and Inkjet-Printed Optical Waveguides. IEEE Trans. Instrum. Meas. 2019, 68, 1582–1589. [Google Scholar] [CrossRef]
- Tavoletta, I.; Arcadio, F.; Renzullo, L.P.; Oliva, G.; Del Prete, D.; Verolla, D.; Marzano, C.; Alberti, G.; Pesavento, M.; Zeni, L.; et al. Splitter-Based Sensors Realized via POFs Coupled by a Micro-Trench Filled with a Molecularly Imprinted Polymer. Sensors 2024, 24, 3928. [Google Scholar] [CrossRef]
- Arcadio, F.; Prete, D.D.; Zeni, L.; Pesavento, M.; Alberti, G.; Marletta, V.; Andò, B.; Cennamo, N. Optical Waveguides Based on a Core of Molecularly Imprinted Polymers: An Efficient Approach for Chemical Sensing. IEEE Sens. J. 2024, 24, 27411–27420. [Google Scholar] [CrossRef]
- Arcadio, F.; Oliveira, R.; Prete, D.D.; Minardo, A.; Zeni, L.; Bilro, L.; Cennamo, N.; Nogueira, R.N. A Novel Microchannel-in-a-Fiber Plasmonic Sensor. IEEE Sens. J. 2023, 23, 11648–11655. [Google Scholar] [CrossRef]
- Pesavento, M.; Marchetti, S.; De Maria, L.; Zeni, L.; Cennamo, N. Sensing by Molecularly Imprinted Polymer: Evaluation of the Binding Properties with Different Techniques. Sensors 2019, 19, 1344. [Google Scholar] [CrossRef]
- Renzullo, L.P.; Tavoletta, I.; Alberti, G.; Zeni, L.; Pesavento, M.; Cennamo, N. Plasmonic Optical Fiber Sensors and Molecularly Imprinted Polymers for Glyphosate Detection at an Ultra-Wide Range. Chemosensors 2024, 12, 142. [Google Scholar] [CrossRef]
- Thompson, M.; Ellison, S.L.R.; Wood, R. Harmonized Guidelines for Single-Laboratory Validation of Methods of Analysis (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 835–855. [Google Scholar] [CrossRef]
- Cennamo, N.; De Maria, L.; D’Agostino, G.; Zeni, L.; Pesavento, M. Monitoring of Low Levels of Furfural in Power Transformer Oil with a Sensor System Based on a POF-MIP Platform. Sensors 2015, 15, 8499–8511. [Google Scholar] [CrossRef]
- Alberti, G.; Arcadio, F.; Pesavento, M.; Marzano, C.; Zeni, L.; Zeid, N.A.; Cennamo, N. Detection of 2-Furaldehyde in Milk by MIP-Based POF Chips Combined with an SPR-POF Sensor. Sensors 2022, 22, 8289. [Google Scholar] [CrossRef]



| ΔI912 nm, c0 [a.u.] | ΔI912 nm, cmax [a.u.] | K [nM] | n | Statistics | |||||
|---|---|---|---|---|---|---|---|---|---|
| Value | St. Dev. | Value | St. Dev. | Value | St. Dev. | Value | St. Dev. | Reduced Chi-Sqr | Adj. R-Square |
| 22 | 6 | 106 | 7 | 23 | 10 | 1 | 0 | 0.24 | 0.96 |
| Analyte | Tested Concentration | ΔI912 nm | Maximum Standard Deviation |
|---|---|---|---|
| Atrazine (ATZ) | 740 nM | 3.8 | 14 a.u. |
| 5-hydroxymethylfurfural (5-HMF) | 740 nM | 4.3 | 14 a.u. |
| 2-furaldehyde (2-FAL) | 74 nM | 81.3 | 14 a.u. |
| Sensor Configuration | Range of Detection | LOD | Ref. |
|---|---|---|---|
| SPR–POF–MIP sensor | 0.12–60.4 μM | 0.042 μM | [31] |
| MIP-filled single-micro-hole SPR–POF sensor | 1–100 nM | 0.42 nM | [32] |
| MIP-filled three-micro-hole SPR–POF sensor | 1–3500 nM | 1 nM | [32] |
| Inkjet-printed platform (longitudinal configuration) PET-lines-MIP and POFs sensor | 0.6–60 μM | 0.32 μM | [33] |
| MIP-Splitter-based sensor | 0.52–52 μM | 0.52 μM | [34] |
| MIP-core waveguide | 0.01–1.2 μM | 10 nM | [35] |
| Micro OF-MIP-OF sensor | 1.5–150 nM | 1.5 nM (minimum experimentally detected concentration) | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitruzzella, R.; Marzano, C.; Arcadio, F.; Sequeira, F.; Cutaia, A.; Cardoso Novo, C.; Oliveira, R.; Pesavento, M.; Zeni, L.; Nogueira, R.N.; et al. Silica Optical Fibers Connected via a Micro MIP-Core Waveguide to Build Optical-Chemical Sensors. Chemosensors 2025, 13, 139. https://doi.org/10.3390/chemosensors13040139
Pitruzzella R, Marzano C, Arcadio F, Sequeira F, Cutaia A, Cardoso Novo C, Oliveira R, Pesavento M, Zeni L, Nogueira RN, et al. Silica Optical Fibers Connected via a Micro MIP-Core Waveguide to Build Optical-Chemical Sensors. Chemosensors. 2025; 13(4):139. https://doi.org/10.3390/chemosensors13040139
Chicago/Turabian StylePitruzzella, Rosalba, Chiara Marzano, Francesco Arcadio, Filipa Sequeira, Alessandra Cutaia, Catarina Cardoso Novo, Ricardo Oliveira, Maria Pesavento, Luigi Zeni, Rogerio Nunes Nogueira, and et al. 2025. "Silica Optical Fibers Connected via a Micro MIP-Core Waveguide to Build Optical-Chemical Sensors" Chemosensors 13, no. 4: 139. https://doi.org/10.3390/chemosensors13040139
APA StylePitruzzella, R., Marzano, C., Arcadio, F., Sequeira, F., Cutaia, A., Cardoso Novo, C., Oliveira, R., Pesavento, M., Zeni, L., Nogueira, R. N., Cennamo, N., & Alberti, G. (2025). Silica Optical Fibers Connected via a Micro MIP-Core Waveguide to Build Optical-Chemical Sensors. Chemosensors, 13(4), 139. https://doi.org/10.3390/chemosensors13040139










